Abstract
Targeted drug delivery for brain tumor treatment is one of the important objectives in nanomedicine. Human glioblastoma is the most frequent and aggressive type of brain tumors. The preferential expression of membrane protein connexin 43 (Cx43) and brain-specific anion transporter (BSAT1) in the tumor and peritumoral area is a key component for targeted drug delivery. The purpose of this study was to design cisplatin-loaded nanogels conjugated with monoclonal antibodies to Cx43 and BSAT1 for treatment of intracranial gliomas 101/8. MRI volumetric analysis of tumor-bearing rats indicated significantly reduced tumor volume with cisplatin-loaded targeted-nanogel treatment compared to other formulations. The median survival of rats treated with targeted nanogels conjugated with specific mAbs against extracellular loops of Cx43 and BSAT1 were 27 and 26.6 days higher than that in control group, respectively. For the first time we demonstrated the efficiency of mAb-targeted cisplatin-loaded nanogels in the experimental model of glioma 101/8. This approach could facilitate the development of new drug delivery systems for the treatment of gliomas.
Introduction
It is difficult to imagine the progress in the chemotherapy of gliomas without the development of drug delivery systems, which improve pharmacokinetic characteristics of drugs and control their release at the tumor site. Nanocontainers designed to prolong the circulation time of drugs in the blood, reduce their systemic toxicity and improve their bioavailability. This approach is especially important in the case of alkylating platinum-containing cytostatic drugs, which display a pronounced non-specific toxicity (Hartmann & Lipp, Citation2003). The undesirable side effects of drugs could be significantly reduced by encapsulation of them into liposomes (Chekhonin et al., Citation2012), micelles (Oberoi et al., Citation2012), polymeric nanoparticles (Wohlfart et al., Citation2011; Steiniger et al., Citation2004) and other nanoparticles (Peer et al., Citation2007). Nanogels – nanoscale hydrogels based on hydrophilic or amphiphilic polymeric network – are one of the promising nanocontainers for platinum-based drugs (Kabanov & Vinogradov, Citation2009; Nukolova et al., Citation2011a). They are characterized by high loading capacity, tunable internal volume and high stability. Moreover, different targeting groups can be conjugated to nanogel surface to enhance drug delivery to particular organ or tissue (Chekhonin et al., Citation2012; Peer et al., Citation2007; Nukolova et al., Citation2011a,b).
Two proteins, connexin 43 (Cx43) and brain-specific anion transporter 1 (BSAT1), are promising targeted antigens for drug delivery to gliomas. Cx43 is the main structural component of gap junctions of normal and reactive astrocytes (Prochnow & Dermietzel, Citation2008), presumably significantly affecting the development of invasive glial tumors (Bates et al., Citation2007; Oliveira et al., Citation2005). BSAT1 is an organic anion transporter specific for cerebral microvascular endothelial cells (Li et al., Citation2004) that regulates transendothelial transport of thyroxine (T4) and some other amphiphilic anions through blood–brain barrier (Sugiyama et al., Citation2003). Previously we obtained the monoclonal antibodies (mAb) against the recombinant extracellular fragment of E2 of Cx43 interacting with the antigen in the native conformation (Baklaushev et al., Citation2009) and mAbs against the extracellular fragment of BSAT1 (Baklaushev et al., Citation2012). Using obtained mAbs we have shown that protein Cx43 over-expressed in astrocytes and glioma cells in the peritumoral invasion zone as well as BSAT1 expressed in peritumoral area. Moreover, intravenously administered fluorescent-labeled mAbCx43 were considerably accumulated in these cells of rat brain (Baklaushev et al., Citation2011). Thus, mAbs against the extracellular fragments of BSAT1 and Cx43 could be promising targeting groups for selective drug delivery to glioma cells in vivo.
The purpose of this study was to estimate the therapeutic efficacy of cisplatin-loaded nanogels conjugated with mAbs against proteins associated with high-grade glioma 101/8.
Methods
Materials
Poly(ethylene glycol)-b-poly(methacrylic acid) (PEG-b-PMAA) diblock copolymer (Mw/Mn = 1.16, PEG 5.5 kDa, PMAA 15.5 kDa) and MAL-PEG(7500)-NH2 were from Polymer Source Inc. (Montreal, QC, Canada) and Creative PEGWorks (Winston Salem, NC), respectively. Amicon YM-30 centrifugal filters (MWCO 30 kDa), 1,2-ethylenediamine, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), ethylenediaminetetraacetic acid (EDTA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), cis-dichlorodiamminoplatinum (II) (cisplatin, CDDP) and other chemicals were from Sigma–Aldrich (St Louis, MO, USA). Anti-Cx43 and anti-BSAT1 monoclonal antibodies (Mw 150 kDa) were obtained as described earlier (Baklaushev et al., Citation2009, Citation2012). Fetal bovine serum (FBS), RPMI 1640 medium, penicillin, streptomycin, Trypsine-EDTA (0.5% trypsin, 5.3 mM EDTA tetrasodium salt), Alexa Fluor 488 goat anti-mouse IgG and other were from Invitrogen (Carlsbad, CA, USA).
Synthesis of drug-loaded targeted nanogels
Nanogels were synthesized using a block ionomer complex of the PEG-b-PMAA/Ca2+ as a template (Nukolova et al., Citation2011b), prepared form aqueous solutions of PEG-b-PMAA and CaCl2 at a molar ratio of [Ca2+]/[COO] = 1.3. Then chains of the PMAA were cross-linked overnight at room temperature (r.t.) using 1,2-ethylenediamine and EDC ([EDC]/[1,2-ethylenediamine] = 2; [COOH]/[EDC] = 5). The nanogels were extensively dialyzed (MWCO, 3.5 kDa) and concentration of carboxylic groups was determined by potentiometric titration.
Monoclonal antibodies against the E2 extracellular fragments of Cx43 and BSAT1 (MAbCx43 and MAbBSAT1) were purified from ascites of Balb/c mice using affinity chromatography on protein-A-agarose, and dialyzed against phosphate buffered saline (PBS, pH 7.4) (Baklaushev et al., Citation2009, Citation2012). Obtained mAbs were thiolated with a tenfold molar excess of Traut’s reagent (0.1 M borate buffer, 5 mM EDTA, pH 8) for 45 min at r.t. After purification of thiolated mAbs (Zeba spin desalting columns, Thermo Scientific, IL, USA), they were incubated with a tenfold molar excess of MAL-PEG-NH2 overnight at 4 °C in PBS. The obtained PEGylated antibodies were purified from excess of PEG-chains by centrifugation (Amicon YM-30) and re-dispersed in PBS.
Finally, activated nanogels (a fivefold excess of EDC to carboxyl groups of nanogels, 5 min, r.t.) were added to PEGylated antibodies and mixed overnight at 4 °C in PBS. Then size exclusion chromatography was used to separate the targeted nanogels from unbound mAbs and side-reaction products (0.5 ml/min, Sepharose CL-6B, PBS, pH 7.4). The amount of attached mAbs was determined by micro bicinchoninic acid (BCA) protein colorimetric assay. Nanogels were loaded with cisplatin ([cisplatin]/[COOH] = 0.5) as described earlier (Nukolova et al., Citation2011b). Unbound cisplatin was removed by ultrafiltration using centrifugal filters Amicon YM-30. The obtained cisplatin-loaded nanogels were stored in the dark at 4 °C until use. The concentration of platinum was measured by X-ray fluorescence analysis using a REspect X-ray fluorescent spectrometer (Tolokonnikov, Russia) with molybdenum as an internal standard.
The specific activity of conjugated antibodies was tested by enzyme-linked immunosorbent assay (ELISA) with immobilized recombinant antigens (extracellular fragments of Cx43 and BSAT1). Polyclonal A3682 antibodies labeled with peroxidase (Sigma–Aldridge, USA) were used as secondary antibodies, the immunoperoxidase reaction was visualized using a ready-to-use TMB solution (Invitrogen, USA).
For in vitro visualization nanogels were labeled with Alexa Fluor 488 succinimidyl ester (Invitrogen) and C6 glioma cells were labeled with with Lysotracker Red DND-99 (Invitrogen) according manufacturer’s procedure. Labeled nanogels were added to monolayer culture of pre-stained glioma cells in DMEM with 10% FBS and after 30 min of incubation cells were analyzed by confocal microscopy.
In vitro cytotoxicity
Cytotoxicity of nanogels was studied in C6 glioma cells using the MTS reagent (Invitrogen) according to the manufacturer’s protocol. C6 cells (60–70% confluent monolayer) were treated with drug-loaded nanogels at decreasing concentrations beginning from 0.5 mg/ml for 24 h. The cell viability was assessed on an Enspire plate analyzer (Perkin Elmer).
Animals
All experiments were carried out using adult female Wistar rats with a body weight of 200 ± 30 g obtained from the Stolbovaya Nursery of the Russian Academy of Sciences (Moscow region, Russia). The rats were caged in groups of six and acclimatized for 1 week. They received standard laboratory food and water ad libitum. The animals were kept and treated in accordance with the Guidelines on Laboratory Practices in the Russian Federation adopted by the Ministry of Health of the Russian Federation (Order 267, 19 June 2003), and the principles of bioethics adopted by the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes (Strasbourg, 1986).
Intracranial inoculation of rat glioma 101/8
The intracranial implantation of the tumor, rat glioblastoma 101/8, was performed using fresh tumor tissue as described by Khalansky et al. (Steiniger et al., Citation2004). To induce the tumor, a piece of tumor tissue (∼106 tumor cells) from the frozen stock was inoculated into the right striatum using a tuberculin syringe (B. Braun, Melsungen, Germany) with a glass needle. Animals were deeply anaesthetized by intraperitoneal injection of 100 mg/kg ketamine and 10 mg/kg xylazine. Through a midline sagittal incision, a burr hole 1.5 mm in diameter was made with a dental drill 2 mm lateral to the sagittal midline and 2 mm posterior to the right coronal suture. Surgical glue (Turbo 2000 Kleber Universal, Boldt Co, Wermelskirchen, Germany) was used to close the scalp incision. By the end of experiments or at the first clinical signs of brain damage, euthanasia was performed by injection of overdose of ketamine (300 mg/kg).
Histology
The animals with intrastriatal gliomas were euthanized and transcardially perfused with cold 4% paraformaldehyde dissolved in PBS. The brain was postfixed in the same solution overnight. The postfixed brain was embedded in paraffin, and serial paraffin sections were made using a rotary microtome (Leica Microsysytems, USA). The slices were stained with hematoxylin–eosin and histochemical analysis was performed using Autostainer XL Leica ST5010 device (Leica Microsystems, USA).
Immunofluorescense assay
About 60-μm sections of paraformaldehyde-fixed rat brains with glioma were made using Microme HM 650 V vibratome. Double immunocytochemical analysis of sections was performed using mAbs against Cx43, BSAT1 and vascular endothelial growth factor and polyclonal rabbit antibodies against glial fibrillary acidic protein (GFAP) (Serbsky National Research Center for Social and Forensic Psychiatry), an astroglial marker of intermediate filaments. The sections were incubated in a cocktail consisting of monoclonal and polyclonal antibodies (0.5 μg/ml) and then visualized using Goat Antimouse Alexa Fluor 488 and Goat Antirabbit Alexa Fluor 633 (Invitrogen, USA), respectively. Fluorescence in the sections was detected by Nikon A1 confocal laser scanning microscope (Nikon, Japan).
Magnetic resonance imaging
Magnetic resonance imaging (MRI) was performed using a ClinScan MRI scanner (Bruker BioSpin) with field strength of 7 T. For obtain T2-WIs in the coronary plane, we used a fat suppression sequence with the following turbo spin-echo parameters: TR/TE = 4000/43 ms; slice thickness, 0.5 mm; matrix, 288 × 320; FOV = 40 mm. For T2-WIs in the transverse plane, the parameters were as follows: TR/TE = 5110/57 ms; slice thickness, 0.5 mm; matrix, 288 × 320; FOV = 40 mm. SWIs in the transverse plane were obtained using a special pulse sequence based on gradient echo with the following parameters: TR/TE = 33/19 ms; FA = 19°; slice thickness, 0.5 mm; matrix, 320 × 320; FOV = 40 mm.
The volumetry of the glioma and its changes with time were analyzed in T2-WIs with the use of the ImageJ software package (Wayne Rasband, National Institute of Mental Health, Bethesda, MD, USA). Initially, the area of glioma was measured separately in each slice, and then, the volume of the entire tumor was calculated as V = (S1 + ⋯ + Sn) × (h + d), where V is the volume, S1, … Sn are the measured areas of individual slices, h is the slice thickness and d is the gap between the slices. Subsequently, we found the averaged volume from the results of calculations for two orthogonal planes (coronary and transversal). Statistical analysis of volumetric data was performed using the non-parametric Mann–Whitney test for two independent groups. Differences were considered significant at p ≤ 0.05; the minimum number of data for the comparison was n = 3.
In vivo experiments
Brain MRI was performed for all rats (n = 47) on the 5th day after the implantation of glioma cells. Only animals with confirmed glioma growth were used in subsequent experiments. Rats with glioma 101/8 were divided into six groups, in which the animals received cisplatin (CDDP group, n = 8), non-targeted nanogels with cisplatin (NG/CDDP, n = 6); CDDP-nanogels conjugated with non-specific mouse IgG (IgG-NG/CDDP, n = 6) and CDDP-nanogels conjugated with mAbs against Cx43 (MAbCx43-NG/CDDP, n = 7) and BSAT1 (MAbBSAT1-NG/CDDP, n = 7). The control group of rats (Dextrose, n = 10) received a 5% glucose solution, which served as a solvent for the free and nanogel-incorporated cisplatin. All formulations were injected into the femoral vein under ketamine anesthesia (100 mg/kg) on days 5, 10 and 15 after the implantation of gliomas at a cisplatin equivalent dose of 5 mg/kg. Control animals received an equivalent volume of dexrtose. The results of in vivo experiments were evaluated by an increase in the lifespan, as well as the time course of glioma growth using MRI in treated animals as compared with control ones.
Results
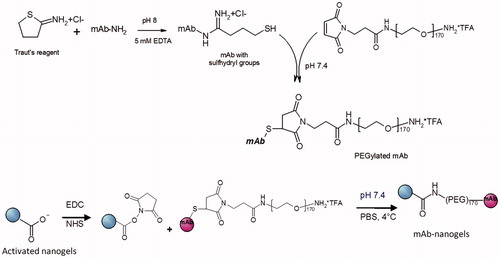
We synthesized the nanogels with the desired degree of cross-linking (cross-linked 20% of carboxylic groups of PMAA chains). The surface of nanogels were further modified with different mAbs via flexible PEG linker and loaded with anticancer drug – cisplatin (). Briefly, thiolated mAbs were conjugated with PEG, purified and conjugated to nanogels. Unbound antibodies and by-products were removed by gel filtration chromatography (Sepharose CL-6B) and mAb-nanogels were concentrated. Due to the colloidal nature of the nanogel, the conventional solution-state 1H NMR does not permit to determine the precise structure of nanogels, and signals of mAb and nanogels overlap. Nevertheless the typical NMR spectrum contains the broad peak at ∼0.5–2.5 ppm, which belongs to the backbone protons of PMAA chains, and the signal at 3.7 ppm, which corresponds to protons of PEG. The mAb modified cross-linked nanogels had average diameter of 120–130 nm, low polydispersity indices (PdI < 0.13) and formed stable aqueous dispersion. These negatively charged nanogels (ζ-potential = −15 ± 5 mV) were efficiently loaded with cisplatin: loading capacity was 30–35% and entrapment efficiency was ∼45% (Nukolova et al., Citation2011b). The drug release from nanogels was sustained, with 16.5 ± 2.1% of release in 24 h, 29.9 ± 2.8% in 3 days and 47.8 ± 3.1% in a week. Practically the same results were obtained for nanogels modified with non-specific IgG (size 125 ± 5 nm, PdI 0.12 ± 0.05, ζ-potential −12 ± 3 mV). The mAb-conjugated nanogels contained ∼100 μg mAbs per mg of nanogel as determined by BCA assay, considering the background of non-conjugated nanogels. Targeted nanogels had spherical morphology (Supplementary Figure 1) and remained stable in PBS (pH 7.4, 4 °C) in a wide range of concentrations (up to 5 mg/ml), exhibiting no aggregation for several weeks. Immunochemical activity of antibodies decreased with each stage of the synthesis as determined by ELISA. However, the activity of final conjugated antibodies was >60% of the original activity of the free monoclonal antibodies even after 1 month of storage at 4 °C. In further experiments, we selected only antibody-conjugated nanogels that reacted with the antigen in a dilution of at least 1:1000.
Internalization of mAb-targeted nanogels labeled with Alexa Fluor 488 was studied by laser scanning confocal microscopy on glioma C6 cells using Lysotracker Red DND-99 for detection of cell organelles (late endosomes and lysosomes) (Supplementary Figure 2). All nanogels at concentrations 1–20 μg/ml could penetrate the cell membrane after incubation of fluorescent-labeled nanogels with cells for 30 min. Fluorescent-labeled nanogels conjugated with non-specific IgG accumulated in all cells but with low intensity of intercellular signal. On the contrary, mAbCx43-nanogels had better accumulation in Cx-43 positive glioma cells, suggesting targeted delivery of nanogels.
Analysis of glioma C6 cells viability revealed that cytotoxicity of targeted CDDP-loaded nanogels was higher than that of non-targeted nanogels. Thus, IC50 for MAbCx43-nanogels/CDDP and nanogels/CDDP were 20.3 ± 0.6 µg/ml and 29.5 ± 1.7 µg/ml, respectively. Cytotoxicity of free CDDP greatly decreased after encapsulation in nanogels (IC50 2.5 µg/ml). The experimental model of intracranial glioma 101/8 was designed to test various chemotherapeutic agents and is considered one of the most adequate models of glioblastoma multiforme (Chekhonin et al., Citation2012). In our experiments, the rate of successful glioma implantation was at least 95%. The growth of glioma was confirmed by MRI and led to animal death in 100% of cases; the median survival of rats after the tumor implantation was 15 days ().
Table 1. The main characteristics of survival of rats in the experimental groups.
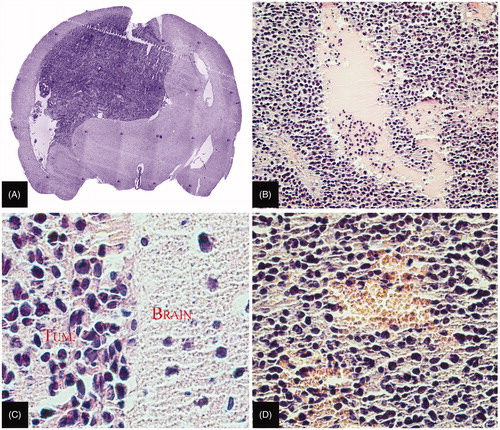
The tissues of glioma 101/8 were examined by routine histological methods and immunohistochemical analysis for the expression of target antigens (Cx43 and BSAT1) (for more details of target antigens see Supplementary Figures 3 and 4). Histological analysis of the growing intrastriatic gliomas (5–15 days) demonstrated all the signs of glioblastoma multiforme: cell polymorphism, pericellular edema, hemorrhage, central necrosis and rapidly developing intracranial dislocation ().
Figure 2. Histological analysis of experimental glioma 101/8. (A) General view of the glioma (×5). (B) Foci of necrosis in the central part of the glioma (×100). (C) Polymorphism of glioma cell nuclei (×400). (D) Hemorrhage in the central part of the glioma (×200). Paraffin sections stained with hematoxylin–eosin.
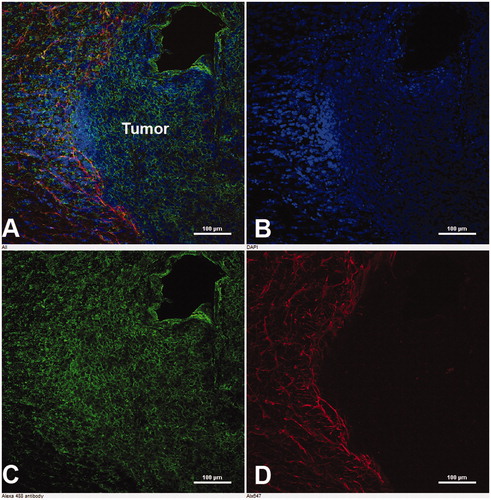
Immunohistochemical analysis showed a high expression of targeted proteins in glioma 101/8. As seen from , Cx43 was predominantly located in the peritumoral area, and BSAT1 in the peritumoral area and the central part of the glioma. The high expression of Cx43 and BSAT1 in the tumor and peritumoral area can promote the effective therapy of gliomas by drug-loaded nanocontainers targeted with mAbs against these proteins.
Figure 3. Immunohistochemical analysis of BSAT1 on brain slices of the experimental glioma 101/8. (A) Merged image. (B) Nuclei of cells stained with DAPI. (C) Immunofluorescence of BSAT1 in the tumor. (D) Immunofluorescence of GFAP in the peritumoral area of the astroglial bank. Laser scanning confocal microscopy. Scale bar, 100 µm.
Cisplatin-loaded nanoparticles (cisplatin-loaded non-targeted nanogels (NG/CDDP), cisplatin-loaded non-specific targeted nanogels (IgG-NG/CDDP), cisplatin-loaded nanogels conjugated with mAbs against Cx43 and BSAT1, respectively (Cx43-NG/CDDP and BSAT1-NG/CDDP) and controls (free cisplatin, dextrose) were intravenously administered after confirmation of brain tumor by MRI. The main characteristics of animal survival in the experimental groups are shown in and .
Figure 4 Changes in the body weight of animals in experimental groups. The groups of animals received the following preparations: DEXTROSE (the control group); CDDP, free cisplatin; NG/CDDP, non-targeted nanogels with cisplatin; IgG-NG/CDDP, nanogels with cisplatin conjugated with non-specific IgG; MAbCx43-NG/CDDP, cisplatin nanogels with mAbs against Cx43; MAbBSAT1-NG/CDDP, cisplatin nanogels with mAbs against BSAT1.
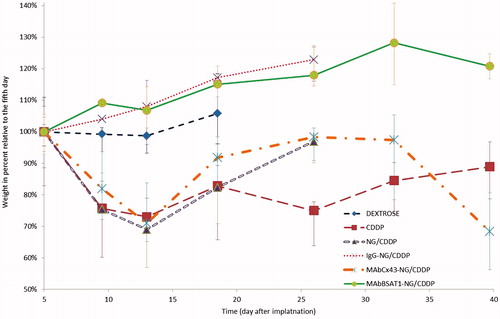
Figure 5. Survival curves of the rats after treatment with cisplatin encapsulated in nanocontainers. The groups of animals received the following preparations: DEXTROSE (the control group), dextrose (placebo); CDDP, free cisplatin; NG/CDDP, non-targeted nanogels; MAbCx43-NG/CDDP, nanogels with mAbs against Cx43; MAbBSAT1-NG/CDDP, nanogels with mAbs against BSAT1; IgG-NG/CDDP, nanogels with non-specific IgG.
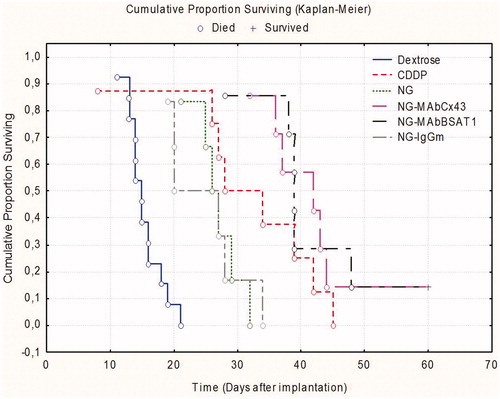
Figure 6. Morphometric analysis of gliomas in the animal groups treated with placebo (Dextrose); non-targeted nanogels (NG/CDDP); nanogels with non-specific IgG (IgG-NG/CDDP); nanogels with mAbs against Cx43 (MAbCx43-NG/CDDP) and nanogels with mAbs against BSAT1 (MAbBSAT1-NG/CDDP).
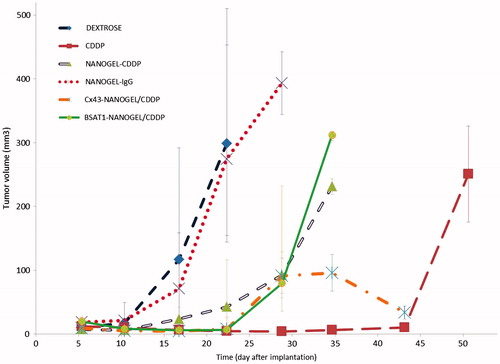
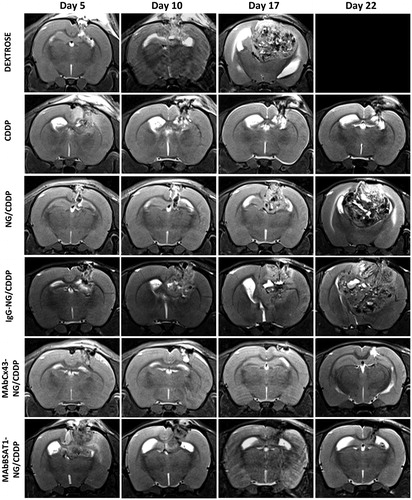
Figure 7. The dynamic MRI of the rat brain after treatment with cisplatin and non-targeted and targeted nanogels loaded with cisplatin. The abbreviations are the same as in . See the text for details.
All animals treated with 5% Dextrose died within 21 days. The death of the animals was due to extensive swelling of the brain, which was often caused by massive hemorrhage into the tumor. If glioma grew into the ventricular system, the intracranial dislocation was exacerbated by development of severe occlusive hydrocephalus (Supplementary Figure 5). The median survival of animals treated with free cisplatin at a dose of 5 mg/kg was increased significantly compared to Dextrose group. However, on days 5–15, the general condition of animals form free cisplatin group was objectively worse than that of placebo group (). Weight loss, decreased motor activity and signs of damage to the central and peripheral nervous system were detected in animals treated with cisplatin. Note that mortality in cisplatin group began even earlier than in placebo group (). However, after 15–20 days, the condition of some animals stabilized, and their lifespan increased to 40–45 days. As a result, the median survival in cisplatin group was significantly higher compared to Dextrose group.
The median survivals of animals receiving cisplatin-loaded nanogels without antibodies or conjugated with non-specific IgG (11.5 and 8.5 days, respectively) were higher than in placebo group. These values were not significantly different from each other or from that in CDDP group. However, the general condition of animals receiving cisplatin encapsulated in nanogels was significantly better than that from cisplatin group. These animals lost less weight, and their physical activity remained relatively intact until the development of symptoms of intracranial dislocation on days 22–27.
The median survivals of the rats in groups receiving nanogels conjugated with mAbs against Cx43 and BSAT1 were higher than that in all other studied groups (27 and 26.6 days, respectively) ( and ). This indicated that treatment of high-grade gliomas 101/8 with targeted cisplatin-loaded nanogels was significantly effective than treatment with non-targeted nanogels or free cisplatin.
Magnetic resonance imaging studies of glioma were performed on days 5, 10 and 15 and weekly afterwards. The volume of the glioma was determined using axial and coronary scans. Morphometric analysis demonstrated that the differences in the glioma volume were insignificant in the animals treated with NG/CDDP, IgG-NG/CDDP and placebo (). The animals treated with targeted nanogels loaded with cisplatin (MAbCx43-NG/CDDP, MAbBSAT1-NG/CDDP) had significantly lower glioma volume compared to the control group receiving 5% dextrose up to the 30th day of the study ( and ). Glioma volumes of animals treated with free cisplatin did not differ significantly from MAbCx43-NG/CDDP and MAbBSAT1-NG/CDDP groups. However, at the later stages of the experiment the distinct trend towards glioma growth was observed in cisplatin group ().
Discussion
Nanogels were prepared by the template-assisted method involving condensation of PEG-b-PMAA by Ca2+ ions, followed by chemical cross-linking of the polyion chains in the cores (Nukolova et al., Citation2011a,b). These soft polymeric materials formed monodisperse spherical nanoparticles with a diameter of ∼120–140 nm and a negative zeta-potential (−20 ± 5 mV). Nanogels can be loaded with chemotherapeutics and exhibit a high loading capacity (cisplatin, 30–35%; doxorubicin, 40–45%) (Kabanov & Vinogradov, Citation2009). Ionic strength and pH are important for efficient drug encapsulation into nanogels. Thus, we loaded cisplatin in nanogels at pH 9 and salt free conditions (Oberoi et al., Citation2011) to prevent displacement of the drug by low molecular weight counterion (such as chloride, phosphate, carbonate and acetate anions) and enlarge the inner cavity of nanogels. After encapsulation of cisplatin in nanogels, mAbs were successfully conjugated to the nanogel surface via the PEG linker. The resultant targeted nanoparticles had similar particle size and charge (123 ± 5 nm, PdI = 0.12 ± 0.05, −15 ± 3 mV) compared with free nanogels. One of the main concerns of antibody modifications was the prevention of their affinity to molecular target. Immunochemical activity of mAbs decreased by ∼10% with each step of the synthesis, probably, due to modification of groups responsible for the binding. Moreover, reduced total activity of the final conjugated antibodies can be due to their shielding by PEG or other conjugated mAbs. However, immunochemical activity of Cx43-NG and BSAT1-NG was >60% of initial activity of the free mAbs and was sufficient for further in vivo experiments.
We studied the efficacy of glioma 101/8 treatment using cisplatin-loaded nanogels. Rat glioma 101/8 was obtained by implantation of solid dimethylbenzanthracene pills into the cerebellum of female Wistar rats. Afterwards, rat glioma 101/8 was maintained by intracerebral passages and was stored in a cryopreserved state in the collection of the Research Institute of Human Morphology of the Russian Academy of Medical Sciences. The tumor was originally diagnosed as an astrocytoma. However, after multiple passages, the tumor acquired a stable structure of glioblastoma (high-grade glioma) and was sensitive to chemotherapy (Steiniger et al., Citation2004). Histological and immunohistochemical studies suggested that this experimental model of glioma is suitable for testing of new cytotoxic drugs. The orthotopic location of glioma allows to consider the different phenomena, such as penetration of the drug through the endothelial barrier in neoplastic cerebral microvessels, development of interstitial edema and increase in the hydraulic pressure in the confined space of the skull. This permits a more adequate extrapolation of experimental data to human intracerebral tumors.
Reduction of the cisplatin systemic toxicity was the first objective of our study. Significant differences in body weight loss of animals treated with free cisplatin and cisplatin-loaded nanogels clearly indicated a better tolerability of cisplatin-loaded nanogels in this model (). Despite the differences in the systemic toxicity, animals from free cisplatin group had a slightly higher median survival rate than animals from non-targeted nanogels or non-specific IgG-nanogels groups. This may be due to a lower passive penetration of nanogels (with an average diameter of 120 nm) through the endothelial barrier into intracranial tumors compared to free cisplatin. This assumption is also supported by rapid development of glioma in rats treated with NG/CDDP and IgG-NG/CDDP than that from CDDP group, as determined by morphometric analysis (). To increase the efficiency of drug delivery to the glioma, nanogels were conjugated with mAbs against the targeted proteins Cx43 and BSAT1, which are highly expressed in tumor and peritumoral area (Baklaushev et al., Citation2009; Oliveira et al., Citation2005; Baklaushev et al., Citation2011, Citation2012; Yusubalieva et al., Citation2012). MRI data confirmed that this approach significantly decreased the tumor growth (). Probably, the interaction of antibodies with the targeted surface proteins facilitated the adhesion of drug-loaded nanogels and their subsequent internalization. These selective interactions could prevent passive reverse diffusion of nanogels along the concentration gradient. As a result, animals treated with CDDP-loaded Cx43-NG and BSAT1-NG had prolonged survival time compared with all other groups (). These data suggest that the targeted delivery of CDDP-loaded nanogels to glioma was more effective in comparison with passive penetration and accumulation of CDDP-nanogel as well as free cisplatin.
Conclusion
Therefore, we successfully synthesized the targeted nanogels for cisplatin delivery to glioma. CDDP-loaded mAbCx43- and BSAT1-conjugated nanogels exhibited enhanced tumor growth inhibition and increased in the lifespanof animals in comparison with other investigated formulations. This approach could facilitate the development of new platforms for the treatment of gliomas.
Supplement Figure 1-5 and Table 1
Download PDF (748.4 KB)Acknowledgements
We thank M.A. Abakumov, T.O. Abakumova, A. V. Leopold, F.A. Koshkin, K.V. Novikova, A.N. Gabashvili and S.A. Cherepanov, students of the Department of Medicinal Nanobiotechnology of Pirogov Russian State Medical University, as well as K.P. Ionova and A.V. Makarov, researchers of the Department of Fundamental and Applied Neurobiology of the Serbsky National Research Centre for Social and Forensic Psychiatry, for their assistance in the study.
Declaration of interest
Authors state no conflict of interests. The study was supported by grants from RFBR (11-04-01806, 13-04-40202 -Н КОМФИ) and the President of Russia for Young Scientists (МК-7114.2012.7), the Russian Federation Ministry of Science and Education grant 11.G34.31.0004.
References
- Baklaushev VP, Gurina OI, Yusubalieva GM, et al. (2009). Immunofluorescent analysis of connexin-43 using monoclonal antibodies to its extracellular domain. Bull Exp Biol Med 148:725–30
- Baklaushev VP, Kardashova KSh, Gurina OI, et al. (2012). Cloning and expression of brain-specific anion transporter BSAT1 and isolation of monoclonal antibodies to its extracellular fragment. Bull Exp Biol Med 152:734–8
- Baklaushev VP, Yusubalieva GM, Tsitrin EB, et al. (2011). Visualization of connexin 43-positive cells of glioma and the periglioma zone by means of intravenously injected monoclonal antibodies. Drug Deliv 18:331–7
- Bates DC, Sin WC, Aftab Q, Naus CC. (2007). Connexin43 enhances glioma invasion by a mechanism involving the carboxy terminus. Glia 55:1554–64
- Chekhonin VP, Baklaushev VP, Yusubalieva GM, et al. (2012). Targeted delivery of liposomal nanocontainers to the peritumoral zone of glioma by means of monoclonal antibodies against GFAP and the extracellular loop of Cx43. Nanomedicine 8:63–70
- Hartmann JT, Lipp HP. (2003). Toxicity of platinum compounds. Expert Opin Pharmacother 4:889–901
- Kabanov AV, Vinogradov SV. (2009). Nanogels as pharmaceutical carriers: finite networks of infinite capabilities. Angew Chem Int Ed Engl 48:5418–29
- Li JY, Boado RJ, Pardridge WM. (2004). Blood-brain barrier genomics. J Cereb Blood Flow Metab 21:61–8
- Nukolova NV, Oberoi HS, Cohen SM, et al. (2011a). Folate-decorated nanogels for targeted therapy of ovarian cancer. Biomaterials 32:5417–26
- Nukolova NV, Yang Z, Kim JO, et al. (2011b). Polyelectrolyte nanogels decorated with monoclonal antibody for targeted drug delivery. React Funct Polym 71:315–23
- Oberoi HS, Laquer FC, Marky LA, et al. (2011). Core cross-linked block ionomer micelles as pH-responsive carriers for cis-diamminedichloroplatinum(II). J Control Release 153:64–72
- Oberoi HS, Nukolova NV, Laquer FC, et al. (2012). Cisplatin-loaded core cross-linked micelles: comparative pharmacokinetics, antitumor activity, and toxicity in mice. Int J Nanomed 7:2557–71
- Oliveira R, Christov C, Guillamo JS, et al. (2005). Contribution of gap junctional communication between tumor cells and astroglia to the invasion of the brain parenchyma by human glioblastomas. BMC Cell Biol 6:7
- Peer D, Karp JM, Hong S, et al. (2007). Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2:751–60
- Prochnow N, Dermietzel R. (2008). Connexons and cell adhesion: a romantic phase. Histochem Cell Biol 130:71–7
- Steiniger SC, Kreuter J, Khalansky AS, et al. (2004). Chemotherapy of glioblastoma in rats using doxorubicin-loaded nanoparticles. Int J Cancer 109:759–67
- Sugiyama D, Kusuhara H, Taniguchi H, et al. (2003). Functional characterization of rat brain-specific organic anion transporter (Oatp14) at the blood-brain barrier: high affinity transporter for thyroxine. J Biol Chem 278):43489–95
- Wohlfart S, Khalansky AS, Bernreuther C, et al. (2011). Treatment of glioblastoma with poly(isohexyl cyanoacrylate) nanoparticles. Int J Pharm 415:244–51
- Yusubalieva GM, Baklaushev VP, Gurina OI, et al. (2012). Antitumor effects of monoclonal antibodies to connexin 43 extracellular fragment in induced low-differentiated glioma. Bull Exp Biol Med 153:163–9