Abstract
Context: An oral dosage form containing floating bioadhesive gastroretentive microspheres forms a stomach-specific drug delivery system for the treatment of Helicobacter pylori.
Objectives: To prepare and evaluate controlled release floating bioadhesive gastroretentive chitosan-coated amoxicillin trihydrate-loaded Caesalpinia pulcherrima galactomannan (CPG)-alginate beads (CCA-CPG-A), for H. pylori eradication.
Materials and methods: CCA-CPG-A beads were prepared by ionotropic gelation, using 23 factorial design with quantity of drug, combination of CPG with sodium alginate and concentration of calcium chloride as variables. Beads facilitated mucoadhesion to gastric mucosa with floating nature caused by chitosan coating for wide distribution throughout GIT. Developed beads were evaluated for characteristics like beads size-morphology, entrapment efficiency, DSC, XRD, FTIR, swelling ratio, in vitro mucoadhesion, in vitro drug release, in vitro floating and in vitro H. pylori growth inhibition studies. CCA-CPG-A beads were studied in Wistar rats for in vivo gastric mucoadhesion, in vivo H. pylori growth inhibition studies using PCR amplification of isolated DNA, rapid urease test.
Result: Developed beads possess drug release of 79–92%, entrapment efficiency of 65–89%, mucoadhesion of 61–89%. In vivo mucoadhesion study showed more than 85% mucoadhesion of beads even after 7th hour. In vitro–in vivo growth inhibition study showed complete eradication of H. pylori.
Discussion: CPG-alginate and chitosan in beads interacts with gastric mucosubstrate surface for prolonged gastric residence with floating bioadhesion mechanism for H. pylori eradication in rats.
Conclusion: Floating bioadhesive CCA-CPG-A beads offer a promising drug delivery system for H. pylori eradication at lower dose, reduced adverse effect and enhance bioavailability.
Introduction
Marshall and Warren noticed Helicobacter pylori (H. pylori) in 1982, which is now known as a worldwide distributed major gastric pathogen. From last few decades in most of the countries, risk of peptic ulcer and gastric cancer has been increased enormously due to H. pylori infection (Rajinikanth et al., Citation2008). As per International Agency for Research on Cancer and World Health Organization, H. pylori infection (as a group 1 carcinogen) is carcinogenic to humans (Badhan et al., Citation2009). Helicobacter pylori are microaerophilic rod-shaped, spiral, gram-negative bacterium with multiple flagella, a prevalent human specific causative pathogen in peptic ulcers, chronic active gastritis, gastric and duodenal ulcers, and gastric adenocarcinoma (Wu et al., Citation2000; Patel & Patel, Citation2007). However, the mechanisms by which H. pylori may origin gastric diseases and carcinogenesis are still hypothetical (Patel & Patel, Citation2007). Several virulence factors supports for the colonization and survival of Helicobacter species in the aggressive environment of the stomach such as different enzymes including urease, catalase, and superoxide dismutase, the flagella, and a number of adhesive proteins. Helicobacter pylori exist mainly on the luminal surface of the gastric mucosa under gelatinous layer of mucosa, is very motile. High levels of urease produced by H. pylori protects the bacterium from the acidic condition of stomach by altering surrounding pH to slightly alkaline, assist to survive at low pH, is important in bacterial colonization and essential for the motility, and adhesion (Labigne & de Rease, Citation1996; Umamaheswari et al., Citation2002; Adebisi et al., Citation2011).
The existing therapy for the eradication of H. pylori includes treatment of proton pump inhibitors with antibiotics and has some inadequacy such as low concentration of the antibiotic reaching the bacteria beneath the mucosa, short residence time of the antibiotic in the stomach, deprived patient compliance to take many different drug products and enhanced bacterial resistance due to higher multiple dosage of antibiotics. Helicobacter pylori eradication treatment is not all the time successful and exhibit some injurious with side effects, expenditure of therapy are the major limitations of the therapy. Therefore, distributing drug at the site of infection for an extended period of time, healing a gastric or peptic ulcer, recovering the stability of antibiotics in gastric environment, piercing through gastric mucus layer to act effectively and prevention of repetition are the few approaches to improve the efficacy of antibiotic therapy (Rajinikanth et al., Citation2008; Narkar et al., Citation2010; Gattani et al., Citation2010). Thus, Gastroretentive Drug Delivery System (GRDDS) can be developed to perk up the effectiveness of antibiotic therapy for prolonged gastric residence and probable longer drug release in the ecological niche of the bacterium. GRDDS based on site-specific floating and bioadhesive system is designed to control and improve the shortcomings of conventional drug delivery vehicles, and distribute antimicrobials to the infected cell lines. The floating microspheres may provide extended and more consistent release of drug with wide distribution throughout GIT whereas mucoadhesive DDS may hold on to the mucus surface and extend residence time which results in an effective localized drug concentration. Thus synergistic effect between mucoadhesive and floating system, i.e. mucoadhesive-floating systems eradicate H. pylori provoked infected sites more effectively and may provide to optimize antibiotic monotherapy of H. pylori based infections (Chitnis et al., Citation1991; Umamaheswari et al., Citation2002; Adebisi et al., Citation2011; Singh et al., Citation2012). Controlled-release gastroretentive drug delivery has been considered an effective way for attaining a smooth plasma drug profile, while localizing the formulation in the region of maximal absorption (Singh et al., Citation2012).
Natural polymer like Caesalpinia pulcherrima galactomannan (CPG) are isolated from endosperm of C. pulcherrima seeds, particularly Leguminosae having (1→4)-β-d-mannopyranosyl residues decorated by (1→6) linked α-d-galactopyranosyl residues with M/G ratio of 2.80 (Thombre & Gide, Citation2013).
Amoxicillin is a orally absorbed semisynthetic α-amino-hydroxybenzylpenicillin broad spectrum antibiotic which is widely applied in a standard treatment of gastric and duodenal ulcers associated with H. pylori infection along with a second antibiotics and proton pump inhibitors, known as triple therapies proved to be effective in clinical application. But still these triple therapies cannot give complete eradication of H. pylori due to short gastric residence time of the dosage forms, ineffective gastric mucosal antimicrobial concentration where H. pylori survive, degradation of Amoxicillin at gastric pH. Amoxicillin is the model drug for the treatment against H. pylori. As it is acid-soluble in nature, it is very difficult to control its drug release for an extended period in the gastric environment of stomach (Liu et al., 2004; Babu et al., Citation2010; Arora et al., Citation2011).
In the present study, beads were prepared using sodium alginate–CPG and chitosan by ionotropic gelation method. Therefore, it would be beneficial to develop a floating mucoadhesive system of Amoxicillin Trihydrate using sodium alginate–CPG polymer blend with chitosan coating for oral use, which might facilitate an intimate contact with the absorbing surfaces of mucous membranes (mucoadhesion) and the controlled release of drug from drug-loaded sodium alginate–CPG beads with wide distribution throughout GIT by means of floating nature was maintained due to chitosan coating. Thus, the gastric residence with prolonged drug release of drug at the targeted site in a controlled rate could be achieved to maximize the therapeutic effect. The chitosan-coated amoxicillin trihydrate-loaded CPG-alginate beads (CCA-CPG-A) were characterized by in vitro and in vivo tests and factorial design was used to optimize the variables.
Material and methods
Amoxicillin trihydrate was obtained as a gift sample from Cachet Pharmaceutical Pvt. Ltd., (Bhiwadi, Rajasthan, India). The seeds of C. pulcherrima plant were obtained from Nasik, Maharashtra, India. All other chemicals were of analytical grade and used as received.
Isolation and evaluation of C. pulcherrima galactomannan
In our previous part of our study, the C. pulcherrima galactomannan was isolated and evaluated for various in vitro and in vivo parameters like moisture content, protein content, elemental analyses, acute toxicity study. The polymer was also evaluated for biocompatibility study in our previous study. (Thombre & Gide, Citation2013; Suryawanshi et al., Citation2014).
Preparation of microspheres
The CPG–alginate beads containing amoxicillin trihydrate were prepared using calcium chloride (CaCl2) as a cross-linking agent by ionotropic gelation method and coated with chitosan. Briefly, sodium alginate and CPG aqueous dispersions were prepared separately using double-distilled water. These dispersions were well mixed with stirring for 10 min at 1000 rpm using a magnetic stirrer (Remi Motors, Mumbai, India). Different concentrations of the drug (700–900 mg) and calcium carbonate (0.5–2.0%) were dissolved/dispersed uniformly in 50 mL of CPG–alginate solution below 40 °C under continuous stirring. The final mixtures were homogenized for 15 min at 1000 rpm using a homogenizer to obtain uniform dispersion. The homogeneous bubble-free solution containing different amounts of drug and calcium carbonate was extruded dropwise through 18 G syringe needle into the 100 mL of calcium chloride (5–7 %) with chitosan dissolved in it at various concentrations (0.3–0.6%) with constant stirring (100 rpm) and curing time 5 min to provide sufficient mechanical strength. After 15 min, beads were collected by decantation, washed repeatedly using double distilled water and dried at 25–30 °C for 24 h. The dried chitosan-coated amoxicillin CPG-alginate beads (CA-CPG-A) were stored in a desiccator until used. All formulations were prepared in triplicate. A total of eight formulations were prepared and the assigned formulation codes are given in (Rajinikanth & Mishra, Citation2007).
Table 1. Experimental variables of factorial design with their coded levels and actual values of CCA-CPG-A beads, results of % drug release, % drug entrapment efficiency, % mucoadhesion, swelling ratio and in vitro floating ability.
Factorial design experiments
A 23 randomized reduced factorial design was used in the present study where three factors each at two levels were evaluated to find the interaction between the selected variables. The variables selected were quantity of drug (X1), concentration of polymer concentration CPG–alginate (X2) and concentration of calcium chloride (X3) at two different levels. The matrix of the design including investigated responses, i.e. % drug release, DEE (%), and mucoadhesion (%) are shown in . Design-Expert® Version 7.0.0 software (Stat-Ease Inc., Minneapolis, USA) was used for the generation and evaluation of the statistical experimental design. The effects of independent variables upon investigated responses were modeled using the following quadratic mathematical model:
(1)
where Y is the dependent variable, β0 is the arithmetic mean response of the eight runs, β1, β2 and β3 are the estimated coefficients for the independent factors X1, X2 and X3, respectively. β12, β13, β23 and β123 are the estimated coefficients for the interaction X1X2, X1X3, X2X3 and X1X2X3, respectively. The main effect terms (X1, X2 and X3) represent the average result of changing one factor at a time from its low to high value. The interaction terms (X1X2X3) show how the response changes when three factors are simultaneously changed. The effects of the variables are interpreted considering the magnitude of coefficient and the mathematical sign it carries (Bolton & Bon, Citation2010). The optimization technique requires minimum experimentation and time, thus proving to be far more effective and cost-effective than the conventional methods of formulating dosage forms (Singh et al., Citation2011).
The adequacy of fitted model was checked by the analysis of variance (ANOVA). The main and interaction effects were represented as response surface curves too. Each sample was tested in triplicate for all the experiments. The results of all experiments were expressed as mean ± SD. In all tests, values of p < 0.05 were regarded as significant.
Yield and entrapment efficiency of beads
The percentage yield of different formulations was recorded by weighing the dried beads. For determination of entrapment efficiency, 100 mg of accurately weighed CCA-CPG-A beads were crushed in a glass mortar and then the powdered microspheres were suspended in 10 mL of 0.1 N HCl (pH 1.2). After 24 hours, the solution was filtered and the filtrate was analyzed for drug content at the maximum wavelength of 272 nm using UV-Visible double-beam spectrophotometer (JASCO V-630, Ahmedabad, India). Each sample determination was made in triplicate.
Morphology and particle size analysis
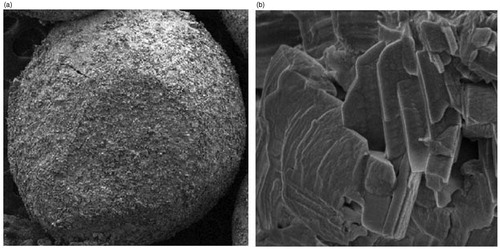
Particle size of the prepared CA-CPG-A beads was determined using an optical microscope (Model BH-2, Olympus, Tokyo, Japan) fitted with a stage and an ocular micrometer. Fifteen to twenty dried beads were measured for calculating the mean diameter of beads. The particle size, shape and morphological characteristics of the CCA-CPG-A beads were assessed with the help of scanning electron microscopy (SEM, JEOL, JSM-7600F A Electron Microscope, Peabody, MA, USA), resolution of 1.0 nm (15 kv) with magnification of × 25 to 1 000 000. Images of the samples were recorded by coating the samples with a thin film of Ag–Pd alloy by vapor deposition technique to render them electrically conductive.
Thermal analysis
Differential scanning calorimetry (DSC) of amoxicillin trihydrate, CPG, sodium alginate and CCA-CPG-A beads was performed. DSC studies were performed for the structural, crystal and physical state characterization of drug. The DSC measurements were performed on a Shimadzu DSC (DSC 60, Tokyo, Japan), with thermal analyzer. Accurately weighed samples (about 3 mg) were placed in an aluminum pan and then hermetically sealed with an aluminum lid. The system was purged with nitrogen gas at a flow rate of 50 ml/min, and heating was performed from 50 °C to 400 °C at a rate of 10 °C/min.
Powder X-ray diffraction
The crystallinities of amoxicillin trihydrate, CPG, sodium alginate and CCA-CPG-A beads were evaluated and recorded using X-ray diffractometer at room temperature with a step size of 0.02 on Model ‘X’Pert ‘PRO’, PANalytica (Almelo, Netherlands) at 40 kV, 30 mA. XRD patterns were recorded in the 2θ range of 10–70°C using CuKα radiation of λ = 0.1542 nm.
Fourier transform infrared analysis
Fourier transform infrared analysis (FTIR) measurements of amoxicillin trihydrate, CPG, sodium alginate and CCA-CPG-A beads of different batches were obtained on JASCO V5300 FTIR (Tokyo, Japan). The pellets were prepared on KBr-press (Spectra Lab, Pune, India) under hydraulic pressure of 150 kg/cm2. The spectra were scanned over the wave number range of 3600 to 400 cm−1 at ambient temperature.
Swelling study
Chitosan-coated amoxicillin-loaded CPG-alginate beads were studied for swelling characteristics. One hundred milligrams of beads were placed in the wire basket of the USP-26 dissolution test apparatus II (Electrolab TDT-06P, Mumbai, India). The basket containing beads were put in a beaker containing 100 ml of 0.1 N HCl (pH 1.2), maintained at 37 °C. The beads were periodically removed at predetermined intervals and weighed (Ibrahim et al., 2002). Then the swelling ratio was calculated as per the following formula:
(2)
In vitro drug release studies
The drug release study was carried out using USP XXIV basket apparatus (Electrolab, TDT-06P, India) at 37 °C ± 0.5 °C and at 100 rpm using 900 mL of 0.1 N HCl (pH 1.2) as a dissolution medium (n = 3) as per USP XXVI dissolution test prescribed for amoxicillin tablets. CA-CPG-A beads equivalent to 100 mg of amoxicillin were used for the test. Five milliliters of sample solution was withdrawn at predetermined time intervals, filtered through a 0.45 μm membrane filter, diluted suitably, and analyzed spectrophotometrically. An equal amount of fresh dissolution medium was replaced immediately after withdrawal of the test sample. The absorbance of the resulting solution was then measured with a UV spectrophotometer at 272n nm (Vasir et al., Citation2003).
In vitro mucoadhesion
The in vitro mucoadhesion of beads was carried out by modifying the method described by Ranga Rao and others (Rao & Buri, Citation1989; Patil & Murthy, Citation2006; Patil & Sawant, 2008) using goat gastric mucosa. A freshly cut piece of goat gastric mucosa (2 cm2) was obtained and then cleaned by washing with physiological isotonic saline solution. The microspheres were placed on goat gastric mucosa after fixing to the polyethylene support. The mucosa was then placed in the desiccator to maintain at more than 80% RH at room temperature for 30 min to allow the polymer to hydrate and to prevent drying of the mucus. The mucosa was then observed under microscope, and the number of beads attached to the particular area was counted. After 30 min, the polyethylene support was introduced into a plastic tube cut in circular manner and held in an inclined position at an angle of 45°. Mucosa was washed thoroughly for 5 min with 0.1 N HCL solution, pH 1.2. Tissue was again observed under microscope to see the number of beads remaining in the same field area. The adhesion number was determined by the following equation:
(3)
where Na is the adhesion number, N0 is the total number of particles in a particular area, and N is the number of particles attached to the mucosa after washing (Liu et al., Citation2005; Jain et al., 2012).
In vitro floating properties of CCA-CPG-A beads
The in vitro floating study was performed using a USP 24 dissolution apparatus II having 500 mL of simulated gastric fluid (SGF, pH 1.2). The medium temperature was kept at 37 ± 0.5 °C. The floating beads (1.0 g beads) were soaked in the dissolution medium and the medium was agitated with a paddle at 50 rpm. After agitation, the beads that floated on the surface of the medium and those that settled down at the bottom of the flask were recovered separately. The floating percentage was estimated (EI-Gibaly, 2002; Rajinikanth et al., Citation2007).
Ex vivo evaluation of gastric mucoadhesiveness of beads
The optimized batch of CCA-CPG-A beads was selected for the in vivo mucoadhesivity study. Three groups (six Wistar rats in each group) of albino rats (200–300 g) of either sex were fasted for 24 h before the experiments, however they were allowed free access to water. Ten beads were orally administered with 2 ml of water to conscious rats. The rats were kept fasted and killed after 2, 4, and 7 h. The beads that remained adhered to the gastrointestinal tract were counted. The gastric mucoadhesion was expressed as the percentage of beads remaining in the stomach after perfusion (Umamaheshwari et al., Citation2004; Nerkar et al., Citation2010). In vivo gastric mucoadhesiveness of beads on rat was carried out in compliance with the protocol of Institutional animal ethical committee as per animal ethical committee of Bhujbal Knowledge City, MET’s institute of pharmacy, Adgaon, Nasik, Maharashtra, India (Registration number: 1344/ac/10/2011-2012/CPCSEA under CPCSEA, India).
Stability studies
The optimized CCA-CPG-A beads batch was subjected for the stability studies as per ICH (1996) specifications, at two different storage conditions, i.e. RT, 25 ± 2 °C/60 ± 5 % RH, and 40 ± 2 °C/75 ± 5 % RH for a period of three months. The samples were packaged in high-density polyethylene (HDPE) bottles with tamper-evident closures and were placed in stability chambers (CHM 6S, Programmable environmental test chamber, M/s Remi, Mumbai, India). The samples were withdrawn at periodic intervals and analyzed for parameters like dissolution performance, entrapment efficiency, buoyancy and bioadhesive characteristics (Singh et al., Citation2012).
In vitro H. pylori growth inhibition studies
The bacterial strain used in the study was isolated from a hospitalized human patient (age 47 years) with gastric ulcer in Wockhardt Hospital, Nasik, India. In vitro growth inhibition studies were performed for chitosan-coated amoxicillin trihydrate-loaded CPG-Alginate beads using a broth culture of H. pylori. The H. pylori broth culture was incubated in a brain–heart infusion containing 0.25% yeast extract and 10% fetal calf serum and supplemented with 0.4% Campylobacter-selective supplement (Skirrow supplement). The H. pylori strain was grown in Brucella broth at 37 °C for 7 days in a microaerophilic atmosphere (5% O2, 10% CO2, and 85% N2). Growth of the bacteria was monitored by measuring the optical density of broth cultures spectrophotometrically at 600 nm. The number of bacteria was determined by optical density with one optimal density unit corresponding to110 colony-forming units (CFU) mL−1. The colonies were identified as H. pylori by morphology and urease activity (Nagahara et al., Citation1998). To study the effect of formulations on H. pylori growth inhibition, 10 mL of nutrient broth was inoculated with a loopful of the H. pylori from stock culture to make a final culture of 100 CFU mL−1 (Rajinikanth et al., Citation2007; Nerkar et al., Citation2010). Amoxicillin suspension and optimized CCA-CPG-A beads were incubated at 37 °C in a microaerophilic atmosphere. Amoxicillin was used at a concentration of 8 μg/mL (this concentration is approximately fourfold of the reported MIC50 for H. pylori urease) (Mergaud et al., Citation1991). The culture containing tubes were shaken at 100 rpm at 37 °C in a microaerobic atmosphere in an incubator. Hundred microliters of nutrient broth sample were removed at various time intervals and serial dilutions were plated on modified Skirrow’s medium. The agar plates were incubated for 4 days at 37 °C under microaerobic conditions. The viable cell counts for each sample were calculated by counting the number of colonies on the agar plates (Rajinikanth et al., Citation2007; Patel & Patel, Citation2007; Nerkar et al., Citation2010).
In vivo H. pylori growth inhibition studies
The bacterial strain used in vitro study was applied in this study.
Animals
Male Wistar rats (230–250 grams) were procured from Haffkine Biopharma Ltd, Mumbai, India. The animals were housed in groups of 4 in solid bottom polypropylene cages. They were maintained at 24 °C ± 1 °C, with relative humidity of 45–55% and 12:12 h dark/light cycle. The animals were acclimatized for a period of two weeks and were kept under pathogen-free conditions. The animals had free access to standard pellet chow (Amrut Feeds, Pranav Agro Industry Ltd, Sangli, India) throughout the experimental protocol, with the exception of overnight fasting before induction of the ulcer. The animals were provided with filtered water. The pharmacological protocol on male Wistar rats was carried out in compliance with the protocol of Institutional animal ethical committee as per animal ethical committee of Bhujbal Knowledge City, MET’s institute of pharmacy, Adgaon, Nasik, Maharashtra, India (Registration number: 1344/ac/10/2011-2012/CPCSEA under CPCSEA, India).
The animals were divided into 5 treatment groups having 18 animals in each group. Drugs CCA-CPG-A beads suspended in 0.5% w/w Sodium C.M.C. was administered at a dose of 120 mg/kg/day p.o. A triple drug regimen of (clarithromycin 25 mg/kg + amoxycillin 50 mg/kg + omeprazole 20 mg/kg) (CAO) suspended in 0.5% w/w sodium C.M.C. was administered p.o. The control group of animals was administered 1 ml of 0.5% w/w sodium C.M.C. At the end of 4th, 7th and 10th week, six animals were sacrificed to determine the infection status (Ghosh et al., Citation2009).
Induction of ulcers and H. pylori infection
Ulcers were induced using a method modified from that described by Kim et al. (Citation2005). Briefly, Naproxen sodium was administered (30 mg/kg.p.o) for three consecutive days. Once the ulcers were induced, the animals were infected with H. pylori as suspension in brucella broth media. The number of bacteria/ml was adjusted to 108 cfu/ml using McFarland’s turbidity standards (Ibrahim et al., 2006). One milliliter of H. pylori suspension was administered for 3 consecutive days to the ulcerated animals to induce infection. The treatment with drugs was initiated 1 week after H. pylori infection. The rats were housed in meshed cages to prevent coprophagy (Kim et al., Citation2005; Ghosh et al., Citation2012).
Log CFU/gram of gastric tissue
Preparation of culture plates
The culture plates were prepared with 7% FCS or Fresh sheep blood. Antibiotic solutions were separately prepared. Amphoterecin 20 mg/ml, vancomycin 6 mg/ml, and polymyxin B 50 units/ml were prepared. To prepare one liter of media 1000 μL of Vancomycin, 150 μL of amphoterecin and 50 μL of Polymyxin B was added. For growing H. pylori, 25 mL of antibiotic incorporated media was poured on to presterilized culture plates under sterile conditions in a laminar air flow. The media was sufficiently cooled (Koga et al., Citation1996).
Sacrifice of the rats and preparation of the homogenate
After the final administration, the rats were fasted for 1 day, killed, and samples were obtained by incising the mucosa of the pylorus and antrum region of the stomach. The sample of 200 mg from the pyloric antrum portion of rat was minced using a sterile surgical blade and homogenized under aseptic conditions in presterilized plastic mortar and pestle. Each stomach was processed in a separate set of mortar and pestle (Koga et al., Citation1996).
Inoculation of the homogenate on the culture plates
Each sample (200 mg) was mixed with 2 mL of sterile fetal bovine serum broth in presterilized plastic mortar and pestle. One milliliter of homogenate was added to 9 ml of brucella broth in a test tube (dilution 1:10), and subsequently serially diluted using sterile brucella broth till dilution of 1:108 was achieved. 100 µl of these dilutions were inoculated onto brucella agar plates incorporated with antibiotics (previously mentioned). These plates were incubated under microaerophilic conditions (85% N2, 10% CO2, 5% O2) at 37 °C for five days. Growing H. pylori colonies were identified by morphology (small grey translucent water droplet like colonies) and confirmed by positive rapid urease reaction. Rats were considered not infected when no H. pylori colony on the plate on which the stomach was cultured was detected. Numbers of colony forming units (CFU) were expressed as log CFU per gram of gastric tissue (Koga et al., Citation1996; Nerkar et al., Citation2010).
Determination of H. pylori infection status
A small portion of the pylorus of the isolated stomach was used for rapid urease test, determination of log CFU/gram of gastric tissue and extraction of DNA was done by amplification of 16 sr RNA gene by polymerase chain reaction. The rest of the stomach was used to determine the histopathological changes (Koga et al., Citation1996; Ghosh et al., Citation2009).
Rapid urease test
The rapid urease test solution was prepared by dissolving 10 g of urea in 100 ml millipore water followed by autoclaving. 0.002 g of phenol red was added into the solution as indicator. The pH of the solution was adjusted to 6. The isolated pyloric tissue was immediately immersed in RUT solution. The change of color from yellow to red within 10 minutes indicated the presence of H. pylori (Vaira et al., Citation2007).
DNA Isolation
DNA isolation from gastric tissue was performed according to standard cetyl tri methyl ammonium bromide method mentioned by Tiwari et al. (Citation2005). Briefly, the tissue samples were suspended in 250μL of digestion buffer II. {0.1 M NaCl, 0.01 M Tris-HCl (pH 8.0), 0.25 M EDTA (pH 8.0), 1% SDS} containing 100μg/ml of proteinase k (Vivantis, India). To this, 250μl of digestion buffer I {0.1 M NaCl, 0.01 M Tris-HCl (pH 8.0), 0.25 M EDTA (pH 8.0)} was added and incubated at 56 °C overnight. DNA was extracted with an equal volume of phenol chloroform and precipitated with 0.6 volume iso-propanol. The DNA pellets were washed thrice with 80%, 75% and 70% ethanol, respectively, and finally resuspended in 50–100μl of sterile water for injection. All the steps were performed in aseptic conditions to minimize contamination. PCR amplification of 16 S rRNA gene: PCR amplification was performed according to protocol mentioned by Tiwari et al. (Citation2005). Briefly, 2μL of the template DNA isolated from gastric tissue was added to 18μL of the reaction mixture containing 1X PCR buffer {50 mM KCl, 10 mM Tris- HCl (pH 8.3), 1.5% (vol/vol) Triton X-100}, 1.5 mM MgCl2, 200uM concentrations of each dNTPs, 10pMol of each primer, & 1U of Taq polymerase. The following thermal cycle steps were used in the PCR amplification: initial denaturation at 96°C for 5 minutes, 40 cycles with 1 cycle consisting of 94°C for 1 minute, 56°C for 1 second, 72 °C for 2 minutes. The final cycle comprised of a 6-minute extension step to ensure full extension of the PCR products. PCR amplification was performed in a thermal cycler (Eppendorf). DNA of the ATCC 26695 type strain was used as positive control in each batch of PCR assays while negative control consisted of all the reagents of the master mix excluding the template DNA. The primers were 16 s r RNA F and 16 s r RNA R (Tiwari et al., Citation2005). The PCR-amplified products were analyzed by agarose gel electrophoresis. 10 μL of each amplified product was added to 3μL of loading buffer (20 mL of glycerol 50%, 25 mg of bromophenol blue, 3 drops of 1 N NaOH) and subjected to electrophoresis in a 2% agarose gel. The gel was examined in gel documentation instrument (Bio Rad, Hercules, USA) and image was captured (Tiwari et al., Citation2005).
Histopathological studies
The pylorus portion of the stomach was fixed in formalin and 4 µM sections were stained with eosin haematoxylin stain to determine the presence or absence of H. pylori induced congestion, inflammation. The images were captured at 40 × magnification.
Infection status
The infection was determined at the end of 4th, 7th and 10th week in the animals using all the three above mentioned techniques. The infection of H. pylori was ameliorated up to varying extents in the different treatment groups of animals. CAO-treated animals demonstrated absence of infection at 4th, 7th and 10th week whereas the control group of animals showed the presence of infection throughout the treatment regimen.
Result and discussion
CPG was isolated from raw seeds of C. pulcherrima and the average yield of dried CPG was found 29 ± 3% of the total weight of the seeds. The CCA-CPG-A beads were prepared by ionotropic gelation method using CaCl2 as cross-linking agent. When dispersion mixture of sodium alginate, CPG, and amoxicillin trihydrate was dropped into the solutions containing Ca2+ ions, ionotropically gelled CPG–alginate beads containing amoxicillin were formed instantaneously due to an electrostatic ionic interaction between negatively charged-COO− groups of sodium alginate and positively charged Ca2+ ions. Actually, Ca2+ ions are accommodated in the interstices of two polyuronate chains having a close ion-pair interaction with COO− anions of the sodium alginate and sufficient coordination by other electronegative oxygen atoms. Thus, the prepared CPG-alginate beads were coated with chitosan.
Statistical analysis
Statistical analysis covers a set of experiment and makes certain accurate and persuasive interpretation of results of a study which determine the response variables, conducting appropriate statistical tests to select best possible model, fitting mathematical models to the data, and determining the values of independent formulation variables to produce optimum response. The present study applied a factorial design which is one of the popular statistical experimental design to optimize the formulation as well as to discover the interactions, if any, between the factors chosen (Singh et al., Citation2005).
To study the effects of independent variables on its attributes and performance, a 23 full factorial design was applied. The independent variables such as concentration of drug, polymer ratio and concentration of cross-linking agent at two levels were evaluated for their effect on % drug release, % entrapment efficiency and % mucoadhesion. The responses obtained are given in . The values of investigated responses measured for all trial formulations were fitted in the 23 factorial design to get model equations for responses analyzed in this investigation. These models were evaluated statistically by applying one-way ANOVA (p < 0.05). All the responses studied were largely affected by the variables chosen as reflected from the results of regression analysis and ANOVA.
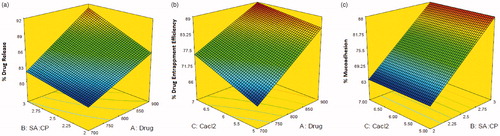
The % drug release of CCA-CPG-A beads () was found in the range of 79.65–92.48%. The result of regression analysis indicated that the drug release from the formulation was significantly affected by the variables of the study (r2 = 0.9596). A comparison of the magnitudes of the regression coefficient brings forth the predominant effect of the polymer ratio, drug concentration. The response surface curve generated out of the results () pointed out the increment of drug release effect with increase in gel viscosity due to polymer concentration, and increasing drug concentration. The high polymeric ratio increases the drug entrapment also.
Figure 1. Response surface curve depicting the effect of factorial variables on (a) %drug release, (b) %drug entrapment efficiency, and (c) % mucoadhesion.
The regression equation for % drug release was:
(4)
indicates a significant influence (r2 = 0.9937) of the variables on the entrapment efficiency, which varied from 65.12 ± 0.24 to 89.07 ± 0.18%. The entrapment efficiency increases with increase in cross linking agent and polymer ratio.
The regression equation for entrapment efficiency was:
(5)
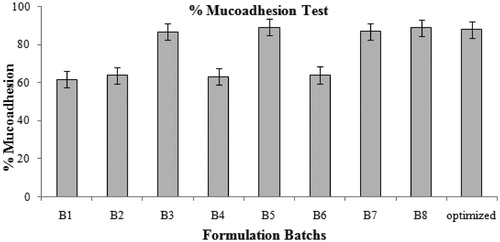
In vitro mucoadhesion results of formulation was observed to be in the range of 61.74 ± 0.21 to 89.12 ± 0.15% (). The mucoadhesivity increasesa with increase in polymer ratio that interact with mucosubstrate on the surface of the stomach and prolonged residence in the stomach
(6)
The optimized chitosan-coated CPG-A beads containing amoxicillin trihydrate (F–O) were formulated using selected process variable settings by numerical analysis according to the 23 factorial design and also evaluated for drug release for 8 h(%), DEE (%), and mucoadhesion (MA %) (). The desirable ranges of the independable variables (factors) were restricted to setting used for the formulation of optimized CCA-CPG-A beads was amoxicillin trihydrate (X1) = 900, CPG–alginate (X2) = 3.0 and concentration of calcium chloride (X3) = 7.0 %.The optimized formulation showed Drug release after 8 h of 90.03 ± 1.02, DEE of 87.66 ± 0.33%, and MA % of 88.16 ± 0.088 % with small percentage error values of −1.2991, −1.3615 and 0.3300 %, respectively. Percentage error evaluation helped to establish the validity of generated model equations and to describe the domain of applicability of optimization model. The determined low percentage error values indicates that mathematical models obtained from the full 23 factorial design were well fitted.
As per the three-dimensional response surface plot, rise in polymer ratio and drug level increases % drug release (). DEE increases with increase of polymer sodium alginate to CPG ratio and CaCl2 concentration (). The viscosity of the polymeric solution increases with rise in polymer ration which results into cohesive interactions among polymer and reduce diffusion and percolation of drug, thus enhances extent of drug entrapment due to formation of larger beads. The % mucoadhesion increases with increase in polymer concentration while it is independent of calcium chloride concentration.
Yield and entrapment efficiency of beads
The yield of CCA-CPG-A beads obtained using Ionotropic gelation method was found to be in the range of 78–92 % w/w. The entrapment efficiency was in the range of 65.12 ± 0.24 to 89.07 ± 0.18% w/w ().
The three-dimensional response surface plot relating DEE is presented in .
DEE increases with increase of polymer sodium alginate to CPG ratio (). However, the chitosan-coated CPG-alginate beads have outer rough surface with a number of minor wrinkles. This is due to the increasing thickness of chitosan coat formed over the beads which may encapsulate a larger amount of drug. The yield value as well as drug entrapment for chitosan-coated CPG-alginate beads obtained with decreased solubility of drug due to chitosan coating.
Morphology and particle size analysis
The scanning electron micrographs (SEM) of the beads are shown in and (). The SEM results revealed that all the chitosan-coated amoxicillin-loaded CPG-alginate beads were discrete and spherical in shape with rough outer surface and (). However, the CCA-CPG-A beads had outer rough surface with a number of minor wrinkles (). The formation of a thin layer coat of chitosan over the beads caused several wrinkles during the drying due to dehydration of the chitosan membrane surrounding the beads (). The bead diameter varied from 0.68 ± 0.0360 to 1.23 ± 0.08544 mm for different batches. The results indicate that the rise in amount of polymers and calcium carbonate increases the size of beads proportionally. This increases micro-viscosity of the polymeric dispersion with increase in polymer concentration, which finally led to the formation of bigger beads. The mean diameter of prepared beads slightly increased with an increase in drug loading and chitosan concentration. This could be featured to drug solubility in water and formation of a thin chitosan coating over the beads due to its ionic interaction, respectively.
Thermal analysis
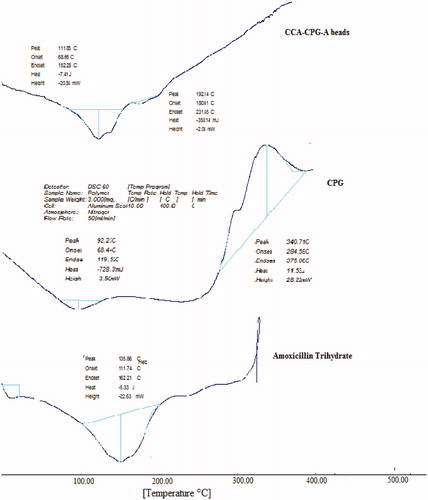
The DSC thermogram showed a sharp endothermic peak at 135.86 °C for pure amoxicillin trihydrate as the melting point of the drug (). The thermal transition for polymers can be seen at 92.27 °C, which is attributed to the melting point of the polymer. In the DSC thermogram of the drug-loaded beads, the endothermic peak was observed at 111.85 °C (). The evaluation of the thermograms clearly revealed no physical interaction between the polymer and the drug in the beads. The analysis of thermograms revealed no physical interaction between the polymer and the drug in the prepared beads.
Powder X-ray diffraction
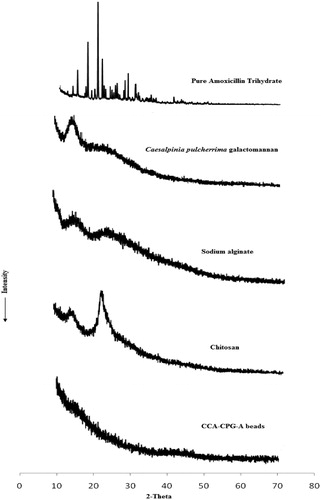
PXRD of chitosan-coated CPG-alginate beads is shown in . The intensity of peaks referred for the crystalline nature of pure drug was decreased significantly in the formulations which can be due to the effect of polymer or formulation process. PXRD of amoxicillin showed characteristic peaks at about 12.2°, 15.18°, 18.08°, 18.12°, 19.38° and 26.74° (2θ). Significant reduction in peak intensities was observed in X-ray diffraction pattern of beads when compared with pure drug; PXRD of CPG and sodium alginate shows peak at 12.5° and 12.2° (2θ). PXRD of chitosan-coated CPG-alginate beads shows the absence of characteristic peaks, indicating reduction in crystallinity of drug in the presence of thick coating of chitosan.
Fourier transform infrared analysis
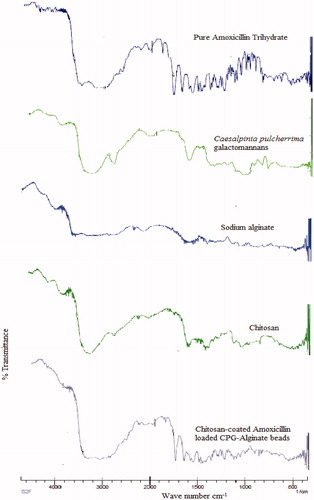
FTIR spectroscopic analysis was performed to confirm the compatibility of amoxicillin with polymers used to prepare chitosan-coated CPG-alginate beads. The FTIR spectra of CPG, sodium alginate, chitosan, optimised CCA-CPG-A beads, and pure amoxicillin were shown in . FTIR spectrum of pure amoxicillin showed characteristic peaks at 1249.87 cm−1 (C-C stretching), 1313.52 cm−1 (C-N stretching), 1573.91 cm−1 (C-H stretching), 1,614 cm−1 (C = O stretching), 1687 cm−1 (–CONH2 stretching), 1776 cm−1 (-COOH stretching), 3325 cm−1 (sec. –NH or –OH) and some prominent bands like 846–567 cm−1 (–CH aromatic ring bending and heteroaromatics). Characteristic peaks of drug were also present in the FTIR spectrum of beads with some broadening and reduction in intensity, indicating the absence of chemical interactions between drug and polymer after production of beads.
Swelling study
For estimating the swelling rato, the beads (∼100) were suspended in 100 mL of simulated gastric fluid (pH 1.2). The particle size was monitored by microscopy technique every 1 h using an optical microscope. The increase in particle size of the microspheres was noted for up to 8 h, and the swelling ratio was calculated. In acidic media, swelling of positively charged matrix takes place due to electrostatic repulsion between like charges and the osmotic effect of bound counterions. Chitosan-coated beads leads to more protonation of amino groups in acidic medium and showed linear increase in swelling of beads due to the combined effect of both the polymers. The swelling ratio for microspheres of batches B1 to B8 is reported in The optimized formulation showed swelling ratio of 11.93 ± 0.251%.
In vitro drug release studies
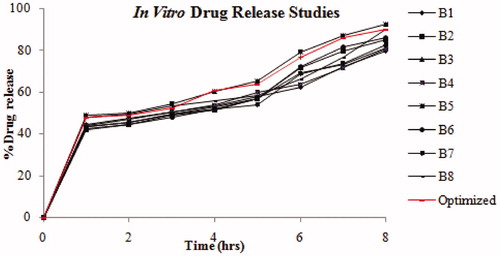
The in vitro drug release profiles of CCA-CPG-A beads with different polymer and drug concentrations are shown in . The rate and extent of amoxicillin from beads characterized with rise in polymeric matrix density and diffusion path length that the drug molecules have to traverse (by formation of bigger sized beads). The drug release from these beads was characterized by an initial phase of high release due to burst effect and good solubility of drug at acidic pH. However, as gelation proceeded (cross-linking of polymer dispersion with Ca2+ ions from calcium carbonate), the remaining drug was released at a slower rate followed by a phase of moderate release. This bi-phasic pattern of release is a characteristic feature of matrix diffusion kinetics. The initial burst effect from the chitosan-coated beads was considerably reduced as chitosan coating over the beads resulted in better incorporation efficiency with formation of a thick coating layer around the beads and increases the distance travelled by the drug molecule through the chitosan coat. This could be the reason for the observed decrease in the burst effect. The internal ionotropic gelation effect of calcium carbonate prolongs the release of drug from bead. In acidic medium, the calcium carbonate dissolves and the ionized Ca2+ ions then promote internal gelation by cross-linking with the polymer and retarding the drug release from polymeric matrix. In addition, the increased crystallinity of drug during chitosan coating may contribute for slow drug release with mucoadhesion to gastric mucosa. When the chitosan-coated amoxicillin-loaded CPG-alginate beads were evaluated for drug release in 0.1 N HCl, pH 1.2, the beads showed more than 50% drug release at the end of 1 h. The best suited batch, Batch 5 of chitosan-coated beads showed controlled drug release of 92.48 ± 0.015 % over a period of 8 h following the Peppas model (r2 = 0.957). The optimized batch of beads confirmed controlled drug release with diffusion mechanism of 90.03 ± 1.02 % over a period of 8 h following the Peppas model (r2 = 0.957) with desirability.
In vitro mucoadhesion
Mucoadhesive properties of amoxicillin containing chitosan-coated CPG-alginate beads increased with increased concentration of polymeric dispersion and drug due to increased CPG alginate–chitosan cross-linking and, in turn, spherical matrix can interact with mucosubstrate on the surface of the stomach, leading to prolonged residence in the stomach () ().
In vitro floating properties
The time the formulation took to emerge on the medium surface (floating lag time) and the percentage of floating beads on the dissolution medium (SGF, pH 1.2) surface were evaluated and are shown in . Beads in acidic media forms gelation and gel barrier at the bead surface occurs due to cross-linking by Ca2+ ions. The calcium carbonate effervesced to release carbon dioxide and calcium ions, which was entrapped in the gel network to generate floating of the formulation. The calcium ion reacts with polymeric solution to form cross-linked three-dimensional gel network which controls further diffusion of carbon dioxide and drug molecules, and resulted in rise of floating time and drug release. The beads demonstrated good floating ability (72–87% of floating) due to presence of calcium carbonate as a gas-forming agent. The floating lag time of beads was found in the range of 4–10 min. The floating lag time of beads reduces with increase in the calcium carbonate concentration that increases the duration of floating (). Thus increase in the Ca2+ concentration subsequently increases evolution of CO2 and reduces the floating lag time and increases duration of floating. The beads containing 2.0% of calcium carbonate showed a good floatability (100%). All the batches of prepared beads were floated for > 8 h in SGF pH 1.2. The beads remained buoyant even after the buoyancy test period.
Table 2. Stability study of CCA-CPG-A beads.
Ex vivo evaluation of gastric mucoadhesiveness of beads
Various types of interaction forces between mucoadhesive materials and the mucus surface decides mucoadhesion such as Van der Waals forces, hydrogen bonding, mechanical interpenetration, electrostatic attraction, and entanglement. These interactions can be evaluated using several methods by in vitro and in vivo methods. The adhesive property of the beads increases with increase in polymer concentration with chitosan coating. The mechanism of gelation involves the formation of double helical junction zones followed by aggregation of double helical segments to form a three dimensional network by complexation with cations and hydrogen bonding with water. Chitosan hydrogel can be formed by covalent cross-linking or ionic cross-linking. Chitosan is a well-known polycation that reacts with negatively charged components, either ions or molecules, leading to the formation of polyelectrolyte complex through ionic bridges between polymer chains. Their mucoadhesive property is mainly based on the ionic interaction with anionic substructures of mucous layer.
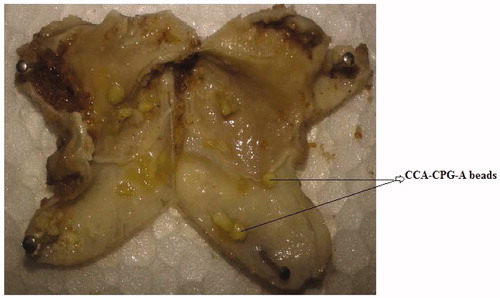
On increasing the polymer-to-drug ratio, the % mucoadhesion also increases because more amount of polymer results protonation of amino groups, which are responsible for binding with sialic acid groups in mucus membrane and thus results in increase in mucoadhesive properties of chitosan-coated beads. The mucoadhesivity of the beads in the gastrointestinal tract were studied on stomach mucosa after 7 h. The percentage of beads remaining in the stomach at 2, 4 and 7 h after administration was found to be more than 85% (F test, p < 0.01). This might indicate that the amoxicillin containing CCA-CPG-A beads had better mucoadhesive effect in the gastrointestinal tract and might stay longer in the stomach for more effective H. pylori clearance ().
Stability studies
The stability study suggested that the optimized formulation were stable at 25 ± 2 °C/60 ± 5 % RH, and 40 ± 2 °C/75 ± 5 % RH for a period of 3 months as there was no significant change in dissolution performance, entrapment efficiency, In vitro floating properties and In vitro mucoadhesion on storage at the respective conditions ().
In vitro H. pylori growth inhibition studies
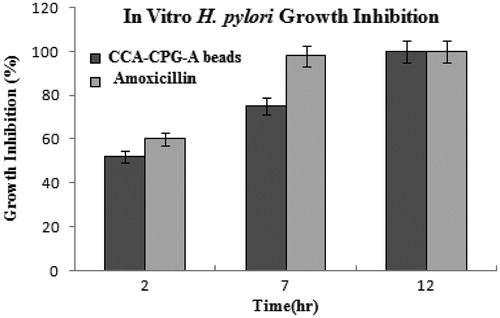
The optimized batch of amoxicillin containing chitosan-coated CPG-alginate beads were evaluated for in vitro H. pylori growth inhibition studies are given in . The antimicrobial effect of amoxicillin and beads were determined as of percentage growth inhibition, which was calculated as the ratio of colony counts of a given mixture against that of tubes containing H. pylori alone. The percentage growth inhibition values reduced progressively with increasing incubation time. The percentage growth inhibition for chitosan-coated CPG-alginate beads was found to be 52 ± 3% and 75 ± 4.15 % at the end of 2 and 7 h, respectively, whereas pure amoxicillin showed relatively more growth inhibition of about 60 ± 1.68 % and 98 ± 0.60 % after 2 and 7 h, respectively. But, the typical gastric residence of pure drug may be up to 2 h only. The optimized batch showed complete growth inhibition after 12 h of incubation.
Nagahara et al. suggested that the topical action of amoxicillin played an important role in the clearance of H. pylori from the results that the amoxicillin mucoadhesive microsphere resided in the stomach for a longer period of time than did amoxicillin suspension and that the microspheres and amoxicillin suspension showed equivalent AUCs. In present study we confirmed that floating mucoadhesive chitosan-coated CPG-alginate beads eradicates H.pylori at low drug concentration than powder did. Clinically, the dose of amoxicillin for eradicating H. pylori in triple therapy was very high, that may cause many adverse effects. Thus floating mucoadhesive chitosan-coated CPG-alginate beads microspheres may provide therapeutic concentration at a much lower dose, which may significantly reduce the adverse effects. Finally, the results suggested that chitosan-coated CPG-alginate beads prolonged drug release and gastric retention and proved better H. pylori growth inhibition than plain drug.
In vivo H. pylori growth inhibition studies
In vivo H. pylori clearance study of prepared CCA-CPG-A beads was carried out with an animal model – male Wistar rats infected with human H. pylori. The polymerase chain reaction and the RUT results identically demonstrated that beads were able to inhibit the infection of the gastric mucosa as at the end of the 10th week.At the end of 4th,7th and 10th week, the infection was studied in the animals using rapid urease test, and histopathologically. The infection of H. pylori was improved up to varying extents in the different treatment groups of animals (). CAO-treated animals demonstrated the absence of infection at 4th, 7th and 10th week whereas the control group of animals showed the presence of infection throughout the treatment regimen. The amoxicillin containing chitosan-coated CPG-alginate beads eradicated the H. pylori infection in a dose and time dependent manner. At a dose of 50 mg/kg/day of beads, 33.33% (2/6) animals were found H. pylori positive after 4 weeks of treatment and 16.66 % (1/6) animals were found H. pylori positive after seventh while 0% (0/6) of the animals were found to be infected at the end of 4th, 7th and 10th week of treatment.
The mean bacterial count in Wistar rat stomach after oral administration of the control groups, CAO and beads under fed condition at dose of 50 mg/kg once daily up to 10th week in H. pylori infected Wistar rat stomach is presented in .
Table 3. Evaluation of H. pylori infection in rats by Rapid Urease Test (RUT) during the treatment with chitosan-coated amoxicillin-loaded CPG-alginate beads regimen of 10 weeks.
The control group with no receipt of drug colonized viable bacteria around 8 log CFU/gram of gastric tissue in stomach. The bacterial load was decreased with rise in administration of the CAO and complete H. pylori clearance was obtained from the 4th week of the treatment. The bead reduces the bacterial load with 3.3 ± 0. 0.2646 log CFU/g of gastric tissue in stomach at 4th week of the treatment, which was found to be 0.7767 ± 0.1102 log CFU/gram of gastric tissue in stomach at 7th week of the treatment and complete H. pylori clearance was obtained at 10th week of the treatment. This indicates that the increased residence time of antibiotics in the form of beads achieves high concentrations of antibiotics in the stomach which is necessary to establish bactericidal activity that resides under the layer of gastric mucus.
The residence time of the beads increases in the stomach due to its floatability and mucoadhesion property which enhances beads retention in the stomach to establish H. pylori eradication at the dose near to the standard dose.
The mean bacterial count after 7th week of treatment with amoxicillin containing chitosan-coated CPG-alginate beads with dose of 50 mg/kg around 16.66 % of H. pylori inhibition was obtained which was almost equal to that of standard at the dose of 30 mg/kg, which is significantly (p < 0.01) lower than that of standard. This result indicates that the stomach specific floating mucoadhesive drug delivery (beads) of amoxicillin eradicates H. pylori effectively at low doses.
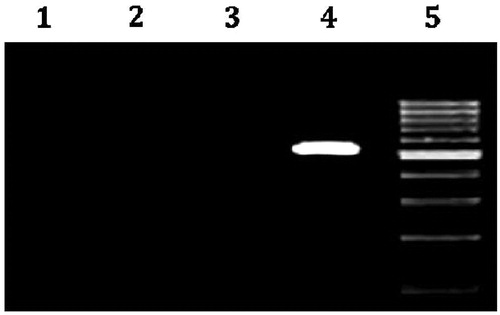
The in vivo H. pylori growth inhibition was further conformed by PCR technique, which detects bacteria more sensitively than microbial culture method. The infection can be determined by the isolation of DNA of H. pylori from the gastric tissue and amplifying the specific genes using DNA as the template and suitable primers along with appropriate thermal cycles. Cetyl tri methyl ammonium bromide (CTAB) method is a reproducible method to segregate bacterial DNA from the gastric tissue. The isolated DNA when subjected to PCR with suitable primers shows the presence of the 16 S rRNA gene which serves as a marker for the presence of H. pylori. 16 S rRNA and hrgA genes serve as markers of H. pylori and hence serve as reproducible diagnostic tool. In the present investigation, 16 s r RNA gene was successfully amplified in animals in which the infection persisted but PCR negative results confirmed the amelioration of the infection among the standard and test drug (beads) treated animals. Agarose gel image showed successful amplification of 534 base pair product of 16 sr RNA gene from the DNA isolated from the pyloric antrum of the H. pylori infected animals. The PCR sample of DNA band was clearly detectable in the original DNA extract at positive control group (DNA from strain ATCC 26695) and was undetected in the original DNA extract at standard group as well as at amoxicillin containing chitosan-coated CPG-alginate beads group (). Thus, amoxicillin containing chitosan-coated CPG-alginate beads might be a promising drug delivery system for the H. pylori clearance due to its floating-bioadhesive property with therapeutic concentration at a much lower dose, which may considerably reduce the adverse effects.
Figure 10. Agarose gel image showing successful polymerase chain reaction (PCR) amplification of 16 S rRNA Gene of H. pylori-infected male Wistar rat antrum. Lane1: Amplicon using SWFI(sterile water for injection) instead of DNA (−ve control). Lane 2: Amplicons using the DNA isolated from the gastric mucosa of infected animals treated with standard Clarithromycin +amoxicillin + Omeprazole(CAO). Lane 3: Amplicons using the DNA isolated from the gastric mucosa of infected animals treated with CCA-CPG-A beads. Lane 4: Amplicons using the DNA isolated from the gastric mucosa of control group of animals (product size 534 base pairs) (+ve control). Lane 5: DNA Ladder 100 base pair.
Conclusion
The current study presented an outline of formulating controlled release drug therapy for the eradication of H. pylori using chitosan-coated amoxicillin-loaded CPG-alginate beads. The floating and bioadhesive properties of the prepared formulations were detected from the results obtained from the IVIV buoyancy and mucoadhesive studies. The beads showed good floating with mucoadhesion for the complete growth inhibition of H. pylori. Thus the stomach specific floating mucoadhesive drug delivery of amoxicillin can be a promising drug delivery system for the treatment of H. pylori infection in peptic ulcer in desired therapeutic concentration at lower dose with reduced adverse effect.
Acknowledgements
Authors would like to acknowledge to Trustees, Bhujbal Knowledge City, MET’s institute of pharmacy, Adgaon, Nasik, Maharashtra, India, for providing the necessary facilities to carry out this work. The authors are thankful to Dr. S. L. Bodhankar and Dr. Pinaki Ghosh, Department of Pharmacology, Poona College of Pharmacy, Bharati Vidyapeeth University, Erandwane, Pune, Maharashtra, 411038, India, for providing precious guidance in in vivo H. pylori studies. We are also thankful to the Dr. Santosh. K. Tiwari of Centre for Liver Research and Diagnostics, Owaisi Hospital, Hyderabad, for valuable guidance in molecular biology techniques in the investigation. We thank Mr Nilesh Kulkarni from Department of Condensed Matter Physics and Materials Science of the Tata Institute of Fundamental Research (TIFR), Mumbai, for XRD facilities.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Adebisi A and Conway BR. (2011). Floating drug delivery systems for prolonging gastric residence time: a review. Curr Drug Deliv 8:494–510
- Arora S, Gupta S, Narang RK, Budhiraja RD. (2011). Amoxicillin loaded chitosan–alginate polyelectrolyte complex nanoparticles as mucopenetrating delivery system for H. pylori. Scientia Pharm 79:673–94
- Babu RJ, Sathigari S, Kumar MT, Pandit JK. (2010). Formulation of controlled release gellan gum macro beads of amoxicillin. Curr Drug Deliv 7:36–43
- Badhan AC, Mashru RC, Shah PP, et al. (2009). Development and evaluation of sustained release gastroretentive minimatrices for effective treatment of H. pylori infection. AAPS PharmSciTech 10:459–67
- Bolton S, Bon C. (2010). Pharmaceutical statistics (practical and clinical applications). 5th edn. : Informa Healthcare
- Chitnis VS, Malshe VS, Lalla JK. (1991). Bioadhesive polymers synthesis, evaluation and application in controlled release tablets. Drug Dev Ind Pharm 17:879–92
- EI-Gibaly I. (2002). Development and in vitro evaluation of novel floating chitosan microcapsules for oral use: comparison with non-floating chitosan microspheres. Int J Pharm 249:7–21
- Gattani SG, Savaliya PJ, Belgamwar VS. (2010). Floating-mucoadhesive beads of clarithromycin for the treatment of Helicobacter pylori infection. Chem Pharm Bull 58:782–7
- Ghosh P, Badgujar L, Ajmera A, et al. (2009). Effect of ethanolic extract of Psoralea corylifolia on H. pylori Infection in laboratory rats. Pharmacologyonline 2:557–69
- Ghosh P, Bhise KS, Paradkar AR, et al. (2012). Reduced ulcerogenic potential and antiarthritic effect of chitosan–naproxen sodium complexes. AAPS PharmSciTech 13:896–902
- Jain N, Gulat N, Kumar D, Nagaich U. (2012). Microspheres: mucoadhesion based controlled drug delivery system. RGUHS J Pharm Sci 3:28–40
- Kim HJ, Kim SY, Song GG, et al. (2005). Protective effect of astaxanthin on naproxen induced gastric antral ulceration in rats. Eur J Pharmacol. 514:53–9
- Koga T, Kawada H, Utsui Y, et al. (1996). In-vitro and in-vivo antibacterial activity of plaunotol, a cytoprotective antiulcer agent, against Helicobacter pylori. J Antimicrobial Chemother 37:919–29
- Labigne A, de Rease H. (1996). Determinants of Helicobacter pylori pathogenicity. Infect. Agents Dis 5:191–202
- Liu Z, Lu W, Qian L, et al. (2005). In vitro and in vivo studies on mucoadhesive microspheres of amoxicillin. J Control Release 102:135–44
- Mergaud F, Trimoulet P, Lamouliatte H, Boyanova L. (1991). Bactericidal effect of amoxicillin on Helicobacter pylori in an in vitro model using epithelial cells. Antimicrob Agents Chemother 35:869–72
- Nagahara N, Akiyama Y, Tada M, et al. (1998). Mucoadhesive microspheres containing amoxycillin for clearance of Helicobacter pylori. Antimicrob Agents Chemother 42:2492–4
- Narkar M, Sher P, Pawar A. (2010). Stomach-specific controlled release gellan beads of acid-soluble drug prepared by ionotropic gelation method. AAPS PharmSciTech 11:267–77
- Patel JK, Patel MM. (2007). Stomach specific anti-helicobacter pylori therapy: preparation and evaluation of amoxicillin-loaded chitosan mucoadhesive microspheres. Curr Drug Deliv 4:41–50
- Patil SB, Murthy RSR. (2006). Preparation and in vitro evaluation of mucoadhesive chitosan microspheres of amlodipine besylate for nasal administration. Indian J Pharm Sci 68:64–7
- Patil SB, Sawant KK. (2008). Mucoadhesive microspheres: a promising tool in drug delivery. Curr Drug Deliv 5:312–18
- Rajinikanth P, Mishra B. (2007). Preparation and in vitro characterization of gellan based floating beads of acetohydroxamic acid for eradication of H. pylori. Acta Pharm 57:413–27
- Rajinikanth P, Karunagaran L, Balasubramaniam J, Mishra B. (2008). Formulation and evaluation of clarithromycin microspheres for eradication of Helicobacter pylori. Chem Pharm Bull 56:1658–64
- Ranga Rao KV, Buri P. (1989). A novel in situ method to test polymers and coated microparticles for bioadhesion. Int J Pharm 52:265–70
- Singh B, Kapil R, Dhawan S, Garg B. (2012). Systematic formulation development of once-a-day gastroretentive controlled release tablets of rivastigmine using optimized polymer blends. J Drug Deliv Sci Technol 22:511–21
- Singh B, Chakkal SK, Ahuja N. (2006). Formulation and optimization of controlled release mucoadhesive tablets of atenolol using response surface methodology. AAPS PharmSciTech 7:E1–10
- Singh B, Garg B, Chaturvedi SC, et al. (2012). Formulation development of gastroretentive tablets of lamivudine using the floating-bioadhesive potential of optimized polymer blends. J Pharm Pharmacol 64:654–69
- Singh B, Kapil R, Nandi M, Ahuja N. (2011). Developing oral drug delivery systems using formulation by design: vital precepts, retrospect and prospects. Expert Opin Drug Deliv 8:1341–60
- Singh B, Kumar R, Ahuja N. (2005). Optimizing drug delivery systems using systematic “design of experiments.” Part I: fundamental aspects. Crit Rev Ther Drug Carrier Syst 22:27–105
- Suryawanshi SR, Thakare NP, More DP, Thombre NA. (2014). Bioavailability enhancement of ondansetron after nasal administration of Caesalpinia pulcherrima-based microspheres. Drug Delivery. Posted online on 26 Nov 2013. doi:10.3109/10717544.2013.860205):1–9
- Thombre NA, Gide PS. (2013). Rheological characterization of galactomannans extracted from seeds of Caesalpinia pulcherrima. Carbohydrate Polym 94:547–54
- Tiwari SK, Khan AA, Ahmad KS, et al. (2005). Rapid diagnosis of H. pylori infection in dyspeptic patients using salivary secretion: a non invasive approach. Singapore Med J 46:224–8
- Umamaheswari RB, Jain S, Tripathi PK, et al. (2002). Floating bioadhesive microspheres containing acetohydroxamic acid for clearance of Helicobacter pylori. Drug Deliv 9:223–31
- Umamaheswari RB, Ramteke S, Jain NK. (2004). Anti–Helicobacter pylori effect of mucoadhesive nanoparticles bearing Amoxicillin in experimental gerbils model. AAPS PharmSciTech 5:60–8
- Vaira D, Holton J, Chaira R. (2007). Diagnosis of H. pylori: invasive and non invasive tests. Best Pract Res Clin Gastroenterol 21:299–313
- Vasir JK, Tambwekar K, Garg S. (2003). Bioadhesive microspheres as a controlled drug delivery system. Int J Pharm 255:13–32
- Wu H, Shi HT, Wang LJX. (2000). Resistance of Helicobacter pylori to metronidazole, tetracycline and amoxiycillin. J Antimicrob Chemother 46:121–3