Abstract
Context: Hen egg low-density lipoprotein (heLDL), which is present in large quantities in egg yolk, share a high identity with human apolipoprotein B-100 precursor.
Objective: This study investigated the use of heLDL as a macrophage-targeted drug delivery carrier against intracellular Staphylococcus aureus.
Methods: Rifapentine (RPT) was incorporated into heLDL (RPT–heLDL). Staphylococcus aureus ATCC 29740 and human U937 macrophage were used as intracellular infection models.
Results and conclusion: The loading efficiency of RPT into the heLDL was 66.10 ± 2.28 μg RPT/mg heLDL. Fluorescence microscopy and oil red O staining results indicated RPT–heLDL can be taken up by U937 macrophages. The cell viability (MTT assay) was increased when the concentration of heLDL was <150 μg/mL. Unloaded heLDL (100 μg/mL) can inhibit the growth of intracellular S. aureus compared with the untreated control group after 18 h incubation. RPT–heLDL (6.6 μg/mL RPT, 100 μg/mL heLDL) eliminated 94% of intracellular S. aureus, whereas the corresponding dose of free RPT (6.6 μg/mL) induced an 87% reduction. The in vitro results of the current study indicated that heLDL might be used as a suitable drug carrier for targeting human macrophages.
Introduction
Staphylococcus aureus can invade a variety of different cell types, including macrophage cells (Craven & Anderson, Citation1984; Foster, Citation2009). Chronic diseases caused by S. aureus (e.g. mastitis) are difficult to cure completely and often relapse. One of the reasons for the difficulties associated with the treatment of these diseases is that some antibiotics, especially those that are water soluble or ionizable compounds, are only poorly cell permeable, and it is therefore difficult for these compounds to reach the therapeutic concentrations required to efficiently eliminate the intracellular S. aureus (Barcia-Macay et al., Citation2006). Long-term and high-dose antibiotic treatment regimens are not only costly but can also lead to the occurrence of pronounced side effects, contaminate the environment and induce antibiotic resistance (Barcia-Macay et al., Citation2006).
Macrophage-targeted drug delivery carriers, such as liposomes, nanoparticles and microspheres, have been studied as potential delivery systems for eliminating intracellular pathogenic bacteria. These systems could be used not only to promote the delivery of therapeutic agents but also to increase the penetration of these materials into macrophages (Couvreur et al., Citation1991; Imbuluzqueta et al., Citation2010). There are, however, concerns surrounding the practical application of these drug delivery systems, including their stability, biocompatibility and immunogenicity. Low-density lipoprotein (LDL) is an endogenous cholesterol carrier, which has been reported to be stable, biocompatible, biodegradable and nonimmunogenic (Corbin & Zheng, Citation2007). These properties make LDL an attractive drug carrier. The core lipid structures (i.e. triglycerides and cholesterol esters) of this material provide an ideal domain for loading highly lipophilic drugs, whereas their phospholipid monolayer and apolipoprotein shell allow for incorporating hydrophilic drugs (Hamidi et al., Citation2006). The difficulties associated with the isolation of large quantities of LDL from plasma and several related biosafety problems, however, have so far prevented the application of LDL as a drug delivery carrier in research and clinical settings.
Hen egg low-density lipoprotein (heLDL) is present in large quantities (68%) in egg yolk and could be used as a potential replacement for plasma LDL. The apolipoproteins in heLDL share a high identity (49%) and homology (69%) with human apolipoprotein B-100 precursor (Jolivet et al., Citation2008). Compared with human plasma LDL, heLDL is more stable and easy to isolate in large quantities, and its quality can be readily controlled. Furthermore, the biosafety of heLDL can be controlled by the production of germ-free eggs (i.e. eggs free of specific contaminants such as parasites, bacteria, mycoplasma and viruses) (Moran, Citation2009). Germ-free eggs have already been widely used in the manufacture of various biological substances for pharmaceutical use in people and animals, such as antibodies, vaccines and proteins. To the best of our knowledge, the use of heLDL as a drug delivery agent has not yet been studied. In the current article, we have developed an in vitro model using human U937 macrophage cells infected with S. aureus ATCC 29740 to test the intracellular bactericidal activity of rifapentine (RPT) loaded in heLDL (RPT–heLDL).
Materials and methods
Purification of heLDL from hen egg yolk
All of the chemicals used in the current study were purchased from Sigma-Aldrich (St. Louis, Missouri, USA), unless noted otherwise. The LDL was purified from hen egg yolk as described in Moussa et al. (Citation2002). Briefly, the yolk was separated from fresh hen eggs and diluted with an equal volume of a 0.15 M NaCl solution. The resulting mixture was then stirred for 1 h at 4 °C. This solution was then centrifuged twice at 10 000 × g for 45 min at 4 °C to collect the supernatant. The supernatant was mixed with ammonium sulfate (40%), and the pH of the mixture was then adjusted to 8.7. The mixture was stirred for 1 h at 4 °C before being centrifuged at 10 000 × g for 45 min to remove the sediment. The supernatant was then dialyzed for at least 6 h against sterilized deionized water (the water was replaced every 2 h) and centrifuged at 10 000 × g for 45 min at 4 °C. The supernatant rich in LDL was then collected and mixed with an equal volume of 0.1 M Tris–HCl buffer (pH 7). The protein content was determined by BCA™ Protein Assay Kit (Pierce Biotechnology, Thermo, Rockford, IL) using bovine serum albumin as a standard.
Incorporation of RPT into LDL
An RPT solution (500 μg/mL) was prepared at 4 °C by dissolving 0.025 g of RPT in 2 mL of 95% ethanol containing 15 mM sodium hydroxide. Following the dissolution of RPT, a PBS consisting of 137 mM NaCl, 3 mM KCl, 8 mM Na2HPO4 and 1.5 mM KH2PO4 was added to the mixture to bring the final volume to 50 mL.
For loading, 4 mL of the RPT solution (500 μg/mL) and 2 mL of the heLDL solution (1000 μg/mL) were mixed and incubated at 37 °C for 6 h in the absence of light. The mixture was then dialyzed against a PBS at 4 °C for 20 h to remove any free RPT (the PBS being changed every 4 h).
Determining the concentration of RPT in LDL
RPT was extracted from heLDL–RPT using a method described in Carr et al. (Citation1991) and Tian Yan (Citation2007) for the extraction of RPT from blood plasma. Briefly, 0.5 mL of a heLDL–RPT solution was made alkaline via the addition of 0.5 mL of a 10 M sodium hydroxide solution, before being extracted with 4 mL of ethyl acetate. The concentration of RPT was determined by spectrophotometric analysis (Mariappan et al., Citation2004). A standard curve for RPT was constructed over the range of 15–250 μg/mL at a wavelength of 474 nm.
Particle characterization
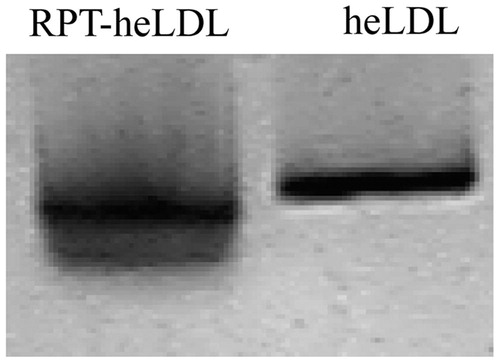
The apolipoprotein compositions of heLDL and RPT–heLDL were determined by 4–20% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE). The stacking gels contained 4% polyacrylamide.
The similarities between heLDL and human plasma LDL were determined by Western blot. The proteins were transferred from SDS–PAGE gel on to a nitrocellulose filter and tested with a goat antihuman LDL antibody (1:10 000 dilution), followed by horseradish peroxidase (HRP)–conjugated donkey antigoat secondary antibody (1:5000 dilution). The detection process was performed with a chemiluminescence reagent (Tiangen, Nanjing, China).
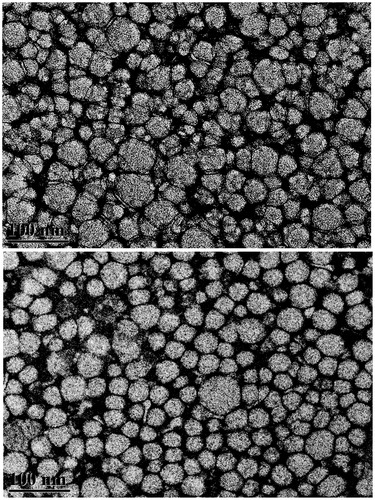
The particle characteristics of heLDL and RPT–heLDL were investigated by transmission electron microscopy at ×45 000 magnification and by agarose gel electrophoresis (Sparks & Phillips, Citation1992).
Cytotoxicity of RPT–heLDL on human U937 macrophages
Human monocyte-like U937 cells (ATCC, Manassas, VA) were maintained in RPMI 1640 medium (Gibco, Invitrogen, Carlsbad, CA), which had been supplemented with 10% heat-inactivated fetal bovine serum (Gibco), 100 U/mL of penicillin and 100 μg/mL of streptomycin (Gibco) in a humidified atmosphere of 5% CO2 at 37 °C. To differentiate the cell line into macrophage-like cells, 106 cells were incubated in 2 mL of RPMI 1640 complete medium with 10 ng/mL of phorbol 12-myristate 13-acetate for 48 h (Lessig et al., Citation2011). The experiments were performed in six-well plates (Becton, Dickinson and Company, Franklin Lakes, NJ). Following the removal of the nonadherent cells, 2 mL of RPMI 1640 containing 2% of fetal calf serum and different concentrations of RPT–heLDL was added and incubated for 18 h. The medium was then removed, and the cells were washed twice with DPBS. Two milliliters of RPMI 1640 containing 0.5 mg/mL 3-[4,5-dimethylthiazol-2-yl]-3,5-diphenyl tetrazolium bromide dye (MTT reagent) was added to each well. Following 4 h of incubation, the supernatants were discarded and 1 mL of dimethyl sulfoxide was added to dissolve the crystals over a period of 5 min. The plates were then shaken and read at 550 nm in a microtiter plate reader (Synergy HT, Biotek Inc, Winooski, VT). The cell viability values were calculated from the absorbance values of the cells incubated with different concentrations of the RPT–heLDL dispersions against the absorbance values of the cells incubated with the RPMI 1640 containing 2% of fetal calf serum (negative control).
Uptake of RPT–heLDL by human U937 macrophages
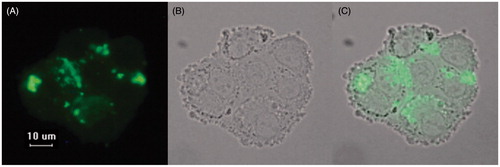
Fluorescein isothiocyanate (FITC) labeling of RPT–heLDL was carried out according to the procedure described by Han et al. (Citation2006). Briefly, 1000 μL of 2 mg/mL RPT–heLDL was mixed with 100 μL of 10 mg/mL FITC (in acetone), and the resulting mixture was incubated at 37 °C in the dark for 20 min. Excess FITC was removed using a 500 μL NanoSep centrifugal ultrafiltration devices with a 100 kDa molecular weight cut-off (PALL Corp., Portsmouth, Hants, UK). The mixture was dialyzed against PBS in the dark for 48 h, with the PBS being changed every 12 h. The protein content of the FITC-labeled RPT–heLDL was determined using a BCA™ Protein Assay Kit (Pierce Biotechnology, Thermoscientific, Rockford, IL). Differentiated U937 cells were incubated with 50 μg/mL of FITC-labeled RPT–heLDL at 37 °C for 18 h. After three washes with PBS to remove extracellular RPT–heLDL, the cells were detached by scraping and 10 μL samples were spread onto glass slides and photographed with an Olympus BX41 fluorescence microscope (Olympus America Inc., Melville, NY).
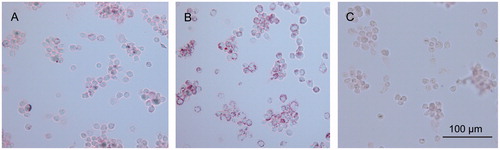
U937 cells were differentiated and incubated with 100 or 200 μg/mL RPT–heLDL according to the MTT assay described earlier. Cells were washed with DPBS, fixed with 10% neutral buffered formalin for 30 min and stained with a saturated concentration of oil red O in 60% isopropanol for 5 min at room temperature. After washed with PBS, slides were mounted with glycerogelatin and photographed with a Leica DM LB microscope (Leica Microsystems Inc., Deerfield, Illinois, USA). For quantitation, 800 μL isopropanol was added to elute the incorporated stain and the optical density was determined at 510 nm.
Bacterial strain
Staphylococcus aureus ATCC29740 was grown from frozen stock by streaking onto tryptic soy agar and incubating for 16 h at 37 °C.
Intracellular antibacterial effects
Staphylococcus aureus colonies from the tryptic soy agar plate were suspended in PBS and washed three times. The optical density of the bacterial suspension was then adjusted to 0.15 at 550 nm equivalent to 5 × 108 CFU/mL. U937 cells were differentiated according to the MTT assay described earlier. Aliquots (20 μL) of the bacterial suspension were then added at a ratio of 1:10 the multiplicity of infection. Following 60 min of infection, the cells were washed five times with DPBS to eliminate nonphagocytosed bacteria. RPMI 1640 (2 mL) containing 100 μg/mL of RPT–heLDL was added to the infected cells. The corresponding concentrations of heLDL were 100 μg/mL, with RPT concentrations equivalent to 6.6 μg/mL. The same concentrations of free RPT and heLDL were used as controls. Nontreated infected cells were also studied. Following 18 h of incubation, the cells were washed three times with PBS and lysed. Viable counts of S. aureus in the lysates were determined at the beginning (0 h) and at the end of the incubation (18 h) via a 10-fold dilution series and plating in duplicate on tryptic soy agar. The experiments were repeated four times, with each treatment being conducted in triplicate.
Statistical analysis
The bacterial numbers were transformed to the corresponding log 10 values for statistical analysis. Differences in the values were identified by the least square means method using the GLM of SAS 9.0 (Statistical Analysis Systems Institute, Cary, NC). p value < 0.05 was considered significant.
Results
Properties of RPT–heLDL
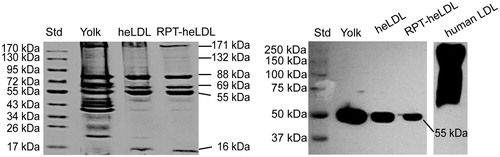
The loading efficiency of RPT into the heLDL was determined to be 66.10 ± 2.28 μg RPT/mg heLDL. The results of SDS–PAGE electrophoresis revealed six identical bands in the RPT–heLDL as well as heLDL (), which indicated that the incorporation of RPT did not lead to the degradation of heLDL apolipoproteins. The molecular masses of the protein bands were near 171, 132, 88, 69, 55, and 16 kDa. Western blot analysis revealed that the protein band of the heLDL in the RPT–heLDL and egg yolk near 55 kDa could bind to the goat antihuman LDL antibody ().
Figure 1. SDS–PAGE and Western blot analyses. heLDL: hen egg yolk low-density lipoprotein; RPT: rifapentine; stacking and running gels: 4 and 4–20% polyacrylamide, respectively; for immunoblot: goat antihuman LDL antibody (1:10 000) (Sigma Aldrich), followed by horse-radish peroxidase (HRP)–conjugated donkey antigoat secondary antibody (1:5000).
The transmission electron microscopy results showed that the average size of the RPT–heLDL was 33 ± 6.8 nm, which was similar to that of the unloaded heLDL (33 ± 9.1 nm) (). These results suggested that the incorporation of the RPT did not significantly affect the particle size of heLDL. The RPT–heLDL moved faster than the heLDL on 0.6% agarose gel (), indicating that some of the negatively charged RPT may have been attached to the surface or inserted into the phospholipid monolayer of the heLDL.
Cytotoxicity and uptake of RPT–heLDL
The cytotoxicity of RPT–heLDL was determined using an MTT assay (). The reduced production of formazan indicated a reduction in cell viability. No significant cytotoxic effects were observed for the RPT–heLDL at concentrations <150 μg/mL. The viabilities of the cells incubated with RPT–heLDL at concentrations of 25, 50 and 100 μg/mL were >100%. This overproduction of formazan was attributed to the activation of the metabolic status of the cells.
Table 1. Viability of human U937 macrophages (MTT assay) after 18 h of incubation with different concentrations of RPT–heLDL.
The uptake of RPT–heLDL into the U937 cells was evaluated by fluorescence microscopy () and oil red O staining (). clearly shows that the FITC-labeled protein entered the U937 macrophages. Following the oil red O staining process, positive red lipid particles were found in most of the cells that had been treated with RPT–heLDL (), whereas no lipid droplets were found in the control group (). The intracellular accumulation of lipids was correlated with the concentration of RPT–heLDL, and the oil red O concentrations measured at 510 nm were 0.30 ± 0.013, 0.47 ± 0.011 and 0.20 ± 0.017, which corresponded to 100 and 200 μg/mL of RPT–heLDL and the control group, respectively.
Intracellular bactericidal activities
Both RPT–heLDL and free RPT induced a significant (p < 0.05) reduction in intracellular S. aureus count (). RPT–heLDL (6.6 μg/mL RPT, 100 μg/mL heLDL) eliminated 94% of intracellular S. aureus, whereas the corresponding dose of free RPT (6.6 μg/mL) induced an 87% reduction. However, the bactericidal activity between RPT–heLDL conjugate and free RPT did not have any statistically significant difference. Interestingly, unloaded heLDL (100 μg/mL) inhibited the growth of intracellular S. aureus compared with the untreated control group after 18 h incubation (7.05 ± 0.26 versus 8.71 ± 0.08 log CFU).
Table 2. Effect of RPT–heLDL against intracellular S. aureus infection in human U937 macrophages.
Discussion
The loading efficiency of RPT (66 μg/mg) assayed in the current study was comparable to that described by Aboutaleb et al. (2012), who reported a loading efficiency of up to 50 μg/mg for the loading of rifampin into solid lipid nanoparticles. Moussa et al. (Citation2002) described the appearance of six major bands from the heLDL on 10% PAGE gel with molecular weights of 175, 140, 80, 65, 60 and 15 kDa. In the current study, six major protein bands were identified on 4–20% PAGE gel and molecular weights similar to those described previously by Moussa et al. (Citation2002). Jolivet et al. (Citation2008) reported that the apolipoproteins in heLDL were similar in their identity (49%) and homology (69%) to human apolipoprotein B-100 (apoB-100) precursor. We demonstrated goat antihuman LDL antibody could react with 55 kDa apolipoprotein in heLDL, supporting the previous findings.
The addition of fluorescently labeled LDL to cells and oil red O staining are classical methods for evaluating the uptake of lipoproteins in macrophages (e.g. human THP-1, human monocyte-derived macrophages and murine macrophage cell lines) (Scholz et al., Citation2004; Moore & Freeman, Citation2006; Xu et al., Citation2010). The results of the current study revealed that the FITC-labeled protein successfully entered the cell and that oil red O–stained lipid droplets were well distributed throughout the cytosol of the U937 macrophages. These results indicated that the RPT–heLDL conjugate had entered the cells successfully. The scavenger receptor–mediated uptake of chemically modified lipoproteins by macrophages, including oxidized and acetylated LDL, was found to be concentration dependent (Xu et al., Citation2010). Our oil red O–staining results revealed a similar pattern to that of the uptake of RPT–heLDL by U937 macrophage, which also correlated well with the concentration. The LDL receptor and CD36 were identified as the major receptors on the macrophage mediating the phagocytosis of human plasma LDL (Frostegard et al., Citation1990; Murphy et al., Citation2005; Sun et al., Citation2007). Further studies are required to understand whether these receptors were involved in the phagocytosis of heLDL. Viability of the U937 macrophage was increased at concentrations of no more than 150 μg/mL. Mine & Nolan (Citation2004) also reported that heLDL could modulate immunity and promote the growth of human U937 monocyte-like cells and U937-derived macrophage-like cells (Frostegard et al., Citation1990; Mine & Nolan, Citation2004).
Staphylococcus aureus could easily invade professional phagocytes and persists in the acidic lysosome compartment for several days (Imbuluzqueta et al., Citation2010). Rifapentine and rifampin, which show enforced bactericidal activity in acidic conditions, have been applied extensively in combinational antimicrobial therapies against intracellular S. aureus infections (Forrest & Tamura, Citation2010). The in vitro infection experiments in the current study demonstrated that the incorporation of RPT into heLDL led to a significant reduction of intramacrophage S. aureus. HeLDL as a drug delivery carrier could help drugs to penetrate the cell membrane to improve their intracellular activity. We did not find statistically significant difference in the bactericidal activity between RPT–heLDL conjugate and free RPT. One possible reason may be free RPT could penetrate the cell membrane easily when it is added to the cell culture directly.
In the current study, heLDL inhibited the growth of S. aureus at 100 μg/mL. Brady et al. (2002) also reported that lipoproteins of hen egg yolk could inhibit the growth of Streptococcus mutans in vitro. The observed inhibition was attributed in part to the bactericidal activity of the fatty acids in the lipoproteins and the immunity-enhancing effect of heLDL on the U937 macrophage.
In addition, the acute toxicity of RPT–heLDL was studied in mice by injecting the material into the tail vein at different doses in the range of 1.7 to 6.79 mg RPT per kg of body weight (the corresponding heLDL doses were in the range of 23 to 92 mg/kg). The results showed that 100% of the mice survived at doses of 6.79 mg/kg RPT–92 mg/kg heLDL (data not shown). These results have provided support for further studies on animal models using RPT–heLDL for intracellular S. aureus treatment.
Conclusions
RPT–heLDL can be taken up by U937 macrophage, increase the cell viability and induce a significant reduction of intramacrophage bacterial count. The current study has demonstrated the potential feasibility of heLDL as a macrophage-targeted drug delivery carrier.
Declaration of interest
None of the authors has any real or perceived conflict of interest. This project was supported by the Major Program of the Inner Mongolian Natural Science Foundation (Grant No. 2010ZD07).
References
- Aboutaleb E, Noori M, Gandomi N, et al. (2012). Improved antimycobacterial activity of rifampin using solid lipid nanoparticles. International Nano Letters 2:1–8
- Barcia-Macay M, Seral C, Mingeot-Leclercq M-P, et al. (2006). Pharmacodynamic evaluation of the intracellular activities of antibiotics against Staphylococcus aureus in a model of THP-1 macrophages. Antimicrob Agents Chemother 50:841–51
- Brady D, Gaines S, Fenelon L, et al. (2002). A lipoprotein-derived antimicrobial factor from hen-egg yolk is active against Streptococcus species. J Food Sci 67:3096–103
- Carr RA, Foster RT, Bhanji NH. (1991). Stereospecific high-performance liquid chromatographic assay of sotalol in plasma. Pharm Res 8:1195–8
- Corbin IR, Zheng G. (2007). Mimicking nature’s nanocarrier: synthetic low-density lipoprotein-like nanoparticles for cancer-drug delivery. Nanomedicine (London, England) 2:375–80
- Couvreur P, Fattal E, Andremont A. (1991). Liposomes and nanoparticles in the treatment of intracellular bacterial infections. Pharm Res 8:1079–86
- Craven N, Anderson JC. (1984). Phagocytosis of Staphylococcus aureus by bovine mammary gland macrophages and intracellular protection from antibiotic action in vitro and in vivo. J Dairy Res 51:513–23
- Forrest GN, Tamura K. (2010). Rifampin combination therapy for nonmycobacterial infections. Clin Microbiol Rev 23:14–34
- Foster TJ. (2009). Colonization and infection of the human host by staphylococci: adhesion, survival and immune evasion. Vet Dermatol 20:456–70
- Frostegard J, Hamsten A, Gidlund M, Nilsson J. (1990). Low density lipoprotein-induced growth of U937 cells: a novel method to determine the receptor binding of low density lipoprotein. J Lipid Res 31:37–44
- Hamidi M, Foroozesh M, Zarrin A. (2006). Lipoproteins: from physiological roles to drug delivery potentials. Crit Rev Ther Drug Carrier Syst 23:497–523
- Han R, Caswell CC, Lukomska E, et al. (2006). Binding of the low-density lipoprotein by streptococcal collagen-like protein Scl1 of Streptococcus pyogenes. Mol Microbiol 61:351–67
- Imbuluzqueta E, Gamazo C, Ariza J, Blanco-Prieto MJ. (2010). Drug delivery systems for potential treatment of intracellular bacterial infections. Front Biosci 15:397–417
- Jolivet P, Boulard Cl, Chardot T, Anton M. (2008). New insights into the structure of apolipoprotein B from low-density lipoproteins and identification of a novel YGP-like protein in hen egg yolk. J Agr Food Chem 56:5871–9
- Lessig J, Neu B, Glander HJ, et al. (2011). Phagocytotic competence of differentiated U937 cells for colloidal drug delivery systems in immune cells. Inflammation 34:99–110
- Mariappan TT, Jindal KC, Singh S. (2004). Overestimation of rifampicin during colorimetric analysis of anti-tuberculosis products containing isoniazid due to formation of isonicotinyl hydrazone. J Pharm Biomed Anal 36:905–8
- Mine Y, Nolan JK. (2004). Biologically active hen egg components in human health and disease. J Poult Sci 41:1–29
- Moore KJ, Freeman MW. (2006). Scavenger receptors in atherosclerosis: beyond lipid uptake. Arterioscler Thromb Vasc Biol 26:1702–11
- Moran L. (Ovagen International Limited., County Mayo). Methods of producing avian eggs and birds of specified germ-free status. US Patent 6718909 B2, July 2, 2009
- Moussa M, Martinet V, Trimeche A, et al. (2002). Low density lipoproteins extracted from hen egg yolk by an easy method: cryoprotective effect on frozen–thawed bull semen. Theriogenology 57:1695–706
- Murphy JE, Tedbury PR, Homer-Vanniasinkam S, et al. (2005). Biochemistry and cell biology of mammalian scavenger receptors. Atherosclerosis 182:1–15
- Scholz H, Aukrust P, Damas JK, et al. (2004). 8-Isoprostane increases scavenger receptor A and matrix metalloproteinase activity in THP-1 macrophages, resulting in long-lived foam cells. Eur J Clin Invest 34:451–8
- Sparks DL, Phillips MC. (1992). Quantitative measurement of lipoprotein surface charge by agarose gel electrophoresis. J Lipid Res 33:123–30
- Sun B, Boyanovsky BB, Connelly MA, et al. (2007). Distinct mechanisms for OxLDL uptake and cellular trafficking by class B scavenger receptors CD36 and SR-BI. J Lipid Res 48:2560–70
- Tian Yan TG, Jiang Ni, Tian Ye, et al. (2007). Study of rabbit plasma concentration of rifampicin suppositories by UV–visible assay. J Dalian Med Univ 29:33–5
- Xu S, Huang Y, Xie Y, et al. (2010). Evaluation of foam cell formation in cultured macrophages: an improved method with oil red O staining and DiI-oxLDL uptake. Cytotechnology 62:473–81