Abstract
KOB extracts are a polyherbal medicine had been prescribed for the treatment of hyperhydrosis and allergic diseases such as allergic asthma and rhinitis in oriental clinics. Therefore, the pharmacokinetic studies of the KOB extract administered orally to normal rats and rhinitis-induced rats to understand the correlation of the efficacy and plasma concentration of KOB in patients of allergic rhinitis in future were performed. The study was conducted according to administration for pure baicalin in normal rats, baicalin in KOB extract in normal rats and rhinitis-induced rats. Baicalin in rat plasma was analyzed and validated by HPLC analysis. The interday precision based on the standard deviation of replicates of quality control samples ranged from 3.6% to 7.9% with accuracy ranging from 92.9% to 101.2% for baicalin. Based on validated analysis, pharmacokinetic study was carried out. Pure baicalin in normal rats and baicalin in KOB extract in normal rats showed bimodal curves due to direct absorption and glucuronidation. The Tmax, Cmax and AUC of pure baicalin in normal rats or baicalin in KOB extract in normal rats were 12 h, 0.68 µg/ml and 9.85 µg h/ml, respectively, or 12 h, 0.46 µg/ml and 6.36 µg h/ml, respectively. The analytical method showed excellent sensitivity, precision and accuracy, being successfully employed in a pharmacokinetic study of polyherbal medicine, KOB extract. Allergic-induced condition did not affect the pharmacokinetics of KOB extracts, suggesting KOB extracts did not require dosage adjustment in subjects with allergic-induced diseases.
Introduction
Allergic rhinitis is among the world’s most common chronic diseases. Over the past several decades, the global prevalence of allergic rhinitis has increased, with the result that it now affects ∼24% of the population in many European countries (Bousquet & Khaltaev, Citation2007). The main symptoms of allergic rhinitis include nasal congestion, rhinorrhea, itching and sneezing as well as non-nasal symptoms such as burning, watery eyes or itching ears and palate (Sibbald & Rink, Citation1991; Bauchau & Durham, Citation2004; Grubbe et al., Citation2009). These symptoms can interfere with cognitive and emotional functioning and have been shown to exact a considerable toll on patients’ quality of life (Bachert, Citation2001; Canonica et al., Citation2007; Pradalier et al., Citation2007). Thus, treatment for allergic rhinitis is an important health care issue. It is used for decongestion agents or antihistamine agents to reduce allergic rhinitis symptoms.
Recently, the studies of effect of KOB03 on allergic response in ovalbumin-induced allergic rhinitis and on mast cell-mediated allergic inflammatory reactions in mast cells were reported (Jung et al., Citation2012a,Citationb). KOB03 is a polyherbal medicine prescribed in oriental clinics for the treatment of hyperhidrosis (Heo, Citation1985) and allergic diseases such as allergic asthma and rhinitis (Kim et al., Citation1991; Na et al., Citation2004; Shin, Citation2005).
Pharmacokinetic studies are useful for understanding the actions and interactions of drugs with regard to efficacy and toxicity and for evaluating the rationality and compatibility of herbs and/or prescriptions (Chen et al., Citation2002; Wang et al., Citation2008). Owing to the complexity of chemicals in compound prescriptions, representative compounds may be chosen as markers to be used to investigate the pharmacokinetics of the whole prescription (Rath et al., Citation2004; Liao et al., Citation2005), allowing the interactions of herbs or prescriptions to be clarified based on the pharmacokinetic behavior of the selected compounds.
In this study, we calculated the pharmacokinetic parameter of the baicalin in KOB extract and compared the pharmacokinetic behavior of the KOB extract administered orally to normal and rhinitis-induced model rats to understand the correlation of the efficacy and plasma concentration effect of KOB extract in patients of allergic rhinitis in future.
Materials and methods
Materials
Baicalin was isolated from Scutellariae Radix (Scutellaria bacicalensis Georgi), provided from Prof. Y. H. Kim of Chungnam National University (Daejeon, Korea). The purity of baicalin was at least 86%, as confirmed by high-performance liquid chromatography (HPLC). HPLC-grade methanol was purchased from Samchun Chemical Co., Ltd. (Seoul, Korea). All water was purified via ultra-filtration.
Animals
Male Sprague–Dawley rats (240–270 g) were purchased from Central Lab. Animal Inc. (Seoul, Korea). Rats were housed in an air conditioned room at a temperature of 23 ± 2 °C with a relative humidity of 55 ± 10%, provided with water and standard food and acclimated in the laboratory for at least a week prior to experimentation. Rats were fasted for 12 h before oral administration with the exception of free access to water. Allergic model rats were established through rats were given an intraperitoneal injection of 8 mg/kg body weight of the mast cell degranulator, compound 48/80 (Jung et al., Citation2013).
Instrumentation
High-performance liquid chromatography was performed with a HP Agilent 1100 series HPLC system (Agilent Technology, Santa Clara, CA) equipped UV detector. A microcentrifuge 1730 MR (Gyrozen Co., Ltd., Daejeon, Korea), an electronic balance (Mettler-Toledo International Inc., Switzerland), a vortex mixer (Scientific Industries Inc., NY) and a nitrogen dryer (EYELA Co., Tokyo, Japan) were also used.
Preparation of KOB extract
KOB extracts was received from Hanpoong Pharm & Food Co., Ltd. (Jeonju, Korea). Briefly, water of 20-fold volume was added to the mixture of Atractylodis Rhizoma Alba (Atractylodes japonica Koidz), Astragali Radix (Astragalus membranaceus Bunge), Saposhnikoviae Radix (Saposhnikovia divaricata Schischkin), Osterici Radix (Ostericum koreanum maximowicz) and Scutellariae Radix (Scutellaria bacicalensis Georgi) (8:6:4:4:4) and then heated at ∼95∼100 °C for 4 h. An extract was filtered using gauze and vacuum-dried.
Chromatography
Baicalin, as an index substance of KOB extract, was analyzed by the HPLC method. HPLC was run using an isocratic method on a Luna C18, 5 μm, 250 mm × 4.6 mm column (Phenomenex, Torrance, CA). Column temperature was 30 ± 1 °C and sample temperature was room temperature. The injection volume was 10 µl and the column flow rate was 1.0 ml/min. Mobile phase was the mixture of 35% (v/v) of acetonitrile and 65% (v/v) of water in the presence of 0.5% phosphoric acid. UV detection of baicalin was performed at 277 nm.
Oral administration of extracts and collecting of blood
Baicalin and KOB were dissolved in water. Baicalin was administered at doses of 100 mg/kg (n = 3) by oral gavage in normal rats. KOB was administered at 1667 mg/kg (containing 100 baicalin mg/kg, n = 3) by oral gavage in normal and allergic-induced rats. Blood samples (1 ml) were collected at 0 (to serve as a control), 0.25, 0.5, 1, 2, 4, 8, 12 and 24 h after drug administration, then immediately transferred into 1.5 ml tubes and centrifuged at 16 000rpm for 10 min in 4 °C. The supernatant was stored at −70 °C for further analysis. This animal experiment procedure was approved by the Animal Ethics Board of Chungnam National University.
Plasma pretreatment
Plasma samples were thawed by cooling. Then, 100 µl of plasma was spiked with 50 µl of 1 M KH2PO4 solution and 400 µl of methanol by vortex mixing for 1 min. The mixture was centrifuged at 9000 rpm for 10 min in 4 °C. The supernatant was transferred to a clean tube and evaporated to dryness under a nitrogen gas stream in 50 °C. The residue was dissolved with 100 μl of mobile phase.
Method validation
Preparation of standards
A stock solution of baicalin was prepared in ethanol. Calibration samples were prepared by spiking blank plasma with this stock solution to obtain final concentrations of 1, 2, 5, 10 and 15 μg/ml. All solutions were stored at 4 °C before analysis. Quality control (QC) samples containing various concentrations of baicalin (1, 2 and 10 μg/ml) were prepared in a similar manner.
Precision, accuracy and stability
Intra-day precision was determined by obtaining three concentrations of baicalin in QC samples, 5 times on the same day. Inter-day precision was determined by successive concentration determinations over a 5-day period. Precision is indicated as the coefficient of variation (RSD). Accuracy was calculated from the mean value of the observed concentrations and the theoretical concentrations. Freeze/thaw stability was evaluated by analyzing QC samples at three concentrations after multiple cycles of freezing (at −70 °C) and thawing (at room temperature).
Pharmacokinetic analysis
Pharmacokinetic parameters are expressed as means ± SD. Plasma concentration–time curves were plotted and pharmacokinetic parameters were calculated using WinNonlin Standard Edition software (Ver. 2.1) (Seoul, Korea). Comparison of parameters between standard baicalin and KOB groups in normal rats were carried out by Student’s t-test. Also, the pharmacokinetic parameters of baicalin in normal- and allergic-induced rats were compared.
Results and discussion
HPLC with isocratic elution consisting of acetonitrile–water containing 0.5% phosphoric acid (35:65 v/v) yielded baseline resolution for baicalin. Among the different mobile phase modifications tested, phosphoric acid appeared to have the best separation, as it prevented peak tailing (). Other endogenous plasma constituents interfered with baicalin were confirmed by observing chromatograms derived from processed blank plasma samples. Typical chromatograms of blank plasma and plasma samples containing baicalin are shown in . The retention time of baicalin was 4.7 min, with no endogenous interference or matrix effect.
Figure 1. Representative HPLC chromatograms of baicalin. (A) Mixture of distilled water and acetonitrile (65:35 v/v) and (B) mixture of phosphoric acid in water and acetonitrile (65:35 v/v) as a mobile phase.
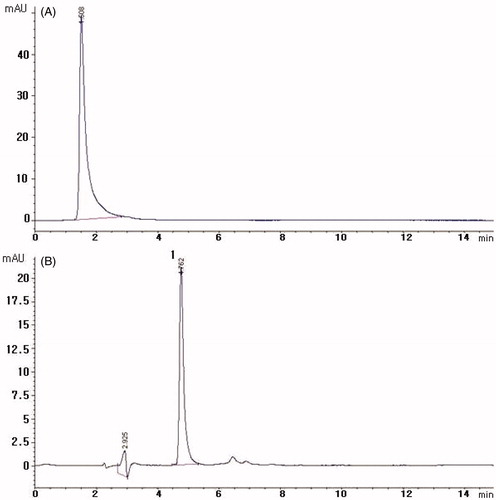
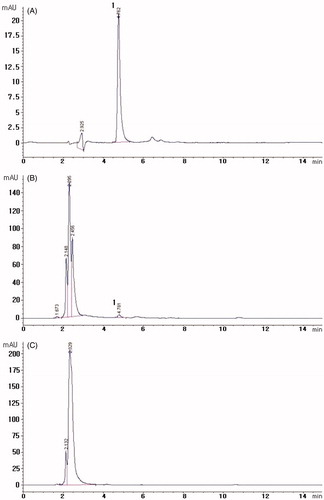
Figure 2. Representative HPLC chromatograms of plasma samples. (A) Chromatogram of stock standard solution, (B) plasma sample after oral administration of baicalin and (C) chromatogram of blank plasma. Peak: 1. Baicalin.
Method validation was performed according to guidelines set by the US FDA (http://www.fda.gov/cder/guidance/index.htm#Biopharmaceutics). The spiked standard samples at five concentration levels over the range of 1.0–15.0 μg/ml were prepared. Calibration curves were generated by plotting chromatographic peak area as a function of baicalin concentration. Peak areas of baicalin in rat plasma displayed good linearity, described by the following regression lines: y = 22.562 x + 0.482 (where y is the peak area and x is the baicalin concentration in μg/ml). QC samples at three concentrations (1.0, 2.0 and 10.0 μg/ml) were analyzed to assess the precision and accuracy of the proposed method. Six replicates were analyzed in each of the three consecutive analytical runs. The accuracy was expressed by the relative error (RE), and the precision was evaluated by the relative standard deviation (RSD). Recovery of extraction procedure was also evaluated at three concentration levels. It was determined by comparing the mean peak areas (n = 5 for each concentration level) obtained from plasma samples spiked before extraction with those from plasma samples spiked after extraction. The intra-day and inter-day precision determined from the replicate analysis of QC samples were <11.2% and 7.9%, respectively. The accuracy of the method ranged from 92.7% to 103.7%. The results are listed in , which indicate good analytical characteristics of this assay. To evaluate sample stability after freeze–thaw cycles and at room temperature, six replicates of QC samples at each of 1.0, 2.0 and 10.0 μg/ml concentrations were subjected to three freeze–thaw (−80 to 25 °C) cycles. In the freezing–thaw stability test, the concentrations of baicalin were between 99.4% and 122.0% of the initial values ().
Table 1. The intra-day and inter-day precision and accuracy value for baicalin in blank plasma.
Table 2. Stability of baicalin in blank plasma according to the repeat of freezing and thawing.
The validated method was successfully applied to the pharmacokinetic study of baicalin in normal rats after the oral administration of baicalin and KOB extracts, and in allergic-induced rats after oral administration of KOB extracts. In normal rats, pharmacokinetic profile of pure baicalin and baicalin-contained KOB extracts showed two plasma concentration peaks of baicalin (). It was reported that generally flavone glucuronides show bimodal pharmacokinetic profile (Lu et al., Citation2007; Tong et al., Citation2012). The first absorption occurred immediately within 1 h. This absorption was considered owing to the direct absorption. The second absorption occurred at 8–12 h. This absorption probably occurred through glucuronidation. During glucuronidation, enteric circulation and enterohepatic circulation may affect the second peak (Lu et al., Citation2007; Tong et al., Citation2012). When standard baicalin was administered orally, normal rats showed 0.683 μg/ml of Cmax and normal rats administered with KOB extracts showed 0.457 μg/ml of Cmax. However, allergic-induced rat group administered with KOB extracts showed 1.991 μg/ml of Cmax, suggesting faster absorption in sick condition than the normal condition. This correlated with the publication that the validated method has been successfully applied for comparing pharmacokinetic profiles of analytes in normal and Alzheimer’s disease rat plasma, and a significant increase in the value of Cmax was observed for polygalaxanthone III and ginsenoside Rd in the model group after oral administration of traditional Chinese medicine, Kai–Xin–San (KXS), compared to the normal group (Lv et al., Citation2013). However, AUC in normal group administered with KOB extracts showed 6.37 μg h/ml and allergic-induced group administered with KOB extracts showed the value of 6.25 μg h/ml of AUC. This result had a correlation with a publication that the pharmacokinetic parameters except for Cmax and AUC were not affected by the administration dose. As well as, those except for Cmax were not affected by the thyroid function (Okamura et al., Citation1986) (). Taken together, allergic-induced condition did not affect the pharmacokinetics of KOB extracts, suggesting KOB extracts did not require dosage adjustment in subjects with allergic-induced diseases (Tebas et al., Citation2008; Graefe-Mody et al., Citation2012).
Figure 3. Plasma concentration–time curves. (A) Baicalin standard in normal rats, (B) KOB extracts to normal rats and (C) KOB extracts to allergic-induced rats.
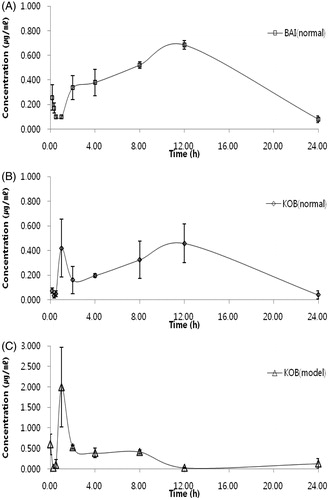
Table 3. Pharmacokinetic parameters of marker compounds in rat plasma.
Conclusion
The analytical method showed excellent sensitivity, precision and accuracy, being successfully employed in the pharmacokinetic study of polyherbal medicine, KOB extract. Allergic-induced condition did not affect the pharmacokinetics of KOB extracts, suggesting KOB extracts did not require dosage adjustment in subjects with allergic-induced diseases.
Declaration of interest
The authors report no declarations of interest. This work was supported by grants from the Ministry of Health & Welfare, Republic of Korea, for the development of herbal medicine drugs for allergic rhinitis (F090002 to Y.-K.P.) and by the Priority Research Centers Program (2009-0093815) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology.
References
- Bachert C. (2001). Decongestant efficacy of desloratadine in patients with seasonal allergic rhinitis. Allergy 56:14–20
- Bauchau V, Durham SR. (2004). Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J 24:758–64
- Bousquet J, Khaltaev N. (2007). Global surveillance, prevention and control of chronic respiratory diseases: a comprehensive approach, Geneva, Switzerland: World Health Organization, 1–155
- Canonica GW, Bousquet J, Mullol J, et al. (2007). A survey of the burden of allergic rhinitis in Europe. Allergy 62:17–25
- Chen LC, Chou MH, Lin MF, Yang LL. (2002). Pharmacokinetics of paeoniflorin after oral administration of Shao-yao Gan-chao Tang in mice. Jpn J Pharmacol 88:250–5
- Graefe-Mody U, Rose P, Retlich S, et al. (2012). Pharmacokinetics of linagliptin in subjects with hepatic impairment. Br J Clin Pharmacol 74:75–85
- Grubbe RE, Lumry WR, Anolik R. (2009). Efficacy and safety of desloratadine/pseudoephedrine combination vs its components in seasonal allergic rhinitis. J Investig Allergol Clin Immunol 19:117–24
- Heo J. (1985). Dongui Bogam. Seoul, Republic of Korea: Dae Sung Publisher Co, 123
- Jung HW, Jung JK, Cho CW, et al. (2012a). Antiallergic effect of KOB03, a polyherbal medicine, on mast cell-mediated allergic response in ovalbumin-induced allergic rhinitis mouse and human mast cells. J Ethnopharmacol 142:684–93
- Jung HW, Jung JK, Kim YH, et al. (2012b). Effect of KOB03, a polyherbal medicine, on ovalbumin-induced allergic rhinitis in guinea pigs. Chin Med 7:1–10
- Jung HW, Jung JK, Park YK. (2013). Comparison of the efficacy of KOB03, ketotifen, and montelukast in an experimental mouse model of allergic rhinitis. Int Immunopharmacol 16:254–60
- Kim CM, Heo MY, Kim HP, et al. (1991). Pharmacological activities of water extracts of Umbelliferae plants. Arch Pharm Res 14:87–92
- Liao QF, Yang W, Jia Y, et al. (2005). LC-MS determination and pharmacokinetic studies of ursolic acid in rat plasma after administration of the traditional Chinese medicine preparation Lu-Ying extract. J Pharm Soc Jpn 125:509–15
- Lu T, Song J, Huang F, et al. (2007). Comparative pharmacokinetics of baicalin after oral administration of pure baicalin, Radix scutellariae extract and Huang-Lian-Jie-Du-Tang to rats. J Ethnopharmacol 110:412–8
- Lv C, Li Q, Zhang Y, et al. (2013). A UFLC-MS/MS method with a switching ionization mode for simultaneous quantitation of polygalaxanthone III, four ginsenosides and tumulosic acid in rat plasma: application to a comparative pharmacokinetic study in normal and Alzheimer’s disease rats. J Mass Spectrom 48:904–13
- Na HJ, Jeong HJ, Hwang CY, et al. (2004). Effect of Boo Yong-Tang on mast cell-mediated allergic reaction. Immunopharm Immunotoxicol 26:445–54
- Okamura Y, Shigemasa C, Tatsuhara T. (1986). Pharmacokinetics of methimazole in normal subjects and hyperthyroid patients. Endocrinol Jpn 33:605–15
- Pradalier A, Neukirch C, Dreyfus I, Devillier P. (2007). Desloratadine improves quality of life and symptom severity in patients with allergic rhinitis. Allergy 62:1331–4
- Rath K, Taxis K, Walz G, et al. (2004). Pharmacokinetic study of artemisinin after oral intake of a traditional preparation of Artemisia Annua L. (Annual Worm wood). Am J Trop Med Hyg 70:128–32
- Shin S. (2005). In vitro effects of essential oils from Ostericum koreanum against antibiotic-resistant Salmonella spp. Arch Pharm Res 28:765–9
- Sibbald B, Rink E. (1991). Epidemiology of seasonal and perennial rhinitis: clinical presentation and medical history. Thorax 46:895–901
- Tebas P, Bellos N, Lucasti C, et al. (2008). Enfuvirtide does not require dose adjustment in patients with chronic kidney failure: results of a pharmacokinetic study of enfuvirtide in HIV-1-infected patients with impaired kidney function. J Acquir Immune Defic Syndr 47:342–5
- Tong L, Wang M, Zhang L, et al. (2012). Simultaneous determination of baicalin, wogonoside, baicalein, wogonin, oroxylin A and chrysin of Radix scutellariae extract in rat plasma by liquid chromatography tandem mass spectrometry. J Pharm Biomed Anal 70:6–12
- Wang CH, Wang R, Cheng XM, et al. (2008). Comparative pharmacokinetic study of paeoniflorin after oral administration of decoction of Radix Paeoniae Rubra and Radix Paeoniae Alba in rats. J Ethnopharmacol 117:467–72
