Abstract
Tricyclic antidepressants, as doxepin hydrochloride (DH), may have analgesic local effect due to its biochemical mechanism of action. Delivery of DH directly to the oral cavity could be an interesting alternative for toothache due to its analgesic local effect. One problem associated with the mucosal administration routes is the short residence time of the dosage form on the mucosal membranes. In this sense, we have developed new doxepin mucoadhesive films able of reducing pain and increasing the effectiveness of treatment. For this purpose, we tested three different polymers: chitosan, sodium hydroxypropylmethylcellulose (HPMC) and sodium carboxymethylcellulose (SCMC) in film elaboration. The results obtained show that all films are hydrophilic matrices that absorb water when placed in an aqueous media. All the films hydrated very quickly, reaching high percentage of swelling after just few minutes (5 min for SCMC, 2 min for HPMC and 30 min for chitosan). Moreover, the SCMC and HPMC films were dissolved whereas chitosan was not dissolved. Dissolution also leads to viscous liquids with a higher retention time over mucosal surfaces what may lead to adhesive interactions. In vitro permeation studies showed that for all the formulations studied, SCMC (19.91%), HPMC (69.5%) and chitosan (24.17%), the percentage of drug permeated increased compared to the drug solution (8.26%). Specifically the HPMC film presents greater amounts of doxepin permeated (49.27 ± 4.47 µg/cm2).
Introduction
Toothache is one of the most important causes of the increased demand of drugs for pain treatment, e. g. analgesics, local anesthetics, vasoconstrictors and drug combinations. It is well known that continued use of these drugs has drawbacks due to the high number of side-effects: headaches, sickness, dizziness, sleepiness, gastrointestinal irritation, anxiety, nervousness. Another alternative to analgesics are tricyclic antidepressants, such as doxepin hydrochloride (DH), since there are studies (Korzeniewska-Rybicka & Plaznik, Citation1998; Onghena & Van Houdenhove, Citation1998; Sudoh et al., Citation2003; Micó et al., Citation2006; McCleane, Citation2007) reporting that they may have analgesic local effect due to its biochemical action, as they are able to increase the amount of noradrenaline and serotonin in the synaptic space. Oral administration of DH also has side effects related to its main function as antidepressant, especially when it is systemically administered (e.g. sublingual administration). However, delivery of DH directly to the oral cavity could be an interesting alternative for toothache due to its analgesic local effect. It has also attracted particular attention due to high patient compliance and unique physiological features: saliva, pH, fluid volume, enzyme activity and the permeability of oral mucosa. For drug delivery systems designed for extended release in the oral cavity, the structure and turnover of the mucosal surface is also a determinant of performance (Patel et al., Citation2011).
One problem associated with the mucosal administration routes is the short residence time of the dosage form on the mucosal membranes (Patel et al., Citation2011). In this sense, retentive mucoadhesive formulations have been reported to be an alternative to the conventional oral medications as they can be readily attached to the buccal cavity, retained for a longer period of time and they can be removed at any time. Mucoadhesive drug delivery systems, including matrix tablets, films, layered systems, discs, microspheres, ointments and hydrogel systems, have been studied and reported by several research groups (Martín del Valle et al., Citation2009; Satheesh et al., Citation2009; Giovino et al., Citation2012; Kianfar et al., Citation2012; Boateng et al., Citation2013; Kianfar et al., Citation2013; Meher et al., Citation2013). Buccal films are preferable over any other system in terms of flexibility and comfort (Perioli et al., Citation2004). They also offer advantages over creams and ointments since they provide a measured dose of drug to the application site, thus reducing pain and increasing treatment effectiveness (Peh & Wong, Citation1999).
Mucoadhesive formulations use polymers as adhesive components. These polymers form viscous liquids when hydrated, increasing their retention time over mucosal surfaces what may lead to interaction between polymers chain and the oral mucose. For this purpose, bioadhesive polymers should possess certain physicochemical features including hydrophilicity, numerous hydrogen bond-forming groups, flexibility for interpenetration with mucus and ephitelial tissue, and viscoelastic properties (Sudhakar et al., Citation2006). Thus, the adequate selection of the polymer is crucial for the correct delivery of drugs in mucoadhesive formulations.
The objective of this study was to develop new DH-based mucoadhesive films indicated for dental pain treatment. For this purpose, we tested three different polymers: chitosan, sodium hydroxypropylmethylcellulose and sodium carboxymethylcellulose. The different transbuccal patches obtained were characterized. We also tested the homogeneity and reproducibility under laboratory conditions. Finally, the DH release and permeation profiles of different formulations were compared using Franz-type diffusion cells.
Materials and methods
Materials
Doxepin hydrochloride (DH) was provided by Fagron Iberica S.A.U. (Barcelona, Spain) and met the requirements of the European Pharmacopoeia. Films were prepared using three film-forming and mucoadhesive polymers: chitosan with degree of deacetylation between 75 and 85 % and molecular weight comprised between 190 kDa and 375 kDa (Sigma–Aldrich, Valencia, Spain), hydroxypropylmethyl cellulose (HPMC) with molecular weight 86 kDa (Methocel K 15 M EP, Palex S.A., Jaen, Spain) and carboxymethylcellulose sodium salt (SCMC) with molecular weight 90–700 kDa (Guinama, Valencia, Spain). The plasticizer was glycerol (Fagron, Barcelona, Spain). Polyvinyl pyrrolidone (PVP) was supplied by BASF (Barcelona, Spain) and acetic acid by Panreac (Barcelona, Spain). Water used to prepare the patches was of Milli-Q quality (Milli-Q Academic, Millipore, France).
Methods
Preparation of mucoadhesive films
Initially, films were prepared using either ionic polymers (Andrews et al., Citation2009) as SCMC (Tsutsumi et al., Citation1994) and chitosan (Sawayanaga et al., Citation1982) and non-ionic polymer as HPMC (Kumar & Himmelstein, Citation1995). For their preparation a solvent evaporation process was used. To improve the performance of the patch and release characteristics, glycerol and polyvinyl pyrrolidone were used as plasticizers. The procedure consisted in dispersing SCMC and HPMC polymers in water (at 4% and 3% respectively) and chitosan in an acetic acid aqueous solution (1.5 % (v/v) to increase the solubility of the polymer which is insoluble in water (Rinaudo, Citation2006)). Then, adequate plasticizers were added under continuous stirring to obtain a suitable viscosity dispersion. The mixtures were poured into petri dish (5 g/petri dish), then stored at 4 °C for 48 h to remove all the air bubbles entrapped and finally dried at 37 °C during 3 h or 24 h for SCMC and HPMC films respectively and at 25 °C during 24 h for chitosan film. In all cases patches were obtained uniform, homogeneous and free from bubbles. In SCMC and chitosan films polymer incorporation takes place prior to the preparation of the gel whereas HPMC is added to the gel previously prepared. Composition of films is shown in .
Table 1. Composition of the bucoadhesives films.
Film thickness
The thickness of the patches was determined using electronic digital caliper Powerfix, model Z22855, version 06/2007 produced by Paget Trading Ltd., c/o Paget Services 65–66 Woodrow London SE 18 5DH UK. We used three films and six measurements were made of each at diametrically opposite points.
Content uniformity
About 0.5 g of each film were added in a plastic bottle with screw cap containing 10 ml of phosphate buffer. All the samples were studied in triplicate. Once prepared, the vials were sonicated for a variable time depending on the type of patch (30 min for HPMC, 60 min for Chitosan and 150 min for SCMC). Then they were kept in a thermostatic bath at 25 °C and stirring at 100 rpm for 24 h. Finally, the samples were filtered and analyzed spectrophotometrically.
Swelling studies
Each film was cut into strips of 2 cm2 and weighted (W1). Samples were allowed to swell on the surface of agar plate and then kept in an incubator maintained at 37 °C. Increase in the weight and size of the patches was determined at preset time intervals. After this time the films were removed from the agar and weighted (W2). The percent swelling, % S, was calculated using the following equation:
where W2 is the weight of the swollen patch after time and W1 is the original patch weight at zero time. The study was performed in triplicate for each type of transbuccal patch (Nafee et al., Citation2003; Perioli et al., Citation2004; Don et al., Citation2008; Derle et al., Citation2009).
Water vapour permeability (WVP)
Five cylindrical glass containers of the same type and size, provided with only one circular opening, were used. The containers were filled up with 20 ml of water and the opening was covered with the films without siliconized paper. The area available for vapour permeation was 4.42 × 10−3 m2. All the containers were maintained at the temperature of 37 °C for 24 h. The weight of each container was recorded 1 h before the beginning of the test and 1 h after its end. The WVP was calculated using the following equation:
where W is the mean loss in weight (g) of the containers and A (m2) is the area of the exposed surface (Minghetti et al., Citation1997; Padula et al., Citation2003).
Microphotographic study
The characterisation of size, shape and surface properties of the patches was carried out through scanning electron microscopy, using a Hitachi S-5–10 scanning microscope (Tokyo, Japan). Prior to examination, samples were mounted on an aluminium stub using a double sided adhesive tape and making it electrically conductive by coating with a thin layer of gold (approximately 20 nm) in vacuum.
Calorimetric analysis
Differential scanning calorimetry (DSC) was performed on the DH, different polymers and other components of the patches using a Mettler (mod. FP80) differential scanning calorimeter equipped with an oven (mod. FP85). The samples, each weighing between 5 and 7 mg were weighed into aluminium pans and sealed. DSC runs were performed at a heating rate of 5 °C/min over a temperature of 25–400 °C. Peak temperature, glassy transition and heat of fusion were determined in every sample using the software FP89 v 4.0 (Toledo, Spain).
In vitro release study from doxepin loaded film
Franz-type diffusion cells have been used in these types of studies (Franz, Citation1975; Kierstan et al., Citation2001). The FDC-400 cell (Vidra–Foc, Barcelona, Spain) used in this experiment consisted of two compartments with a membrane clamped between the donor and receiver chambers. The receptor phase was phosphate-buffered saline, at a chosen pH of 6.8, considered appropriate, given that this is the pH encountered in the buccal mucosa, where the drug is absorbed after oral cavity administration 600 µl samples were collected at predetermined time intervals and replaced with and equal volume of phosphate-buffered saline. Doxepine hydrocloride was determined by spectophotometric method at λmax = 293 nm. The membranes used were 47 mm in diameter and 0.45 μm in pore size. Subsequent to tests on membranes of both methylcellulose (Teknokroma, Sant Cugat del Valles, Barcelona, Spain) and nylon (Waters Corporation, Barcelona, Spain). The nylon variety were selected for our diffusion studies, given that these were found to offer the least resistance to the diffusion of the active principle.
In vitro permeation study from doxepin-loaded film
The permeation study of bucoadhesivos patches was performed in Franz diffusion cells. For this, we used reverse chicken skin after removal of superficial fat content, such as mucosal tissue model. The panicles of chicken skin were stored at 4 °C in phosphate buffer solution at pH 6.9, for a time not more than 24 h (Wong et al., Citation1999). These skin panicles were mounted in stainless steel discs 4 cm diameter and a central aperture of 2 cm diameter, aperture which allows exposure of the mucosa during the release assay. These discs are composed of two parts so that the skin is perfectly subjected to said support for its use in the proposed trial. The sampling procedure is the same as in the previous section.
Partition coefficient determination
The partition coefficient of DH was determined using n-octanol as orgaic solvent and phosphate buffer adjusted to pH 6.8 as aqueous phase. Phase volume ratio was 5:5 ml. After equilibrating for 24 h in water bath at 25 °C, the aqueous phase was separated by centrifugation (5200 rpm for 15 min) and assayed for DH content.
Six replicates were carried out. The partition coefficient was calculated as the ratio of organic concentration (estimated by difference between initial and final concentration) to final aqueous concentration.
Analytical methods
The concentration of DH was measured by UV-spectrophotometry at 293 nm (λmax). The method was previously validated and verified for accuracy, precision and linearity. A Perkin–Elmer UV-Vis Lambda 40 UV-spectrophotometer was used to take all measurements.
Statistical analysis
Statistical analysis of the experimental data in order to determine their differences were made using one-way analysis of variance (ANOVA) test. Differences were considered to be significant when p < 0.05.
Results and discussion
Lipophylicity of doxepin
Lipophility is one of the most important physicochemical parameters of the active molecules expressing permeation ability through the biological membranes (Qin et al., Citation2002; Kweon et al., Citation2003). Classical partition coefficients (p) obtained using n-octanol as the organic solvent show a pH dependency as predicted by the basic nature of Doxepine with a pKa of nine. The obtained p value expressed as decimal logarithm was 1.12 ± 0.02 for pH 6.8 ± 0.1. Theoretical background concerning transbuccal penetration would indicate this pH value for buccal membrane. The p value falls within the proper range for potential drugs to penetrate the lipid phase of the buccal membrane (Dearden & Bresnen, Citation1988; Taylor, Citation1990; Earll, Citation1999; Yener et al., Citation2010).
Films characterization
Once satisfied that doxepin hydrochloride is a drug capable of crossing the buccal membrane and given its powerful local analgesic effect, it could be used in the treatment of dental pain. For this purpose, we have developed new doxepin mucoadhesive films able of reducing pain and increasing the effectiveness of treatment. In this study, specifically, three mucoadhesive polymers as HPMC, SCMC and chitosan were used to develop mucoadhesive films. Moreover, the polymers were chosen from a series of trials on the base of the chemical compatibility, organoleptic properties and adhesiveness appearance.
The results of films characterization are shown in . According to these results, there were no statistically significant differences between the thickness of the film in all cases (94.44 ± 4.81 µm SCMC, 92.78 ± 0.96 µm HPMC and 92.22 ± 2.55 µm chitosan p > 0.05). In all cases thickness was suitable for application in the oral cavity (Perioli et al., Citation2004). The drug content per gram of transbuccal systems was 1.18 ± 0.06 mg, 2.80 ± 0.17 mg and 3.05 ± 0.20 mg for SCMC, HPMC and chitosan, respectively. Additionally, uniformity was observed in drug content distribution as shown by the standard deviation of the dose obtained in each type of bucoadhesive film. This meets the standards set by the Real Farmacopea Española about maximum deviation of 10%. The thickness and content uniformity are essential parameters for the evaluation of the dosage form in order to achieve a formulation with uniformity and consistency.
Table 2. The results of thickness, drug content, percentage of hydration and water vapour permeability of each formulation (all the values are presented as Mean ± S.D.; n = 18, 3, 3, 6, respectively).
The percentages of swelling and WVP values measured for the different films are shown in . All the films hydrated very quickly, reaching high percentage of swelling after just few minutes (5 min for SCMC, 2 min for HPMC and 30 min for chitosan). There were not statistically significant differences between SCMC and chitosan (64.04% ± 2.03 versus 67.30% ± 2.45; p > 0.05). In contrast, HPMC showed a lower percentage of swelling compared to chitosan or SCMC (55.91% ± 2.26; p < 0.05). However, the SCMC film was dissolved in a maximum time of 5 min and HPMC film was dissolved in a maximum time of 3 min whereas chitosan was not dissolved, the chitosan films increase their weight and size by 150%. This can cause discomfort to the patient. The swelling of these films is due to its physicochemical properties such as being cationic, hemostatic and insoluble at high pH. Chitosan is insoluble in most organic solvents, chitosan is readily soluble in dilute acidic solutions below pH 6.0 due to the quaternisation of the amine groups that have a pKa value of 6.3 making chitosan a water-soluble cationic polyelectrolyte. The presence of the amino groups indicates that pH substantially alters the charged state and properties of chitosan. At low pH, these amines get protonated and become positively charged and that makes chitosan a water-soluble cationic polyelectrolyte. On the other hand, as the pH increases above six, chitosan’s amines become deprotonated and the polymer loses its charge and becomes insoluble. The soluble-insoluble transition occurs at its pKa value around pH between six and six point five. As the pKa value is highly dependent on the degree of N-deacetylation, the solubility of chitosan is dependent on the degree of deacetylation and the method of deacetylation used. Apart from the degree of deacetylation, the molecular weight is also an important parameter that significantly affects the solubility and other properties (Dasha et al., Citation2011). In contrast, the SCMC and HPMC films did not show this increment, what could make them more adequate for patient comfort. In relation to the results obtained in the WVP, SCMC film (78.09 ± 1.24 g/m2, 24 h) and chitosan film (76.20 ± 4.21 g/m2, 2 h) were more permeable than HPMC (43.76 ± 1.17 g/m2, 24 h). These results corroborate those obtained in the swelling study, in which we observed a higher percentage of moisture in the films of SCMC and chitosan. Moreover, according to the method proposed by the British Pharmacopeia, the three systems studied were not very permeable to water vapor, since the limit at which a permeable material is considered is 500 g/m2 per 24 h.
The results obtained show that HPMC and SCMC polymers form hydrophilic matrices that absorb water when placed in an aqueous media. This may be supplied by saliva, which may also act as the dissolution medium. Bioadhesive formulations are often water soluble and in a dry form they attract water from biological surfaces leading to a strong interaction. These materials also form viscous liquids when hydrated with increasing their retention time over mucosal surfaces what may lead to adhesive interactions (Batchelor, Citation2004). The duration of adhesion depends on the amount of water at the interface between the film and the buccal mucosa. Excessive water reduces the duration of adhesion (Sudhakar et al., Citation2006) so the rapid water absorbency may cause the shortening of the duration of adhesion. But it is believed that the faster the rate of absorption of water, the shorter the time required for the material to obtain initial adhesion.
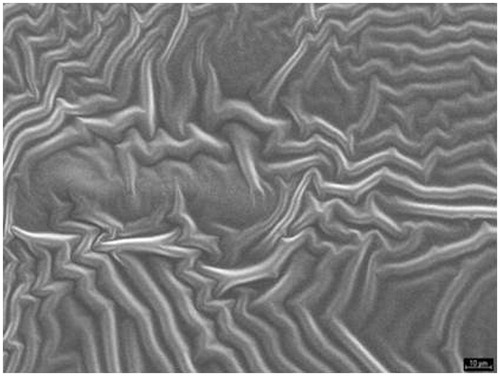
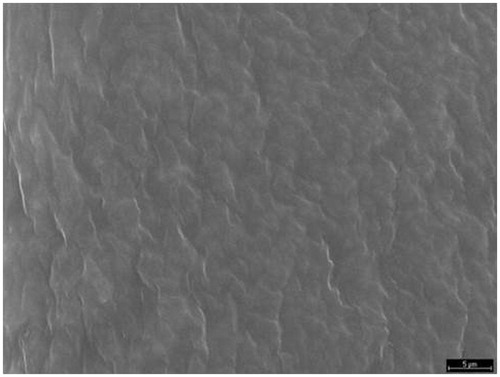
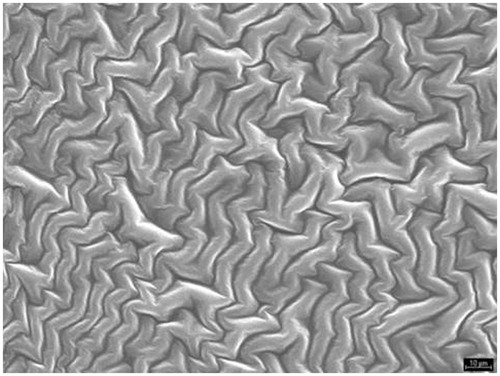
For a given drug the release kinetics from the polymer matrix could be governed by the buccal film structure. The morphology film has been studied by SEM. The SEM study () shows that film formed by a matrices structure are homogeneous, uniform and intact, without pores or cracks in their structure (Villalobos et al., Citation2005). This three-dimensional structure was similar to the film of SCMC and chitosan, although the structure was denser in the second one. Both polymers are ionic in nature as opposed to HPMC, which is a non-ionic polymer, and therefore its structure showed a different appearance () (Sánchez-González et al., Citation2009).
Calorimetric study
Differential scanning calorimetry (DSC) studies were performed to investigate chemical interactions between drug and the components films because the mechanical properties of hydrophilic polymers are affected by these interactions. The determination of glass transition temperature (Tg) is difficult because the mucoadhesive films contain a moisture associated and because they are complexe structures. Therefore the Tg is not a sharp transition even in fully optimised systems and may occur over temperature range, being a further difficulty that the Tg may overlap the moisture loss endotherm.
As shown in the thermogram for each film (), there was an endothermic transition in the temperature range between 30 °C and 120 °C, which is attributed to dehydration by the loss of the bonds between water molecules and polymer chains. At the end of the calorimetric curve was evidence from other thermal process of 240 °C, which is attributed to the decomposition of biopolymers. The physical mixtures with the same proportions as the films show similar behaviour (). Moreover, DSC curves did not show the characteristic peak of the drug (), most likely due to a poor relationship with the other excipients.
Figure 4. DSC thermogram of bucoadhesives films: (a) SCMC (black line), (b) HPMC (red line) and (c) chitosan (green line).
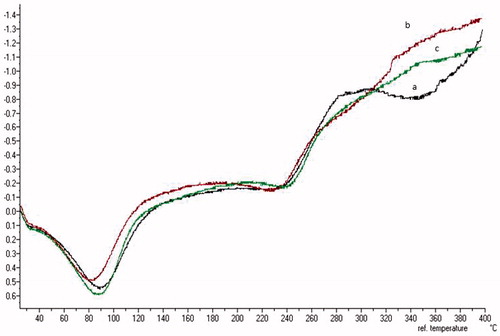
Figure 5. DSC thermograms of physical mixtures with the same proportions as the films (a) SCMC (black line), (b) HPMC (red line) and (c) chitosan (green line). Graph inset is DSC thermogram of the DH.
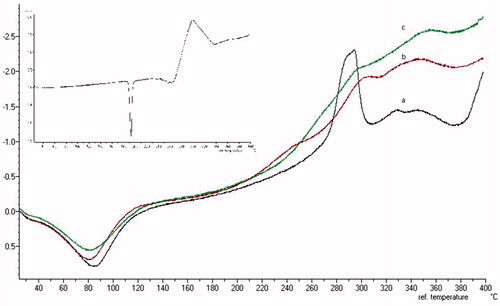
The enthalpies needed for endothermic transition were different between films (281 J/g SCMC, 251 J/g and 281 J/g chitosan) and physical mixture of components (369 J/g SCMC, 342 J/g and 326 J/g chitosan). In all cases, we observed a decrease in the enthalpy values for the mucoadhesive films, probably due to the presence of water. We also observed similar dehydration enthalpies for SCMC and chitosan polymers. However, the HPMC film showed a trend to lower enthalpy values, thus suggesting a lower energy supplied to remove water from the stand. This result, together with a similar trend showed in swelling analysis, confirms that SCMC and chitosan films present a higher absorption capacity of the water molecule in their structure (Ford, Citation1999; Ritthidej et al., Citation2002).
In vitro release study from doxepin-loaded film
As a previous step to the drug release study, membrane selection processes were studied in order to arrive at a reliable assessment of the influence of the films in doxepin hydrochloride release (Morales et al., Citation2004). Two types of synthetic membranes were tested: nylon (1488.9 µg released in 2 h) and methylcellulose (1444.3 µg released in 2 h). It was clear that the nylon membrane was that which offers least resistance, allowing a greater quantity of the drug to pass through it. This membrane was therefore chosen for the ensuing release study of mucoadhesive films with doxepin hydrochloride.
The release study was carried out over a period of no longer than 6 h, given that formulations cannot remain for longer periods within the buccal membrane. On the other hand, it should be taken into account that some of the components that constitute the formula may be soluble within the buffer, and as in the case of doxepin, will pass through the membrane. In parallel, release tests using a control formulation without the drug were carried out, in which components were observed to pass through the membrane and to contribute to absorbency of the samples at 293 nm. However, its contribution was found to be identical within the time interval studied, and the appropriate corrections were carried out. In such a way, it was possible to be sure that the quantities obtained only corresponded to quantity of released doxepin. Finally, all extracted samples were analysed spectrophotometrically, in order to determine the quantities released.
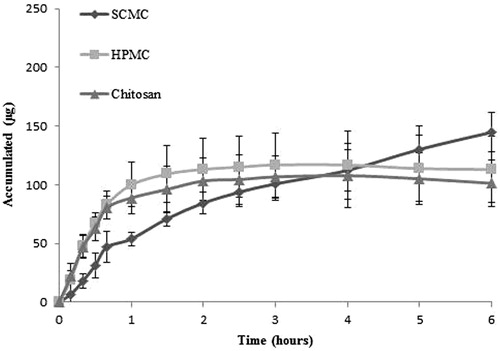
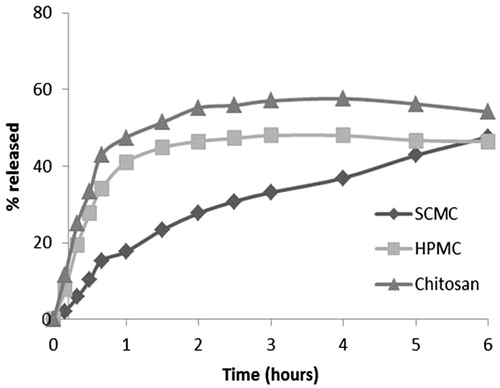
, shows that the HPMC film and chitosan film present practically identical behavioural patterns during of the test and there are not differences statistically significant (p > 0.005). On the other hand, the SCMC film was that which releases the drug at a slower rate, although at the end of the testing period, it can be seen that the quantities of released drug were in fact similar for all films.
In this regard, the percentages of the quantities of released DH past the first 6 h of the test were similar for SCMC and HPMC films being 46.4%, however the release continues for 24 h with percentages of 63.5% and 58.2% respectively (). These two results were very similar, and after being subjected to statistical analysis (ANOVA), the release profiles among two formulations did not show any statistically significant differences (p > 0.05). On the other hand, chitosan film release maximum yields 58.2% of the drug to 3 h of starting the test and this value did not change during the same. This behaviour can be explained by the fact that SCMC and HPMC polymers form hydrophilic matrices that forms viscous liquids when hydrated with aqueous media, as confirmed by previous swelling studies. It is therefore possible to make an accurate assessment of the influence of the swelling behaviour on released amounts. On completion of the test, it can be seen that chitosan film were not dissolved and the drug was released for less time (3 h versus 6 h).
Study of the drug permeation
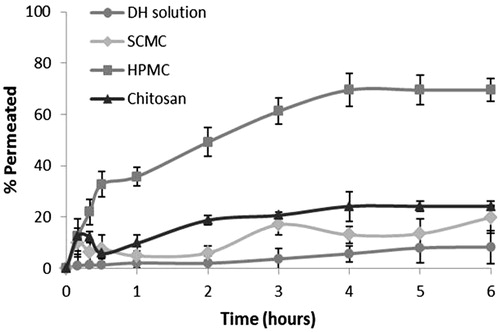
Ex vivo drug permeation was studied in order to understand the permeation pattern of drug through biological membrane. The permeation release from bucoadhesive films is controlled by the chemical properties of drug, type of delivery films as well as the physiological and physicochemical properties of the buccal membrane (Rao et al., Citation2000). Therefore, the in vitro permeation studies are predictive of in vivo performance of a drug. shows that since the three formulations studied, SCMC (19.91%), HPMC (69.5%) and chitosan (24.17%) was achieved to increase the percentage of drug permeated with respect to the drug solution (8.26%). As for the permeation of doxepin from different formulations, we conclude that there are not statistically significant differences (p > 0.005) between the amounts of doxepin permeated per cm2 of buccal mucosa for the SCMC (17.76 ± 1.31 µg/cm2) and chitosan systems (10.98 ± 0.26 µg/cm2). However, there were statistically significant differences compared to HPMC films (49.27 ± 4.47 µg/cm2), in spite of the fact that in vitro test showed that the amount of released DH was the same in the three formulations. Taking these results into account, it could be concluded that the permeation from the polymer matrix could be explained by the buccal film structure. This three-dimensional structure is similar to the film of SCMC and chitosan and both polymers are ionic as opposed to HPMC, which is a non-ionic polymer, and therefore its structure shows a different appearance. In addition, the results obtained in swelling, WVP and DSC studies show differences between ionic polymer film and HPMC film. The critical parameter seems to be the buccal film structure (Melero et al., Citation2008), influencing in the adhesive contact and the drug permeation.
Conclusions
The present study have shown that the polymers tested exhibits good film forming ability for the formulation of transmucosal delivery films, due to their properties of swelling and thickness well as the other results obtained in the characterization of the films as three-dimensional structure or calorimetric studies. Since the three formulations studied achieved increase in the percentage of drug permeated with respect to the drug solution. However, HPMC film provided greater amounts of doxepin permeated, although the percentage of DH released in the in vitro studies is similar from three films. Taking these results into account, it could be concluded that the permeation from the polymer matrix could be governed by the buccal film structure. Therefore doxepin hydrocloride films achieved the desired objective for the administration in the treatment of dental pain, presenting HPMC film the better characteristics.
Acknowledgements
The authors are very much grateful to Professor Ana C. Calpena from the department of Pharmaceutical Technology of the University of Barcelona for their valuable suggestions in this work.
Declaration of interest
Herminia Castan is recipient of a grant from the Foundation Empresa-Universidad de Granada. The rest of the authors report no declaration of interest.
References
- Andrews GP, Laverty TP, Jones DS. (2009). Mucoadhesive polymeric platforms for controlled drug delivery. Eur J Pharm Biopharm 71:505–18
- Batchelor H. (2004). Novel bioadhesive formulations in drug delivery, the drug delivery companies report. Tech In Overviews 17:1–4
- Boateng J, Mani J, Kianfar F. (2013). Improving drug loading of mucosal solvent cast films using combination of hydrophilic polymers with amoxicillin and paracetamol as model drugs. Bio Med Res Int 2013:1–8
- Dasha M, Chiellini F, Ottenbriteb RM, Chiellini E. (2011). Chitosan:a versatile semi-synthetic polymer in biomedical applications. Prog Poly Sc 36:981–1014
- Dearden JC, Bresnen GM. (1988). The measurement of partition coefficients. Quant Struct Act Relat 7:133–4
- Derle D, Joshi O, Pawar A, et al. (2009). Effect of tablet excipients on mucoadhesive properties of polyoxyethylene and carbopol 971 P. Int J Pharm Pharm Sci 1:198–205
- Don TM, Huang ML, Chiu AC, et al. (2008). Preparation of thermo-responsive acrylic hydrogels useful for the application in transdermal drug delivery systems. Mater Chem Phys 107:266–73
- Earll M. (1999). A guide to Log P and pKa measurements and their use. C chem MRS, 3–4
- Ford JL. (1999). Thermal analysis of hydroxypropylmethylcellulose and methylcellulose: powders, gels and matrix tablets. Int J Pharm 179:209–28
- Franz TJ. (1975). Percutaneous absorption on the relevance of in vitro data. J Invest Dermatol 64:190–5
- Giovino C, Ayensu I, Tetteh J, Boateng JS. (2012). Development and characterisation of chitosan films impregnated with insulin loaded PEG-b-PLA nanoparticles (NPs): a potential approach for buccal delivery of macromolecules. Int J Pharm 428:143–51
- Kianfar F, Ayensu I, Boateng JS. (2013). Development and physico-mechanical characterization of carrageenan and poloxamer-based lyophilized matrix as a potential buccal drug delivery system. Drug Dev Ind Pharm 40:361–9
- Kianfar F, Chowdhry BZ, Antonijevic MD, Boateng JS. (2012). Novel films for drug delivery via the buccal mucosa using model soluble and insoluble drugs. Drug Dev Ind Pharm 38:1207–20
- Kierstan KT, Beeper AE, Mitchell JC, et al. (2001). UV-spectrophotometry study of membrane transport processes with a novel diffusion cell. Int J Pharm 229:87–94
- Korzeniewska-Rybicka I, Plaznik A. (1998). Analgesic effect of antidepressant drugs. Pharmacol Biochem Behav 59:331–8
- Kumar S, Himmelstein KJ. (1995). Modification of in situ gelling behavior of carbopol solution by hydroxypropylmethyl cellulose. J Pharm Sci 84:344–8
- Kweon DK, Song SB, Park YY. (2003). Preparation of water-soluble chitosan/heparin complex and its application as wound healing accelerator. Biomat 24:1595–601
- Martín del Valle EM, Galan MA, Carbonell RG. (2009). Drug delivery technologies: the way forward in the new decade. Ind Eng Chem Res 48:2475–86
- McCleane G. (2007). Analgésicos por vía tópica. Anesthesiol Clin N A 25:825–39
- Meher JG, Tarai M, Yadav NP, et al. (2013). Development and characterization of cellulose-polymethacrylate mucoadhesive film for buccal delivery of carvedilol. Carbohydr Polym 96:172–80
- Melero A, Garrigues TM, Almudever P, et al. (2008). Nortriptiline hydrochloride skin absortion: development of a transdermal patch. Eur J Pharm Biopharm 69:588–96
- Micó JA, Ardid D, Berrocoso E, Eschalier A. (2006). Antidepressants and pain. Tren Pharmacol Sci 27:348–54
- Minghetti P, Cilurzo F, Liberti V, Montanari L. (1997). Dermal therapeutic systems permeable to water vapour. Int J Pharm 158:165–72
- Morales ME, Gallardo V, Calpena AC, et al. (2004). Comparative study of morphine diffusion from sustained release polymeric suspensions. J Cont Rel 95:75–81
- Nafee NA, Ismail FA, Boraie NA. (2003). Mucoadhesive buccal patches of miconazole nitrate: in vitro/in vivo performance and effect of ageing. Int J Pharm 264:1–14
- Onghena P, Van Houdenhove B. (1998). The antidepressant-induced analgesic effects. Baillière Clin Anaes 12:53–68
- Padula C, Colombo G, Nicoli S, et al. (2003). Bioadhesive film for the transdermal delivery of lidocaine: in vitro and in vivo behavior. J Cont Rel 88:277–85
- Patel VF, Liu F, Brown MB. (2011). Advances in oral transmucosal drug delivery. J Cont Rel 153:106–16
- Peh KK, Wong CF. (1999). Polymeric films as a vehicle for buccal delivery: swelling, mechanical and bioadhesive properties. J Pharm Pharm Sci 2:53–61
- Perioli L, Ambrogi V, Angelici F, et al. (2004). Development of mucoadhesive patches for buccal administration of ibuprofen. J Control Release 99:73–82
- Qin C, Du Y, Xiao L, et al. (2002). Enzymic preparation of water-soluble chitosan and their antitumor activity. Int J Boil Macromol 31:111–7
- Rao PR, Ramakrishna S, Diwan PV. (2000). Drug release kinetics from polymeric films containing propanolol hydrochloride for transdermal use. Pharm Dev Techno 5:465–72
- Rinaudo M. (2006). Chitin and chitosan: properties and applications. Prog Poly Sc 31:603–32
- Ritthidej GC, Phaechamud T, Koizumi T. (2002). Moist heat treatment on physicochemical change of chitosan salt flims. Int J Pharm 232:11–22
- Sánchez-González L, Vargas M, González-Martínez C, et al. (2009). Characterization of edible films based on hydroxypropylmethylcellulose and tea tree essential oil. Food Hydrocol 23:2102–9
- Satheesh Madhav NV, Shakya AK, Shakya P, Singh K. (2009). Orotransmucosal drug delivery systems: a review. J Cont Rel 140: 2–11
- Sawayanaga Y, Nambu N, Nagai T. (1982). Permeation of drugs through chitosan membranes. Chem Pharm Bull 30:3297–301
- Sudhakar Y, Kuotsu K, Bandyopadhyay AK. (2006). Buccal bioadhesive drug delivery —:a promising option for orally less efficient drugs. J Cont Rel 114:15–40
- Sudoh Y, Cahoon EE, Gerner P. (2003). Tricyclic antidepressant as long acting local anesthetics. Pain 103:49–55
- Taylor PJ. (1990). Hydrophobic properties of drugs in comprehensive medical chemistry. In: Ramsden CA, ed.. Quantitative drug design. Pergamon Press, Oxford, 241–94
- Tsutsumi K, Tahayama K, Machida Y, et al. (1994). Formulation of buccal mucoadhesive dosage form of ergotamine tartrate. S.T.P. Pharm Sci 4:230–4
- Villalobos R, Chanona J, Hernández P, et al. (2005). Gloss and transparency of hydroxypropyl methylcellulose films containing surfactants as affected by their microstructure. Food Hydrocol 19:53–61
- Wong CF, Yuen KH, Peh KK. (1999). Formulation and evaluation of controlled release Eudragit buccal patches. Int J Pharm 178:11–22
- Yener G, Üner M, Gönüllü Ü, et al. (2010). Design of Meloxicam and Lornoxicam transdermal patches: preparation, physical characterization, ex vivo and in vivo studies. Chem Pharm Bull 58:1466–73