Abstract
Skin is the largest organ of the human body and plays the most important role in protecting against pathogen and foreign matter. Three important modes such as topical, regional and transdermal are widely used for delivery of various dosage forms. Among these modes, the topical dosage forms are preferred because it provides local therapeutic activity when applied to the skin or mucous membranes. Additives or pharmaceutical excipients (non-drug component of dosage form) are used as inactive ingredients in dosage form or tools for structuring dosage forms. The main use of topical dosage form additives are controling the extent of absorption, maintaining the viscosity, improving the stability as well as organoleptic property and increasing the bulk of the formulation. The overall goal of this article is to provide the clinician with information related to the topical dosage form additives and their current major applications against various diseases.
Introduction
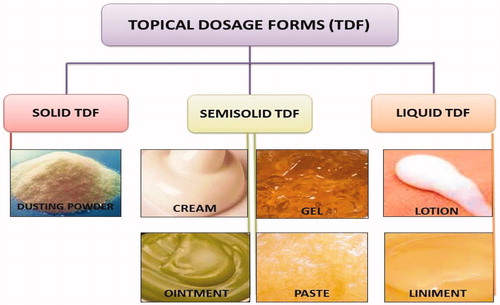
Skin is often known as largest organ of the human body. Skin is composed of three primary layers – epidermis, dermis and hypodermis. The skin is an ever-changing organ that contains many specialized cells and structures. It plays the most important role in protecting against pathogens. It gathers sensory information from the environment and plays an active role in the immune system protecting us from disease. It is also involved in maintaining the proper temperature for the body to function well. Natural and synthetic cosmetics are frequently used to treat the appearance of the face and condition of the skin (Dominguez-Huttinger et al., Citation2013). Three important modes such as topical, regional and transdermal are widely used for delivery of various dosage forms. Among these modes, the topical dosage forms are preferred because are protective, emollient and deliver therapeutic agents to exert local activity when applied to the skin or mucous membranes. Topical dosage forms are classified into three major categories such as solid (dusting powder), liquid (lotion, liniment) and semi-liquid (ointment, paste, cream and gel) (Chuo & Kaminska, Citation2009). Additives or pharmaceutical excipients (nondrug component of dosage form) are used as inactive ingredients in dosage form or tools for structuring dosage forms. The main use of topical dosage form additives are to control the extent of absorption, maintain the viscosity, improve the stability as well as organoleptic property and increase the bulk of the formulation. Topical dosage forms are widely used for delivery of various bio-active molecules to the target site as well as improve the bioavailability of encapsulated dug substance (Andersson et al., Citation2013).
Modes of delivery to the skin
Three important modes such as topical, regional and transdermal are widely used for delivery of various dosage forms ().
Figure 1. Various modes of drug delivery to the skin (A) topical (B) regional and (C) transdermal delivery.
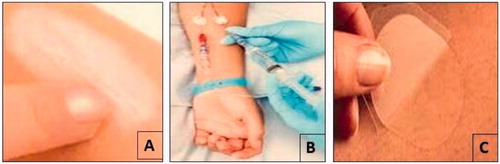
Topical delivery
Topical delivery can be defined as the application of a drug containing formulation to the skin. It is used mainly to directly treat cutaneous disorders such as acne or the cutaneous manifestations of a universal disease such as psoriasis. The main goal of this delivery is confining the pharmacological or other effect of the drug to the surface of the skin or within the skin. Various formulations such as foams, sprays, medicated powders, solution or others forms are widely used by topical route (Pando et al., Citation2013).
Regional delivery
Regional delivery is defined as the application of a drug containing formulation to the skin for treating or alleviating disease symptoms in deep tissues. The main aim of this delivery is to effect the pharmacological actions of the drug within musculature, vasculature joints, and other, beneath and around the site of application. Regional activity requires percutaneous absorption and deposition as well as regional concenttrations are considered to be higher than systemic administration at the same total body exposure to the drug. Various formulations such as ointments, creams, large adhesive patches, plasters, poultices and cataplasms are widely used by topical route (Crommelin et al., Citation2003).
Transdermal delivery
Transdermal delivery can be defined as the application of a drug to the skin for treat systemic disease. The main aim of this delivery is achieving systemically active levels of the drug. The main important requisite for this delivery is percutaneous absorption with appreciable systemic drug accumulation. Although such traditional dosage forms as ointments can be employed in this kind of therapy (e.g. nitro-glycerin ointments) due to no local accumulation of drug (Mazzitelli et al., Citation2013).
Topical dosage form
Among of these modes, the topical dosage forms are preferred because they are protective, emollient and delivered therapeutic agents to exert local activity when applied to the skin or mucous membranes. Topical dosage forms are classified into three major categories ().
Solid topical dosage form
Dusting powder
It is a finely divided insoluble powder containing ingredients such as talc, zinc oxide or starch used on the skin or on wounds especially for allaying irritation or absorbing moisture, which discourage bacterial growth and some used for their lubricant properties.
Semisolid topical dosage form
Cream
It is a semisolid emulsion formulation for application to the skin or mucous membranes. Water in oil (w/o) emulsion type creams are less greasy and good spreadability as comparison to ointments whereas oil in water (o/w) emulsion creams readily rub into the skin is termed as vanishing cream and is readily removed by water.
Ointment
It is a greasy semisolid preparation of dissolved or dispersed drug. Ointment bases influence topical drug bioavailability due to their occlusive properties of the stratum corneum, which enhances the flux of drug across the skin and they affect drug dissolution and drug partitioning within or from the ointment to the skin, respectively.
Gel
Gels are transparent or translucent semisolid preparations of one or more active ingredients in suitable hydrophilic or hydrophobic bases. Gels may be clear or opaque, and polar hydroalcoholic or nonpolar. Gels are prepared by either a fusion process or a special procedure necessitated by the gelling agents, humectants and preservatives (Garg et al., Citation2013).
Paste
It is a stiff preparation containing a high proportion of finely powdered solid such as starch, zinc oxide, calcium carbonate and talc. Pastes are less greasy than ointment.
Liquid topical dosage form
Lotion
A lotion is a low- to medium- viscosity topical preparation intended for application to unbroken skin. Lotions are applied to external skin with bare hands, a clean cloth, cotton wool or gauze and provide cooling effects to the skin by evaporation of solvents.
Liniments
The liniments are liquid or semiliquid preparations meant for application to the skin. Applied to skin with friction and rubbing of the skin. They act as rubefacient, soothing or stimulant. The vehicle may be alcohol, oil or soap based.
Additives of topical dosage forms
Additives or pharmaceutical excipients (nondrug component of dosage form) are used as inactive ingredients in dosage form or tools for structuring dosage forms. Generally, they have little or no therapeutic value but are useful in the manufacturing and compounding of various pharmaceutical dosage forms. Some ideal properties or characteristics of additives are: (1) they must be non-toxic, (2) commercially available in acceptable grade, (3) acceptably cheap, (4) physically and chemically stable and (5) not be contraindicated and must be color compatible (Park et al., Citation2013). The main use of topical additives are control the extent of absorption, maintain the viscosity as well as stability of dosage form, improve the organoleptic property and increase the bulk of the formulation. Additives are classified according to nature of solvent, base, diluents, vehicles and patient acceptability (Luo et al., Citation2012). shows classification of additives according to requirements or applications.
Table 1. Classification of additives according to requirements or applications.
Additives in dusting powder
Dusting powder is designed for application to the unbroken or broken skin. Easy powder flow ability and spread ability are two important parameters that are always considered at the time of manufacturing and evaluation of topical powder. The dusting powders should adhere to the skin providing good covering and adsorptive property, absolutely free from irritant effect, and should protect the skin from drying and irritation (Kircik & Del Rosso, Citation2010). Dusting powders are generally prepared by mixing two or more ingredients. one of which must be starch, talc or kaolin as one of the ingredients of the formulation. Due to the chemically inert nature of talc and kaolin, they are the most commonly used ingredients in the formulation of dusting powder. Sometimes, crust formation may take place in the presence of significant quantities of fluids (Mariappan et al., Citation2013). shows the important properties and their use of various additives used in the formulation of topical powder. Topical dusting powders are widely used for drug delivery to the target site. Some of the important applications associated with topical drug delivery through dusting powder shown in .
Table 2. Properties and use of additives in topical powder formulation.
Table 3. Applications of topical dusting powder dosage form in drug delivery.
Additives in cream and paste
It is a semisolid emulsion formulation for application to the skin or mucus membranes (Parnami et al., Citation2013) Emulsifying agent used in cream formulation as additive (a) w/o emulsifying agent and (b) o/w emulsifying agent. and shows the important properties and their use of various additives used in the formulation of w/o and o/w topical cream, respectively. Topical creams as well as paste are widely used for drug delivery to the target site. Some of the important applications associated with topical creams and paste are shown in and , respectively.
Table 4. Properties and use of additive in topical cream (w/o emulsifying agent – cleansing cream) formulation.
Table 5. Properties and use of additive in topical cream (with emulsifying agent – vanishing cream) formulation.
Table 6. Applications of topical cream dosage form in drug delivery.
Table 7. Applications of topical paste dosage form in drug delivery.
Additives in ointment
An ointment is a semisolid, homogenous, viscous preparation, which is intended for external application to the skin or mucus membranes. Ointments are used topically on a variety of body surfaces. They usually have moisturizing property, and are good for dry skin, as well as have a low risk of sensitization due to their having few ingredients beyond the base oil or fat, and low irritation risk (El-On et al., Citation2007). They are often disliked by patients due to greasiness. The vehicle of an ointment is known as the ointment base.
Ointment base
An ideal ointment base should possess the following properties such as should be pharmaceutically well-designed, should have a low manifestation of irritation, should be companionable with other ingredients, easily washable with water, secure on storage, have a low sensitization catalog and should release the medicament efficiently at the site of application (Fellinger et al., Citation2013). The USP recognizes four general classes of ointment bases to be used therapeutically or as vehicles for active ingredients.
Oleaginous bases
These bases are composed entirely of lipophilic materials. They are anhydrous, hydrophobic, insoluble in water or not removable by water. The major advantages of these bases are that they are inexpensive, nonreacting, nonirritating, good emollient, have protective and occlusive properties and are not water washable. However, they suffer from major drawbacks such as greasy nature, not removed easily with washing, and cannot absorb water, and therefore most liquid ingredients are difficult to incorporate into hydrocarbon bases (Hidaka et al., Citation2006). shows a list of materials used as oleaginous bases.
Table 8. List of materials used as oleaginous bases.
Absorption bases
Absorption bases are known to take up several times their own weights of water but not the absorption of medicament from the bases. Theycan be classified into two subgroups: anhydrous absorption bases and water-in-oil emulsions. The advantages of absorption bases are that they are good protective, occlusive and emollient properties, can absorb liquids and do not wash off easily so they hold incorporated medications in contact with the skin. Chemical stability problems, microbial growth and poor patient acceptance are the major drawbacks associated with these bases. Hydrophilic petrolatum and anhydrous lanolin of USP are examples of monograph on absorption bases (Das & Kumar, Citation2013).
Emulsion bases
W/O emulsion bases
Lanolin and cold cream are examples of ointments without emulsion bases. They are used as emollients. The aqueous phase hydrates the skin. The oily phase forms an occlusive covering which prevents loss of water by evaporation. The main drawback of ointments without emulsion base is their greasy and sticky nature. Example w/o emulsion base is cold cream (Ozsoy et al., Citation2004).
O/W emulsion base
Hydrophilic ointment and vanishing cream are type of o/w emulsion base. They are easily removed with water. They are non-greasy and non-sticky. Example of o/w emulsion is vanishing cream, which is often used as a cosmetic (Noda et al., Citation2011).
Water soluble bases
Water soluble bases are also known as greaseless ointment bases. They consist of water soluble ingredients such as polyethylene glycol polymer. Polyethylene glycol is known as carbowax. Carbowax is water soluble, nonvolatile and inert. Some water washable bases are also prepared by glyceryl monostearae (GMS), cellulose derivative, sodium alginate, bentonite and carbopol 934. The main advantages of water soluble bases are leaving no oil residue, easily removed by washing and absorbs some water or alcohol, but these bases can be irritating, especially on denuded or abraded skin or mucus membranes. Compatibility problems have been found with incorporated drugs that are subject to oxidation (Jain et al., Citation2010).
Preservatives in ointment
The antimicrobial compounds and their quantities is a crucial parameter to prevent contamination and spoilage of ointment bases by microorganisms such as bacteria, virus and fungi. Various antimicrobial compounds show irritation or toxicity to the tissue. For instance, methyl and propyl parabens are irritant to nasal passages. Boric acid and quaternary ammonium compounds or phenyl mercuric nitrates are toxic as well as irritant to nasal tissues. On some occassions, the preservatives reducing the antimicrobial compounds availability for antimicrobial action by making the complex. In the presence of Tween 80, methylparaben, benzalkonium chloride, benzoic acid etc. are inactivated to a significant extent. The partition coefficient of the antimicrobial compound between aqueous and oily phases plays an important role in bactericidal activity (Kempf et al., Citation2011).
Antioxidants in ointment
Antioxidants should be included to prevent the oxidative degradation of the base from the oxidation. It may be more desirable to select two antioxidants instead of one. The concentration of antioxidants would depend upon their partition coefficients between the aqueous and oily phases if both the phases are present in a base. Generally, compounds like butylated hydroxy anisole, propyl gallate, nor-dihydroguaiaretic acid etc. are used in ointment bases (Gupta, Citation2012).
Chelating agents
The traces of metallic ions are likely to catalyze oxidative degradations, so small amounts of substances such as citric acid, maleic acid; phosphoric acid etc. may be added to chelate the metallic ions (Sugawara et al., Citation2007).
Perfumes
Essential oils from plant materials are used as perfumes in ointment bases. The floral group blends such as jasmine, rose, lily and gardenia are widely used in bases.
Topical ointments are widely used for drug delivery to the target site. Some of the important applications associated with topical ointments are shown in .
Table 9. Applications of topical ointment dosage form in drug delivery.
Additives in gels
Gels are semisolid preparations of solution or dispersion of one or more active ingredients as well as hydrocolloidal substances (gelling agent). Gelling agents (also known as solidifiers or stabilizers and thickening agents) exhibit pseudoplastic property and gives a thixotrophic consistency to the gel. Gelling agents are more soluble in cold water (e.g. methylcellulose and poloxamers) than hot water (e.g. bentonite, gelatin and sodium carboxymethylcellulose). Gelling agents are used in concentrations of 0.5–10% for easy addition to the active drug before the gel is formed and maintain the viscosity of the gelling agents in the gelling layer within a range of about 1000 cps to about 100 000 cps (Chiang et al., Citation2013). shows the important properties and their use of various additives used in the formulation of topical gel. Topical gels are widely used for drug delivery to the target site. Some of the important applications associated with topical gel shown in .
Table 10. Properties and use of additives in topical gel formulation.
Table 11. Applications of topical gel dosage form in drug delivery.
Additives in lotions
Lotions are usually suspensions of solids in an aqueous medium. It is a low to medium viscosity topical preparation intended for application to unbroken skin. They are thicker and emollient in nature in comparison to solution. They are usually oil mixed with water, and more often than not have less alcohol than solutions. The main advantages of this delivery system are avoidance of first pass metabolism, easy to apply, improved patient compliance, improved physiological and pharmacological response, avoidance of gastro-intestinal incompatibility and ability to easily terminate medications when needed (Meyer et al., Citation2008). However, various disadvantages are associated with these systems such as skin irritation or contact dermatitis, which may occur due to the drug and/or additives, poor permeability of some drugs, chances of allergic reactions and sometime drugs are not absorbed through the skin due to larger particle size. shows the important properties and their use of various additive used in the formulation of topical lotion (Hussain Shah et al., Citation2013). Topical lotions are widely used for drug delivery to the target site. Some of the important applications associated with topical lotions shown in .
Table 12. Properties and use of additives in topical lotion formulation.
Table 13. Applications of topical lotion dosage form in drug delivery.
Additives in liniments
Liniments are alcoholic or oil-based solutions or emulsions formulations, containing therapeutic active agents mainly used for external application. These formulations are also known as embrocations because they are rubbed onto the affected area with friction. Liniments should not be applied to skin that is bruised or broken. Presence of the oil base or soap base providing ease of application and massage on affected areas. Alcoholic liniments are generally used due to their various characteristics property such as excellent rubefacient, counterirritant, mildly astringent and penetrating effects. At the time of massage, oily liniments are more useful and exhibit milder action. Fixed oils are thick, viscous liquids and contain unsaturated fatty acids which cause good penetration into the skin. shows the important properties and their use of various additives used in the formulation of topical liniments (Oplander et al., Citation2012). Topical liniments are widely used for drug delivery to the target site. Some of the important applications associated with topical liniments shown in .
Table 14. Properties and use of additives in topical liniment formulation.
Table 15. Applications of topical liniment dosage form in drug delivery.
Conclusion
Topical dosages form such as ointment, cream, paste, gel, lotion, powder and liniments place a very important role in pharmaceutical as well as cosmetics fields. Additives of topical dosage forms show their excellent role in dosage form design, dosage form stability, controlled drug release pattern and deliver the drug to the target place for the longer duration of period. Now-a-days, topical dosage forms are the first choice as delivery systems in pharmaceutical and other fields.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Abdel-Mottaleb MM, Moulari B, Beduneau A, et al. (2012). Nanoparticles enhance therapeutic outcome in inflamed skin therapy. Eur J Pharm Biopharm 82:151–7
- Abdul L Abdul R, Sukul RR, Nazish S. (2010). Anti-inflammatory and antihistaminic study of a Unani eye drop formulation. Ophthalmol Eye Dis 2:17–22
- Aggarwal N, Goindi S. (2013). Preparation and in vivo evaluation of solid lipid nanoparticles of griseofulvin for dermal use. J Biomed Nanotechnol 9:564–76
- Agrawal Y, Petkar KC, Sawant KK. (2010). Development, evaluation and clinical studies of Acitretin loaded nanostructured lipid carriers for topical treatment of psoriasis. Int J Pharm 401:93–102
- Ahmed TA, Ibrahim HM, Ibrahim F, et al. (2011). In vitro release, rheological, and stability studies of mefenamic acid coprecipitates in topical formulations. Pharm Dev Technol 16:497–510
- Ahuja M, Dhake AS, Sharma SK, Majumdar DK. (2008). Topical ocular delivery of NSAIDs. AAPS J 10:229–41
- Amaral LF, Camilo NS, Pereda MD, et al. (2011). Evaluation of antimicrobial effectiveness of C-8 xylitol monoester as an alternative preservative for cosmetic products. Int J Cosmet Sci 33:391–7
- Ambrogi V, Perioli L, Pagano C, et al. (2010). Econazole nitrate-loaded MCM-41 for an antifungal topical powder formulation. J Pharm Sci 99:4738–45
- Andersson M, Holm L, Ridderstrale Y, et al. (2013). Morphology and composition of the spider major ampullate gland and dragline silk. Biomacromolecules 14:2945–52
- Andreassi L, Giannetti A, Milani M. (2003). Efficacy of betamethasone valerate mousse in comparison with standard therapies on scalp psoriasis: an open, multicentre, randomized, controlled, cross-over study on 241 patients. Br J Dermatol 148:134–8
- Aquino RP, Auriemma G, Mencherini T, et al. (2013). Design and production of gentamicin/dextrans microparticles by supercritical assisted atomisation for the treatment of wound bacterial infections. Int J Pharm 440:188–94
- Asakawa M, Maekawa T, Mori K, et al. (1989). [A new clinical trial in intravesical chemotherapy with instillation of peplomycin preparation as an emulsion in hydroxypropylcellulosum – preliminary study of patients with bladder tumor]. Hinyokika Kiyo 35:39–42
- Asha BM, Revathi M, Yadav A, Sakthivel N. (2012). Purification and characterization of a thermophilic cellulase from a novel cellulolytic strain, Paenibacillus barcinonensis. J Microbiol Biotechnol 22:1501–9
- Badilli U, Sen T, Tarimci N. (2011). Microparticulate based topical delivery system of clobetasol propionate. AAPS PharmSciTech 12:949–57
- Bartley J. (2013). Should chitosan and tranexamic acid be combined for improved hemostasis after sinus surgery? Med Hypotheses 81:1036–8
- Bavarsad N, Fazly Bazzaz BS, Khamesipour A, Jaafari MR. (2012). Colloidal, in vitro and in vivo anti-leishmanial properties of transfersomes containing paromomycin sulfate in susceptible BALB/c mice. Acta Trop 124:33–41
- Belov SV, Borik MA, Danileiko Iu K, et al. (2013). [New bipolar electrosurgical tools based on zirconium dioxide]. Med Tekh 2:20–4
- Bhatia A, Singh B, Wadhwa S, et al. (2013). Novel phospholipid-based topical formulations of tamoxifen: evaluation for antipsoriatic activity using mouse-tail model. Pharm Dev Technol 19:160–3
- Bhatt B, Koland M, Shama KP. (2013). Investigation of the ex vivo and in vivo iontophoretic delivery of aceclofenac from topical gels in Albino rats. Int J Pharm Investig 3:105–11
- Bhattacharyya S, Agrawal A, Knabe C, Ducheyne P. (2013). Sol-gel silica controlled release thin films for the inhibition of methicillin-resistant Staphylococcus aureus. Biomaterials 35:509–17
- Bikkad ML, Nathani AH, Mandlik SK, et al. (2013). Halobetasol propionate-loaded solid lipid nanoparticles (SLN) for skin targeting by topical delivery. J Liposome Res. [Epub ahead of print]
- Birkenfeld B, Parafiniuk M, Bielecka-Grzela S, et al. (2011). The penetration of topically applied ointment containing hyaluronic acid in rabbit tissues. Pol J Vet Sci 14:621–7
- Brown RS, Edwards D, Walsh-Chocolaad T, Childs RW. (2013). Topical tacrolimus with custom trays in the treatment of severe oral chronic graft-versus-host disease refractory to a potent topical steroid therapy: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol 115:e26–30
- Campbell SM, Pye A, Horton S, et al. (2007). A clinical investigation to determine the effect of pressure injection on the penetration of topical methyl aminolevulinate into nodular basal cell carcinoma of the skin. J Environ Pathol Toxicol Oncol 26:295–303
- Castro GA, Oliveira CA, Mahecha GA, Ferreira LA. (2011). Comedolytic effect and reduced skin irritation of a new formulation of all-trans retinoic acid-loaded solid lipid nanoparticles for topical treatment of acne. Arch Dermatol Res 303:513–20
- Cevc G, Blume G. (2001). New, highly efficient formulation of diclofenac for the topical, transdermal administration in ultradeformable drug carriers, Transfersomes. Biochim Biophys Acta 1514:191–205
- Cevc G, Blume G. (2004). Hydrocortisone and dexamethasone in very deformable drug carriers have increased biological potency, prolonged effect, and reduced therapeutic dosage. Biochim Biophys Acta 1663:61–73
- Chan TY. (1996). Potential dangers from topical preparations containing methyl salicylate. Hum Exp Toxicol 15:747–50
- Chen L, Zhou Y, Wang X, et al. (2013). Enhanced oil-mineral aggregation with modified bentonite. Water Sci Technol 67:1581–9
- Chiang WS, Fratini E, Ridi F, et al. (2013). Microstructural changes of globules in calcium-silicate-hydrate gels with and without additives determined by small-angle neutron and X-ray scattering. J Colloid Interface Sci 398:67–73
- Cho EB, Kim H, Kim D. (2009). Effect of morphology and pore size of sulfonated mesoporous benzene-silicas in the preparation of poly(vinyl alcohol)-based hybrid nanocomposite membranes for direct methanol fuel cell application. J Phys Chem B 113:9770–8
- Chuo Y, Kaminska B. (2009). Sensor layer of a multiparameter single-point integrated system. IEEE Trans Biomed Circuits Syst 3:229–40
- Cohen B, Pinkas O, Foox M, Zilberman M. (2013). Gelatin-alginate novel tissue adhesives and their formulation-strength effects. Acta Biomater 9:9004–11
- Cohen DL, Lipton JI, Bonassar LJ, Lipson H. (2010). Additive manufacturing for in situ repair of osteochondral defects. Biofabrication 2:035004
- Conaghan PG, Dickson J, Bolten W, et al. (2013). A multicentre, randomized, placebo- and active-controlled trial comparing the efficacy and safety of topical ketoprofen in Transfersome gel (IDEA-033) with ketoprofen-free vehicle (TDT 064) and oral celecoxib for knee pain associated with osteoarthritis. Rheumatology 52:1303–12
- Crommelin DJ, Storm G, Jiskoot W, et al. (2003). Nanotechnological approaches for the delivery of macromolecules. J Control Release 87:81–8
- Das A, Kumar GS. (2013). Binding of the plant alkaloid aristololactam-beta-d-glucoside and antitumor antibiotic daunomycin to single stranded polyribonucleotides. Biochim Biophys Acta 1830:4708–18
- De Jong EM, Menke HE, Van Praag MC, Van De Kerkhof PC. (1999). Dystrophic psoriatic fingernails treated with 1% 5-fluorouracil in a nail penetration-enhancing vehicle: a double-blind study. Dermatology 199:313–18
- Delany EG, Fagan CL, Gundala S, et al. (2013). NHC-catalysed aerobic aldehyde-esterifications with alcohols: no additives or cocatalysts required. Chem Commun 49:6510–12
- Derry S, Sven-Rice A, Cole P, et al. (2013). Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2:CD007393
- Dev RK, Bali V, Pathak K. (2011). Novel microbially triggered colon specific delivery system of 5-fluorouracil: statistical optimization, in vitro, in vivo, cytotoxic and stability assessment. Int J Pharm 411:142–51
- Dominguez-Huttinger E, Ono M, Barahona M, Tanaka RJ. (2013). Risk factor-dependent dynamics of atopic dermatitis: modelling multi-scale regulation of epithelium homeostasis. Interface Focus 3:20120090
- Ein SH, Langer JC. (2012). Delayed management of giant omphalocele using silver sulfadiazine cream: an 18-year experience. J Pediatr Surg 47:494–500
- El-Badry M, Fetih G, Shakeel F. (2014). Comparative topical delivery of antifungal drug croconazole using liposome and micro-emulsion-based gel formulations. Drug Deliv 21:34–43
- El-On J, Bazarsky E, Sneir R. (2007). Leishmania major: in vitro and in vivo anti-leishmanial activity of paromomycin ointment (Leshcutan) combined with the immunomodulator Imiquimod. Exp Parasitol 116:156–62
- Elewski BE. (2007). Percutaneous absorption kinetics of topical metronidazole formulations in vitro in the human cadaver skin model. Adv Ther 24:239–46
- Elsaid N, Jackson TL, Gunic M, Somavarapu S. (2012). Positively charged amphiphilic chitosan derivative for the transscleral delivery of rapamycin. Invest Ophthalmol Vis Sci 53:8105–11
- Erdogan M, Wright JR, Jr, McAlister VC. (2002). Liposomal tacrolimus lotion as a novel topical agent for treatment of immune-mediated skin disorders: experimental studies in a murine model. Br J Dermatol 146:964–7
- Farber MK, Angelo TE, Castells M, Tsen LC. (2010). Anesthetic management of a patient with an allergy to propylene glycol and parabens. Anesth Analg 110:839–42
- Farzi M, Emam-Djomeh Z, Mohammadifar MA. (2013). A comparative study on the emulsifying properties of various species of gum tragacanth. Int J Biol Macromol 57:76–82
- Faure H, Exbrayat C, Garnier A, et al. (1998). Influence of the ‘directions for use' on positivity rates of the Hemoccult II test. Eur J Gastroenterol Hepatol 10:1021–4
- Feldman SR, Yentzer BA. (2009). Topical clobetasol propionate in the treatment of psoriasis: a review of newer formulations. Am J Clin Dermatol 10:397–406
- Fellinger C, Hemmer W, Wantke F, et al. (2013). Severe allergic dermatitis caused by lanolin alcohol as part of an ointment base in propolis cream. Contact Dermatitis 68:59–61
- Fernandez-Campos F, Clares Naveros B, Lopez Serrano O, et al. (2013). Evaluation of novel nystatin nanoemulsion for skin candidosis infections. Mycoses 56:70–81
- Fouad SA, Basalious EB, El-Nabarawi MA, Tayel SA. (2013). Microemulsion and poloxamer microemulsion-based gel for sustained transdermal delivery of diclofenac epolamine using in-skin drug depot: in vitro/in vivo evaluation. Int J Pharm 453:569–78
- Franz TJ, Parsell DA, Halualani RM, et al. (1999). Betamethasone valerate foam 0.12%: a novel vehicle with enhanced delivery and efficacy. Int J Dermatol 38:628–32
- Gao Y, Katz DF. (2013). Multicompartmental pharmacokinetic model of tenofovir delivery by a vaginal gel. PLoS One 8:e74404
- Garg BJ, Saraswat A, Bhatia A, Katare OP. (2010). Topical treatment in vitiligo and the potential uses of new drug delivery systems. Indian J Dermatol Venereol Leprol 76:231–8
- Garg T, Singh O, Arora S, Murthy R. (2012). Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst 29:1–63
- Garg T, Singh S, Goyal AK. (2013). Stimuli-sensitive hydrogels: an excellent carrier for drug and cell delivery. Crit Rev Ther Drug Carrier Syst 30:369–409
- Geraud B, Bocquet L, Barentin C. (2013). Confined flows of a polymer microgel. Eur Phys J E Soft Matter 36:30–3
- Goindi S, Kumar G, Kumar N, Kaur A. (2013). Development of novel elastic vesicle-based topical formulation of cetirizine dihydrochloride for treatment of atopic dermatitis. AAPS PharmSciTech 14:1284–93
- Gomes-Filho JE, Duarte PC, De Oliveira CB, et al. (2012). Tissue reaction to a triantibiotic paste used for endodontic tissue self-regeneration of nonvital immature permanent teeth. J Endod 38:91–4
- Goswami D, Basu JK, De S. (2013). Lipase applications in oil hydrolysis with a case study on castor oil: a review. Crit Rev Biotechnol 33:81–96
- Gratieri T, Pujol-Bello E, Gelfuso GM, et al. (2013). Iontophoretic transport kinetics of ketorolac in vitro and in vivo: Demonstrating local enhanced topical drug delivery to muscle. Eur J Pharm Biopharm. In press
- Gruning N, Muller-Goymann CC. (2008). Physicochemical characterisation of a novel thermogelling formulation for percutaneous penetration of 5-aminolevulinic acid. J Pharm Sci 97:2311–23
- Gupta VD. (2012). Chemical stability of pentoxifylline in a topical cream. Int J Pharm Compd 16:80–1
- Harada S, Horisawa E, Kano S, Sugibayashi K. (2011). Formulation study of topically applied O/W lotion containing vitamin D3 derivative, focusing on skin permeability of the drug. Drug Dev Ind Pharm 37:917–25
- Harrison LI, Stoesz JD, Battiste JL, et al. (2009). A pharmaceutical comparison of different commercially available imiquimod 5% cream products. J Dermatolog Treat 20:1–5
- Herzog J L, Solomon JA, Draelos Z, et al. (2011). A randomized, double-blind, vehicle-controlled crossover study to determine the anti-pruritic efficacy, safety and local dermal tolerability of a topical formulation (srd174 cream) of the long-acting opiod antagonist nalmefene in subjects with atopic dermatitis. J Drugs Dermatol 10:853–60
- Hidaka N, Suemaru K, Aimoto T, Araki H. (2006). Effect of simultaneous insertion of oleaginous base on the absorption and on the anticonvulsant effect of diazepam suppository. Biol Pharm Bull 29:705–8
- Hilten R, Speir R, Kastner J, Das KC. (2011). Production of aromatic green gasoline additives via catalytic pyrolysis of acidulated peanut oil soap stock. Bioresour Technol 102:8288–94
- Huang H, Deng M, Jin H, et al. (2013). Preventing intra-abdominal adhesions with a sodium hyaluronate carboxymethylcellulose membrane enabled visualization of hepatic microcirculation. Int J Surg 11:935–43
- Huang YB, Lee KF, Huang CT, et al. (2010). The effect of component of cream for topical delivery of hesperetin. Chem Pharm Bull (Tokyo) 58:611–14
- Hui X, Hewitt PG, Poblete N, et al. (1998). In vivo bioavailability and metabolism of topical diclofenac lotion in human volunteers. Pharm Res 15:1589–95
- Hussain Shah SN, Hussain T, Ullah Khan I, et al. (2013). Formulation study of topically applied lotion: in vitro and in vivo evaluation. Bioimpacts 3:11–19
- Iena Ia M. (1993). The therapeutic properties of aloe. Lik Sprava 2:142–5
- Jain A, Zheng W, Garad S, et al. (2010). Importance of early characterization of physicochemical properties in developing high-dose intravenous infusion regimens for poorly water-soluble compounds. PDA J Pharm Sci Technol 64:517–26
- Jansen MM, Verzijl JM, Burger DM, Hekster YA. (2013). Controlled release of morphine from a poloxamer 407 gel. Int J Pharm 452:266–9
- Jin ZL, Tada A, Sugimoto N, et al. (2006). [Analysis of constituents in urushi wax, a natural food additive]. Shokuhin Eiseigaku Zasshi 47:167–72
- Johnson W Jr, Bergfeld WF, Belsito DV, et al. (2011). Amended safety assessment of Sesamum indicum (sesame) seed oil, hydrogenated sesame seed oil, Sesamum indicum (sesame) oil unsaponifiables, and sodium sesameseedate. Int J Toxicol 30:40S–53S
- Kang C, Shin SC. (2012). Development of prilocaine gels for enhanced local anesthetic action. Arch Pharm Res 35:1197–204
- Kempf M, Kimble RM, Cuttle L. (2011). Cytotoxicity testing of burn wound dressings, ointments and creams: a method using polycarbonate cell culture inserts on a cell culture system. Burns 37:994–1000
- Keshtkaran M, Mohammadifar MA, Asadi GH, et al. (2013). Effect of gum tragacanth on rheological and physical properties of a flavored milk drink made with date syrup. J Dairy Sci 96:4794–803
- Khlamova OG, Shul'diakov AA, Lepilin AV. (2011). [Improvement of herpetic stomatitis therapy in patients with chronic tonsillitis]. Antibiot Khimioter 56:34–6
- Khozeimeh F, Shahtalebi MA, Noori M, Savabi O. (2010). Comparative evaluation of ketoconazole tablet and topical ketoconazole 2% in orabase in treatment of Candida-infected denture stomatitis. J Contemp Dent Pract 11:017–24
- Kimball AB, Kaczvinsky JR, Li J, et al. (2010). Reduction in the appearance of facial hyperpigmentation after use of moisturizers with a combination of topical niacinamide and N-acetyl glucosamine: results of a randomized, double-blind, vehicle-controlled trial. Br J Dermatol 162:435–41
- Kircik L, Del Rosso JQ. (2010). Comparative efficacy and safety results of topical hemostatic powder and sterile compressed foam sponge in second intention healing following Mohs micrographic surgery. J Drugs Dermatol 9:137–40
- Kiyoyama T, Tokuda Y, Shiiki S, et al. (2009). Isopropyl alcohol compared with isopropyl alcohol plus povidone-iodine as skin preparation for prevention of blood culture contamination. J Clin Microbiol 47:54–8
- Komeh-Nkrumah SA, Nanjundaiah SM, Rajaiah R, et al. (2012). Topical dermal application of essential oils attenuates the severity of adjuvant arthritis in Lewis rats. Phytother Res 26:54–9
- Koseki T, Onishi H, Takahashi Y, et al. (2008). Development of novel fast-disintegrating tablets by direct compression using sucrose stearic acid ester as a disintegration-accelerating agent. Chem Pharm Bull (Tokyo) 56:1384–8
- Kulhari H, Pooja D, Narayan H, et al. (2011). Design and evaluation of ocusert for controlled delivery of flurbiprofen sodium. Curr Eye Res 36:436–41
- Lazareva EB, Spiridonova TG, Chernega EN, et al. (2002). [Topical pectins for the treatment of burn wounds]. Antibiot Khimioter 47:9–13
- Leung DY, Hanifin JM, Pariser DM, et al. (2009). Effects of pimecrolimus cream 1% in the treatment of patients with atopic dermatitis who demonstrate a clinical insensitivity to topical corticosteroids: a randomized, multicentre vehicle-controlled trial. Br J Dermatol 161:435–43
- Li G, Fan Y, Fan C, et al. (2012a). Tacrolimus-loaded ethosomes: physicochemical characterization and in vivo evaluation. Eur J Pharm Biopharm 82:49–57
- Li S, Qiu YQ, Zhang SH, Gao YH. (2012b). A novel transdermal fomulation of 18beta-glycyrrhetic acid with lysine for improving bioavailability and efficacy. Skin Pharmacol Physiol 25:257–68
- Lorenz-Baath K, Korotkov VS, Lierse Von Gostomski C, Sieber SA. (2012). In vitro percutaneous absorption of 14C-labeled beta-lactone promises topical delivery of new bacterial virulence inhibitors. Chem Med Chem 7:1490–5
- Luo X, Li J, Lin X. (2012). Effect of gelatinization and additives on morphology and thermal behavior of corn starch/PVA blend films. Carbohydr Polym 90:1595–600
- Malik R, Venkatesh KS, Dwivedi AK, Misra A. (2012). Episodic transdermal delivery of testosterone. Mol Pharm 9:1537–43
- Marchal J, Pifferi F, Aujard F. (2013). Resveratrol in mammals: effects on aging biomarkers, age-related diseases, and life span. Ann NY Acad Sci 1290:67–73
- Marchiori MC, Rascovetzki RH, Ourique AF, et al. (2013). Improved tretinoin photostability in a topical nanomedicine replacing original liquid suspension with spray-dried powder with no loss of effectiveness. Drug Dev Ind Pharm 39:579–86
- Mariappan R, Manninen P, Massicotte EM, Bhatia A. (2013). Circulatory collapse after topical application of vancomycin powder during spine surgery. J Neurosurg Spine 19:381–3
- Masoumi HR, Kassim A, Basri M, Abdullah DK. (2011). Determining optimum conditions for lipase-catalyzed synthesis of triethanolamine (TEA)-based esterquat cationic surfactant by a Taguchi robust design method. Molecules 16:4672–80
- Mazzitelli S, Pagano C, Giusepponi D, et al. (2013). Hydrogel blends with adjustable properties as patches for transdermal delivery. Int J Pharm 454:47–57
- Menter A, Vamvakias G, Jorizzo J. (2008). One-week treatment with once-daily fluorouracil cream 0.5% in participants with actinic keratoses. Cutis 81:509–16
- Meyer J, Marshall B, Gacula M Jr, Rheins L. (2008). Evaluation of additive effects of hydrolyzed jojoba (Simmondsia chinensis) esters and glycerol: a preliminary study. J Cosmet Dermatol 7:268–74
- Min YW, Park SU, Jang YS, et al. (2012). Effect of composite yogurt enriched with acacia fiber and Bifidobacterium lactis. World J Gastroenterol 18:4563–9
- Mitra SK, Sundaram R, Venkataranganna MV, et al. (2000). Anti-inflammatory, antioxidant and antimicrobial activity of Ophthacare brand, an herbal eye drops. Phytomedicine 7:123–7
- Mitri K, Shegokar R, Gohla S, et al. (2011). Lipid nanocarriers for dermal delivery of lutein: preparation, characterization, stability and performance. Int J Pharm 414:267–75
- Mohammadi G, Nokhodchi A, Barzegar-Jalali M, et al. (2011). Physicochemical and anti-bacterial performance characterization of clarithromycin nanoparticles as colloidal drug delivery system. Colloids Surf B Biointerfaces 88:39–44
- Montazerolghaem M, Engqvist H, Karlsson Ott M. (2013). Sustained release of simvastatin from premixed injectable calcium phosphate cement. J Biomed Mater Res A 102:340–7
- Moseke C, Bayer C, Vorndran E, et al. (2012). Low temperature fabrication of spherical brushite granules by cement paste emulsion. J Mater Sci Mater Med 23:2631–7
- Moussa A, Noureddine D, Sm H, et al. (2012). Additive potential of ginger starch on antifungal potency of honey against Candida albicans. Asian Pac J Trop Biomed 2:253–5
- Mouzakis DE, Papadopoulos TD, Polyzois GL, Griniari PG. (2010). Dynamic mechanical properties of a maxillofacial silicone elastomer incorporating a ZnO additive: the effect of artificial aging. J Craniofac Surg 21:1867–71
- Naganathan S, Razak HA, Hamid SN. (2010). Effect of kaolin addition on the performance of controlled low-strength material using industrial waste incineration bottom ash. Waste Manag Res 28:848–60
- Nair AB, Singh K, Al-Dhubiab BE, et al. (2013). Skin uptake and clearance of ciclopirox following topical application. Biopharm Drug Dispos. doi: 10.1002/bdd.1866. [Epub ahead of print]
- Nakamura S, Ishihara M, Takikawa M, et al. (2011). Increased survival of free fat grafts and vascularization in rats with local delivery of fragmin/protamine microparticles containing FGF-2 (F/P MP-F). J Biomed Mater Res B Appl Biomater 96:234–41
- Naldi L, Yawalkar N, Kaszuba A, et al. (2011). Efficacy and safety of the Betamethasone valerate 0.1% plaster in mild-to-moderate chronic plaque psoriasis: a randomized, parallel-group, active-controlled, phase III study. Am J Clin Dermatol 12:191–201
- Nayak AK, Pal D, Pradhan J, Hasnain MS. (2013). Fenugreek seed mucilage-alginate mucoadhesive beads of metformin HCl: Design, optimization and evaluation. Int J Biol Macromol 54:144–54
- Noda Y, Watanabe K, Sanagawa A, et al. (2011). Physicochemical properties of macrogol ointment and emulsion ointment blend developed for regulation of water absorption. Int J Pharm 419:131–6
- Oplander C, Volkmar CM, Paunel-Gorgulu A, et al. (2012). Dermal application of nitric oxide releasing acidified nitrite-containing liniments significantly reduces blood pressure in humans. Nitric Oxide 26:132–40
- Ozcan I, Azizoglu E, Senyigit T, et al. (2013). Enhanced dermal delivery of diflucortolone valerate using lecithin/chitosan nanoparticles: in-vitro and in-vivo evaluations. Int J Nanomed 8:461–75
- Ozsoy Y, Gungor S, Cevher E. (2004). Vehicle effects on in vitro release of tiaprofenic acid from different topical formulations. Farmaco 59:563–6
- Pacios MG, Silva C, Lopez ME, Cecilia M. (2012). Antibacterial action of calcium hydroxide vehicles and calcium hydroxide pastes. J Investig Clin Dent 3:264–70
- Paller AS, Nimmagadda S, Schachner L, et al. (2003). Fluocinolone acetonide 0.01% in peanut oil: therapy for childhood atopic dermatitis even in patients who are peanut sensitive. J Am Acad Dermatol 48:569–77
- Panagiotopoulos IA, Pasias S, Bakker RR, et al. (2013). Biodiesel and biohydrogen production from cotton-seed cake in a biorefinery concept. Bioresour Technol 136:78–86
- Pando D, Caddeo C, Manconi M, et al. (2013). Nanodesign of olein vesicles for the topical delivery of the antioxidant resveratrol. J Pharm Pharmacol 65:1158–67
- Park JK, Walker B, Seo JH. (2013). Controlling Polarity of Organic Bulk Heterojunction Field-Effect Transistors via Solvent Additives. ACS Appl Mater Interfaces 5:4575–80
- Parnami N, Garg T, Rath G, Goyal AK. (2013). Development and characterization of nanocarriers for topical treatment of psoriasis by using combination therapy. Artif Cells Nanomed Biotechnol. [Epub ahead of print]
- Patel H, Raval G, Nazari M, Heerklotz H. (2010). Effects of glycerol and urea on micellization membrane partitioning and solubilization by a non-ionic surfactant. Biophys Chem 150:119–28
- Pauporte M, Maibach H, Lowe N, et al. (2004). Fluocinolone acetonide topical oil for scalp psoriasis. J Dermatolog Treat 15:360–4
- Pople PV, Singh KK. (2012). Targeting tacrolimus to deeper layers of skin with improved safety for treatment of atopic dermatitis – part II: in vivo assessment of dermatopharmacokinetics, biodistribution and efficacy. Int J Pharm 434:70–9
- Pople PV, Singh KK. (2013). Development and evaluation of colloidal modified nanolipid carrier: application to topical delivery of tacrolimus, part II – in vivo assessment, drug targeting, efficacy, and safety in treatment for atopic dermatitis. Eur J Pharm Biopharm 84:72–83
- Puri R, Jain S. (2012). Ethogel topical formulation for increasing the local bioavailability of 5-fluorouracil: a mechanistic study. Anticancer Drugs 23:923–34
- Qiao Y, Huang Z, Li Q, et al. (2007). Developmental changes of the FAS and HSL mRNA expression and their effects on the content of intramuscular fat in Kazak and Xinjiang sheep. J Genet Genomics 34:909–17
- Rainsford KD, Kean WF, Ehrlich GE. (2008). Review of the pharmaceutical properties and clinical effects of the topical NSAID formulation, diclofenac epolamine. Curr Med Res Opin 24:2967–92
- Remane Y, Leopold CS, Maibach HI. (2006). Percutaneous penetration of methyl nicotinate from ointments using the laser Doppler technique:bioequivalence and enhancer effects. J Pharmacokinet Pharmacodyn 33:719–35
- Rhoades J, Gialagkolidou K, Gogou M, et al. (2013). Oregano essential oil as an antimicrobial additive to detergent for hand washing and food contact surface cleaning. J Appl Microbiol 115:987–94
- Romantsov MG, Kovalevskii MA, Iaremenko AI, et al. (2009). [Up-to-date approach to treatment of inflammatory infections in the maxillofacial region]. Antibiot Khimioter 54:52–7
- Rooney M, Pfister W, Mahoney M, et al. (2009). Long-term, multicenter study of the safety and efficacy of topical alprostadil cream in male patients with erectile dysfunction. J Sex Med 6:520–34
- Sabale V, Vora S. (2012). Formulation and evaluation of microemulsion-based hydrogel for topical delivery. Int J Pharm Investig 2:140–9
- Sabzevari A, Adibkia K, Hashemi H, et al. (2013). Polymeric triamcinolone acetonide nanoparticles as a new alternative in the treatment of uveitis: in vitro and in vivo studies. Eur J Pharm Biopharm 84:63–71
- Salgado A, Raposo S, Marto J, et al. (2013). Mometasone furoate hydrogel for scalp use: in vitro and in vivo evaluation. Pharm Dev Technol 19:618–22
- Sanchez-Lozada LG, Mu W, Roncal C, et al. (2010). Comparison of free fructose and glucose to sucrose in the ability to cause fatty liver. Eur J Nutr 49:1–9
- Sankar V, Praveen C, Prasanth KG, et al. (2009). Formulation and evaluation of a proniosome hydrocortisone gel in comparison with a commercial cream. Pharmazie 64:731–4
- Saukkoriipi JJ, Laasonen K. (2008). Density functional studies of the hydrolysis of aluminum (chloro)hydroxide in water with CPMD and COSMO. J Phys Chem A 112:10873–80
- Sawyer J, Febbraro S, Masud S, et al. (2009). Heated lidocaine/tetracaine patch (Synera, Rapydan) compared with lidocaine/prilocaine cream (EMLA) for topical anaesthesia before vascular access. Br J Anaesth 102:210–15
- Seka MA, Van Dewiele T, Verstraete W. (2002). Full-scale evaluation of a multi-component additive for efficient control of activated sludge filamentous bulking. Environ Technol 23:67–72
- Senyigit T, Tekmen I, Sonmez U, et al. (2011). Deoxycholate hydrogels of betamethasone-17-valerate intended for topical use: in vitro and in vivo evaluation. Int J Pharm 403:123–9
- Shahzad Y, Khan Q, Hussain T, Shah SN. (2013). Influence of cellulose derivative and ethylene glycol on optimization of lornoxicam transdermal formulation. Int J Biol Macromol 61:26–32
- Shalonov PM, Dadabaev TD, Khalilov KhN. (1989). [Treatment of burn wounds with dibunol liniment]. Klin Khir 3:53–4
- Shalviri A, Sharma AC, Patel D, Sayani A. (2011). Low-surfactant microemulsions for enhanced topical delivery of poorly soluble drugs. J Pharm Pharm Sci 14:315–24
- Shatalov VN, Korman DB, Krutova TV, et al. (1988). [Antiulcer activity of dibunol in experimental stomach and duodenal ulcers]. Farmakol Toksikol 51:60–4
- Shimoyama T, Miyagi Y, Itoh K, Kobayashi M. (2013). Effect of storage temperature on gelation of oral methylcellulose formulation. Yakugaku Zasshi 133:719–25
- Shirsand S, Para M, Nagendrakumar D, et al. (2012). Formulation and evaluation of Ketoconazole niosomal gel drug delivery system. Int J Pharm Investig 2:201–7
- Shishu, Rajan S, Kamalpreet. (2009). Development of novel microemulsion-based topical formulations of acyclovir for the treatment of cutaneous herpetic infections. AAPS PharmSciTech 10:559–65
- Shul'diakov AA, Barkhatova TS, Satarova SA, Perminova TA. (2012a). [Herpetic infection in patients with psoriasis: the improvement of therapy]. Klin Med (Mosk) 90:61–3
- Shul'diakov AA, Barkhatova TS, Zubareva EV, et al. (2012b). [Topical immunomodulation in the treatment of herpetic infections in HIV-infected patients]. Eksp Klin Farmakol 75:35–7
- Siddoju S, Sachdeva V, Friden PM, Banga AK. (2012). Evaluation of acyclovir cream and gel formulations for transdermal iontophoretic delivery. Ther Deliv 3:327–38
- Smith JM, Rastogi R, Teller RS, et al. (2013). Intravaginal ring eluting tenofovir disoproxil fumarate completely protects macaques from multiple vaginal simian-HIV challenges. Proc Natl Acad Sci USA 110:16145–50
- Soboleva LA, Shul'diakov AA, Khlamova OG, Romantsov MG. (2011). [Improvement of treatment of inflammatory diseases in oral cavity]. Eksp Klin Farmakol 74:41–4
- Soltanipoor F, Delaram M, Taavoni S, Haghani H. (2012). The effect of olive oil on prevention of striae gravidarum: a randomized controlled clinical trial. Complement Ther Med 20:263–6
- Song J, Liu L, Li P, Xiong G. (2012). Short-range interactions between surfactants silica species and EDTA(4)-salt during self-assembly of siliceous mesoporous molecular sieve: a UV Raman study. Spectrochim Acta A Mol Biomol Spectrosc 97:616–24
- Spraker MK, Gisoldi EM, Siegfried EC, et al. (2006). Topical miconazole nitrate ointment in the treatment of diaper dermatitis complicated by candidiasis. Cutis 77:113–20
- Stein L. (2005). Clinical studies of a new vehicle formulation for topical corticosteroids in the treatment of psoriasis. J Am Acad Dermatol 53:S39–49
- Stocker ME, Houghton K. (2001). Anaesthetic drug costs in a district general hospital day surgery unit. Ambul Surg 9:87–9
- Stough D, Bucko AD, Vamvakias G, et al. (2008). Fluorouracil cream 0.5% for the treatment of actinic keratoses on the face and anterior scalp: interim results of an 18-month open-label study. J Clin Aesthet Dermatol 1:16–21
- Stratton SP, Alberts DS, Einspahr JG, et al. (2010). A phase 2a study of topical perillyl alcohol cream for chemoprevention of skin cancer. Cancer Prev Res (Phila) 3:160–9
- Sugawara K, Takayanagi T, Kamiya N, et al. (2007). Voltammetric sensing of sugar by an electrode covered with wheat germ agglutinin/chitin film. Talanta 71:1637–41
- Sugiura H, Uehara M, Hoshino N, Yamaji A. (2001). An open study of a lotion formulation to improve tolerance of tacrolimus in facial atopic dermatitis. Br J Dermatol 145:795–8
- Sul GD, Park HJ, Bae JH, et al. (2013). Preventive effects of multi-lamellar emulsion on low potency topical steroid induced local adverse effect. Ann Dermatol 25:5–11
- Suwannateep N, Wanichwecharungruang S, Fluhr J, et al. (2013). Comparison of two encapsulated curcumin particular systems contained in different formulations with regard to in vitro skin penetration. Skin Res Technol 19:1–9
- Tang Y, Zhou C, Luan J, Hu J. (2012). Improvement of length of survival of expanded flap by application of topical papaverine cream. J Plast Surg Hand Surg 46:389–92
- Tanriverdi ST, Ozer O. (2013). Novel topical formulations of Terbinafine-HCl for treatment of onychomycosis. Eur J Pharm Sci 48:628–36
- Vaghasiya H, Kumar A, Sawant K. (2013). Development of solid lipid nanoparticles based controlled release system for topical delivery of terbinafine hydrochloride. Eur J Pharm Sci 49:311–22
- Valenzuela P, Simon JA. (2012). Nanoparticle delivery for transdermal HRT. Nanomedicine 8:S83–9
- Van Hoogdalem EJ, Terpstra IJ, Baven AL. (1996). Evaluation of the effect of zinc acetate on the stratum corneum penetration kinetics of erythromycin in healthy male volunteers. Skin Pharmacol 9:104–10
- Venkataraman M, Nagarsenker M. (2013). Silver sulfadiazine nanosystems for burn therapy. AAPS PharmSciTech 14:254–64
- Vijay J, Sahadevan J, Prabhakaran R, Gilhotra RM. (2012). Formulation and Evaluation of Cephalexin Extended-release Matrix Tablets Using Hydroxy Propyl Methyl Cellulose as Rate-controlling Polymer. J Young Pharm 4:3–12
- Vorndran E, Geffers M, Ewald A, et al. (2013). Ready-to-use injectable calcium phosphate bone cement paste as drug carrier. Acta Biomater 9:9558–67
- Wang KX, Zhang LH, Peng JL, et al. (2005). Effect of liniment levamisole on cellular immune functions of patients with chronic hepatitis B. World J Gastroenterol 11:7208–10
- Wang W, Zhang P, Shan W, et al. (2013a). A novel chitosan-based thermosensitive hydrogel containing doxorubicin liposomes for topical cancer therapy. J Biomater Sci Polym Ed 24:1649–59
- Wang X, Liu G, Ma J, et al. (2013b). In situ gel-forming system: an attractive alternative for nasal drug delivery. Crit Rev Ther Drug Carrier Syst 30:411–34
- Wang Y, Li L, Li H, et al. (2010). Transdermal permeation of geniposide in the herbal complex liniment in vivo and in vitro. Int J Pharm 392:72–7
- Weisshaar E, Dunker N, Gollnick H. (2003). Topical capsaicin therapy in humans with hemodialysis-related pruritus. Neurosci Lett 345:192–4
- Weisshaar E, Ziethen B, Gollnick H. (2000). Lack of efficacy of topical capsaicin in serotonin-induced itch. Skin Pharmacol Appl Skin Physiol 13:1–8
- Wikiera A, Mika M. (2013). [Structure and properties of pectin]. Postepy Biochem 59:89–94
- Yuan X, Capomacchia AC. (2013). Influence of physicochemical properties on the in vitro skin permeation of the enantiomers, racemate, and eutectics of ibuprofen for enhanced transdermal drug delivery. J Pharm Sci 102:1957–69
- Zakzewski CA, Wasilewski J, Cawley P, Ford W. (1998). Transdermal delivery of regular insulin to chronic diabetic rats: effect of skin preparation and electrical enhancement. J Control Release 50:267–72
- Zampieri AL, Ferreira FS, Resende EC, et al. (2013). Biodegradable polymeric nanocapsules based on poly(DL-lactide) for genistein topical delivery: obtention, characterization and skin permeation studies. J Biomed Nanotechnol 9:527–34
- Zhang YT, Zhao JH, Zhang SJ, et al. (2011). Enhanced transdermal delivery of evodiamine and rutaecarpine using microemulsion. Int J Nanomedicine 6:2469–82
- Zhou H, Agarwal AK, Goel VK, Bhaduri SB. (2013). Microwave assisted preparation of magnesium phosphate cement (MPC) for orthopedic applications: a novel solution to the exothermicity problem. Mater Sci Eng C Mater Biol Appl 33:4288–94
- Zhu F, Wang S, Wang YJ. (2013). Physical properties and enzyme susceptibility of rice and high-amylose maize starch mixtures. J Sci Food Agric 93:3100–6
- Zivkovich AH, Feldman SR. (2009). Are ointments better than other vehicles for corticosteroid treatment of psoriasis? J Drugs Dermatol 8:570–2
- Zulfakar MH, Abdelouahab N, Heard CM. (2010). Enhanced topical delivery and ex vivo anti-inflammatory activity from a betamethasone dipropionate formulation containing fish oil. Inflamm Res 59:23–30