Abstract
When polyethylene glycol (PEG)ylated liposomes were repeatedly injected into the same animal, the second dose of liposomes would rapidly clear from the bloodstream and enhance accumulation in the liver and spleen, and this phenomenon is called “accelerated blood clearance (ABC)”. There are many factors known to influence ABC phenomenon, in this study, we mainly focused on the effects of different phospholipids (PL) types and animal models. The effects of PL types on ABC phenomenon were examined by repeating injection of PEGylated liposomes prepared by five different types of PL (hydrogenated soy phosphatidylcholine, egg sphingomyelin, soybean phosphatidycholin, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine and egg phosphatidycholin) in rats. Dramatically, repeated injection of different types of PL could induce ABC phenomenon altogether. Both t1/2 and AUC of experimental group (EG) were lower significantly than those of control group (CG). Our results also showed that the liver accumulation of second dose increased significantly (p < 0.01) in all EG as compared that of CG. Interestingly, ABC phenomenon of liposomes prepared by unsaturated PL was more obvious than that of saturated PL. All the first dose could induce the antibody (anti-PEG IgM) level increasing significantly (p < 0.01). For different animal models, we found that after repeated injection of PEGylated liposomes, rats, mice, rabbits and guinea pigs could produce ABC phenomenon. Various PL types and animal models could all produce the ABC phenomenon. However, their extent of accelerated clearance differed. ABC phenomenon is possibly a ubiquitous immune phenomenon in life.
Introduction
Conventional liposomes (CLs) are drug delivery carriers that are easily phagocytized by the mononuclear phagocytic system (MPS) and rapidly eliminated from the blood circulation. To prolong the circulation time in vivo and enhance targeted drug delivery, CLs are usually modified with hydrophilic and flexible polyethylene glycol (PEG). The study on PEGylated liposomes and related products is still attracting research attention, although many clinical trials have been conducted over the years.
PEGylated liposomes have many advantages and some disadvantages according to an in-depth study. The structure of PEG is very stable and it can form a steric hindrance on liposome surfaces to prevent the cellular uptake of liposomes. “Endosomal escape” of PEGylated liposomes is highly difficult to achieve even when the liposomes enter cells. The “PEG dilemma” phenomenon would result in the loss of biological activity of protein or gene that is encapsulated in liposomes (Hatakeyama et al., Citation2011; Kibria et al., Citation2011). Recent studies suggested that when PEGylated liposomes are repeatedly injected into the same animal, the first dose of liposomes can reduce the circulation time in the bloodstream and enhance the hepatic and splenic accumulations of the second dose. This phenomenon is called “accelerated blood clearance (ABC)” (Dams et al., 2000; Laverman et al., Citation2001; Park, Citation2013). PEGylated liposomes usually need to be injected repeatedly in clinical applications, but experimental pharmacokinetic data on duplicate injections of PEGylated liposomes are relatively scarce. It is generally believed that liposomes modified with PEG have no or lower immunogenicity, but the behavior of PEGylated liposomes in vivo is more complicated than expected.
The ABC phenomenon is a major flaw in the clinical application of PEGylated liposomes. When toxic drugs are encapsulated in liposomes, the enhanced accumulation in the liver and spleen of the second dose of PEGylated liposomes can lead to apoptosis and necrosis of MPS cells. Liver Kupffer cells need at least 2 weeks to recover, and the lack of Kupffer cells may cause bacteremia, which is deadly for cancer patients. The ABC phenomenon can also decrease drug bioavailability and gene therapy. When PEGylated liposome-encapsulated oligonucleotide, pDNA, or RNA is repeatedly injected into the mice, a strong immune response is induced. The circulation time of the preparation decreases, and the death rate of mice significantly increases (Semple et al., Citation2005). Thus, an in-depth investigation on the ABC phenomenon is necessary.
In recent years, the influencing factors of the ABC phenomenon were studied extensively in related literature (Ishida et al., 2006a; Cui et al., Citation2008; Ma et al., Citation2012). These influencing factors included lipid dose, PEG-distearoylphosphatidylethanolamine (PEG-DSPE) concentration, molecular weight of PEG, particle size and zeta potential of liposomes, different preparation types (liposomes, nanoparticles, micelles and emulsions), and model drugs (adriamycin, topotean, mitoxantrone and trace or fluorescent labeling). However, the two main factors, namely, phospholipid (PL) types and animal models, have not yet been studied.
Ishida et al. (Citation2005) found that when the first PL dose is larger than 1 μmol·kg−1, the second injection of PEGylated liposomes cannot induce the ABC phenomenon. By contrast, Laverman et al. (Citation2001) reported that an intravenous (i.v.) injection of different PL doses of PEGylated liposomes (i.e. 0.05, 0.5 or 5 μmol·kg−1) as a first dose can cause the second specific dose (5 μmol·kg−1) of injected PEGylated liposomes to induce the ABC phenomenon. These two aforementioned studies presented contrasting effects of the first dose on the ABC phenomenon. The former study used hydrogenated egg phosphatidylcholine (HEPC), whereas the latter study used partially hydrogenated egg phosphatidylcholine. In this study on ABC phenomenon, HEPC, cholesterol and PEG-DSPE were usually used to prepare PEGylated liposomes (Laverman et al., Citation2001; Ishida et al., Citation2005, 2006b), or hydrogenated soybean phosphatidylcholine (HSPC) was adopted instead of HEPC (Ishida et al., Citation2006a). As of this writing, no experimental studies have demonstrated the effects of PL types on the ABC phenomenon, and these effects are worthy of further investigation.
Dams et al. (2000) found that rhesus monkeys and rats, but not mice, can produce the ABC phenomenon by repeated injections of PEGylated liposomes. Ishida et al. (Citation2004) showed that an intense ABC phenomenon can be induced in mice. Tagami et al. (Citation2009) used mice as an animal model to investigate the ABC phenomenon and proved that repeated injections of liposome-encapsulated siRNA in mice can induce an intense ABC phenomenon. Coins et al. (Citation1998) reported that repeated injections of PEGylated liposomes in rabbits at a 6-week interval result in similar pharmacokinetic parameters of liposomes between the second and first injections, which indicated that no ABC phenomenon occurred. This result was consistent with Dams’ conclusion that the ABC phenomenon only occurred within a relatively short interval time (4 weeks) between the two injections. However, the aforementioned study failed to prove if rabbits can produce the ABC phenomenon because a shorter injection interval was not adopted. Oussoren & Storm (Citation1997, Citation1999) reported that rats injected four times successively at an interval of 1 or 2 days results in the absence of change in pharmacokinetic parameters. This finding was in agreement with the study of Dams, and showed that the ABC phenomenon is significant at an interval of 5 to 7 days between the two injections. Effects of different species of animals on the ABC phenomenon exist, but no comprehensive data are available to elucidate this phenomenon theoretically.
This study systematically compared the effects of five different types of PL [egg phosphatidylcholine (EPC), soybean phosphatidylcholine (SPC), egg sphingomyelin (ESM), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) and HSPC] and four animal models (rats, mice, rabbits and cavies) on the ABC phenomenon, and provided some references for the study of the ABC phenomenon.
Materials and methods
Materials
HSPC, ESM, SPC and DPPC were purchased from Lipids Company (Ludwigshafen, Germany). EPC and PEG-DSPE were purchased from Shanghai Advanced Vehicle Technology Co. Ltd. (Shanghai, China). Cholesterol and bovine serum albumin (BSA) were obtained from Amresco Biosharp (Solon, USA). Calcein and trihydroxymethyl aminomethane (Tris) were obtained from Bodi Reagent Factory (Tianjin, China). Horseradish peroxidase (HRP)-conjugated antibodies (goat anti-rat immunoglobulin M (IgM) antibody HRP conjugate, rabbit anti-mouse IgM antibody HRP conjugate, mouse anti-rabbit IgM antibody HRP conjugate and rabbit anti-cavy IgM antibody HRP conjugate) were purchased from Beijing Biosynthesis Biotechnology Co. Ltd. (Beijing, China). o-Phenylenediamine was purchased from Sigma-Aldrich (St. Louis, USA). All other chemicals were of analytical reagent grade and used without further purification.
Animal
Male Wistar rats (200 g to 250 g), male KM mice (20 g to 25 g), male Japanese white rabbits (2.0 kg to 2.5 kg), and male Dunkin–Hartley guinea pigs (450 g to 500 g) were provided by the Dalian Medical University Experimental Animal Center. These animals had free access to water and food. All experiments were carried out in accordance with the guidelines of the local Animal Welfare Committee.
Preparation of empty CLs and calcein liposomes
In this study, two kinds of liposomes were prepared, namely, the “empty” PEGylated liposomes for the first injection and PEGylated calcein liposomes for the second injection. The “empty” liposomes were prepared by the lipid film hydration method as previously described (Bangham & Lea, Citation1978). In brief, a mixture of PL and cholesterol in a molar ratio of 3:2 was dissolved in chloroform. The solution was evaporated dry to form the lipid film at 50 °C. The resulting lipid film was hydrated in PBS or calcium acetate solution at 65 °C. To obtain large unilamellar vesicles of uniform size, the suspensions were passed through 0.45 and 0.22 μm filter membranes after sonication with an ultrasonic cell disruptor (JY92-2D probe sonicator; Ningbo, China).
The calcium acetate gradient method was used to prepare calcein liposomes (Clerc & Barenholz, Citation1995; Xu et al., Citation2010). To remove the external aqueous phase of calcium acetate, the obtained liposome suspensions hydrated with calcium acetate solution were ultrafiltrated through stirred ultrafiltration cells (Amicon stirred cell 8010, Millipore Co., USA). The remaining liposomes on the ultrafiltration membrane were resuspended in sodium sulfate buffer. An appropriate amount of calcein was added to the liposomes and incubated at 65 °C for 1 h. The suspension was centrifuged at 1500 × g for 10 min to remove free calcein precipitation.
ESM was dissolved in ethanol in the liposome preparation process and then by rotary evaporation to obtain the liposomes after hydration because ESM is insoluble in chloroform.
Preparation of PEGylated liposomes
The post-injection method was used to prepare the long-circulating liposomes (Iden & Allen, Citation2001; Visser et al., Citation2005; Steenpaβ et al., Citation2006). An appropriate amount of PEG-DSPE was dissolved in chloroform. After the chloroform was removed with an evaporator, the lipid film was hydrated in 5 mL of 0.01 M PBS at 37 °C, and PEG-DSPE micelles were finally obtained. “Empty” liposomes or calcein liposomes were incubated with PEG-DSPE micelle solution (lipids: PEG-DSPE = 100:6, molar ratio) at 65 °C for 2 h, and the resulting PEGylated liposome suspension was extruded in 0.22 μm polycarbonate membranes.
The liposome diameters were measured using a Nano 2590 Malvern particle size analyzer (Malvern, UK). Ultrafiltration was used to determine the encapsulation efficiency (EE) of calcein liposomes. The calcein concentration was analyzed using a model F-7000 fluorescence spectrophotometer (Hitachi Inc., Japan) at excitation and at emission wavelengths of 490 and 512 nm, respectively.
Influence of different PL types on ABC phenomenon
The dosing regimen for liposomes for injection is presented in . Male Wistar rats were randomly divided into 10 groups for pharmacokinetics and biodistribution studies of PEGylated liposomes made of different PL types. The first injection was at a dose of 0.1 μmol PL/kg via the caudal vein. The interval between the two injections was 6 days. For the second injection, calcein-loaded PEGylated liposomes were injected intravenously at a dose of 5 μmol PL/kg. At different time points following the i.v. injection, blood samples were obtained via eye puncture. After withdrawing the last blood sample at 4 h, the livers and spleens were excised and rinsed in cold normal saline (NS). Finally, the water on the organ surface was absorbed with filter paper.
Table 1. The injection project of effect of different type of phospholipids on ABC phenomenon in rats.
Analytical procedure of the plasma and tissue samples
Before analysis, the blood samples were treated as follows: 0.5 mL of blood was placed in a test tube coated with heparin, and the supernatant plasma was obtained by centrifuging the mixture at 2000 × g for 10 min. NS was added to the quantitative tissues to gain 10% tissue homogenate, and the mixture was separated by centrifugation at 2000 × g for 10 min. An appropriate amount of plasma supernatant or tissue homogenate was placed into a 5 mL volumetric flask, and PBS (pH 7.4) containing 10% Triton X-100 was added to volume. The plasma and tissue samples were assayed by fluorescence spectrophotometry as previously described (Xu et al., Citation2010).
Effects of different animal models on ABC phenomenon
Rats, mice, rabbits and guinea pigs were randomly divided into eight groups. These animals were administered repeated injections of PEGylated liposomes made of EPC. The first injection was liposomes at a dose of 0.1 μmol PL/kg for the experimental group (EG) or NS for the control group (CG) via the caudal vein (rats and mice) or marginal ear vein (rabbits and guinea pigs). Six days after the first injection, calcein-labeled PEGylated liposomes were administered by i.v. injection at a dose of 5 μmol PL/kg. At different time points following the i.v. injection, blood samples were obtained via eye ground vein (rats and mice), marginal ear vein (rabbits) or plantar vein (guinea pigs). Rats, guinea pigs and rabbits were sacrificed after withdrawing the last blood sample at 4 h, whereas mice were sacrificed at each time point. The plasma and tissue samples of different animal models were processed as described above.
Detemination of IgM
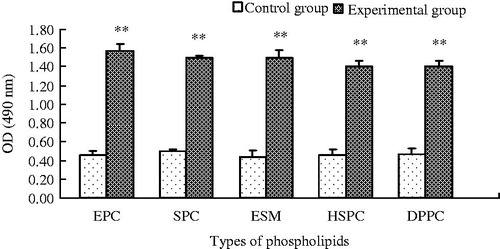
Serum samples were collected 6 days after injecting a single dose of empty PEGylated liposomes or NS, and the serum collected before the first injection was used as a control. IgM quantification was performed using a slightly modified published procedure (Ma et al., Citation2012). In brief, 50 µL of ethanol solution of PEG-DSPE (0.2 mM) was added to a 96-well plate, and the lipid-coated plates were allowed to air dry completely. Then, 100 µL of Tris-buffered saline (50 mM Tris, pH 8.0) containing 1% BSA was added to each well as blocking solution. After 30 min of incubation, the wells were washed five times with Tris-buffered saline containing 0.05% Tween 20. The serum samples were diluted 100-fold with 50 mM Tris saline containing 1% BSA and 0.05% Tween 20. The diluted serum samples (100 µL) were added to each well, incubated for 30 min, and washed five times. A total of 100 µL of HRP-conjugated Ab (1 μg/mL) (goat anti-rat IgM-HRP conjugate, rabbit anti-mouse IgM-HRP conjugate, mouse anti-rabbit IgM-HRP conjugate or rabbit anti-cavy IgM-HRP conjugate) in the diluent agent (as described above) was added to the wells. After incubation for 30 min, the wells were washed five times. o-Phenylenediamine (100 μL; 1 mg/mL) was added as the developer to initiate coloration. After 15 min of incubation, 100 μL of 2 M H2SO4 was added to terminate the reaction, and the absorbance was measured at 490 nm using a microplate reader (Multiskan Ascent®, Thermo Labsystems, USA). All incubations were performed at room temperature.
Statistical analysis
Statistical comparisons were performed by Student’s t-test for two groups and one-way ANOVA for multiple groups. The p < 0.05 was considered statistically significant.
Results
Characteristics of liposomes
shows that the particle sizes of CLs for the first injection and the calcein liposomes for the second injection were lower than 120 nm, and the polydispersity index was below 0.3. This result indicates that these liposomes possessed a relatively uniform particle diameter. All the EEs of calcein liposomes were greater than 16%, and the liposomes were suitable for in vivo experiments in animals.
Table 2. Mean particle sizes and entrapment efficiency of the prepared liposomes (n = 6).
ABC phenomenon in repeated injections of PEGylated liposomes made of different PL types in rats
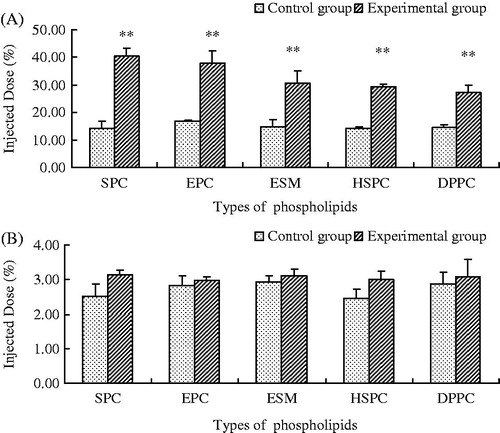
The remaining second injection of PEGylated calcein liposomes in rat plasma at different times is shown in . The blood circulation time of the CG was comparatively long, and calcein still presented a high concentration in the plasma after 4 h. However, the calcein concentration significantly decreased for the EG, especially for the liposomes made of unsaturated PL (EPC, SPC and ESM). The calcein concentration was almost undetected after 4 h. shows the biodistribution of liver and spleen of the second injection of PEGylated liposomes after 4 h. Compared with the CG, the liver accumulation of liposomes of the EG significantly increased (p < 0.01), whereas the spleen accumulation also increased but no significant difference was observed (p > 0.05). Thus, repeated injections of PEGylated liposomes made of five different PL types in rats could all induce the significant ABC phenomenon.
Figure 1. Blood clearance (0–4 h) of calcein in rats after a second injection of PEGylated liposomes made of different types of phospholipid (n = 6).
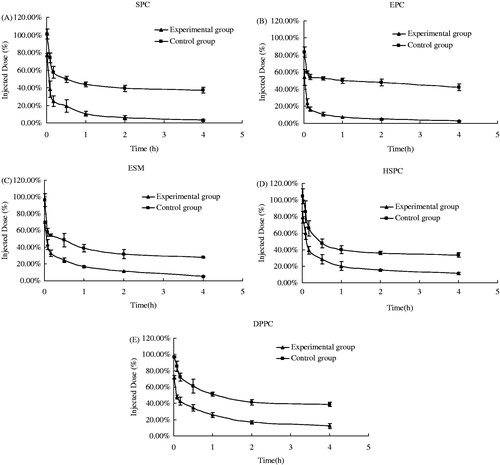
Figure 2. Liver (A) and spleen (B) biodistribution (4 h) of calcein in rats after a second injection of PEGylated liposomes made of different types of phospholipid (n = 6) (**p < 0.01).
These repeated injections showed various degrees in inducing the ABC phenomenon in rats. After repeated injections of unsaturated PL liposomes made of SPC, EPC or ESM, the degree of decrease in half-life (t1/2) and AUC was significantly higher than that of saturated PL (DPPC or HSPC) in rats (p < 0.05; ).
Table 3. Change of pharmacokinetics parameters in rat after repeated injections of PEGylated liposomes made of different types of phospholipids (n = 6).
Change in anti-PEG IgM after repeated injections of PEGylated liposomes made of different PLs
As shown in , PEG-DSPE-modified liposomes made of different PL types could induce a significant increase in anti-PEG IgM in rats (p < 0.01). The increase in antibody levels 6 days after the first injection of PEGylated liposomes made of unsaturated PL (SPC, EPC and ESM) was slightly higher than that of the two saturated PLs (DPPC and HSPC), and no significant difference was observed.
Pharmacokinetics and tissue distribution after repeated injections of PEGylated liposomes made of EPC in rats
When rats received repeated injections of PEGylated liposomes, liposomes prepared by unsaturated PL produced a more obvious ABC phenomenon. For easy comparison, EPC was selected for the preparation of liposomes in investigating the ABC phenomenon of different animal models. As shown in and 2 and , the plasma clearance rate significantly increased after repeated injections of EPC liposomes in rats. Compared with the CG, AUC and t1/2 decreased by 5.25 and 5.61 times (p < 0.01), respectively. Calcein accumulation in liver and spleen increased. These results show that an obvious ABC phenomenon could be observed after repeated injections of PEGylated liposomes in rats.
Pharmacokinetics and tissue distribution after repeated injections of PEGylated liposomes made of EPC in mice
The blood circulation time in mice of the CG was relatively long, and calcein still presented a high concentration at 4 h (). For the EG, the plasma clearance rate significantly increased, and calcein showed a significantly descending trend. The remaining amount of calcein in the EG was only about 20% after 4 h in vivo, which was 30% lower than that in the CG.
Figure 4. Blood clearance (0–4 h) (A) and liver (B) and spleen (C) biodistribution (4 h) of calcein in mice after a second injection of PEGylated EPC liposomes (n = 6) (*p < 0.05, **p < 0.01).
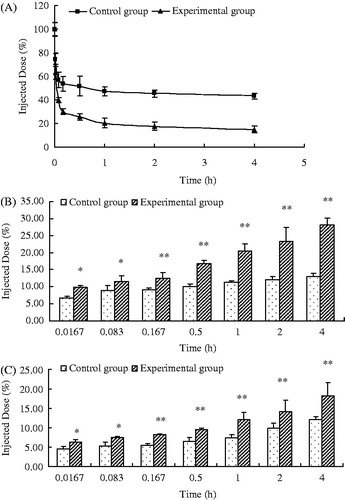
As shown in , the amount of calcein accumulation in the liver and spleen of the EG at each time point increased than that of the CG (p < 0.05 or <0.01). Thus, repeated injections of PEGylated liposomes in mice could produce an obvious ABC phenomenon.
Pharmacokinetics and tissue distribution after repeated injections of PEGylated liposomes made of EPC in rabbits
shows the pharmacokinetic changes in rabbits. The significant influence of repeated injections of PEGylated liposomes to the blood clearance was easily observable. Calcein still showed a high concentration in the CG 4 h after the second injection. The plasma elimination rate of the EG significantly increased than that of the CG. The remaining amounts of calcein were only about 12% for the EG and 45% for the CG.
Figure 5. Blood clearance (0–4 h) (A) and biodistribution (4 h) (B) of calcein in rabbits after a second injection of PEGylated EPC liposomes (n = 6) (**p < 0.01).
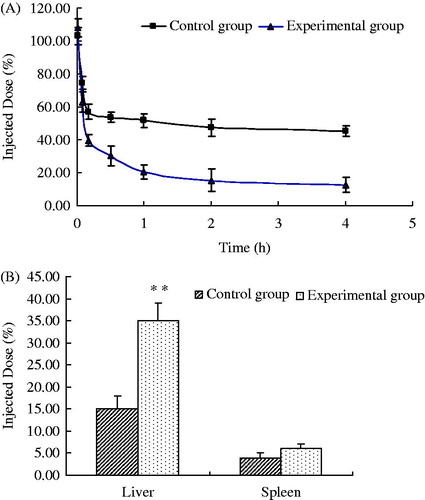
The tissue distribution in rabbits is shown in . Four hours after the second i.v. injection of PEGylated liposomes, calcein accumulation in the liver and spleen of the EG increased than that of the CG. The accumulation in the spleen was less than that in the liver, but no significant difference was observed (p > 0.05). These results prove that repeated injections of PEGylated liposomes in rabbits could induce an obvious ABC phenomenon.
Pharmacokinetics and tissue distribution after repeated injections of PEGylated liposomes made of EPC in guinea pigs
As shown in , calcein still showed a high concentration in the CG 4 h after the injection of the second dose. The plasma elimination rate of the EG significantly increased than that of the CG. The remaining amounts of calcein were only about 12% and 35% for EG and CG, respectively.
Figure 6. Blood clearance (0–4 h) and biodistribution (4 h) of calcein in guinea pigs after a second injection of PEGylated EPC liposomes (n = 6) (**p < 0.01).
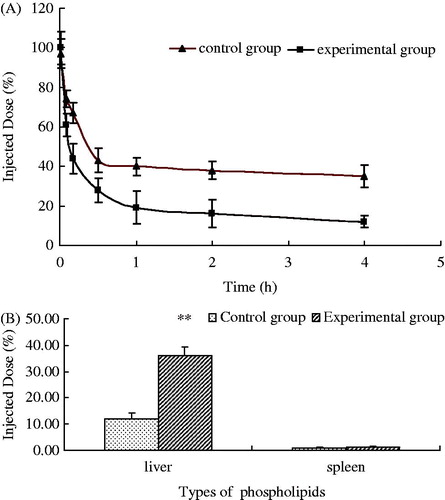
As shown in , calcein accumulation in the liver and spleen in the EG increased than that in the CG, but the increase in accumulation in the spleen was minimal. Thus, repeated injections of PEGylated liposomes in guinea pigs could produce an obvious ABC phenomenon.
Comparison of different animal models on pharmacokinetic parameters
A non-compartment model was used to calculate the pharmacokinetic parameters of different animal models after repeated injections of PEGylated liposomes. Both t1/2 and AUC of the CG were significantly higher than those of the EG (). This result indicates that various animal models in this study could produce the ABC phenomenon after repeated injections of PEGylated liposomes.
Table 4. Change of pharmacokinetics parameters in different animal models after repeated injections of PEGylated liposomes (n = 6).
Anti-PEG IgM response in different animal models after i.v. injection of “Empty” PEGylated liposomes
The level of anti-PEG IgM was assessed by ELISA 6 days after the first injection. As shown in , a single first dose of “empty” PEGylated liposomes induced a significant increase in anti-PEG IgM (p < 0.01). All the animal models in this study produced an immune response after injection of PEGylated liposomes, and the increasing trends of the antibody were consistent with the changes in the pharmacokinetic parameters.
Table 5. Change of anti-PEG IgM level in different animals after a single intravenous injection of empty PEGylated liposomes in different kinds of animals (n = 6).
Discussion
Water-soluble fluorescence probe-calcein
Much literature reported that calcein can be used as water-soluble fluorescence probe to trace microparticle (Paula Corrêa Oliveira Bahia et al., Citation2009; Koga et al., Citation2010). In this study, we adopted calcein to mark the inner water phase of the liposomes to investigate the pharmacokinetic of microparticle formulations. Compared with radioimmunoassay, fluorescence labeling has no radioactive contamination and is easy to perform. So we think that as long as such dynamin changes could be detected, we can try to use calcein to label liposomes. And whether calcein, the fluorescent label used in this study, will produce the same results as the most commonly applied tracer labels still requires further validation.
Effect of different PL types on ABC phenomenon
Given that the purposes of target delivery and therapy differ, many different types of PL were used to prepare liposomes, such as EPC, SPC, ESM, DPPC and HSPC. Liposomes made of different PLs have different physical and chemical properties, which result in the differences in circulation time in vivo and action mechanism. Examining the relationship of PL types with the ABC phenomenon is necessary to guide the correlative research if PEGylated liposomes prepared by all PL types could induce the ABC phenomenon. Previous studies usually used rats as experimental animal models, and an intense ABC phenomenon was observed (Ishida et al., Citation2003, 2006c; Kong et al., Citation2006; Ma et al., Citation2012). However, the conclusions were inconsistent with other animal models. To avoid the perturbation of animal models, we selected rats as the animal model to investigate the effect of different PL types on the ABC phenomenon.
The ABC phenomenon could be affected by the PL dose. When rats were injected with different doses of PEGylated liposomes (0, 0.001, 0.01, 0.1, 1 and 5 μmol PL/kg), followed by an injection with radioactive-labeled PEGylated liposomes 5 days later (5 μmol PL/kg), the second dose of PEGylated liposomes in the blood clearance and liver accumulation increased with decreasing first dose. When the first dose was greater than 1 μmol PL/kg, the blood clearance rate of the second injection of PEGylated liposomes no longer increased (Ishida et al., Citation2005). Thus, 0.1 and 5 μmol PL/kg were determined as the initial and second doses, respectively.
PEGylated liposomes can produce a good long-circulating effect only when the proportion of PEG–lipids is or beyond 5% (including the listed Doxil liposomes) (Basile et al., Citation2012; Zalba et al., Citation2012). In a previous study, repeated injections of 5% PEG-lipid-modified liposomes produce a significant ABC phenomenon. Thus, the PEG–lipid ratio in this study was set at 5%.
Anti-PEG IgM had a t1/2 of 5 days, and when the time interval between the two injections was 5 to 7 days, the ABC phenomenon was reportedly highly intense (Ishida et al., Citation2003, 2006c). Thus, the time interval was set at 6 days to observe the obvious ABC phenomenon in animals.
A recent research indicated that the first injection of PEGylated liposomes can induce anti-PEG IgM production, whereas the second injection of PEGylated liposomes combined with an antibody results in rapid blood clearance (Dams et al., 2000). This study used ELISA to determine the amount of anti-PEG IgM. According to the results of plasma clearance, biodistribution and anti-PEG IgM, repeated injections of liposomes made of five PL types all produced a significant ABC phenomenon. The intense degree of the phenomenon produced by unsaturated PL was also stronger than that by saturated PL. Based on literature, the ABC phenomenon induced by repeated injections of PEGylated liposomes made of partially hydrogenated PL (unsaturated) is more intense in rats than that of PEGylated liposomes made of HSPC or HEPC (Dams et al., 2000; Basile et al., Citation2012; Park, Citation2013). This finding was consistent with our results.
The degree of increase in antibody after injection of PEGylated liposomes made of different PLs was almost the same. Thus, the difference in clearance rate of the second dose possibly did not originate from the change in the antibody, but the second injection of PL type of liposomes. From an immunological perspective, this difference in degree of ABC phenomenon between different PL types may be due to the following reason. Liposomes will combine with opsonin in blood and be phagocytized by MPS after PEGylated liposomes are injected into the animals. Opsonin can promote the phagocytosis of bacteria, antigens and antibodies. Antibodies and complements are the main opsonins in the body in vivo. The main materials of PEGylated liposomes, namely, PL and PEG-DSPE, both have hydrophobic and hydrophilic groups. The combination of anti-PEG IgM, which acts as an opsonin, with PEGylated liposomes mainly depends on the hydrophobic effect with the hydrophobic group on the liposome surface. This combination increases the clearance rate of liposomes from blood and induces the ABC phenomenon. Related studies proved that the ABC phenomenon is related to the hydrophobic groups on the liposome surface (Shiraishi et al., Citation2013). Nevertheless, the hydrophobic effect between antigen and opsonin can be weakened by the bilayer of high mechanical strength in liposomes (Lasic et al., Citation1991; Wang et al., Citation2001). In this study, liposomes consisting of unsaturated PL (SPC, EPC and ESM) possessed poor stability and were easily assembled and oxygenized. By contrast, liposomes made of saturated PL (DPPC and HSPC) were more stable and rigid and had a favorable anti-oxidation ability. The strong rigidity of liposomes reduced the hydrophobic interaction with opsonin and decreased the phagocytosis probability and clearance rate of liposomes from blood. Thus, the degree of ABC phenomenon induced by liposomes made of saturated PL was weaker than that of unsaturated PL, but the exact reason remains unknown.
The foreign molecules, especially particulate antigen material, were swallowed, digested by liver Kupffer cells or delivered to the other immunocytes to be further removed after injection. PEGylated liposomes, as macromolecule antigens, induced the production of anti-PEG IgM after the first injection, and the PEG layer prevented liposomes from being recognized by the immune system. The second injection of PEGylated liposomes was identified by anti-PEG IgM and phagocytized by Kupffer cells. Thus, a considerable second dose of PEGylated liposomes aggregated in the liver. The increase in calcein accumulation in the spleen was not significant after the second dose than that in the liver. This result may be related to the model drug and testing method, and the findings were similar to previous research (Dams et al., 2000; Xu et al., Citation2010).
Effect of different animal models on ABC phenomenon
In different species of mammals, a common life phenomenon exists, especially the most basic life processes, which is the basis of animal experiments for the application of medical research. However, animals do not have the same bodies. Various species of animal models may have different responses to identical factors. This study proved that PEGylated liposomes might be recognized by B cells as a thymus-independent type-2 antigen. Thus, a small difference in the immune system of an animal can cause a significant change in the experimental results. In various experiments on the ABC phenomenon, the results will differ if the animal models vary. The same animal models may also have various results when the parameters such as interval time of injection, PL dose, PEG-DSPE concentration and model drug differ. For example, Dams et al. (2000) reported that the ABC phenomenon is not observed in mice, but Ishida et al. (Citation2004) found a significant ABC phenomenon using the same animal model. Therefore, we evaluated whether different animal models had different responses to the repeated injections of PEGylated liposomes.
The effect of different animal models on the ABC phenomenon was investigated by fixing all the influencing factors of the ABC phenomenon (including the PL type, injected dose, model drug and time interval of injection). In this study, rabbits and guinea pigs were selected as experimental animal models to investigate the ABC phenomenon. Rats and mice were also included in the investigation. It is possible that different animals have different kinetics of triggering the ABC effect, for example the peak effect of rats is 5 to 7 days after initial sensitization, but the peak effect of mice is 10 days (Ishida et al., Citation2004). The injection interval was determined 6 days and the aim is to fix all variables and only compare ABC phenomenon of different animals. Our results proved that all types of animals can produce ABC phenomenon. In the subsequent studies, the authors will further compare the ABC phenomenon of different animals and different injection intervals.
All animals had many distinctions, such as various body sizes, blood volume and immunity. The results show that repeated injections of PEGylated liposomes resulted in the ABC phenomenon in all experimental animals. When all the experimental operations were the same, the ABC phenomenon in rats was significantly stronger than that in other animal models, which may be associated with the different immune systems of various animals. By comparing the blood routine examinations of these four kinds of animal models, the amount of white blood cells (WBC) of rats (1.52 × 1010/L) was higher than that of mice (5.7 × 109/L), rabbits (1.01 × 1010/L) and guinea pigs (9.9 × 109/L). WBC is also called an immune cell, which can be divided into two categories, namely, phagocytic cells and immune cells. The total lymphocytes of rats (9.3 × 109/L) were also higher than those of mice (4.1 × 109/L), rabbits (5.1 × 109/L) and guinea pigs (4.9 × 109/L) (Kong et al., Citation2006; Hu et al., Citation2007; Zhang et al., Citation2010).
Spleen is a primary immune organ and has an important function in the organism. According to literature, the ratios of spleen and body weight in rats, mice, rabbits and guinea pigs were 0.268%, 0.191%, 0.063% and 0.083%, respectively (Yang et al., Citation2003; Xia et al., Citation2009). This ratio could influence the intensity of anti-PEG IgM level. Compared with other animals (rabbits and guinea pigs), rats and mice have a larger proportion of spleen and more immune cells in blood. Thus, rats exhibited the highest changes in anti-PEG IgM levels and the strongest ABC phenomenon among all animal models. In addition, among four animal species, only mice showed that splenic macrophages contribute to the enhanced clearance of second dose. The exact reason for this finding needs further investigation.
The rat is the most widely used animal model on the investigation of the ABC phenomenon, and the data of other animal models were contradictory and incomplete. This study showed that mice, rabbits and guinea pigs could also produce an intense ABC phenomenon. Based on literature, the ABC phenomenon can also be induced in rhesus monkeys (Dams et al., 2000), beagles (Zhao et al., Citation2012; Suzuki et al., Citation2012; Li et al., Citation2012) and other animals. Thus, the ABC phenomenon is a ubiquitous immune phenomenon in life.
Conclusion
In our study, different levels of ABC phenomenon could be induced by repeated injections of PEGylated liposomes prepared by five different types of PLs (HSPC, DPPC, EPC, SPC and ESM). This phenomenon was more obvious in liposomes prepared by unsaturated PL than those by saturated PL. Rats, mice, rabbits and guinea pigs were selected as experimental animal models to study the effects of different animals on the ABC phenomenon. All animals could produce an intense ABC phenomenon after repeated injections of PEGylated liposomes. In this study, the effect of PL type and animal model on the ABC phenomenon was systematically studied, and the reason for generating different ABC phenomena was discussed. We will further investigate the effect of other PL types and animal models on the ABC phenomenon and provide some references for the ABC phenomenon in our future study.
Acknowledgements
This work was supported by National Natural Science Foundation of China (81102394, 81072602) and China Postdoctoral Science Foundation funded project (2013M530899).
References
- Bangham JA, Lea EJA. (1978). The interaction of detergents with bilayer lipid membranes. Biochim Biophys Acta 511:388–96
- Basile L, Passirani C, Huynh NT, et al. (2012). Serum-stable, long-circulating paclitaxel-loaded colloidal carriers decorated with a new amphiphilic PEG derivative. Int J Pharm 426:231–8
- Clerc S, Barenholz Y. (1995). Loading of amphipathic weak acids into liposomes in response to transmembrane calcium acetate gradients. Biochim Biophys Acta 1240:257–65
- Coins B, Philips WT, Klipper R. (1998). Repeat injection studies of technetium-99 m-labeled PEG-liposomes in the same animal. J Liposome Res 8:265–81
- Cui JX, Li CL, Wang CX, et al. (2008). Repeated injection of pegylated liposomal antitumour drugs induces the disappearance of the rapid distribution phase. J Pharm Pharmaco l60:1651–7
- Dams ET, Laverman P, Oyen WJ, et al. (2000). Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J Pharmacol Exp Ther 292:1071–9
- Hatakeyama H, Akita H, Harashima H. (2011). A multifunctional envelope type nano device (MEND) for gene delivery to tumours based on the EPR effect: a strategy for overcoming the PEG dilemma. Adv Drug Deliver Rev 63:152–60
- Hu JW, Lu SM, Che LP, et al. (2007). A probe into normal levels of hematological and biochemical indexes in 10 kinds of common SPF rats and mice. Lab Animal Sci 24:5–10
- Iden DL, Allen TM. (2001). In vitro and in vivo comparison of immunoliposomes made by conventional coupling techniques with those made by a new post-insertion approach. Biochim Biophys Acta 1513:207–16
- Ishida T, Atobe K, Wang XY, et al. (2006a). Accelerated blood clearance of PEGylated liposomes upon repeated injections: effect of doxorubicin-encapsulation and high-dose first injection. J Control Release 115:251–8
- Ishida T, Harada M, Wang XY, et al. (2005). Accelerated blood clearance of PEGylated liposomes following preceding liposome injection: effects of lipid dose and PEG surface-density and chain length of the first-dose liposomes. J Control Release 105:305–17
- Ishida T, Ichihara M, Wang XY, et al. (2006b). Spleen plays an important role in the induction of accelerated blood clearance of PEGylated liposome. J Control Release 115:243–50
- Ishida T, Ichihara M, Wang XY, et al. (2006c). Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposome. J Control Release 112:15–25
- Ishida T, Ichikawa T, Ichihara M, et al. (2004). Effect of the physicochemical properties of initially injected liposomes on the clearance of subsequently injected PEGylated liposomes in mice. J Control Release 95:403–12
- Ishida T, Maeda R, Ichihara M, et al. (2003). Accelerated clearance of PEGylated liposomes in rats after repeated injections. J Control Release 88:35–42
- Kibria G, Hatakeyama H, Ohga N, et al. (2011). Dual-ligand modification of PEGylated liposomes shows better cell selectivity and efficient gene delivery. J Control Release 153:141–8
- Koga K, Takarada N, Takada K. (2010). Nano-sized water-in-oil-in-water emulsion enhances intestinal absorption of calcein, a high solubility and low permeability compound. Eur J Pharm Biopharm 74:223–32
- Kong XF, Hu YL, He ZH, et al. (2006). Normal parameters of blood routine and rheology of two kinds of rabbits. Animal Hus Vet Med 38:49–51
- Lasic DD, Maritin FJ, Gabizon A, et al. (1991). Sterically stabilized liposomes: a hypothesis on the molecular origin of the extended circulation times. Biochem Biophys Acta 1070:187–92
- Laverman P, Carstens MG, Boerman OC, et al. (2001). Factors affecting the accelerated blood clearance of polyethylene glycol-liposomes upon repeated injection. J Pharmacol Exp Ther 298:607–12
- Li CL, Cao JN, Wang YJ, et al. (2012). Accelerated blood clearance of pegylated liposomal topotecan: influence of polyethylene glycol grafting density and animal species. J Pharma Sci 101:3864–76
- Ma YL, Yang Q, Wang L, et al. (2012). Repeated injections of PEGylated liposomal topotecan induces accelerated blood clearance phenomenon in rats. Eur J Pharm Sci 45:539–45
- Oussoren C, Storm G. (1997). Lymphatic uptake and biodistribution of liposomes after subcutaneous injection. III. Influence of surface modification with poly(ethyleneglycol). Pharm Res 14:1479–84
- Oussoren C, Storm G. (1999). Effect of repeated intravenous administration on the circulation kinetics of poly(Ethyleneglycol)-liposomes in rats. J Liposome Res 9:349–55
- Park K. (2013). Mechanistic study on the ABC phenomenon of PEG conjugates. J Control Release 165:234
- Paula Corrêa Oliveira Bahia A, Barbosa Rabelo L, Cristiano Souza W, et al. (2009). Physicochemical and pharmacokinetic characterization of ultradeformable vesicles using calcein as hydrophilic fluorescent marker. Adv Planar Lipid Bilayers Liposomes 9:65–85
- Semple SC, Harasym TO, Clow KA, et al. (2005). Immunogenicity and rapid blood clearance of liposomes containing polyethylene glycol-lipid conjugates and nucleic acid. J Pharmacol Exp Ther 312:1020–6
- Shiraishi K, Hamano M, Ma HL, et al. (2013). Hydrophobic blocks of PEG-conjugates play a significant role in the accelerated blood clearance (ABC) phenomenon. J Control Release 165:183–90
- Steenpaβ T, Lung A, Schubert R. (2006). Tresylated PEG-sterols for coupling of proteins to preformed plain or PEGylated liposomes. Biochim Biophys Acta 1758:20–8
- Suzuki T, Ichihara M, Hyodo K, et al. (2012). Accelerated blood clearance of PEGylated liposomes containing doxorubicin upon repeated administration to dogs. Int J Pharm 436:636–43
- Tagami T, Nakamura K, Shimizu T, et al. (2009). Effect of siRNA in PEG-coated siRNA-lipoplex on anti-PEG IgM production. J Control Release 137:234–40
- Visser CC, Stevanović S, Voorwinden LH, et al. (2005). Targeting liposomes with protein drugs to the blood-brain barrier in vitro. Eur J Pharm Sci 25:299–305
- Wang L, Hou BG, Hou XP, et al. (2001). Effects of different phospholipids on the stabilities of doxorubicin liposomes in vitro and vivo. Acta Pharm Sin 36:444–7
- Xu H, Wang KQ, Deng YH, et al. (2010). Effects of cleavable PEG-cholesterol derivatives on the accelerated blood clearance of PEGylated liposome. Biomaterials 31:4757–63
- Xia JY, Lei PQ, Zeng XL, et al. (2009). Determination to the weight of main organs and biochemical indexes of SPF KM Mice. J Sichuan Phys Sci 31:104–6
- Yang F, Gu ZX, Shi YX, et al. (2003). Determination of main indexes of hematology, organ relative weight and length of head, body and tail in SPF Wistar rats. Lab Animal Sci Manage 20:1–5
- Zalba S, Navarro I, Trocóniz IF, et al. (2012). Application of different methods to formulate PEG-liposomes of oxaliplatin: evaluation in vitro and in vivo. Eur J Pharm Biopharm 81:273–80
- Zhang K, Sun SH, Zhang HM, et al. (2010). Comparison of hematological parameters in germ-free and conventional guinea pigs. Acta Lab Animali Sci Sin 18:176–80
- Zhao YX, Wang L, Yan MN, et al. (2012). Repeated injection of PEGylated solid lipid nanoparticles induces accelerated blood clearance in mice and beagles. Int J Nanomed 7:2891–900