Abstract
Context: Local delivery systems for treatment of intractable inner ear disorders have been attempted by many investigators.
Objective: To evaluate the permeability and safety of a drug delivery system for the inner ear using a poly(2-hydroxyethyl aspartamide) (PHEA) polymersome.
Materials and methods: One-month-old male C57/BL6 mice were used. We administered the same amount of the fluorescent dye, Nile red, into the middle ear in two forms: loaded in PHEA polymersomes (NP group) or diluted in ethanol (NR group). At 1 day after administration, we harvested the cochlea and counted visible red particles in the tissues of cochlea under confocal microscopy and compared the groups. In a safety evaluation, 1 week after the same surgery, we conducted hearing tests and histological evaluations of the bulla and cochlea, and compared the results with those of the sham operation and negative control groups.
Results: In terms of permeability, the number of red particles in the organ of Corti was increased significantly in the NP group, and three subjects in the NP group showed uptake of red particles in inner hair cells. However, there was no statistically significant difference in the observations in the lateral wall or modiolus. In safety tests, the NP and sham-operation groups showed decreased DPOAE responses and mildly swollen middle ear mucosa, compared with the negative control group, which was thought to be the result of postoperative changes.
Conclusions: PHEA nanoparticles may have utility as a drug carrier into the inner ear in terms of both permeability and safety.
Introduction
Hearing loss is the common endpoint of many inner ear disorders, including presbycusis, sudden sensorineural hearing loss (SSNHL), genetic diseases, trauma, exposure to noise and ototoxic medications, and autoimmune inner ear disease. However, the therapeutic options for these diseases are limited. In fact, generally, the therapeutic options are limited to hearing aids and cochlear implants for chronic or slowly progressive hearing loss, and for acute hearing loss, systemic steroids have been the primary therapeutic choice.
Although systemic steroids have met with some success in several inner ear diseases with acute hearing loss, their clinical utility is limited by undesirable side effects arising from the high systemic doses required to achieve sufficient cochlear fluid levels of the drug (Parnes et al., Citation1999). Certain physiological and anatomical features of the inner ear, such as the limited cochlear blood supply and the blood–inner ear barrier, can compromise the availability of systemic drug to target cells within the cochlea. Other disadvantages of systemic therapies are: (1) many drugs have short plasma half-lives; (2) if their molecular weight is high, parenteral resorption is impaired; and (3) there are large inter-individual variations due to variation in body metabolism (McCall et al., Citation2010).
To address these problems, several local delivery systems for the inner ear for treatment of a variety of inner ear disorders have been investigated. The inner ear is particularly well-suited for local drug delivery. It is isolated from the rest of the body by the blood–inner ear barrier, and the perilymph and endolymph fluids permit liquids to reach the entire cochlea rapidly (Juhn et al., 1982; Chen et al., Citation2010). Local delivery to the inner ear bypasses the blood–inner ear barrier, allowing drugs to reach their intended targets more directly with use of lower doses. Thus, a higher concentration of the drug in the inner ear can be obtained, and systemic side effects can be minimized. Presently, there are two main routes for local drug delivery to inner ear: (1) intracochlear or intravestibular drug delivery, and (2) intratympanic (extracochlear) drug application to the round window membrane (Chen et al., Citation2010). Because opening of the human inner ear for the sole purpose of local drug delivery is performed rarely, intracochlear or intravestibular drug delivery is generally limited, whereas intratympanic drug delivery is in widespread use for several inner ear diseases, including idiopathic sudden sensorineural hearing loss and Meniere’s disease (Slattery et al., 2005; Sajjadi & Paparella, 2008).
Among several local delivery systems, nanoparticle-based delivery has been shown by several authors to have advantages as a local drug delivery system (Chen et al., Citation2010; Roy et al., Citation2010). The aims of this study were to develop a drug delivery system for the inner ear using poly(amino acid)-based nanoparticles, and to investigate inner ear permeability and safety after middle-ear administration using various inner ear functional tests and histological examination.
Materials and methods
Design of experiments
First, we evaluated the permeability of the poly(2-hydroxyethyl aspartamide) (PHEA) polymersome into inner ear, and second, we evaluated its safety. In the evaluation of permeability, we divided animals into two groups: the nanoparticle group (NP) and the Nile red group (NR) and administered the same amount of the hydrophobic fluorescent dye, Nile red, into the middle ear in two forms. In the NP group, the dye was loaded in poly(2-hydroxyethyl aspartamide) (PHEA) polymersomes, and in the NR group, it was diluted in ethanol at the same concentration as in the NP group. In the NP group, fluorescein isothiocyanate (FITC) was attached to the polymersome to compare the distribution of the polymersome and Nile red in the cochlea. At 24 h after administration, we removed the cochlea from animals in each group, and harvested three inner ear tissues from the cochlea: the organ of Corti, the lateral wall in the basal turn and the modiolus, according to a previous report (Zhang et al., Citation2010). Using confocal microscopy, we evaluated the permeability of the PHEA polymersome by comparing the red signals of the specimens in each group.
In the safety evaluation, we established two control groups in addition to the NP group: a sham operation group to evaluate the effects of surgery itself, and a negative control group. In the sham operation group, we conducted the same surgery but administered saline into the middle ear, and, in the negative control group, no surgery was conducted. At 1 week after administration of the PHEA polymersome or saline, we evaluated hearing function in the mice by determining distortion product otoacoustic emissions (DPOAE) and auditory brainstem responses (ABRs) in the three groups, and morphological changes in the middle ear and inner ear by light microscopy.
All procedures were performed in accordance with national ethics guidelines. This study was approved by the Institutional Review Board of our hospital (CUMC-2012-0066-03).
Manufacture and characterization of PHEA polymersomes
Poly(succinimide) (PSI) was synthesized by the thermal condensation of L-aspartic acid (Sigma Aldrich, St. Louis, MO) with phosphoric acid (85%, Junsei, Tokyo, Japan) as reported previously (Kang et al., Citation2002), and the ring was opened with 2-aminoethanol (Sigma Aldrich, St. Louis, MO) to give PHEA. Purified PSI (10 g, 103 mmol equiv. as succinimide units) was dissolved in 80 mL of N,N-dimethylformamide (Merck, Darmstadt, Germany; DMF), and then 15 mL of 2-aminoethanol was added drop-wise (1.2 equiv. of succinimide units). The reaction temperature was maintained at 30 °C for 6 h. The reaction mixture was precipitated in 1:1 ethanol/ethyl ether (5 equiv. volumes of DMF), followed by washing with methanol and was dried in vacuo.
FITC-labeled PHEA was prepared by reaction of 1.41 g (1 mmol NH2 group of the aspartic unit) of PHEA-NH2 in DMF (20 mL) with 0.156 g (0.4 mmol) of FITC at room temperature for 12 h. The reaction mixture was dialyzed against distilled water and freeze-dried. Then, the reaction mix (20 mg) and Nile red (1 mg) were dissolved in DMSO/H2O (1:1) and incubated for 12 h. Non-entrapped Nile red was removed by dialysis using a buffer solution. The final loading of Nile red into the PHEA polymersomes was 64 µg/mL. Before middle ear administration, the Nile red-loaded polymersome was diluted to 2 mg/mL with distilled water.
Animals and surgery
One-month-old male C57/BL6 mice were used. To evaluate the permeability of the PHEA polymersome, five mice were allocated to the nanoparticle group (NP) and five to the Nile red group (NR). For the safety evaluation, another 12 mice were divided evenly into three groups: NP group, sham-operation group and negative control group.
Before the surgical procedure, the mice were anesthetized with a mixture of 30 mg/kg Zoletil (Virbac, Carros, France) and 10 mg/kg Rompun (Bayer, Leverkusen, Germany). The animals were placed on a thermo-regulated heated pad in a supine position, and the ventral side of the neck was shaved and sterilized with 70% ethanol. A midline incision was made and the thymus was dissected to expose the posterior belly of the left-side digastric muscle. Then, the posterior belly of the digastric muscle was resected and the left-side bulla was exposed. A hole was made in the bulla with fine forceps, and the hole was widened until the round window niche was exposed. The diameter of the hole made in the bulla was about 2 mm. After finding the niche, a small piece of gelatin sponge (Johnson and Johnson, New Brunswick, New Jersey), which was immersed with 20 µL of PHEA polymersome or dilute Nile red dye, was positioned over that. Then, the hole was blocked with bone wax (B. Braun, Melsungen, Germany), and the wound was closed. After the operation, Rimadyl (1.0 mg/kg, Pfizer, Surrey, UK) was injected to relieve pain. Baytril (10 mg/kg, Orion, Hamburg, Germany) was injected i.p. once per day to prevent potential middle ear infection.
At 24 h after the operation, the bullae were fixed by cardiac perfusion with 4% paraformaldehyde (Merck, Darmstadt, Germany). The isolated cochleae were rinsed with tap water for 1 min to remove potential free PHEA polymersomes remaining on the outer surface and further stored in the fixation solution for 2 h. After washing with PBS, the isolated cochleae were dissected under a stereomicroscope, and the organ of Corti, the lateral wall and the modiolus of basal turn were harvested (). The specimens were stained with 4′,6-diamidino-2-phenylindole (DAPI; 10 mg/mL, Sigma Aldrich, St. Louis, MO) for 10 min at room temperature in the dark. After washing with PBS, the specimens were placed on slides and mounted with Fluoromount (Sigma Aldrich, St. Louis, MO) for confocal microscopy.
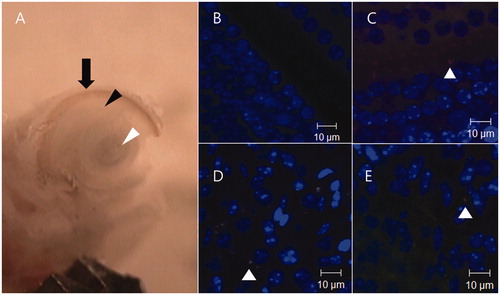
Figure 1. Harvest of inner ear tissues and negative and positive control confocal microscopy images. After drilling out the cochlea wall, the organ of Corti (black arrow head), the lateral wall (arrow), and the modiolus (white arrow head) of basal turn were harvested. Under a confocal microscope, the negative control image of the organ of Corti was acquired after modulating the intensity of the laser to remove auto-fluorescence (B). Twenty minutes after infusion of the nanoparticles to the scala tympani through the round window membrane, positive control images of the organ of Corti (C), modiolus (D), lateral wall (E) were also acquired.
To evaluate safety, 20 µL of PHEA polymersome (NP group) or 0.9 % saline (sham operation group) was administered to the middle ear by the surgical procedure described above. At 1 day after surgery, we opened the bulla of the subjects again, and removed the saline- or nanoparticle-immersed gelatin sponge from the middle ear because it could attenuate sound from outside and produce errors in the hearing tests. At 1 week after surgery, we conducted audiologic tests in the test, sham operation, and negative control groups first, and then removed the cochlea and bullae from the mice for the histological examination.
Evaluation of the permeability of PHEA polymersomes to inner ear
The harvested specimens were observed under a confocal microscope (LSM5 live configuration Variotwo VRGB, Zeiss, Jena, Germany).We used a 415–480 nm bandpass filter for the DAPI signal, a 500–525 nm bandpass filter for FITC, and a 550-nm long-pass filter for Nile red. To remove the effects of auto-fluorescence by tissue in the cochlea, we made a negative control image from a non-treated mouse. Three inner-ear tissues (organ of Corti, lateral wall and modiolus) were harvested from the non-treated mouse in the same way, and by confocal microscopy, we identified the maximum laser intensity that did not elicit a red or green signal from the control specimen ().
After setting the standard laser intensity to control for auto-fluorescence in the three inner ear tissues, we also generated a positive control image. After anesthesia, we made a large hole in the bulla to expose the round window membrane, and PHEA nanoparticles loaded with Nile red were injected into the scala tympani through the round window membrane. Then, 20 min after injection, the cochlea was harvested from the mouse, and inner ear tissues from the cochlea were observed under a confocal microscope using the standard laser intensity ().
Next, we evaluated inner ear tissues from the NP and NR groups using the standard laser intensity. We obtained a 400× magnified image of each tissue, and selected the location in each tissue that showed the most visible red particles using the 550 nm long-pass filter. Then, we enumerated the visible red particles in each image and compared them between the groups.
Evaluation of the safety of PHEA polymersomes in the middle and inner ears
At 1 week after surgery, we performed hearing tests using DPOAE and ABR in the three groups, and the results of NP and sham operation group was compared with that of negative control group. Auditory testing was performed in a soundproof chamber as reported previously (Park et al., Citation2011). Prior to acoustic testing, mice were anesthetized with an intraperitoneal injection of a mixture of 30 mg/kg Zoletil (Virbac, Carros, France) and 10 mg/kg Rompun (Bayer, Darmstadt, Germany) and boosted with one-fifth of the original dose, as required. Mouse body temperature was maintained with a heating pad. In ABR measurement, the evoked ABR thresholds were recorded differentially from the scalp. Responses were recorded using subdermal needle electrodes at the vertex, below the pinna of the left ear (reference), and below the contralateral ear (ground). A click (100 µs duration; 31 Hz) sound was used for threshold measurements. ABR measurements were made using an Intelligent Hearing System (IHS) Smart EP System, running the IHS High-frequency Software (v. 2.33) and using IHS high-frequency transducers (HFT9911-20-0035, IHS, Miami, FL, USA). Evoked potentials were amplified (×200 000), bandpass-filtered (100–3000 Hz), and averaged over 1024 sweeps. Recording epochs comprised the 12 ms following stimulus onset. Thresholds were determined for a broadband click stimuli by decreasing the sound pressure level (SPL) in 10 dB decrements until the lowest level at which a distinct ABR wave pattern recognizable by two of the investigators was reached.
DPOAEs were recorded using the Smart OAE system (v. 4.26; IHS). DPOAE measurements were conducted for pure tones of 6–32 kHz. An Etymotic 10B+ probe was inserted into the external ear canal and used in conjunction with two different types of transducer, depending on the range of the stimulation frequency. An Etymotic ER2 stimulator was used for frequencies ranging from 6 to 16 kHz. For frequencies ranging from 16 to 32 kHz, an IHS high-frequency transducer was used. Stimulus response signals were sampled at a rate of 128 kHz using a 16-bit D/A converter. The L1 amplitude was set to 65 dB SPL and the L2 amplitude to 55 dB SPL. Frequencies were acquired with a ratio of frequency 2 (F2) to frequency 1 (F1) of 1.22. Five stimulation levels, ranging from 65 to 25 dB SPL in 10-dB steps, were used. In total, four blocks were acquired; each block consisted of 32 sweeps.
Finally, we removed the cochleae and bullae from the mice. The bullae were perfused with 4% PFA and maintained in fixative overnight at 4 °C. After rinsing with PBS, the bullae were decalcified with 5% EDTA for 2–4 days. The bullae were dehydrated using ethanol, and embedded in paraffin wax. Embedded bullae were sectioned and stained with hematoxylin and eosin, and we investigated the thickness of the middle ear mucosa and the presence of inflammation by light microscopy. The cochleae were perfused through the round and oval windows with 2% paraformaldehyde and 2% glutaraldehyde in 0.1 M phosphate-buffered saline (PBS, pH 7.4) and incubated in the same fixative overnight at 4 °C. The cochleae were then rinsed with 0.1 M PBS and incubated in 1% osmium tetroxide overnight, followed by immersion in 5% EDTA for 2–4 days. The decalcified cochlea were then dehydrated in ethanol and propylene oxide, embedded in Araldite 502 resin (Electron Microscopy Sciences, Fort Washington, PA) and then sectioned at 5 µm. After staining with toluidine blue and mounting in Permount on microscope slides, five mid-modiolar sections with clearly visible whole organ of Corti (OC) from the base to the apex were chosen for pictures at a magnification of 400×. The ImageJ software (National Institute of Health, Bethesda, MD) was used for morphological observations.
Results
Characteristics of PHEA polymersomes.
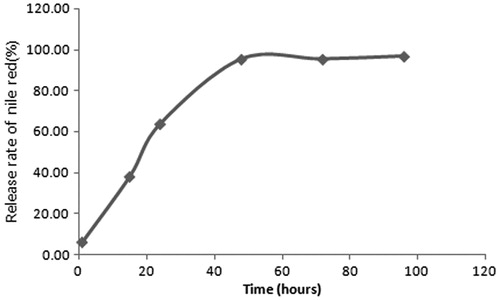
A size and zeta potential measurement of the manufactured PHEA nanoparticles by Dynamic light scattering (NANOPHOX, Sympatec, Clausthal-Zellerfeld, Germany), indicated a hydrodynamic diameter of 27.4 ± 16.2 nm and a zeta potential of −35 ± 4.3 mV. The release profile of Nile red, which was obtained by representing the percentage of nile red release with respect to the amount of the encapsulated Nile red, showed about 100% release of Nile red within 60 hours ().
Evaluation of the permeability of PHEA polymersomes to the inner ear
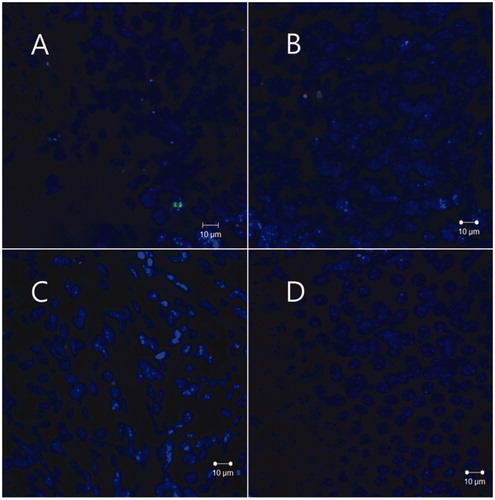
The NP group showed significantly more visible red particles in the organ of Corti than the NR group, and, in particular, three mice in the NP group showed uptake of red particles into inner hair cells, whereas only one mouse in the NR group showed two particles in the supporting cells of the organ of Corti (, ). In the NP group, the distribution of the green FITC signal, which was chemically attached to the outer surface of the polymersome, corresponded with the distribution of the red signal of Nile red ().
Figure 3. Uptake of nanoparticles (arrows) in the inner hair cells 24 h after round window administration. Three mice in the NP group showed uptake of red particles into inner hair cells. A mouse among them, showed evident green signals of FITC (B) and red signals of nile red (C), and the distributions of both signals roughly corresponded in merged image (D). Merged images of the other two mice also showed uptake of nanoparticles into inner hair cells (E, F).
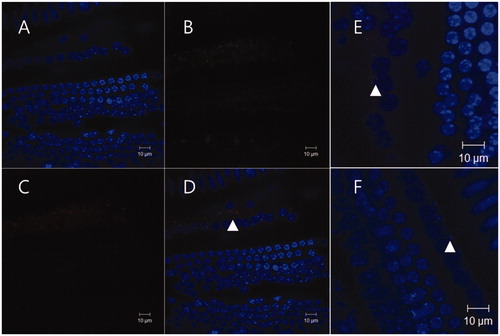
Table 1. Presence of red particles in the organ of Corti, and numbers of visible particles in three inner ear tissues (confocal microscopy images at ×400).
In observations of the lateral wall and modiolus, the mean particle numbers in the two groups showed no difference, but the fluorescence of the red particles was more intense (), and more mice in the NP group exhibited red particles in inner ear tissues than in the NR group (four versus three in the lateral wall, and five versus three in the modiolus).
Evaluation of the safety of PHEA polymersomes in the middle and inner ear
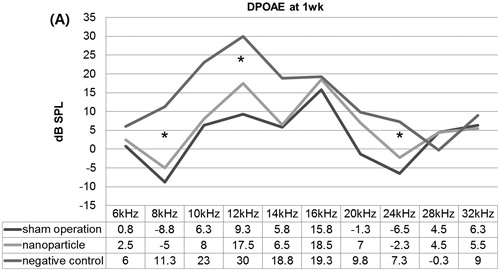
In the ABR test at 1 week after the operation, the three groups showed similar mean thresholds to the click sound: 23.8 ± 2.5 dB SPL in the NP group, 30 ± 4.1 dB SPL in the sham operation group and 26.3 ± 2.5 dB SPL in the negative control group. However, in the DPOAE test at 1 week after the operation, the NP group showed significantly lower DPOAE levels at 8, 12 and 24 kHz versus the negative control, but similar DPOAE levels to the sham operation group ().
Figure 5. Distortion product otoacoustic emissions (DPOAE) at 1 week after the operation. The nanoparticle group showed significantly lower DPOAE levels at 8, 12 and 24 kHz compared with the negative controls, but a similar DPOAE level as the sham operation group. *P <0.05.
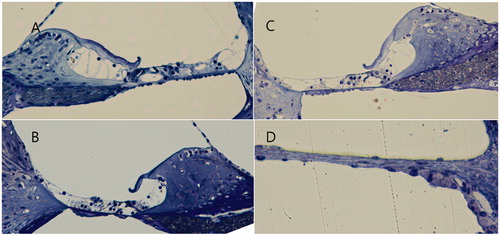
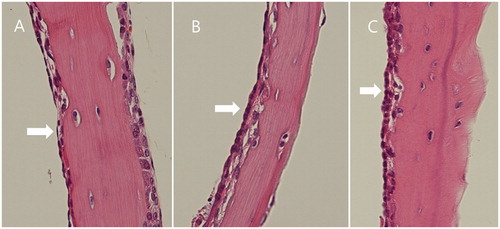
The middle ear mucosae in the NP and sham operation groups appeared mildly swollen, compared with the normal-looking mucosa of the negative control group, but no other signs of inflammation were evident ().
Figure 6. Middle ear mucosa (arrow) at 1 week after the operation. Compared with the negative control (A), the nanoparticle (C) and sham operation (B) groups showed a swollen and thickened mucosa.
Finally, the light microscopy images of the organ of Corti and round window membrane of the NP group showed that the inner ear structures were well preserved at all turns of the cochlea; the round window membrane also showed a normal appearance ().
Discussion
Interest in administering medications to the middle ear for inner ear absorption has resulted in the development of technologies to improve delivery. Current strategies in various stages of development and use include intratympanic injection, the Silverstein Microwick (Micromedics, St. Paul, MN), microcatheter implantation, hydrogels and nanoparticles (McCall et al., Citation2010). Among them, nanoparticle-based drug delivery may offer increased efficacy and reduced drug-associated side effects. The increased efficacy will occur in part as a consequence of the ability to target the drug, within the nanoparticles, to the site within the tissue where the therapeutic effect is required (Gelperina et al., Citation2005). The surface of nanoparticles can also be functionalized by attaching specific antibodies or ligands to the outer surface, to target specific cells or to ease permeation through cell membranes (Roy et al., Citation2010; Yang et al., Citation2008). In recent research, two 12-mer peptides (A665 and A666) with affinity to prestin were identified, and polymersomes covalently labeled with these peptides demonstrated effective targeting to outer hair cells in a rat cochlear explant study (Surovtseva et al., Citation2012). In another study using nanoparticles conjugated with a neurotrophin-derived peptide ligand, specific targeting of nanoparticles to spiral ganglion neurons, Schwann cells, and nerve fibers was shown (Roy et al., Citation2010). In addition to these advantages of nanoparticles, encapsulation of therapeutic agents in nanoparticles can provide controlled release (Guzman et al., Citation1996). Nanoparticles applied locally to the inner ear can diffuse in the inner ear fluids without being cleared from them. The delivery carriers could release loaded drugs through biodegradation or diffusion out of the carriers. Finally, nanoparticles can provide physical protection in vivo to delicate drug structures (Yokoyama et al., Citation1990).
Among the various nanoparticles, we used polymersomes derived from aspartic acid. Polymersomes are produced using amphiphilic synthetic block copolymers to form the vesicle membrane, and have radii ranging from 50 to 1000 nm or more (Discher et al., Citation1999). They have a hydrophilic surface and hydrophobic core, so can readily encapsulate hydrophobic drugs in aqueous solutions. Polymersomes are similar to liposomes, which are vesicles formed from naturally occurring lipids. While having many of the properties of natural liposomes, polymersomes exhibit increased stability and reduced permeability. Furthermore, the use of synthetic polymers enables designers to manipulate the characteristics of the membrane and thus control the permeability, release rates, stability and other properties of the polymersome.
In the present study, we examined the permeability of PHEA nanoparticles to the inner ear after middle-ear administration, and their safety by hearing function tests and histological examination. In the permeability test, our results showed superior permeability into the inner ear versus that of the non-nanoparticle loaded fluorescent dye, especially into the organ of Corti. When a drug reaches the inner ear from the middle ear cavity, it must pass the round window membrane or oval window (Zou et al., Citation2010; Pyykko et al., Citation2011). Although there is some controversy regarding which is the dominant route for the inner ear, the round window membrane seems to be superior (Salt et al., Citation2012). Factors affecting the permeability of a drug through the round window membrane include the size, configuration, concentration, lipid-solubility, and electrical charge of the drug itself, and the thickness of the round window membrane (Goycoolea, Citation2001). Regarding these factors, our drug-loaded polymersome has some weaknesses versus the non-loaded drug, in terms of size and lipid solubility. However, we postulate two reasons for the superior permeability of the PHEA nanoparticles. First, they can prevent precipitation of the hydrophobic drug in the middle ear cavity before it passes through the round window membrane, because they can carry a hydrophobic drug within the core, and dissolve in aqueous solution over a long period. Second, after crossing the round window membrane, they can access the entire cochlea through the perilymph and endolymph fluids, and release the dye over the long-term, rather than being readily absorbed at or near the round window membrane. Also, the absorption of the polymersome into inner hair cells in our study is noteworthy. The organ of Corti is more difficult to reach for a drug in the perilymph than the spiral ganglion or spiral ligament. A recent anatomical study of the cochlea using scanning electron microscopy revealed that the perilymph and fluid spaces in the modiolar periphery form a common system through numerous pores, and, although rare, several pores existed between the scala tympani and spiral ligament. However, the basement membrane showed no opening to the perilymphatic space in that study (Rask-Andersen et al., Citation2006). These results show the relative ease with which drugs in the perilymph can reach the spiral ganglion and the spiral ligament versus the organ of Corti.
In the safety test, at 1 week after the operation, only mild thickening of the middle ear mucosa in mice in the NP group was evident; as a result, the response in DPOAE was decreased. However, based on the similar results in the sham-operation group, this was thought to be an acceptable physical injury due to the surgery itself, rather than toxicity from the nanoparticles. The toxicity of a high concentration of nanoparticles in primary cochlear cell culture has been shown previously (Zhang et al., Citation2011). When nanoparticles occupy a certain volume of the cell, cell function will be impaired – the so-called “nanoparticle overload phenomenon” (Moss & Wong, Citation2006). To maintain the delivery efficacy of nanoparticles, and to lower their toxicity, the drug to nanoparticle loading ratio should be increased. A lower loading ratio requires a higher concentration of nanoparticles to deliver the desired dose of drug to the inner ear. Although the nanoparticle concentration in our study was higher than in the report mentioned above, we did not observe any toxicity, and so the difference in the materials forming the nanoparticles likely influences the toxicity/concentration effect.
As limitations of the current study, the number of subjects was a little small, and long-term toxicities over than 1 week were not evaluated, because the current study was a preliminary study to find out possibilities of the PHEA nanoparticles as a drug delivery system for inner ear. So, we think its efficacy and long-term safety should be verified again in further studies.
Declaration of interest
The authors acknowledge the financial support of the Catholic Medical Center Research Foundation, in program year 2012, and the Catholic University of Korea, Daejeon St. Mary’s Hospital Clinical Research Institute grant, funded by the Catholic University of Korea Daejeon St. Mary’s Hospital (CMCDJ-P-2013-002).
References
- Chen G, Zhang X, Yang F, Mu L. (2010). Disposition of nanoparticle-based delivery system via inner ear administration. Curr Drug Metab 11:886–97
- Discher BM, Won YY, Ege DS, et al. (1999). Polymersomes: tough vesicles made from diblock copolymers. Science 284:1143–6
- Gelperina S, Kisich K, Iseman MD, Heifets L. (2005). The potential advantages of nanoparticle drug delivery systems in chemotherapy of tuberculosis. Am J Respir Crit Care Med 172:1487–90
- Goycoolea MV. (2001). Clinical aspects of round window membrane permeability under normal and pathological conditions. Acta Otolaryngol 121:437–47
- Guzman LA, Labhasetwar V, Song C, et al. (1996). Local intraluminal infusion of biodegradable polymeric nanoparticles. A novel approach for prolonged drug delivery after balloon angioplasty. Circulation 94:1441–8
- Juhn SK, Rybak LP, Fowlks WL. (1982). Transport characteristics of the blood–perilymph barrier. Am J Otolaryngol 3:392–6
- Kang H, Kim JD, Han SH, Chang IS. (2002). Self-aggregates of poly(2-hydroxyethyl aspartamide) copolymers loaded with methotrexate by physical and chemical entrapments. J Control Release 81:135–44
- McCall AA, Swan EE, Borenstein JT, et al. (2010). Drug delivery for treatment of inner ear disease: current state of knowledge. Ear Hear 31:156–65
- Moss OR, Wong VA. (2006). When nanoparticles get in the way: impact of projected area on in vivo and in vitro macrophage function. Inhal Toxicol 18:711–16
- Park SN, Back SA, Choung YH, et al. (2011). alpha-Synuclein deficiency and efferent nerve degeneration in the mouse cochlea: a possible cause of early-onset presbycusis. Neurosci Res 71:303–10
- Parnes LS, Sun AH, Freeman DJ. (1999). Corticosteroid pharmacokinetics in the inner ear fluids: an animal study followed by clinical application. Laryngoscope 109:1–17
- Pyykko I, Zou J, Zhang W, Zhang Y. (2011). Nanoparticle-based delivery for the treatment of inner ear disorders. Curr Opin Otolaryngol Head Neck Surg 19:388–96
- Rask-Andersen H, Schrott-Fischer A, Pfaller K, Glueckert R. (2006). Perilymph/modiolar communication routes in the human cochlea. Ear Hear 27:457–65
- Roy S, Johnston AH, Newman TA, et al. (2010). Cell-specific targeting in the mouse inner ear using nanoparticles conjugated with a neurotrophin-derived peptide ligand: potential tool for drug delivery. Int J Pharm 390:214–24
- Sajjadi H, Paparella MM. (2008). Meniere’s disease. Lancet 372:406–14
- Salt AN, King EB, Hartsock JJ, et al. (2012). Marker entry into vestibular perilymph via the stapes following applications to the round window niche of guinea pigs. Hear Res 283:14–23
- Slattery WH, Fisher LM, Iqbal Z, et al. (2005). Intratympanic steroid injection for treatment of idiopathic sudden hearing loss. Otolaryngol Head Neck Surg 133:251–9
- Surovtseva EV, Johnston AH, Zhang W, et al. (2012). Prestin binding peptides as ligands for targeted polymersome mediated drug delivery to outer hair cells in the inner ear. Int J Pharm 424:121–7
- Yang SR, Kim SB, Joe CO, Kim JD. (2008). Intracellular delivery enhancement of poly(amino acid) drug carriers by oligoarginine conjugation. J Biomed Mater Res A 86:137–48
- Yokoyama M, Miyauchi M, Yamada N, et al. (1990). Characterization and anticancer activity of the micelle-forming polymeric anticancer drug adriamycin-conjugated poly(ethylene glycol)-poly(aspartic acid) block copolymer. Cancer Res 50:1693–700
- Zhang Y, Zhang W, Johnston AH, et al. (2010). Improving the visualization of fluorescently tagged nanoparticles and fluorophore-labeled molecular probes by treatment with CuSO(4) to quench autofluorescence in the rat inner ear. Hear Res 269:1–11
- Zhang Y, Zhang W, Lobler M, et al. (2011). Inner ear biocompatibility of lipid nanocapsules after round window membrane application. Int J Pharm 404:211–19
- Zou J, Sood R, Ranjan S, et al. (2010). Manufacturing and in vivo inner ear visualization of MRI traceable liposome nanoparticles encapsulating gadolinium. J Nanobiotechnol 8:32