?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Effects of chitosan oligomers with different types and varying concentrations on the intestinal and pulmonary absorptions of calcitonin were investigated in rats by an in situ closed loop method and an in vivo pulmonary absorption experiment, respectively. Various chitosan oligomers demonstrated different efficiencies in improving the intestinal and pulmonary absorptions of calcitonin, and chitosan hexamer with the optimal concentration of 0.5% (w/v) showed the greatest absorption enhancing effect. Moreover, pharmacodynamic parameters of calcitonin after its coadministration intrapulmonarily with various chitosan oligomers were consistently larger than that in the intestinal delivery, indicating the superior potential of pulmonary administration for systemic delivery of calcitonin. Furthermore, various chitosan oligomers neither obviously increased release amounts of protein nor activities of lactate dehydrogenase (LDH) in bronchoalveolar lavage fluid (BALF), revealing the safety of these chitosan oligomers to lung tissue. In addition, bioadhesions of various chitosan oligomers were well consistent with their absorption enhancing effects in the absorption experiment, suggesting the contribution of mucoadhesive properties of chitosan oligomers to their absorption improving effects. Taken together, chitosan oligomers, especially chitosan hexamer, can effectively improve the intestinal and pulmonary absorptions of calcitonin partly due to the mucoadhesion between positive chitosan oligomers and negative mucus in the membrane.
Introduction
Chitosan and its derivatives have been well-researched in the past few years due to the favorable characteristics such as biocompatibility, biodegradation, and low toxicity (Illum, Citation1998; Thanou et al., Citation2001; Di et al., Citation2008; Wang et al., Citation2011). Absorptions of several drugs with poor bioavailability across mucosal surfaces had been potentially improved by using of chitosan or its derivatives as desirable excipients (Hirano et al., Citation1990; Dornish et al., Citation1996; Millotti et al., Citation2011; Gupta et al., Citation2013; Na et al., Citation2013; Huang et al., Citation2014). However, most of the chitosan and its derivatives are poorly soluble in water at physiological pH and also have relatively high molecular weight, which consequently limited their applications in the formulation of dosage forms.
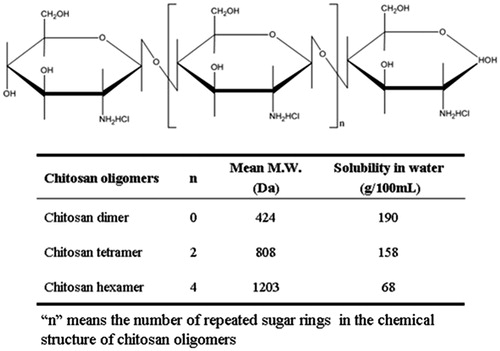
Chitosan oligomers are a novel type of chitosan derivatives and can be prepared by complete deacetylation from chitosan. As shown in , different types of chitosan oligomers including chitosan dimer, tetramer, and hexamer are named according to the number of sugar rings in their chemical structures, and most importantly, they have relatively high solubility in water and low molecular weight compared with conventional chitosan and its derivatives.
Recently, chitosan oligomers have been applied to many fields of drug delivery. It was reported that chitosan–DNA nanoparticles based on ultrapure chitosan oligomers produced substantially higher transgene expression in some cells, demonstrating the excellent potentials of chitosan oligomers as carriers of plasmids for corneal gene delivery (Klausner et al., Citation2010, Citation2012). In contrast, in the area of absorption enhancement technology, absorption enhancing effects of chitosan oligomers on the intestinal absorptions of some poorly absorbable compounds and pulmonary absorption of interferon-α had been demonstrated as useful additives in previous studies (Yamada et al., Citation2005; Gao et al., Citation2008), but their absorption enhancing effects on other therapeutic peptides have not been examined until now. Additionally, the simultaneous comparison of the absorption enhancing potential of chitosan oligomers from different administration route is rarely documented.
Therefore, in the present study, the comparison of absorption-promoting potentials of chitosan oligomers after their intestinal and pulmonary administrations was carried out based on the significant enhancing effects of chitosan oligomers on calcitonin absorption in rats. In addition, we also examined the pulmonary membrane toxicities of various chitosan oligomers by measuring the biological markers including release amounts of protein and LDH activities in BALF. Finally, mucoadhesive characteristics of various chitosan oligomers were evaluated by dynamic light scattering measured in kcps (kilo counts per second) to further explore their contributions to the absorption enhancing mechanisms of chitosan oligomers.
Material and methods
Materials
Various chitosan oligomers including chitosan dimer, tetramer, and hexamer were supplied by Huicheng Bio. Co. (Shanghai, China). Salmon calcitonin was purchased from Aladdin Industrial Inc. (Shanghai, China). Calcium test kit (OCPC method) was obtained by Beijing BHKT Clinical Reagent Co., Ltd (Beijing, China). LDH assay kit was provided from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) and protein assay kit (BCA method) was obtained from Applygen Technologies Inc. (Beijing, China), using bovine serum albumin (BSA) as a standard. All other reagents were of analytical grade.
Preparation of drug solution
Calcitonin with the concentration of 10 µg/mL was prepared by dissolving in isotonic phosphate buffer solution (PBS, pH 7.4) and various chitosan oligomers, used as absorption enhancers, were added to above dosing solution to yield a final concentration of 0.1%, 0.5%, and 1.0%, (w/v). In certain experiments, different solutions containing varying concentrations (0.1%, 0.5%, and 1.0%, w/v) of chitosan oligomers alone were prepared in PBS (pH 7.4) for evaluating the pulmonary membrane toxicity and bioadhesion of chitosan oligomers.
In situ intestinal absorption study
Intestinal absorption of calcitonin was investigated in male SD rats (8–10 weeks, 250–280 g) with an in situ closed loop method, as reported previously (Yamamoto et al., Citation1994; Gao et al., Citation2008). The experiment was performed according to the guidelines of the Animal Ethics Committee at Xi’an Jiaotong University. All rats were fasted overnight before the experiment, but they are allowed to access to water freely. The animals were firstly anesthetized with an intraperitoneal injection of sodium pentobarbital (40 mg/kg body weight) and then the rat abdomen was opened through a midline abdominal incision. After ligating the bile duct of rat, each intestinal segment (small intestine or large intestine) was cannulated and then flushed by PBS (pH 7.4). Immediately thereafter, each dosing solution of calcitonin with or without chitosan oligomers, kept at 37 °C, was administered to the intestinal loop. Finally, blood samples (∼0.25 mL) were taken from jugular vein of rats at time intervals up to 240 min and immediately centrifuged at 12 000 rpm for 5 min to collect the serum fractions, which were stored in ice until determination.
In vivo pulmonary absorption study
The pulmonary absorption of calcitonin in the presence or absence of various chitosan oligomers was evaluated according to the methods reported previously (Enna et al., Citation1972; He et al., Citation2007). The experiments were carried out in accordance with the guidelines of the Animal Ethics Committee at Xi’an Jiaotong University. Male SD rats, weighing 250–280 g, were fasted overnight before the experiment, and anesthetized with sodium pentobarbital (40 mg/kg body weight, i.p.). After the rat had been secured to a procedure, the trachea was exposed and cut transversely halfway between the fourth and fifth tracheal rings caudal to the thyroid cartilage. A polyethylene tube (i.d., 1.5 mm, o.d., 2.5 mm) 2.5 cm in length was gently inserted through the tracheal incision. Thereafter, 100 µL of calcitonin solutions with or without various chitosan oligomers were warmed to 37 °C and then injected into the lung through cannulated tube with a calibrated one hundred syringe. The jugular vein was exposed and blood samples (∼0.25 mL) were taken with heparin rinsed syringes periodically after dosing. Blood samples were immediately centrifuged (12 000 rpm, 4 °C) for 5 min and serum fractions were kept in ice prior to analysis. During experiments, body temperature of animals was maintained at 37 ± 1 °C with heating lamps.
Evaluation of membrane toxicities caused by various chitosan oligomers
To evaluate the pulmonary membrane toxicities of chitosan oligomers, PBS (pH 7.4) with or without various chitosan oligomers were directly administered to rat tracheae in the same manner as was the case of in vivo pulmonary absorption studies, but it was unnecessary to collect the blood sample. 4 h later, the animal was bled from the abdominal aorta under pentobarbital anesthesia and PBS (pH 7.4) was perfused into the rat lung along the trachea. Afterwards, the BALF were collected and then stored in ice immediately until following determination.
Measurements of zeta potential
Zeta potentials of calcitonin solution in the presence or absence of various chitosan oligomers were measured by electrophoretic laser Doppler anemometry at 25 °C with a Malvern Zetasizer – nano zs90 (Malvern Instruments, Malvern, UK). The viscosity and dielectric constant of deionized water were used as calculation parameters.
Measurement of bioadhesions of various chitosan oligomers
Bioadhesions of various chitosan oligomers were examined by dynamic light scattering measured in kcps (kilo count per second) according to recent publication (Sun et al., Citation2010). Briefly, fresh small intestine prepared from male SD rats was excised, rinsed with PBS (pH 7.4) and cut into segments with 4 cm in length. Each segment was incised along the mesentery and spread into 500 µL of freshly prepared solutions including various chitosan oligomers, which was then incubated for 2 h at 37 °C. The intensity of the light scattering signal of resulting solutions was finally determined with a Malvern Zetasizer – nano zs90 (Malvern Instruments, Malvern, UK). The relative reduced kcps percentage to the initial value was calculated to evaluate bioadhesions of various chitosan oligomers.
Data analysis
Plasma calcium level was measured by a Calcium Test Kit with the OCPC method (BHKT Clinical Reagent Co., Ltd, Beijing, China).
The magnitude of hypocalcemic response of calcitonin in the presence or absence of various chitosan oligomers was calculated using the trapezoidal method as the area above the curve (AAC) for 0–4 h and the corresponding decrease in the plasma calcium level (D%) was calculated by a method as described previously using the following equation (Yamamoto et al., Citation1994; Feith et al., Citation2005):
The pharmacological availability (PA%) of calcitonin was calculated as follows:
Statistical analyses
The results were presented as the mean ± S.E. of three samples and the data were assessed using ANOVA test for multiple comparisons. p < 0.05 was considered as the minimum level of significance.
Results and discussion
Effects of various chitosan oligomers on the intestinal and pulmonary absorptions of calcitonin
Calcitonin is a polypeptide hormone of 32 amino acids, with a molecular weight of 3454.93 Da. It acts to reduce blood calcium (Ca2+) and generally can be used therapeutically for the treatment of hypercalcemia or osteoporosis. However, it is well known that absorption of peptide and protein drug including calcitonin is typically poor after non-parenteral administration. On one hand, one reason for the low bioavailability of calcitonin is that it is susceptible to degrade by various peptidase and digestive enzymes in the enteral and other topical sites. On the other hand, relative high molecular weight of calctionin in a sense limits the drug transport across the epithelial cells, thus leading to the poor membrane permeability of calcitonin. Therefore, in the present study, absorption enhancing potential of various chitosan oligomers on the absorptions of poorly absorbable drugs was evaluated by choosing of calcitonin as models of poorly absorbable drugs. Meanwhile, calcium concentration in rat plasma was monitored to evaluate the intestinal or pulmonary absorption of calcitonin characterized by hypocalcemic effect. Plasma calcium concentration–time profiles after intestinal and pulmonary administrations of calcitonin in the presence or absence of various chitosan oligomers are shown in and , respectively, and corresponding pharmacodynamic parameters of calcitonin are summarized in and , respectively.
Figure 2. Effects of various chitosan oligomers on the calcitonin absorption from rat jejunum (a and b) and colon (c and d). Each point represents the mean ± S.E. of three experiments.
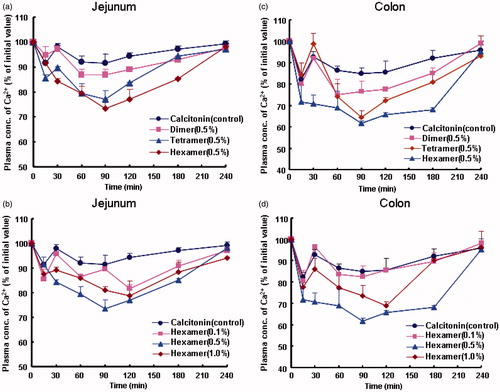
Figure 3. Effects of different types (a) and varying concentrations (b) of chitosan oligomers on the pulmonary absorption of calcitonin. Each point represents the mean ± S.E. of three experiments.
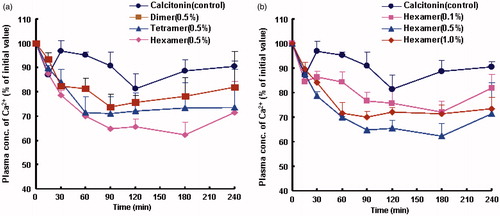
Table 1. Pharmacodynamic parameters of calcitonin after its intestinal administration with or without different types and varying concentrations of chitosan oligomers to rats.
Table 2. Pharmacodynamic parameters of calcitonin after pulmonary administration with or without different types and varying concentrations of chitosan oligomers to rats.
We found that when the PBS (pH 7.4) alone or each chitosan oligomer was delivered to rats, calcium concentration in rat plasma maintained a steady level around the initial value until the terminal time of 4 h (data now shown). This finding indicated that no hypocalcemic effect was induced by PBS (pH 7.4) or chitosan oligomers themselves before the application of drug and additionally confirmed that the surgical operation in rats did not influence obviously the plasma calcium levels in the absorption studies. On the contrary, a slight decrease in the plasma calcium level was observed after intestinal or pulmonary administration of calcitonin alone to rats ( and ), suggesting that a small part of calcitonin could be absorbed following its intestinal or pulmonary delivery in the absence of any chitosan oligomer.
In comparison, calcitonin absorption was significantly improved by various chitosan oligomers after their intestinal and pulmonary administration to rats. For the intestinal delivery, plasma calcium levels were significantly reduced by chitosan hexamer and tetramer after their coadministration with calcitonin to rat intestine and the hypocalcemic effect of chitosan hexamer was greater than that of chitosan tetramer ( and ). In addition, of the applied concentration (0.1%, 0.5%, and 1.0%, w/v), 0.5% (w/v) chitosan hexamer demonstrated the greatest enhancing effects on calcitonin absorption both in the jejunum () and in the colon ().
A similar trend was also found for the pulmonary route. A significant decrease in the plasma calcium level was observed when chitosan hexamer or tetramer was coadministered with calcitonin to rat lung () and relative D%, PA%, and AAC values of calcitonin after pulmonary administration were consistently larger than that in the case of intestinal route, as observed in and . These findings suggested that the hypocalcemic effects of chitosan oligomers on calcitonin absorption from pulmonary delivery seem stronger than that from intestinal route. Two meaningful aspects are proposed to contribute to the superior performance for the pulmonary delivery in comparison with the intestinal route. First, lung possesses special structures, such as extensive vasculature and thin layer alveolar epithelium (0.1–0.2 µm), which results in more efficient drug absorption through alveolar epithelium. Second, the superior absorption improving performance might result from the beneficial properties of lung such as a low intrinsic enzymatic activity, avoidance of first pass metabolism, and minimal contact of peptides and proteins with resident proteases (Hussain et al., Citation2004; Li & Seville, Citation2010), which decreased the loss of calcitonin before its absorption and meanwhile avoided extensive degradation of peptides and proteins by various peptidase and digestive enzymes, thereby leading to the higher bioavailability of calcitonin after pulmonary administration to rats.
It is also important to explain the reason why there is an optimal concentration for chitosan hexamer demonstrating the maximal absorption enhancing effect in the present study. In the previous publications, similar results were also found in the case of PAMAM dendrimers and polyethulenimines (PEI). It was reported that enoxaparin formulation containing 1% G2 PAMAM dendrimer produced a significant increase in anti-factor Χ a level (Cmax). Interestingly, a decrease rather than an increase in anti-factor Χ a level was observed when increasing the concentration of G2 dendrimer to 2% and the optimum charge ratio of enoxaparin–dendrimer was attributed to this phenomenon (Bai et al., Citation2007). Another explanation was elucidated as the possible reason for the lack of a concentration-dependent effect of PEI in increasing nasal absorption of low molecular weight heparin (LMWH). It was pointed out that self-aggregation of PEIs could be occurring, more likely at higher concentrations. As a result, it would form larger size of particles, thus leading to poor contacts with the cell surface and/or inefficient endocytosis (Yang et al., Citation2006).
When we try to find the reason why the absorption enhancing effect of chitosan hexamer lacks concentration dependence in this study, it should be noted that there are some common features between chitosan hexamer and PAMAM dendrimer or PEI. These polymers are positive charged due to the amino groups in their chemical structures and additionally, the model drugs are all negative charged. Therefore, similar to PAMAM dendrimer or PEI, it is possible that when the concentration of chitosan hexamer increased from 0.5% to 1.0% (w/v), an excess amount of unbound cationic charge is already presented. Consequently, binding sites on the cell surface were competed or self-aggregation of chitosan hexamer possibly occurred concomitant with the reduction in the opportunity for chitosan hexamer to contact with cell surface and/or efficient endocytosis, which consequently leads to the decrease in drug absorption. Therefore, it will be reasonable to assume that maximum drug absorption induced by chitosan hexamer with an optimal concentration in the present study was possibly attributed to the optimum charge ratio of hexamer–drug and/or self-aggregation of hexamer at higher concentration, although further studies are required to elucidate it in the future.
Membrane toxicities of chitosan oligomers
Great attentions should be paid to the potential membrane toxicity induced by absorption enhancer due to its inherent toxicity (Maher et al., Citation2009) and the direct link between potency and toxicity (Whitehead et al., Citation2008). Consequently, the evaluation of membrane damage of absorption enhancer becomes an important issue when developing it in clinic.
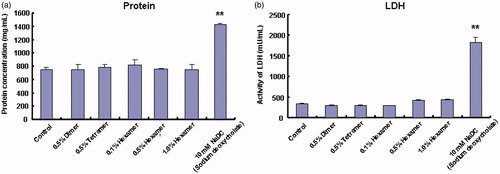
In our previous studies, we evaluated the safety of chitosan oligomers by determining some biological markers including the total protein and LDH activities released from intestinal membranes (Gao et al., Citation2008), but the potential toxicities of these chitosan oligomers for the pulmonary membranes have not been carried out. Therefore, in the present study, evaluation of pulmonary membrane toxicities induced by various chitosan oligomers was performed by measuring the total amount of protein and the activity of LDH released from pulmonary tissue in BALF and relative results are shown in . As demonstrated in , no obvious change was obtained in the amounts of protein and LDH activities in BALF between the PBS (pH 7.4) group and each group of chitosan oligomers with different types and varying concentrations, although sodium deoxycholate (NaDC), used as a positive control, significantly increased the levels of protein and LDH in BALF. These findings indicated that these chitosan oligomers over the applied concentration (0.1–1.0%, w/v) cannot cause any obvious mucosal damage to the pulmonary membranes.
Mechanism of absorption enhancing effects of chitosan oligomers on calcitonin absorption
The mechanism by which chitosan and its derivatives increased the bioavailability of some poorly absorbable drugs was proposed to be a combination of bioadhesion and a transient opening of tight junctions in the membranes (Schipper et al., Citation1997). In our previous studies, we found that 0.5% (w/v) chitosan hexamer moderately decreased the values of transepithelial electrical resistance (TEER) of rat intestinal membranes as compared with control (PBS, pH 7.4), indicating that chitosan oligomers might loosen tight junctions of intestinal epithelium, thereby enhancing drug permeability via a paracellular pathway (Gao et al., Citation2008). However, it should be noted that chitosan and its derivatives are naturally cationic and this inherent mucoadhesion between the chitosan and its derivatives and the negatively mucus also should be one of the absorption enhancing mechanisms. Unfortunately, mucoadhesion-related absorption promoting mechanisms of chitosan oligomers were explored sparsely although many publications have demonstrated the mucosal absorption enhancement induced by chitosan or its derivatives.
Therefore, in this study, bioadhesive characteristics of various chitosan oligomers were investigated upon incubation of various chitosan oligomers with rat small intestine. The bioadhesions was characterized by the corresponding decrease percentage of light-scattering signal intensity measured in kcps with respect to the initial value based on the particle immobility at the intestinal surface by adhesion. This method was previously used to measure the bioadhesion of polyelectrolyte complexes (Sun et al., Citation2010) and the particle concentration in a sample (Mao et al., Citation2006). In the past few years, several mucoadhesion test methods have been reported and experiments were carried out either by using animal mucosa (Hagerstrom & Edsman, Citation2001; Sogias et al., Citation2012), human gastric mucosa (Jackson et al., Citation2001), mucin discs manufactured by compression of mucin (Jones et al., Citation1997) or hydrogels as mucosa-mimetic material (Hall et al., Citation2011). However, due to the similar structure of mucosa between animal and human, animal mucosa offers the advantage to get an impression of the characteristics of adhesive drug formulations for use in humans. Therefore, the use of animal mucosa to study mucoadhesion is still of high importance although it has several disadvantages.
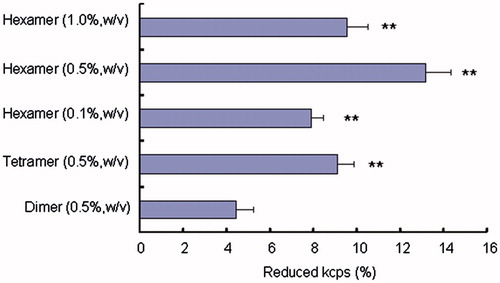
shows the reduced kcps percentage of various chitosan oligomers measured by dynamic light scattering. As shown in , bioadhesion of each chitosan oligomer is quite different in the case of different types and varying concentrations, and chitosan hexamer, among the applied various chitosan oligomers, showed the greatest value of bioadhesion. In addition, when the varying concentrations (0.1%, 0.5%, and 1.0%, w/v) of chitosan hexamer were incubated with the intestine, the reduced kcps percentage attained the maximum in the presence of 0.5% (w/v) chitosan hexamer. The reason for the superior mucoadhesive property of chitosan hexamer in comparison with chitosan tetramer and dimer was not fully understood. However, it may be plausible that chitosan hexamer, which has longer chains and more positive charges due to the existence of amino groups in its molecule as compared with chitosan tetramer and dimer, seems to have higher tendency to interpenetrate and entangle with the mucus protein, thereby resulting in more stronger mucoadhesive interactions between the positive chitosan oligomers and the negative mucin gycoproteins.
Figure 5. Reduced kcps percentage of various chitosan oligomers measured by dynamic light scattering. Data represent the mean ± S.E., n = 3. *p < 0.05, **p < 0.01, compared with the dimer group.
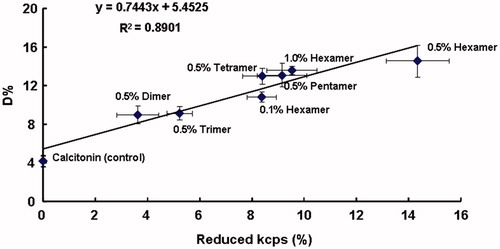
shows the relationship between the bioadhesion of various chitosan oligomers and their absorption enhancing effects on calcitonin absorption. A good linear correlation between the absorption enhancing effects (expressed as D%) and the bioadhesion of chitosan oligomers is shown in , indicating that bioadhesion of chitosan oligomers could in a sense influence the transport of calcitonin across the cell membranes and might also be one of the absorption enhancing mechanisms for chitosan oligomers to improve the poor permeability of calcitonin.
Figure 6. The relationship between the absorption enhancing effects (D%) and the bioadhesions (reduced kcps%) of various chitosan oligomers. Data represent the mean ± S.E., n = 3.
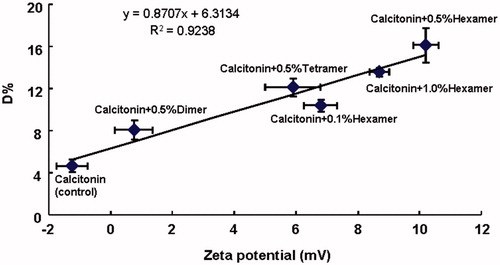
In addition, the difference in the absorption enhancing efficiencies of various chitosan oligomers for calcitonin absorption suggested that absorption enhancing abilities of various chitosan oligomers in a sense depend on the type of chitosan oligomers. It is possible that the chemical structures of enhancers, especially the functional groups in their molecules, affect their absorption efficiencies. Previous studies have reported that absorption enhancing effects of polyamidoamine (PAMAM) dendrimers probably depend on the number of amino groups, since the number of amino groups on the surface of enhancer with higher generation was generally greater than that with lower generation (Dong et al., Citation2010, Citation2011). Therefore, in this study, to demonstrate whether the absorption enhancing effect of chitosan oligomer was influenced by the amino group in their structures, the zeta potentials of calcitonin solutions with or without various chitosan oligomers were estimated and the relationship between the absorption enhancing effect (expressed as D%) of chitosan oligomer and the zeta potential is plotted in . It can be seen from that the zeta potential of calcitonin solution, used as control, was negative in the absence of any chitosan oligomers. However, the magnitude of this negative zeta potential tended to decline and gradually changed to positive as various chitosan oligomers were added to calcitonin solution (), suggesting that the negative charge of calcitonin solution was neutralized by chitosan oligomers. Furthermore, a good linear correlation was also observed between the zeta potential of calcitonin solution in the presence of various chitosan oligomers and the absorption enhancing effect of chitosan oligomers (). This finding indicated that the different zeta potentials caused by different amino group contents of chitosan oligomers could affect drug permeability across the cell membranes. Taken together, the change in the charge of calcitonin solution to positive by the addition of chitosan oligomers may diminish the coulombic repulsion generated between the negatively charged cell membrane and the negatively charged calcitonin, which probably contributes in part to the absorption enhancing mechanism of chitosan oligomers and consequently, triggering the absorption enhancing effects of chitosan oligomers on calcitonin absorption.
Figure 7. The relationship between the absorption enhancing effects (D%) and zeta potential of calcitonin solutions in the presence or absence of various chitosan oligomers. Data represent the mean ± S.E., n = 3.
Taken together, it was plausible to believe that the absorption enhancing mechanisms of chitosan oligomers were possibly related to be a combination of mucoadhesion and an effect on tight junctions in the epithelium.
Conclusion
In conclusion, the present study demonstrated that chitosan oligomers, especially chitosan hexamer (0.5%, w/v), were effective to increase calcitonin absorption in rats from intestinal and pulmonary routes and meanwhile did not cause any obvious membrane damage. Mucoadhesions between the positive chitosan oligomers and negative mucus in the membrane were correlated with the absorption enhancing effects of chitosan oligomers, indicating the contribution of mucoadhesive properties of chitosan oligomers to their absorption improving mechanisms. Therefore, these novel chitosan oligomers are promising for improving the oral or pulmonary absorptions of many therapeutic peptides including calcitonin.
Declaration of interest
This work was supported by grants from the National Natural Science Foundation of China (81102382) and the Fundamental Research Funds for the Central Universities (to Yang Gao and Hailong Zhang). The authors report no declarations of interest.
References
- Bai SH, Thomas CD, Ahsan F. (2007). Dendrimer as a carrier for pulmonary delivery of enoxaparin, a low-molecular weight heparin. J Pharm Sci 96:2090–106
- Di CG, Zambito Y, Zaino C. (2008). Polymeric enhancers of mucosal epithelia permeability: synthesis, transepithelial penetration-enhancing properties, mechanism of action, safety issues. J Pharm Sci 97:1652–80
- Dong ZQ, Katsumi H, Sakane T, Yamamoto A. (2010). Effects of polyamidoamine (PAMAM) dendrimers on the nasal absorption poorly absorbable drugs in rats. Int J Pharm 393:244–52
- Dong ZQ, Abdul HK, Gao Y, et al. (2011). Polyamidoamine dendrimers can improve the pulmonary absorption of insulin and calcitonin in rats. J Pharm Sci 100:1866–78
- Dornish M, Aarnold M, Skaugrud O. (1996). Alginate and chitosan: biodegradable biopolymers in drug delivery systems. Eur J Pharm 182:21–3
- Enna SJ, Schanker LS. (1972). Absorption of drugs from the rat lung. Am J Physiol 223:1227–31
- Feith G, Habib F, Okada N, et al. (2005). Nitric oxide donors can enhance the intestinal transport and absorption of insulin and [Asu1,7]-eel calcitonin in rats. J Control Release 106:287–97
- Gao Y, He L, Katsumi H, et al. (2008). Improvement of intestinal absorption of insulin and water-soluble macromolecular compounds by chitosan oligomers in rats. Int J Pharm 358:70–8
- Gupta S, Jain A, Chakraborty M, et al. (2013). Oral delivery of therapeutic proteins and peptides: a review on recent developments. Drug Deliv 20:237–46
- Hagerstrom H, Edsman K. (2001). Interpretation of mucoadhesive properties of polymers gel preparations using a tensile strength method. J Pharm Pharmacol 53:1589–99
- Hall DJ, Khutoryanskaya OV, Khutoryanskiy VV. (2011). Developing synthetic mucosa-mimetic hydrogels to replace animal experimentation in characterization of mucoadhesive drug delivery systems. Soft Matter 7:9620–3
- He L, Gao Y, Lin YL, et al. (2007). Improvement of pulmonary absorption of insulin and other water-soluble compounds by polyamines in rats. J Control Release 1:94–101
- Hirano S, Seino H, Akiyama Y, Nonaka I. (1990). Chitosan: a biocompatible material for oral and intravenous administration. In: Gebelein, CG, Dunn RL, eds. Progress in biomedical polymers. New York: Plenum Press, 283–9
- Huang AW, Su ZG, Li S, et al. (2014). Oral absorption enhancement of salmon calcitonin by using both N-trimethyl chitosan chloride and oligoarginines-modified liposomes as the carriers. Drug Deliv 21:388–96
- Hussain A, Arnold JJ, Khan MA, Ahsan F. (2004). Absorption enhancers in pulmonary protein delivery. J Control Release 1:15–24
- Illum L. (1998). Chitosan and its use a pharmaceutical excipient. Pharm Res 15:1236–331
- Jackson SJ, Perkins AC. (2001). In vitro assessment of the mucoadhesion of cholestyramine to procine and human gastric mucosa. Eur J Pharm Biopharm 52:121–7
- Jones DS, Woolfson AD, Brown AF. (1997). Textural, viscoelastic and mucoadhesive properties of pharmaceutical gels composed of cellulose polymers. Int J Pharm 151:223–33
- Klausner EA, Zhang Z, Chapman RL, et al. (2010). Ultrapure chitosan oligomers as carriers for forneal gene transfer. Biomaterials 31:1814–20
- Klausner EA, Zhang Z, Won SP, et al. (2012). Corneal gene delivery: chitosan oligomer as a carrier of CpG rich, CpG free or S/MAR plasmid DNA. J Gene Med 14:100–108
- Li HY, Seville PC. (2010). Novel pMDI formulations for pulmonary delivery of proteins. Int J Pharm 1–2:73–8
- Maher S, Leonard TW, Jacobsen J, Brayden DJ. (2009). Safety and efficacy and sodium caprate in promoting oral drug absorption: from in vitro to the clinic. Adv Drug Delivery Rev 61:1427–49
- Mao SR, Bakowsky U, Jintapattanakit A, Kissel T. (2006). Self-assembled polyelectrolyte nanocomplexs between the chitosan derivatives and insulin. J Pharm Sci 95:1035–48
- Millotti G, Perera G, Vigl C, et al. (2011). The use of chitosan-6-mercaptonicotinic acid nanoparticles for oral peptide drug delivery. Drug Deliv 18:190–7
- Na LN, Wang J, Wang LL, Mao SR. (2013). A novel permeation enhancer: N-succinyl chitosan on the intranasal absorption of isosorbide dinitrate in rats. Eur J Pharm Sci 48:301–306
- Schipper NG, Olsson S, Hoogstraate JA, et al. (1997). Chitosan as absorption enhancers for poorly absorbable drugs 2: Mechanism of absorption enhancement. Pharm Res 14:923–9
- Sogias IA, Williams AC, Khutoryanskiy VV. (2012). Chitosan-based mucoadhesive tables for oral delivery of ibuprofen. Int J Pharm 436:602–610
- Sun W, Mao S, Wang YJ, et al. (2010). Bioadhesion and oral absorption of enoxaparin nanocomplexes. Int J Pharm 386:275–81
- Thanou M, Verhoef JC, Junginger HE. (2001). Oral drug absorption enhancement by chitosan and its derivatives. Adv Drug Deliv Rev 52:117–26
- Wang WX, Gao JQ, Liang WQ. (2011). Chitosan-coated liposomes for intracellular oligonucleotides delivery: characteristics and cell uptake behavior. Drug Deliv 18:208–14
- Whitehead K, Karr N, Mitragotri S. (2008). Safe and effective permeation enhancers for oral drug delivery. Pharm Res 25:1782–8
- Yamada K, Odomi M, Okada N, et al. (2005). Chitosan Oligomers as potential and safe absorption enhancers for improving the pulmonary absorption of Interferon-a in rats. J Pharm Sci 94:2432–40
- Yamamoto A, Taniguchi T, Rikyuu K, et al. (1994). Effects of various protease inhibitors on the intestinal absorption and degradation of insulin in rats. Pharm Res 11:1496–500
- Yang TZ, Hussain A, Bai SH, et al. (2006). Positively charged polyethylenimines enhance nasal absorption of the negatively charge drug, low molecular weight heparin. J Control Research 115:289–97