Abstract
Objectives: The efficacy of ketorolac tromethamine (KT) floating alginate beads as a drug delivery system for better control of KT release was investigated. The formulation with the highest drug loading, entrapment efficiency, swelling, buoyancy, and in vitro release would be selected for further in vivo analgesic effect in the mice and pharmacokinetics study in rats compared to the tablet dosage form.
Methods: KT floating alginate beads were prepared by extrusion congealing technique. KT in plasma samples was analyzed using a UPLC MS/MS assay.
Results: The percentage yield, drug loading and encapsulation efficiency were increased proportionally with the hydroxypropylmethyl cellulose (HPMC) polymer amount in the KT floating beads. A reverse relationship was observed between HPMC amount in the beads and the KT in vitro release rate. F3-floating beads were selected, due to its better in vitro results (continued floating for >8 h) than others. A longer analgesic effect was observed for F3 in fed mice as compared to the tablets. After F3 administration to rats, the Cmax (2.2 ± 0.3 µg/ml) was achieved at ∼2 h and the decline in KT concentration was slower. F3 showed a significant increase in the AUC (1.89 fold) in rats as compared to the tablets.
Conclusion: KT was successfully formulated as floating beads with prolonged in vitro release extended to a better in vivo characteristic with higher bioavailability in rats. KT in floating beads shows a superior analgesic effect over tablets, especially in fed mice.
Introduction
Oral delivery remains the preferred route of drug administration with a higher level of patient adherence. However, this route has certain problems such as unpredictable gastric emptying rate, short gastro-intestinal transit time (8–12 h) and existence of a limited absorption window in the gastric region and upper small intestine for several drugs leading to low and variable oral absorption over shorter period of time (Agyilirah et al., Citation1991; Chawla et al., Citation2003; Shivkumar et al., Citation2004).
Various gastro-retentive dosage forms (GRDFs) have been postulated to significantly extend the period of time over which drug can be released and thus prolongs dosing intervals and eventually improving patient compliance. Such system can be retained in the stomach and assist in improving the oral delivery of drugs. Optimal bioavailability would be assured for drugs with an absorption window in a particular gastrointestinal tract using these GRDFs (Rouge et al., Citation1996; Alexander & Juergen, Citation2006). The proposed GRDFs have included, but are not limited to bioadhesive, floating, swelling, expanding, modified shape and other delayed gastric devices drug delivery systems (DDS) (Singh & Kim, Citation2000; Arora et al., Citation2005; Shishu et al., Citation2007).
Floating systems are low density systems that have sufficient buoyancy to float over the gastric contents and remain in the stomach without affecting the gastric emptying rate for a prolonged period. While the system floats over the gastric contents, the drug is released slowly at the desired rate, prolonging the gastric retention time and reducing the fluctuation of drug concentration in plasma (Arora et al., Citation2005; Pahwa et al., 2013).
Several polymers with various viscosity grades such as hydroxylpropylmethyl cellulose (HPMC), Carbopol 934P, Eudragit RL, calcium alginate, chitosan, xanthan gum, guar gum, ethylcellulose and others have been employed in the design of floating systems (Arora et al., Citation2005). Alginate beads have been developed in recent years as a unique vehicle for DDS. Alginate is a hydrophilic polymer, stable in acidic media and easily depredated in alkaline media. These properties have enabled widespread use of alginate beads in GRDFs for sustained release of drugs (Gadad et al., Citation2009). Floating alginate beads could be prepared by reacting carbonate salts present in the alginate matrix with acetic acid, thus producing carbon dioxide gas. The carbon dioxide evolved permeates through the alginate leaving gas bubbles or pores. The most commonly used gas forming agents are sodium bicarbonate and calcium carbonate (Choi et al., Citation2002; Shishu & Nidhi, Citation2007). Hydrophilic polymers (HP) are widely used in floating beads to sustain the release of drugs. Upon contact with water, HP slowly forms thick gel, which retains the integrity of the formulation and promotes sustained release of drugs through the thick gel (Stops et al., Citation2008).
Ketorolac tromethamine (KT) is a non-steroidal anti-inflammatory drug with a biological half-life ranging from 4 to 6 h (Martin & Bustamante, Citation1993; Ravindra & Sabitha, Citation2010). In addition, KT is a weak acid, well absorbed from the upper portion of the duodenum. Therefore, the concept of floating beads would be utilized to minimize the irritant effect of KT, as a weak acidic drug, on the stomach by avoiding direct contact with the mucosa. Furthermore, formulation of floating KT would prolong its gastric residence time and would ensure sustained KT release into the proximal part of the intestine thus providing means of using low dosage for prolonged periods (Sinha & Trehan, Citation2005). At present, a great deal of emphasis is being placed on the development of orally controlled or sustained release forms for KT. This would be beneficial in attaining the required therapeutic efficacy and achieving better tolerance with fewer gastrointestinal side effects. However, to our knowledge, no published data have been developed for a floating dosage form of KT and nor has its pharmacokinetics been monitored in rats. Therefore, the present study was undertaken to investigate the efficacy of KT floating alginate beads as a drug delivery system for better control of KT release using calcium carbonate as a gas-forming agent and using different polymer concentrations. The formulation, with the optimal drug loading, entrapment efficiency, swelling, buoyancy, in vitro release and in vivo analgesic effect in the mice would be selected for a pharmacokinetic study to investigate its in vivo superiority over to conventional tablet dosage form in rats.
Materials and methods
Materials
KT was purchased from (Varda Biotech Ltd., India). Flurbiprofen, mass grade formic acid and ammonium formate were purchased from (Sigma-Aldrich, St. Louis, MO, USA). Sodium alginate, calcium carbonate and glacial acetic acid were purchased from (BDH, UK). Hydroxypropylmethyl cellulose (HPMC) was purchased from (DOW Chemicals, UK). Ketoral® tablets were purchased from (Al-Arab Drug Company, Egypt). All other reagents were of analytical grade and were used as received.
Methods
Preparation of KT-floating alginate beads
Ketorolac floating beads were prepared by extrusion congealing technique introduced by Vani et al. (Citation2010). KT (40 mg) was dissolved in distilled water (5 ml). The solution was dispersed in 95 ml sodium alginate solution (3% w/v) containing HPMC polymer in ratio of (alginate: polymer, 9:1, 7:1, and 5:1 w/w). Calcium carbonate, a gas forming agent, was added to KT-alginate in a 1:1 ratio (w/w). The composition of the prepared formulations is shown in . After degassing, the mixture was added, drop wise, to a 100 ml of 1% (w/v) calcium chloride solution containing 10% (v/v) glacial acetic acid using a 24G needle syringe. The suspended beads were stirred for 10 min with a magnetic stirrer at 50 rpm to improve the mechanical strength of the beads and to allow for completion of the gas production reaction. The formed beads were collected, washed with ethanol and distilled water and air dried for 48 h.
Table 1. Composition of different KT floating beads.
KT floating alginate beads characterization
The percentage of yield, drug loading and entrapment efficiency of KT floating alginate beads were assessed after ensuring dryness of the beads (n = 3) (Pahwa et al., 2013; Gadad et al., Citation2009).
Drug loading was determined by weighing 25 mg of KT prepared beads, dissolved in 50 ml of buffer solution (pH, 1.2), centrifuged, filtered and the filtrate was analyzed at 322 nm using a UV/visible spectrophotometer (Thermo Fisher Scientific Inc., Madison, USA).
Scanning electron microscope (SEM)
An SEM (Metler Toledo, Tokyo, Japan) was used to examine the surface properties and morphology characteristics of the beads.
Determination of swelling properties
A known weight (50 mg) of floated beads was soaked in 100 ml of buffered solution (pH, 1.2) at 37 ± 0.5 °C. The beads were removed at specified time intervals, dried and weighed to determine the swelling ratio. The swelling ratio was determined using the following equation (Shishu & Nidhi, Citation2007).
The experiment was performed in triplicate and the average results were taken.
In vitro evaluation of floating ability
The floating efficacy of KT-alginate beads was measured by suspending 50 mg of the beads in 100 ml of buffered solution (pH, 1.2). The beads were stirred at 100 rpm, and the time of most of the beads to rise to the surface of the solution was recorded as the floating lag time. After 8 h, the layer of floated beads as well as the sinking layer were separately collected by filtration. Beads of both layers were dried at 40 °C until constant weight was achieved. Both fractions of beads were weighed and the buoyancy was determined by the weight ratio of floating particles to the sum of floating and sinking particles (Stops et al., Citation2008; Ravindra & Sabitha, Citation2010).
In vitro release study
The in vitro dissolution of the KT-alginate floating beads was carried out using USP dissolution test apparatus II (Erweka DT-600 GmbH, Germany). Each vessel containing an amount of beads equivalent to 10 mg KT was rotated at 50 rpm in 900 ml of buffered solution (pH, 1.2) maintained at 37 ± 0.5 °C. An aliquot of 5 ml of the solution was withdrawn at predetermined time intervals and replaced with a fresh dissolution medium. The withdrawn samples were analyzed for KT content spectrophotometrically at 322 nm. The release rate of 10 mg of KT powder and KT commercial tablets (Ketoral, 10 mg) were also determined. The experiment was carried out in triplicate and the average values of the released amount were calculated.
Kinetic study
The in vitro release data were analyzed using different kinetic equations; zero-order, first-order kinetics, Higuchi diffusion and Korsmeyer–Peppas models (Martin & Bustamante, Citation1993).
Analgesic effect study in mice
The analgesic effect of the selected KT floating beads’ formulation (F3) was assessed in young Swiss-albino mice by two different methods: hot plate and tail flick (Thanoo et al., Citation1993; Sinha & Trehan, Citation2005). Mice of either sex with an average body weight of 30–40 g were used. The animals were housed at room temperature. Mice were randomly divided into five groups each of six mice as follows: group I, was used as the control and received 1 ml normal saline; group II, was given an aqueous solution of KT (1.1 mg/kg); group III was given Ketoral®; group IV and V were given the selected F3-floating formulation. Animals in group IV were fasted for ∼12 h prior to and during the experiments while animals in group V were kept on standard laboratory pellet-diet but water was available at all times for both groups. Mice were orally dosed 30 min before the beginning of these two tests. KT alginate beads containing 1.1 mg/kg of KT corresponding to 10 mg human dose was given to groups IV and V using an oral tube. All the experiments were performed in accordance to the ethical guidelines established and approved by the committee on the use and care of laboratory animals at King Saud University (Issa et al., Citation2009).
Hot plate method
The mice were placed on Hot Plate Analgesiameter (MK-350 D, Japan), maintained at 51 ± 1 °C. The time was taken by a mouse to begin to jump or lick forepaw was determined and this time was called the reaction time. The trial took ∼25 s to avoid any tissue damage. Analgesic effect was measured at predetermined time intervals (0.5, 1, 2, 3, 4, 5, 6, 7 and 8 h) and the changes in the behavior of the mice in fast and fed state were recorded.
Tail-flick method
The mice were placed on tail-flick apparatus, Tail-Flick Analgesiameter (MK-330B, Japan) using simple restrain device to fix the animal for testing. The tail was placed into a sensing groove and a photo-sensor was located under this groove. The beam that produces the radiant heat stimulus was focused on the area of the distal part of the tail and the strength of the radiant heat lamp was kept constant. The time was taken by a mouse to withdraw the tail was measured. Cut-off reaction time was 10 s to avoid any tissue injury. The reaction time of the analgesic effect was measured at 0.5, 1, 2, 3, 4, 5, 6, 7 and 8 h, and the changes in the behavior of mice were detected (Ibrahim et al., Citation2010).
Pharmacokinetics studies in rats
Animal dosing and sampling scheme
Thirty-two male Wistar rats (175–230 g) were used. Rats were randomly separated into two groups (n = 16) and each group was divided into two subgroups (n = 8) for different sampling time. Each subgroup was marked and housed in one cage. Animals were having free access to food and water during the experiment. For the first group, aliquot of F3 weight was suspended in sterile water for injection (SWFI) and each rat was received an oral dose of 1.1 mg/kg of KT using rats oral tubing. While the second group was received KT (Ketoral®) tablets, crushed and suspended in SWFI and each rat was given an equivalent to 1.1 mg/kg as described above. KT suspensions were vortexed for few seconds before each administration. Blood samples (0.5 ml) were collected in 0.6 ml graduated microtainer, containing lithium heparin, at 0.0, 0.5, 2, and 4 h and at 1, 6, 8, 12 and 24 h from the first and the second subgroups, respectively, after drug administrations. Blood samples were collected from the orbital venous plexus, under light halothane anesthesia. Four to five blood samples were collected from each rat per day to avoid any damage to the eye. Therefore, each data point is the mean of eight replicates. Plasma samples were separated by centrifugation at 4000 rpm for 15 min and stored at −20 °C till assayed.
Chromatographic system and conditions
The analysis was carried out on a Waters Acquity UPLC™ (Waters Corp., Milford, MA). An Acquity UPLC™ BEH C18 column (50 mm × 2.1 mm, 1.7 µm, Waters Corp., Milford, MA) was employed for separation at 40 °C. The gradient elution for UPLC analysis consisted of two solvents: Solvent A: 10% methanol, 90% acetonitrile and 0.2% formic acid and solvent B: 10 mM ammonium formate, 0.2% formic acid and 1% acetonitrile (pH 2.9). The gradient elution began with 30% of solvent A and changed linearly to 65% solvent A within 2.5 min, and changed back to 30% solvent A at 3 min. Throughout the UPLC process the flow rate was set at 0.4 ml/min and the run time was 4 min. All data were collecting and treated using Mass Lynx™ V 4.1 software with QuanLynx™ V 4.1 program (Waters Corp., Milford, MA). The method was validated for selectivity, linearity, precision, accuracy, and carry over, extraction recovery and stability briefly before the beginning of this study according to published assay study (Radwan et al., Citation2010).
Drug analysis
KT concentrations in plasma were measured using a validated specific and sensitive UPLC MS/MS method (Radwan et al., Citation2010). Briefly, samples aliquot of 200 µl were added to a 1.8 ml Eppendorf tube and were spiked with 20 µl of flurbiprofen, the internal standard (IS). The mixture was vortexed for 10 s and 800 µl of acetonitrile were added and the mixture was vortexed for 1 min, centrifuged at 20 000 rpm for 15 min at 10 °C. The supernatant was transferred into a clean glass tube and evaporated to dryness under a gentle stream of nitrogen. The residue was reconstituted in 100 µl of water:acetonitrile (50:50, v/v), vortexed for 1 min, centrifuged at 4000 rpm for 5 min, transferred into a plastic autosampler vial with pre-slit septum (Waters, USA) where 1 µl was injected into the UPLC MS/MS system.
Data and statistical analysis
Plasma concentrations of KT were presented as the mean ± SD and pharmacokinetics parameters were estimated using model-independent methods (Gibaldi & Perrier, Citation1982). The terminal elimination rate constant (k) was estimated by linear regression analysis of the terminal portion of the log-linear plasma concentration–time profile of KT. The terminal elimination half-life (t1/2) was calculated from k using the formula t1/2 = 0.693/k. The mean maximum KT concentration (Cmax) and the time to reach Cmax (Tmax) were derived directly from the individual blood levels. The area under each drug concentration time curve (AUC0–24, µg ml−1 h) to the last data point were calculated by the linear trapezoidal rule and extrapolated to time infinity by the addition of Cn/k where, Cn is concentration of the last measured plasma sample. The apparent body clearance (Cl/F) was calculated using the equation Cl/F = dose/AUC. All statistical differences in data were evaluated by IBM SPSS Statistics 20 using Student t-test, correlation or one way analysis of variance (ANOVA) where p value <0.05 was considered significant.
Results and discussion
Ketorolac floating beads composed of alginate with three different ratios (9:1, 7:1, and 5:1) of sodium alginate: HPMC polymer were prepared by extrusion congealing method as shown in . The data of the physicochemical characterizations include percentage yield, drug loading, drug encapsulation efficiency, floating lag time and % floating after 8 h of KT formulations are presented in . For the three tested formulations (F1, F2 and F3), the percentage yield of KT floating beads is in the range of 88.0 ± 2.6 to 91.0 ± 2.0 while the percentage drug loading in these beads is 80.8 ± 4.3 to 83.8 ± 4.1. Since KT is water soluble, a lower percentage of encapsulation efficiency was observed (69.9 ± 1.6 to 75.58 ± 1.5). Therefore, the percentage yield, drug loading and encapsulation efficiency are affected by the amount of HPMC in the beads. The increase in HPMC amount increased the alginate beads ability to retain more amount of the drug. This is achieved due to the formation of two types of protective layers in the beads leading to a delay in the diffusion of the drug more effectively than a single protective layer formed by sodium alginate alone (Ajit et al., Citation2006).
Table 2. Physicochemical characteristic of different prepared KT floating beadsa.
Scanning electron microscope of KT floating beads
SEM examination of F3-alginate beads of KT showed a smooth spherical shape with a mean diameter of 1190 ± 13.2 µm ().
Floating studies
A lag time in the range of 86.0 ± 9.80 to 155 ± 15.7 s was detected for the KT floating beads. It was noticed that the beads were continued floating for more than 8 h and percentage of floating was from 87.5 ± 2.5 to 90.3 ± 2.5 as shown in .
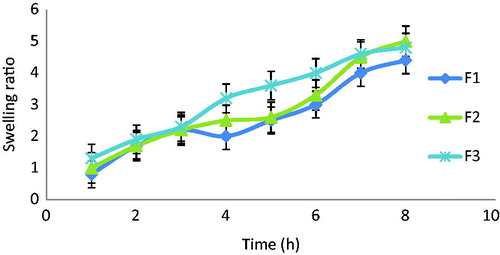
Swelling characteristics of KT floating beads
The swelling ratio of beads describes the amount of water that contained within the hydrogel at equilibrium which is a function of the network structure, hydrophilicity and ionization of the functional groups. shows that the swelling ratios of the prepared formulations were in the order of F3 > F2 > F1 explaining the effect of HPMC amount on the swelling behavior of KT beads. In general, it was evident that, the swelling ratios increased by increasing HPMC polymer concentration. This may be due to the hydrophilicity nature of this cellulose derivative polymer and in addition to the presence of hydroxyl group in the molecules, which plays an important role in water uptake and in matrix integrity of swollen polymer (Borkar et al., Citation2010).
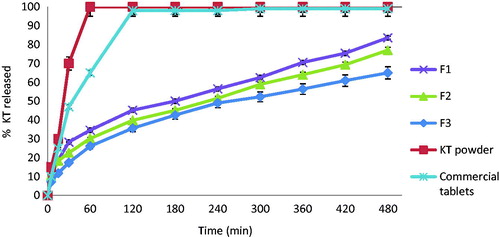
In vitro release studies
The In vitro release of KT from the different prepared floating beads is presented in . KT was completely released from its powder and commercial tablets (Ketoral®) after 50 and 85 min, respectively. Its release from the prepared floating beads was sustained over a longer period of time depending on HPC amount in each formula. For the prepared formulations F1, F2, and F3 containing sodium alginate/HPMC in ratios of 9:1, 7:1 and 5:1, respectively, the lower the sodium alginate to the HPMC in the prepared formulations, the significantly slower the KT release as compared to its release from either the powder or the commercial tablets. The percent release of KT after 8 h from F1, F2, and F3 were 81.2 ± 2.5, 72.2 ± 0.2, and 61.2 ± 0.2, respectively. This finding is in good agreement with Raju et al.’s (Citation2010) conclusion that HPMC as a hydrophilic matrix was capable of forming a strong viscous gel once in contact with an aqueous media, and this could be useful in controlling the delivery of highly water soluble drugs (Raju et al., Citation2010).
In vitro release kinetic study
The release data of KT were fitted to zero, first-order, Higuchi diffusion and Korsmeyer–Peppas kinetic models as presented in . The best fit, with the highest determination coefficient (R2), of KT formulations was achieved with the Higuchi diffusion model followed by Korsmeyer–Peppas release model. Higuchi diffusion model describes drug release from polymeric system by diffusion mechanism and Fickian release behavior (n < 0.5).
Table 3. Kinetic parameters of KT floating beads according to different kinetic models.
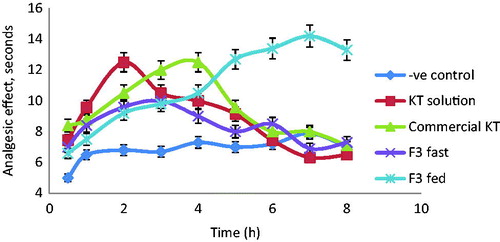
Analgesic effect using hot plate method
The slowest in vitro release KT floating beads formulation F3 (61.23 ± 0.18% released after 8 h) was selected for the in vivo analgesic effect evaluation of KT in mice as compared to the commercial tablets (Ketoral®) and KT solution. In addition, F3 remained buoyant for more than 8 h and showed the highest percent of KT loading and also encapsulation efficiency (). shows the effect of these different formulations on the change of animal’s behavior towards pain stimulant. In the mice, a significant analgesic effect (p < 0.05) was observed with KT among all tested dosage forms after 0.5 h of administration as compared to the control. The oral solutions compared to the control show a maximum analgesic effect (12.6 ± 0.6 s) after 2 h followed by a gradual decrease before its complete stop after 6 h. The same trend was observed with KT commercial tablets and F3 on fasted mice but the maximum analgesic effect was detected after 3 and 4 h of administrations, respectively. In fed mice, however, F3 showed significant maximum analgesic effect (14.3 ± 0.5 s) after 7 h and it was lasted longer (>8 h). These results are in agreement with earlier report of Desai & Bolton (Citation1993). They have found that the GRT of theophylline floating tablets was 1.57 h in fast state while after meal a significant prolongation in GRT (7.15 h) was observed (Desai & Bolton, Citation1993). Therefore, it could be concluded that food intake has a significant effect on improving analgesic profile of drugs in mice. The presence of food in the stomach increases GRT and maintains the beads floated in the stomach for longer period sufficient to release the drug from the formulated dense structure beads. While in fast mice, beads are emptied from the stomach before complete release of the drug dosing (Singh & Kim, Citation1991).
Analgesic effect using tail-flick method
Further evaluation of analgesic effect of the selected F3-floating beads of KT was studied using tail-flick method. Tail withdrawal time (time passed till mice withdraw its tail from the radiant heat source) was taken as the end point. shows a significant analgesic effect (p < 0.05) among KT different dosage forms in mice after 0.5 h of administration as compared to the control. The maximum analgesic effect was observed after 2 and 4 h after the solution and the tablet administrations, respectively. This effect was gradually decreased and completely disappeared after 4 and 5 h of both dosage forms administration, respectively. In the meantime, F3 administration to fast mice shows a maximum analgesic effect (7.6 ± 0.2 s) after 5 h lasted with lower effect for 6 h. However, F3 in the fed mice had a maximum analgesic effect (9.1 ± 0.2 s) after 6 h and its effect was contin ued for >8 h. Therefore, the duration of analgesic effect achieved by KT in F3, in fed mice was doubled compared to the duration of analgesic effect gained by KT solution (group II) and moreover, it was 1.5 fold higher than that obtained by commercial KT tablets (group III).
Table 4. Analgesic effect of KT floating beads (F3), KT solution and commercial KT tablets (Ketoral) in mice using tail-flick method.
Pharmacokinetics of F3 and Ketoral® in rats
Ketorolac mean plasma concentration–time profiles after oral administrations of commercial KT tablets and the selected floating beads, F3 as 1.1 mg/kg of KT are depicted in . The calculated pharmacokinetic parameters of KT of the two dosage forms are presented in . Both dosage forms show the same onset of action. After PO administration of KT tablets, the drug was rapidly absorbed, distributed, and eliminated with t1/2 of 5.8 h. The Cmax (1.7 ± 0.4 µg/ml) was achieved after 2 ± 0.1 h of administration. The mean residence time (MRT) was 8.1 h, and the apparent Cl was 91.3 (ml/h)/kg. However, after F3-floating beads administration, there was a rapid release of KT followed by a slower one. The Cmax (2.24 ± 0.3 µg/ml) was achieved at ∼2 h and the decline was slower than that observed after tablets with t1/2 of 6.8 h. The mean AUC after F3 was 89.3% higher than that after the tablets which indicates that F3 has decreased the apparent Cl of KT (47.9, (ml/h)/kg) since the drug absorption was not affected by floating beads formulation as shown earlier. The MRT of KT beads administration was also higher than the tablets (10.1 h) indicates that the current formulation has improved bioavailability (Frelative) over the tablets in rats. Therefore, the present study demonstrated that the prepared floating beads can attain in the stomach for sufficient period of time to release KT over a prolonged period of time. These results are in a good agreement with the earlier finding of Ritschel et. al. (Citation1991) who reported a significant increase in the relative bioavailability of the floating dosage form of furosemide (42.9%) as compared to the commercial available tablet (33.4%) and enteric coated product (29.5%) (Ritschel et al., Citation1991). Similar observations were established by Ichikawa et al. (Citation2000) who found that floating pills containing P-aminobenzoic acid had 1.61-fold increase in the AUC than non-floating pills (Ichikawa et al., Citation2000). Therefore, it could be concluded that the selected formulation of KT floating beads not only improved the analgesic profile of ketorolac in fed mice but also its oral bioavailability in rats.
Figure 5. KT plasma concentration time profile in rats (mean ± SD, n = 8) after oral administration of Ketoral and floating beads (F3).
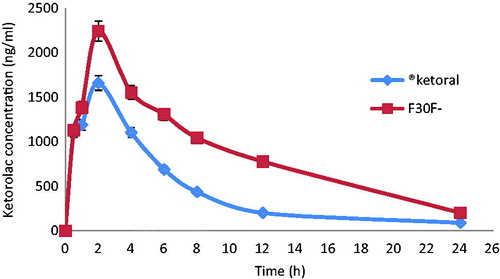
Table 5. Comparative pharmacokinetics parameters of ketorolac tromethamine after oral administration of selected floating beads and commercial tablets in rats (n = 8).
Declaration of interest
This research project was supported by a grant from the “Research Center for Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University. The authors have no conflict of interests to disclose.
References
- Agyilirah GA, Green M, Ducret R. (1991). Evaluation of the gastric retention properties of cross linked polymer coated tablet versus those of a non disintegrating tablets. Int J Pharm 75:241–7
- Ajit P, Sunil A, Sangamesh A, Nadagouda N. (2006). Semi-interpenetrating polymer network microspheres of gelatin and sodium carboxymethyl cellulose for controlled release of ketorolac tromethamine. J Carbohy Poly 65:243–52
- Alexander S, Juergen S. (2006). Gastro-retentive drug delivery systems. Expert Opin Drug Del 3:217–33
- Arora S, Ali J, Ahuja A, et al. (2005). Floating drug delivery system: a Review. AAPS Pharm SciTech 6:372–90
- Borkar S, Suresh R, Sawant V. (2010). An approach to formulate bilayered gastroretentive floating drug delivery system of cefpodoximeproxetil. Int J Chem Tech Res 2:1229–42
- Chawla G, Gupta P, Koradia V, Bansal AK. (2003). A means to address regional variability in intestinal drug absorption. Pharm Tech 27:50–68
- Choi BY, Park HJ, Hwang SJ, Park JB. (2002). Preparation of alginate beads for floating drug delivery system: effects of CO2 gas-forming agents. Int J Pharm 239:81–91
- Desai S, Bolton S. (1993). A floating controlled release drug delivery system: in vitro–in vivo evaluation. J Pharm Res 10:1321–5
- Gadad AP, Patil MB, Naduvinamani SN, et al. (2009). Sodium alginate polymeric floating beads for the delivery of cefpodoximeproxetil. J Appl Poly Sci 114:1921–6
- Gibaldi M, Perrier D. (1982). Pharmacokinetics. 2nd ed. New York (NY): Marcel Dekker, 407–17
- Ibrahim M, Amin M, Fetih G, Abou Ela A. (2010). Formulation and evaluation of ketorolac tromethamine-Eudragit solid dispersions of potential sustained release properties. STP Pharma Pratiques 20:189–200
- Ichikawa M, Kato T, Kawahara M, et al. (2000). A new multiple-unit oral floating dosage system. II: drug delivery via gastric retention. J Control Rel 63:235–59
- Issa M, Mohamed A, Seham B. (2009). Synthesis and pharmacological evaluation of 2-substituted benzo[b]thiophenes as anti-inflammatory and analgesic agents. Eur J Med Chem 44:1718–25
- Martin A, Bustamante P. (1993). Physical pharmacy: physical chemical principles in the pharmaceutical. 4th ed. Philadelphia, London: Lea & Febiger, 575–8
- Pahwa R, Bisht S, Kumar V, Kohli K. (2013). Recent advances in gastric floating drug delivery technology: a review. Curr Drug Deliv 10:286–98
- Radwan M, AlQuadeib B, Aloudah N, AboulEnein H. (2010). Pharmacokinetics of ketorolac loaded to polyethylcyanoacrylate nanoparticles using UPLC MS/MS for its determination in rats. Int J Pharm 397:173–8
- Raju D, Kiran S, Varma M. (2010). Design development and evaluation of extended release tablets of Alfuzosin hydrochloride. J Chem Pharm Res 2:90–6
- Ravindra R, Sabitha R. (2010). Effect of different co-polymers on sodium alginate microcapsules containing isoniazid. Int J Pharm Tech Res 2:2198–203
- Ritschel W, Menon A, Sakr A. (1991). Biopharmaceutic evaluation of furosemide as a potential candidate for a modified release per oral dosage form. J Exp Clin Pharmacol 13:629–36
- Rouge N, Buri P, Deolkar E. (1996). Drug absorption sites in the gastrointestinal tract and dosage forms for site specific drug delivery system. Int J Pharm 36:117–39
- Shishu G, Gupta N, Aggarwal N. (2007). Stomach-specific drug delivery of 5-fluorouracil using floating alginate beads. AAPS PharmSciTech 8:E143–9
- Shishu N, Nidhi A. (2007). Stomach specific drug delivery of 5-fluorouracil using floating alginate beads. J Pharm Sci Tech 8:1–7
- Shivkumar HG, Vishakante G, Wdaand D, et al. (2004). Floating drug delivery system: an innovative approach to prolong gastric retention. Ind J Pharm Educ 38:172–9
- Singh B, Kim K. (1991). Floating drug delivery systems: an approach to oral controlled sustained-release characteristics with P-aminobenzoic acid and Isosorbide dinitrate as model drugs. J Pharm Sci 80:1153–6
- Singh B, Kim K. (2000). Floating drug delivery system: an approach to oral controlled drug delivery via gastric retention. J Control Rel 63:235–59
- Sinha V, Trehan A. (2005). Formulation, characterization, and evaluation of ketorolac tromethamine-loaded biodegradable microspheres. Drug Del 12:133–9
- Stops F, Fell J, Collett J, Martini L. (2008). Floating dosage forms to prolong gastro-retention: the characterization of calcium alginate beads. Int J Pharm 350:301–11
- Thanoo B, Sunny M, Jayakrishnan A. (1993). Oral sustained release drug delivery systems using polycarbonate microspheres capable of floating on the gastric fluid. J Pharma Pharmacol 45:21–4
- Vani M, Meena A, Godwin F. (2010). Design and evaluation of gastro retentive floating beads of ranitidine hydrochloride. Int J Pharm Biomed Sci 1:1–4