Abstract
Objectives: We determined whether sodium cholate (NaCh) could act as a solubilizing agent for the necrosis avid iodine-123-labeled hypericin (123I-Hyp) and investigated biodistribution and targetability of this formulation in rabbits with acute myocardial infarction (AMI).
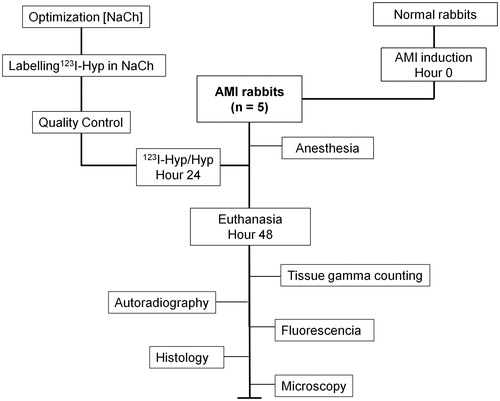
Materials and methods: Solubility of radioiodinated hypericin/hypericin (Hyp) in NaCh solutions was evaluated by microscopy. Hyp with 123I-sodium iodide was performed using hydrogen peroxide as oxidant in 0.06 M NaCh. Radiochemical yield determination and purification were conducted using high performance liquid chromatography. 123I-Hyp was solubilized in 0.06 M NaCh containing 1.9 × 10−4 M Hyp. The formulation was macroscopically inspected and intravenously injected to five rabbits with AMI. At 24 h, biodistribution was evaluated by tissue gamma counting (TGC) and necrosis targetability was assessed by TGC, autoradiography, fluorescence examination and histology.
Results: Microscopically NaCh at 0.06 M shows the best properties for solubilizing the radioiodinated Hyp/Hyp. 123I-Hyp in 0.06 M NaCh was achieved in 85% with radiochemical purity of 99% after purification. NaCh-dissolved 123I-Hyp/Hyp shows no particles. By TGC, animals exhibited higher (p = 0.003) radioactivity accumulation in AMI (0.8 ± 0.2% ID/g) than in normal myocardium (0.05 ± 0.02% ID/g), as confirmed by autoradiography, fluorescence measurement and histology. Among organs, the highest uptake of radioactivity was found in liver (15.7 ± 0.6% ID), large (9.7 ± 1.0% ID) and small (5.9 ± 0.6% ID) intestines.
Conclusion: Necrosis avidity of NaCh-dissolved 123I-Hyp/Hyp and its hepatobiliary excretion were demonstrated. The suitability of NaCh as solubilizing agent of 123I-Hyp for hotspot imaging of AMI was proved.
Introduction
Hypericin (Hyp) is a naturally occurring fluorescent substance that is originally found in the St. Johns Wort (perforatum) plant (Roth, Citation1990) (). Over the past decades, it has been of pharmaceutical interest due to its diverse biological properties (D'Hallewin et al., Citation2002; Avato et al., Citation2004; Sosa et al., Citation2007). The avidity to necrosis is a more recently discovered property of Hyp and derivatives with new theragnostic utilities (Ni et al., Citation2005, Citation2006; Li et al., Citation2011).
Figure 1. Schematic representation of hypericin (Hyp), a highly lipophilic anthraquinone-derivative found in Hypericum perforatum plant. Iodine-123-labeled iodohypericin (123I-Hyp) obtained by labeling Hyp with iodine-123 via aromatic electrophilic substitution. Sodium cholate (NaCH), a bile salt produced by the liver, with strong solubilization capacity of water-insoluble substances.
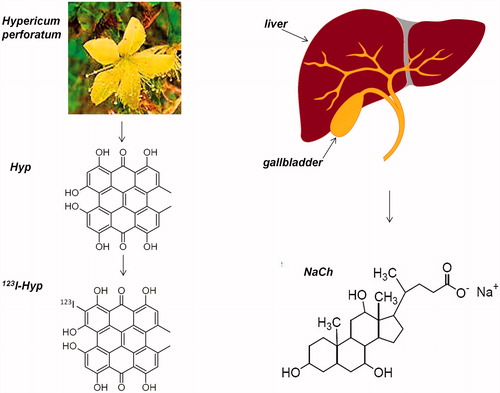
Chemically, Hyp is an anthraquinone derivative (naphthodianthrone) with an amphiphilic structure containing hydrophilic and hydrophobic properties. It dissolves monomolecularly in aqueous alkaline solutions and polar solvents such as ethanol, methanol, pyridine, acetone, ethyl acetate, butanone and dimethylsulfoxide (DMSO) (Theodossiou et al., Citation2009; Karioti & Bilia, Citation2010). In biological media, it has been found in soluble form owing to the complex formation with biological macromolecules, mainly low-density lipoprotein and human serum albumin (Miskovsky, Citation2002). However, its poor biocompatible solubility in water-based formulations is a major limiting factor for its successful launch in market despite its pharmacological potentials (Van De Putte et al., Citation2006). In organic solvents, at the pH interval between 4 and 11, it forms monobasic salts (Weiner & Mazur, Citation1992; Yamazaki et al., Citation1993), which undergo homoassociation in the presence of water. The resulting aggregates lack necrosis targetability and exhibit unwanted retention in organs of the mononuclear phagocyte system (MPS) (Van De Putte et al., Citation2006).
Iodine-123-labeled hypericin (123I-Hyp) is a radioiodinated compound, which is obtained via aromatic electrophilic substitution by replacement of a hydrogen atom into the aromatic structure of Hyp with an oxidized radioactive iodine atom (Bormans et al., Citation2004; Sun & Ni, Citation2009). 123I-Hyp has emerged as a potent necrosis avid agent due to its notable long-lasting uptake in necrotic tissues (Ni et al., Citation2005, Citation2006), making it a potential single-photon emission computed tomography (SPECT) agent for detection and quantification of acute myocardial infarction (AMI) (Fonge et al., Citation2008; Cona et al., Citation2013a). Contrary to conventional drug chemistry, radiopharmacy involves tiny amount of tracers that emit sufficient radiations to generate desired diagnostic signals. Per se, the solubility concerns are negligible. Nevertheless, up to now, the best biological performance of 123I-Hyp has been attained using a mixture of radioiodinated Hyp and unlabeled Hyp in a concentration near 10−3 mol/l, in which Hyp may start to form aggregates in the aqueous medium. Furthermore, the introduction of halogen atoms into a molecule often leads to the derivatives that are more lipophilic and therefore less soluble in water (Sihver et al., Citation2004; Ghaedi et al., Citation2011). As a result, a wrong formulation of a mixture of 123I-Hyp with its parent Hyp could lead to poor imaging signal to background noise ratio due to significant accumulation in MPS organs. To overcome such limitations, different solvents or their combinations currently used for pharmaceutical parenterals have been examined to dissolve the poorly water soluble Hyp and its radioiodinated derivative. However, experimental evidences have shown that these synthetic carriers may result biologically and pharmacologically in adverse effects (Montaguti et al., Citation1994; Ten Tije et al., Citation2003; Laurent et al., Citation2007).
Sodium cholate (NaCh) is the sodium salt of the naturally occurring cholic acid (), which is one of the two major bile acids produced from cholesterol catabolism in the liver. Cholic acid represents about 40% of the total biliary bile acids (Murphy & Signer, Citation1974). Unlike cholic acid, NaCh is a water soluble ionic surfactant whose solubilizing capacity for sparingly soluble undissociated bile acid species (Carey, Citation1984), water-insoluble nutrients (Murphy & Signer, Citation1974) or drugs (Acton, Citation2011) has been reported. Based on these documents, we supposed that this natural surfactant could be a valuable delivery system for the potential cardiac imaging agent 123I-Hyp/Hyp.
In addition, developing preparations of 123I-Hyp/Hyp as “instant kits”, something similar to the commercial 99mTc radiopharmaceuticals, could be attractive and meaningful for clinical applications. To this end, a fast and easy preparation of 123I-Hyp/Hyp solubilized in the biocompatible surfactant NaCh with high labeling yields and without the need for additional purification steps due to the absent undesirable reagents or solvents in the formulation would be required. To test this hypothesis, in this study, we investigated the biodistribution and necrosis avidity of 123I-Hyp/Hyp prepared and dissolved in a NaCh solution in a rabbit model of AMI.
Materials and methods
Drugs and chemicals
Hyp, 1,3,4,6,8,13-hexahydroxy-10,11-dimethylphenanthro[1,10,9,8-opqra]perylene-7,14-dione, with a purity higher than 98% was obtained from Planta Natural Products (Austria; http://www.planta.at/hyper/hyper.htm). Sodium cholate (NaCh) and 30% (w/w) hydrogen peroxide solution (H2O2) were purchased from Sigma-Aldrich (St Louis, MO). All other chemicals and solvents of reagent grade were obtained from commercial sources.
Optimization of the solubilizing agent concentration
Iodine-123-labeled Hyp (123I-Hyp) was prepared and purified as previously described (Cona et al., Citation2013b). A mixture of 123I-Hyp/Hyp (∼1.0 × 10−7 mol/l/1.9 × 10−4 mol/l) was formulated in NaCh solutions at different concentrations (0.01, 0.02, 0.03, 0.04, 0.05 and 0.06 M). The six formulations were prepared at room temperature for 2–3 min with continued shaking to ensure complete mixing. For evaluation of physical properties, each formulation was microscopically examined (Zeiss Axiophot Microscope, Oberkochen, Germany) as in a study with other 123I-Hyp/Hyp formulations (Cona et al., Citation2013c). For stability tests, three vials of each formulation were prepared, kept for 24 h at room temperature in a dark place and then microscopically examined.
Tracer preparation using NaCh as reaction media
Radiolabeling Hyp with 123I-sodium iodide (123I-NaI) (33.3 GBq/ml; GE Healthcare, Diegem, Belgium) was performed with 30% (w/w) H2O2 as oxidizing agent in 0.06 M NaCh solution, 0.5 M sodium phosphate buffer at pH 7.4 and room temperature for 30 min. Radiochemical yield determination and purification were performed by high performance liquid chromatography (HPLC) in a Merck-Hitachi L-6200 Intelligent pump system (Tokyo, Japan) equipped with an XTerra® C18 semi-preparative column 10 mm× 250 mm, 10 µm (Waters, Milford, MA), a Merck-Hitachi L-4200 HP UV/VIS detector at 254 nm coupled in series with a Raytest radioactivity detector (Straubenhardt, Germany). Sample injection was carried out via a Rheodyne injector model 7725 valve. Using a flow rate of 3.0 ml/min, the column was eluted with acetonitrile/0.05 M ammonium acetate buffer pH 7.0 in gradient mode (0 min: 75:25 v/v, 5 min: 80:20 v/v, 25 min: 90:10 v/v and 30 min: 75:25 v/v). Data acquisition and processing were performed with GinaStar acquisition software (version 4.6; Raytest). After purification, solvent removal from the 123I-Hyp fraction was done by evaporation at 70 °C under a gentle stream of nitrogen. Formulation of 123I-Hyp was carried out with 0.06 M NaCh solution containing an excess of Hyp. The resulting solution was then macroscopically inspected for controlling aggregates formation ().
Animal model
The experiment was approved by the current institutional regulations for the use and care of laboratory animals according to European Ethics Committee guidelines (decree 86/609/EEC). Five white male New Zealand rabbits (Animal House, KU Leuven, Belgium) weighing around 3–4 kg were used. A rabbit model of AMI in the left ventricle was prepared, as previously described (Feng et al., Citation2009).
Ex vivo biodistribution study by tissue gamma counting and autoradiography
Twenty-four hours after AMI, the animals were anesthetized by intramuscular administration of Ketalar® (ketamine hydrochloride, Parke-Davis Warner-Lambert, Bornem, Belgium) and Ranpun® (xylazine hydrochloride, Bayer AG, Leverkusen, Germany) at 0.5 ml/kg each. Immediately after, they were intravenously (IV) injected with a dose of 111.0 MBq/0.25 mg/kg of 123I-Hyp/Hyp in 0.06 M NaCh via an ear vein. One day later, the rabbits were sacrificed with an overdose of pentobarbital sodium (Nembutal) (Sanofi Sante Animale, Brussels, Belgium).
Specimens of organs (brain, thyroid, lungs, liver, gallbladder, stomach, spleen, pancreas, kidneys, small and large intestines) and tissues (infarcted and viable myocardium, muscle, bone, blood and skin) were harvested and weighed. Their radioactive contents were measured for one minute using a 2480 Automatic Gamma Counter, Wizard2TM3'' (Perkin Elmer, Waltham, MA). The total administered dose was estimated from the counts of a standard solution of 2.0 µl of 123I-Hyp and the difference in the volume of the dose loaded in the injection syringes before and after 123I-Hyp/Hyp injection. After correction for background radiation and decay, radioactivity in organs or tissues was expressed as percentage of injected dose (%ID) per organ and percentage of injected dose per gram of tissue (%ID/g).
Autoradiography, fluorescence and histology
The hearts of the animals were then cut into 3 mm short-axis sections. The tissue samples were embedded in Tissue Tek medium (Miles, Elkhart, IN 46515) and snap-frozen in pre-cooled isopentane on dry ice. Ten- and 50 µm slices were prepared using a Cryotome (Thermo Fisher, Waltham, MA) and then thaw-mounted onto glass slides, which were further exposed to a super resolution screen (Canberra-Packard, Meridan, CT) for two days. Digital autoradiography imaging of cryosections was carried out using a Cyclone Phosphor Imager scanner (Canberra-Packard). Four regions of interest (ROI) were defined and marked around the infarcts and viable myocardium using Optiquant software version 5.0 (Canberra-Packard). Radioactivity ratios between infarcts and viable myocardium were determined.
Digital pictures of 50-µm tissue sections were acquired using an Olympus IX61 DSU microscope (Tokyo, Japan) under fluorescent mode, at 10 × magnifications. Fifty- and 10-µm tissue sections were stained with hematoxylin and eosin (HE). For macroscopic observations, 50-µm sections were digitally photographed at 4 × magnifications (Canon Digital IXUS 860, Melville, NY 11747). To verify infarcted tissues, 10-µm sections were microscopically inspected at 50–100 × magnifications.
Statistical analysis
Numerical data were expressed as mean ± standard deviation. Statistical analysis was performed with a paired t test using the computer program GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, www.graphpad.com). p Values less than 0.05 were considered statistically significant.
Results
Optimization of the solubilizing agent concentration
Microscopy confirmed aggregate formation of Hyp solubilized in NaCh solutions of concentrations between 0.01 and 0.05 M, which was gradually reduced by increasing the amount of the solubilizing agent. After 24 h storage, no significant changes were noticed in the physical appearance and microscopic profile of each solution. Especially with 0.06 M NaCh, almost no precipitate was microscopically observed; similar to what was previously reported with a less biocompatible formulation (Cona et al., Citation2013c).
Tracer preparation
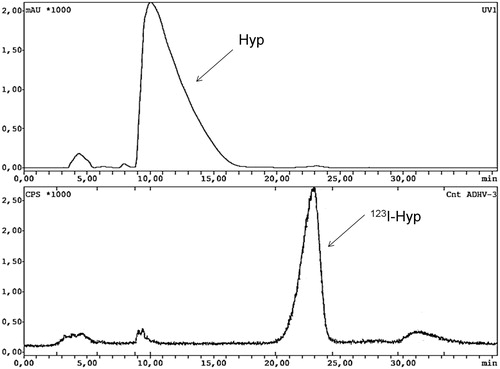
illustrates representative HPLC chromatograms with UV absorbance and radiometric detection of the radioiodination Hyp with 123I-NaI in 0.06 M NaCh, as reaction media. Overall, 123I-Hyp was prepared in a radiolabeling yield of roughly 85% and with a specific activity above 1 GBq/µmol. In the radio-HPLC chromatogram, free iodine-123 at 4.5 min was observed accounting for 5.6% of the total radioactivity. A major peak corresponding to 123I-Hyp eluted with a retention time (RT) of 22.9 min. At 30.2 min, a small peak coming out with 7.00% of the total counts corresponded to di-iodinated Hyp, as confirmed by mass spectrometry. On the UV chromatogram, a broad peak of unlabeled Hyp appeared with an RT of 11.9 min.
Figure 3. HPLC UV-chromatogram of unlabeled hypericin (Hyp) coming out at 10.3 min (upper part) and HPLC radiochromatogram (lower part) of iodine-123-labeled hypericin (123I-Hyp) with a retention time of 22.9 min.
After HPLC purification, the 123I-Hyp exhibited radiochemical purity above 99%. A final solution mixing 123I-Hyp at 370 MBq/ml and 5.2 × 10−3 mol/l of Hyp (0.25 mg/kg) was prepared using 0.06 M NaCh as solvent.
Animal model
The animal model of AMI was successfully established in all rabbits. No animals died due to the anesthesia, transoral intubation and surgical procedures.
Tissue gamma counting
Organ distribution and targetability of 123I-Hyp/Hyp dissolved in 0.06 M NaCh in rabbits with AMI at 24 h post-injection are shown in .
Table 1. Organ distribution of iodine-123 labeled hypericin (123I-Hyp) dissolved in 0.06 M sodium cholate in rabbits (n = 5) with re-perfused myocardial infarction at 24 h post-injection.
Targetability
The results of the ex vivo biodistribution study showed that 123I-Hyp/Hyp in 0.06 M NaCh exhibited a significantly higher (p = 0.003) uptake in the AMI (0.8 ± 0.2%ID/g) than in normal myocardium (0.05 ± 0.02 %ID/g), leading to an infarct-to-normal ratio of 14.9 ± 1.5.
Biodistribution
In all animals, the highest proportion of injected doses (%ID) was found in the liver (15.7 ± 0.6 %), followed by large (9.7 ± 0.9%) and small (5.9 ± 0.6%) intestines, suggesting the hepatobiliary pathway as the main excretion route for 123I-Hyp/Hyp in rabbits. Negligible amounts of 123I-Hyp/Hyp were detected in brain (0.014 ± 0.003 %ID), thyroid (0.047 ± 0.005 %ID) and spleen (0.3 ± 0.1 %ID). Most organs and tissues showed radioactivity concentrations (%ID/g) less than 0.1%. The organ that accumulated the highest concentration was thyroid (0.438 ± 0.091 %ID/g) due to its low mass and natural affinity for iodine. In contrast, skeletal muscle showed high %ID (24.486 ± 4.033 %ID) but low 123I-Hyp/Hyp concentration (0.021 ± 0.007 %ID/g) because of its relatively large mass in the body.
Autoradiography, fluorescence examination and histology
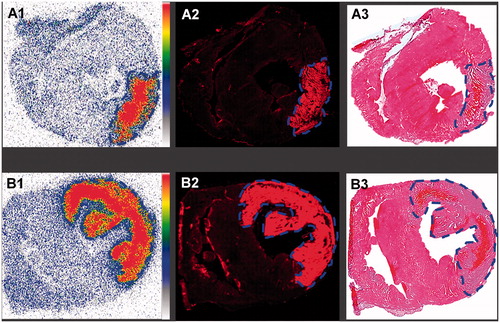
illustrate the autoradiograms of 50-µm tissue sections, the fluorescent images and corresponding HE-stained slice from rabbits with small (upper row) and large (lower row) AMI receiving 123I-Hyp/Hyp in 0.06 M NaCh. High radioactivity uptake in infarcts but the lack of accumulation in normal myocardium was evidenced. By quantitative ROI analysis, necrotic-to-normal myocardium (DLU)/mm2 ratios of 15.3 ± 2.7 were obtained. show the fluorescent images of the abovementioned 50-µm heart sections. Strong fluorescence signals in infarcted areas accumulating 123I-Hyp/Hyp were observed in contrast to the almost no detectable fluorescent signal coming from the normal myocardium, as confirmed by HE-stained histopathology ( of the same slices.
Figure 4. Ex vivo analysis of infarcts and viable heart tissues from rabbits with a small (upper row) and large (lower row) re-perfused acute myocardial infarction (AMI) having received intravenously iodine-123-labeled-hypericin/hypericin (123I-Hyp/Hyp). A1 and B1: autoradiograms of 50-µm thick sections show higher tracer accumulation in infracts than in viable myocardium. The color code bar indicates the coding scheme for the radioactivity. Red color refers areas with the highest 123I-Hyp/Hyp activity, whereas white encodes for the lowest activity. A2 and B2: corresponding low power microscopic images of 50-µm thick sections corroborate the preferential uptake of the highly fluorescent 123I-Hyp/Hyp in AMI contrast to the low fluorescence in viable myocardium. A3 and B3: HE-stained sections confirmed the location of the viable myocardium tissue and the presence of a small (upper row) and large (lower row) lesion of myocardial necrosis characterized by scattered hemorrhage.
Discussion
In nuclear imaging, high signal to background ratios are mandatory for accurate visualization and quantification of AMI. The 123I-Hyp/Hyp mixture has shown excellent properties as a cardiac diagnostics due to its unique and selective necrosis avidity (Fonge et al., Citation2008; Cona et al., Citation2013b). However, its marked lipophilicity and consequent poor solubility in water is a common limitation in parenteral tracer formulation. Furthermore, the incorporation of an iodine atom into the Hyp structure may aggravate the poor water solubility of the tracer. As a result, 123I-Hyp/Hyp may precipitate as insoluble aggregates with distorted biodistribution imposing significant clinical impact in scan interpretation and diagnostic imaging accuracy. This makes essential to elaborate a formulation, in which 123I-Hyp/Hyp are dissolved molecularly for guaranteeing high tracer uptake in infarcts and meanwhile a fast clearance from blood and normal tissues.
For boosting the solubility of Hyp in aqueous solutions, different methods have been being applied over the past decades. The cosolvency method, one of the oldest and most commonly used techniques in roughly 66% of Food and Drug Administration-approved parenteral products, has constituted the preferred solubilizing approach. Based on the addition of water miscible solvents to aqueous systems, this method incorporates the required dose as a true solution in a small volume of liquid. For Hyp, dimethyl sulfoxide (DMSO) (Head et al., Citation2006; Higuchi et al., Citation2008) and polyethylene glycol 400 (PEG-400) (Čavarga et al., Citation2005; Huygens et al., Citation2005) have been chosen as cosolvents due to their strong solubilization capacity and relatively low toxicity. Procyanidins and alcoholic mixtures of 99:1 (v/v) methanol:pyridine or ethanol in water at different proportions have been used to dissolve naphthodianthrones by complex formation (Chung et al., Citation2000; Assadi et al., Citation2011). Inclusion complex by using the cyclic oligosaccharide cyclodextrins and entrapment in phospholipids liposomes have also been exploited (Derycke & de Witte, Citation2002; Nandi et al., Citation2003). Known as drug “containers”, their structures are distinguished by a hydrophilic exterior with a hydrophobic interior that can encapsulate Hyp. The solid dispersion techniques based on the formation of water soluble complexes of Hyp non-covalently bound to polyvinylpyrrolidone of various degrees of polymerization (10.000–40.000 g/mol) have also been applied for clinical purposes (Kubin et al., Citation2008). Solubilization by micelles formation using synthetic surfactants such as polysorbate 80 (Tween 80), Triton X-100, phosphatidylcholine, Cremophor-EL and Cremophor-RH40 has been reported in pharmacological studies under physiological conditions (Senthil et al., Citation1994; Guilhermano et al., Citation2004). In the case of 123I-Hyp/Hyp, so far, the cosolvency method appears the most commonly used due to its rapidness and simplicity in line with the safety regulations for manipulation of radioactive drugs. Preclinical studies using formulations consisting of water/PEG-400 (80/20, v/v) (Ni et al., Citation2006; Li et al., Citation2011; Van de Putte et al., Citation2012) and DMSO/PEG-400/water (25/60/15, v/v/v) (Cona et al., Citation2013a) have been reported. Pure DMSO as carrier of the poorly water soluble 123I-Hyp/Hyp has also been used (Li et al., Citation2012). However, although these synthetic solvents are distinguished for chemical and biological inertia, they may show adverse pharmacological and toxicological effects, especially given undiluted. Solvent-related toxicity problems including cardiovascular effects, hypersensitivity reactions, nephrotoxicity, neurotoxicity, carcinogenicity, teratogenicity, mutagenicity, hemolysis and irritating and sensitizing effects have been reported (Montaguti et al., Citation1994; Ten et al., Citation2003; Laurent et al., Citation2007).
In an attempt to find a more biocompatible delivery system for 123I-Hyp/Hyp, we postulated that sodium cholate (NaCh), a naturally occurring detergent-like biochemical with low toxicity, could be a potential carrier for clinical applications. NaCh is a water-soluble bile salt, which is synthesized in the liver by cytochrome P450-mediated oxidation of cholesterol, stored in the gallbladder and secreted into the intestines to assist food digestion. NaCh is an amphiphilic molecule with a hydrophilic concave region and a hydrophobic convex region making it an ideal surfactant agent. As a result, the NaCh molecule, having limited solubility in any solvent, tends to accumulate at solvent surface. At a certain concentration (0.013–0.018 M) known as critical micellar concentration (CMC), it forms micelles that act as an encapsulating agent for dietary fats, hydrophobic substances and guarantee efficient transport and absorption of poorly water soluble nutrients. Due to the enlargement of the size of the primary micelles caused by the hydrogen bonding forces among the hydrophilic regions, a secondary micellization occurs at higher concentration of NaCh (0.05 M) (O’Connor et al., Citation1983; Conte et al., Citation1984; Santhanalakshmi et al., Citation2001).
Based on earlier studies reporting the strong capacity of NaCh for solubilizing a wide sort of synthetic and natural hydrophobic substances (Carey, Citation1984; Acton, Citation2011), we evaluated the biological profile of 123I-Hyp/Hyp in a NaCh solution in rabbits with AMI. This animal model of infarction was chosen because it has been routinely used to prove the potential application of 123I-Hyp as diagnostic cardiac agent showing reproducible and accurate results (Fonge et al., Citation2008; Feng et al., Citation2013; Cona et al., Citation2013a). As carrier of 123I-Hyp/Hyp, NaCh solutions at different concentrations were prepared for evaluating their solubilizing properties. Because 123I-Hyp is intended for cardiac imaging based on the detection of gamma-ray emissions from the fast decaying radioisotope 123I in the tracer 123I-Hyp/Hyp, in this preliminary study, stability of the formulations over a short period of time (24 h) was studied. The best results were achieved by using 0.06 M NaCh, which is in agreement with a previous study (Ji et al., Citation2013). This could be due to the fact that such a concentration is nearly above the secondary CMC (0.05 M) reported for sodium cholate at 25 °C (O’Connor et al., Citation1983; Conte et al., Citation1984; Santhanalakshmi et al., Citation2001), which favors the 123I-Hyp/Hyp solubilization.
At surfactant concentration above the CMC, the formed micelles are able to accommodate the hydrophobic molecule due to an increase in micelle-bound solutes, thus improving the solubility of the molecule in the medium. Indeed, the relatively high radiochemical yield (> 85%) attained after Hyp radioiodination suggested that most likely 123I-Hyp/Hyp is in molecular form when it is dispersed within the micellar microenvironment of NaCh, as was noticed by microscopic inspection of the formulation before tracer administration. On the contrary, a decrease in radiochemical yield would be expected due to the poor solubility of Hyp in improper formulations (Cona et al., Citation2013c).
Under physiological conditions, both necrosis avidity and tissue distribution of 123I-Hyp/Hyp in 0.06 M NaCh solution exhibited similar patterns to those observed in earlier reports (Fonge et al., Citation2008; Cona et al., Citation2013a). Either by tissue gamma counting, autoradiography or fluorescence examination, a unique avidity for infarcts and almost no accumulation in viable myocardium of 123I-Hyp/Hyp were found in all the animals studied. This is also an evidence of the good solubilization of the hydrophobic 123I-Hyp/Hyp in 0.06 M NaCh. Indeed, the relatively low accumulation in MPS organs such as lungs and spleen indicated the presence of no or minimal aggregate formation of 123I-Hyp/Hyp. Otherwise, prolonged radioactivity retention in such organs would happen due to aggregates entrapment, as was previously reported (Van De Putte et al., Citation2006). In accordance with previous investigations, thyroid and stomach radioactivities were negligible, corroborating the in vivo stability of the radioiodinated Hyp over time (Cona et al., Citation2013a). Similarly, brain retained the lowest level of radioactivity due to the relatively high molecular weight, strong lipophilic character and high protein binding of 123I-Hyp/Hyp, making it unable to cross the brain–blood barrier. As expected, 123I-Hyp/Hyp was excreted from the body mainly via hepatobiliary pathways followed by elimination via intestines.
However, further improvements have to be made before using a NaCh solution to solubilize 123I-Hyp/Hyp in real clinical applications. In previous works (Fonge et al., Citation2008; Cona et al., Citation2013a), less biocompatible solvents (e.g. DMSO and PEG-400) have been used for radiolabeling and formulation of Hyp. Under such circumstances, 123I-Hyp/Hyp has shown higher labeling yields (>95%) after preparation (Cona et al., Citation2013a). Similarly, better radioactivity uptake ratios between necrotic and normal myocardium leading to enhanced quality SPECT images have been also noted. Therefore, in order to get the best NaCh solution for 123I-Hyp/Hyp exhibiting high labeling yields and the absence of particulates in the formulation, parameters directly related to the interactions between NaCh surfactant and 123I-Hyp/Hyp such as solubilizing capacity of micelles, surfactant concentration and CMC have to be optimized under different conditions such as pH and electrolytes addition. In addition, studies on the storage life of both the radioactive and non-radioactive iodinated Hyp/Hyp in NaCh solutions to determine how long the product can be kept in storage under specified conditions have to be determined.
Conclusions
Our study demonstrated a high avidity of 123I Hyp/Hyp in 0.06 M NaCh for necrotic tissues in rabbits with AMI. 123I-Hyp/Hyp was mainly eliminated via the hepatobiliary pathway and secreted into intestines. The suitability of using sodium cholate as a solubilizing agent to formulate the necrosis avid agent 123I-Hyp for hotspot imaging of irreversibly damaged MI was proven. Further optimization on the solubilization process is needed.
Declaration of interest
The authors report no declarations of interest.
This study was partially supported by the grants awarded by an EU project Asia-Link CfP 2006-EuropeAid/123738/C/ACT/Multi-Proposal No. 128-498/111, the KU Leuven Molecular Small Animal Imaging Center MoSAIC (KUL EF/05/08) and KU Leuven the center of excellence In vivo Molecular Imaging Research (IMIR). The corresponding author Ni Y is currently a Bayer Lecture Chair holder.
References
- Acton A. (2011). Issues in pharmacology, pharmacy, drug research, and drug innovation. ScholarlyEditions™ eBook. Atlanta, GA
- Assadi A, Zarrindast MR, Jouyban A, Samini M. (2011). Comparing of the effects of hypericin and synthetic antidepressants on the expression of morphine-induced conditioned place preference. Iran J Pharm Res 10:619–26
- Avato P, Raffo F, Guglielmi G, et al. (2004). Extracts from St. John’s wort and their antimicrobial activity. Phytother Res 18:230–2
- Bormans G, Dieter H, Christiaen A, et al. (2004). Preparation, analysis and biodistribution in mice of iodine-123 labeled derivatives of hypericin. J Labelled Comp Rad 47:191–8
- Carey MC. (1984). Bile acids and bile salts: ionization and solubility properties. Hepatology 4:66S–71S
- Čavarga P, Brezánib P, Fedoročkoc P, et al. (2005). Photoinduced antitumour effect of hypericin can be enhanced by fractionated dosing. Phytomedicine 12:680–3
- Chung PS, Rhee CK, Kim KH, et al. (2000). Intratumoral hypericin and KTP laser therapy for transplanted squamous cell carcinoma. Laryngoscope 110:1312–6
- Cona MM, Feng Y, Li Y, et al. (2013a). Comparative study of Iodine-123-labeled-hypericin and Tc-99m-labeled-hexakis [2-methoxy isobutyl isonitrile] in a rabbit model of myocardial infarction. J Cardiovasc Pharmacol 62:304–11
- Cona MM, Li J, Feng Y, et al. (2013b). Targetability and biodistribution of radioiodinated hypericin: comparison between microdosing and carrier-added preparations. Anticancer Agents Med Chem. 2013 Aug 28. PMID: 24102315. [Epub ahead of print]
- Cona MM, Li JJ, Chen F, et al. (2013c) Radioiodinated hypericin: its biodistribution, necrosis avidity and therapeutic efficacy are influenced by formulation. Pharmaceut Res. 2013 Aug 9; PMID: 23934256. DOI: 10.1007/s11095-013-1159-4
- Conte G, Di Blasi R, Giglio E, et al. (1984). Nuclear magnetic resonance and x-ray studies on micellar aggregates of sodium deoxycholate. J Phys Chem 88:5720–4
- Derycke ASL, de Witte PAM. (2002). Transferrin-mediated targeting of hypericin embedded in sterically stabilized PEG-liposomes. Int J Oncol 20:1181–7
- D'Hallewin MA, Kamuhabwa AR, Roskams T, et al. (2002). Hypericin-based fluorescence diagnosis of bladder carcinoma. BJU Int 89:760–3
- Feng Y, Cona MM, Vunckx K, et al. (2013). Detection and quantification of acute reperfused myocardial infarction in rabbits using DISA-SPECT/CT and 3.0T cardiac MRI. Int J Cardiol 168:4191–8
- Feng Y, Xie Y, Wang H, et al. (2009). A modified rabbit model of reperfused myocardial infarction for cardiac MR imaging research. Int J Cardiovasc Imaging 25:289–98
- Fonge H, Vunckx K, Wang H, et al. (2008). Non-invasive detection and quantification of acute myocardial infarction in rabbits using mono-[123I]iodohypericin µSPECT. Eur Heart J. 29:260–9
- Ghaedi M, Montazerozohori M, Behfar M, Marahel F. (2011). Influence of multiwalled carbon nanotubes on the response performance of carbon paste iodide ion selective electrode based on iron (II) phthalocyanine. Int J Electrochem Sci 6:6074–84
- Guilhermano LG, Ortiz L, Ferigolo M, Barros HM. (2004). Commercially available hypericum perforatum extracts do not decrease immobility of rats in the forced swimming test. Prog Neuropsychopharmacol Biol Psychiatry 28:49–55
- Head CS, Luu Q, Sercarz J, Saxton R. (2006). Photodynamic therapy and tumor imaging of hypericin-treated squamous cell carcinoma. World J Surg Oncol 4:87
- Higuchi A, Yamada H, Yamada E, et al. (2008). Hypericin inhibits pathological retinal neovascularization in a mouse model of oxygen-induced retinopathy. Mol Vis 14:249–54
- Huygens A, Kamuhabwa AR, de Witte PA. (2005). Stability of different formulations and ion pairs of Hypericin. Eur J Pharm Biopharm 59:461–8
- Ji Y, Zhan Y, Jiang C, et al. (2013). Improvement of solubility and targetability of radioiodinated hypericin by using sodium cholate based solvent in rat models of necrosis. J Drug Target. DOI:10.3109/1061186X.2013.867962. [Epub ahead of print]
- Karioti A, Bilia AR. (2010). Hypericins as potential leads for new therapeutics. Int J Mol Sci 11:562–94
- Kubin A, Loew HG, Burner U, et al. (2008). How to make hypericin water-soluble. Pharmazie 63:263–9
- Laurent A, Mottu F, Chapot R, et al. (2007). Cardiovascular effects of selected water-miscible solvents for pharmaceutical injections and embolization materials: a comparative hemodynamic study using a sheep model. PDA J Pharm Sci Technol 61:64–74
- Li J, Cona MM, Chen F, et al. (2012). Exploring theranostic potentials of radioiodinated hypericin in rodent necrosis models. Theranostics 2:1010–9
- Li J, Sun Z, Zhang J, et al. (2011). A dual-targeting anticancer approach: soil and seed principle. Radiology 260:799–807
- Miskovsky P. (2002). Hypericin – a new antiviral and antitumor photosensitizer: mechanism of action and interaction with biological macromolecules. Curr Drug Targets 3:55–84
- Montaguti P, Melloni E, Cavalletti E. (1994). Acute intravenous toxicity of dimethyl sulfoxide, polyethylene glycol 400, dimethylformamide, absolute ethanol, and benzyl alcohol in inbred mouse strains. Arzneimittelforschung 44:566–70
- Murphy GM, Signer E. (1974). Bile acid metabolism in infants and children. Gut 15:151–63
- Nandi I, Bateson M, Bari M, Joshi HN. (2003). Synergistic effect of PEG-400 and cyclodextrin to enhance solubility of progesterone. AAPS PharmSciTech 4:1–5
- Ni Y Bormans G, Marchal G, Verbruggen A. (2005). Tissue infarction and necrosis specific compounds (of hypericin derivatives). PCT/BE2004/000107 Patent application
- Ni Y, Huyghe D, Verbeke K, et al. (2006). First preclinical evaluation of mono-[123I]iodohypericin as a necrosis-avid tracer agent. Eur J Nucl Med Mol Imaging 33:595–601
- O’Connor CJ, Ch'ng BT, Wallace RG. (1983). Studies in bile salt solutions. 1. Surface tension evidence for a stepwise aggregation model. J Colloid Interface Sci 95:410–9
- Roth L. (1990). Hypericum, hypericin: botanik, inhaltsstoffe, wirkung. Landsberg: Ecomed
- Santhanalakshmi J, Shantha Lakshmi G, Aswal VK, Goyal PS. (2001). Small-angle neutron scattering study of sodium cholate and sodium deoxycholate interacting micelles in aqueous medium. J Chem Sci 113:55–62
- Senthil V, Jones LR, Senthil K, Grossweiner LI. (1994). Hypericin photosensitization in aqueous model systems. Photochem Photobiol 59:40–7
- Sihver W, Bier D, Holschbach MH, et al. (2004). Binding of tritiated and radioiodinated ZM241,385 to brain A2A adenosine receptors. Nucl Med Biol 31:173–7
- Sosa S, Pace R, Bornancin A, et al. (2007). Topical anti-inflammatory activity of extracts and compounds from Hypericum perforatum L. J Pharm Pharmacol 59:703–9
- Sun ZP, Ni Y. (2009). Iodogen method for preparation of radioiodinated Hypericin. Patent CN 200910013998
- Ten Tije AJ, Verweij J, Loos WJ, Sparreboom A. (2003). Pharmacological effects of formulation vehicles: implications for cancer chemotherapy. Clin Pharmacokinet 42:665–85
- Theodossiou TA, Hothersall JS, De Witte PA, et al. (2009). The multifaceted photocytotoxic profile of hypericin. Mol Pharmaceutics 6:1775–89
- Van de Putte M, Marysael T, Fonge H, et al. (2012). Radiolabeled iodohypericin as tumor necrosis avid tracer: diagnostic and therapeutic potential. Int J Cancer 131:E129–37
- Van De Putte M, Roskams T, Bormans G, et al. (2006). The impact of aggregation on the biodistribution of hypericin. Int J Oncol 28:655–60
- Weiner L, Y Mazur. (1992). EPR studies of hypericin: photogeneration of free radicals and superoxide. J Chem Soc Perkin Trans 2:1439–42
- Yamazaki T, Ohta N, Yamazaki I, Song PS. (1993). Excited-state properties of hypericin: electronic spectra and fluorescence decay kinetics. J Phys Chem 97:7870–5