Abstract
The conventional formulation of prednisolone is considered to be low in efficacy, primarily on account of their failure in providing and maintaining effective therapeutic drug levels. This study aims to focus on development of a mucoadhesive buccal delivery system with a twofold objective of offering a rapid as well as a prolonged delivery of prednisolone coupled with enhanced therapeutic efficacy. Buccoadhesive films of prednisolone were prepared by solvent-casting method using hydroxyl propyl methyl cellulose (K100), Carbopol 940 and/or Eudragit® NE 40 D. Placebo films possessing the most desirable physicomechanical properties were selected for drug loading. The effect of polymer and its content on film properties, i.e. mucoadhesive strength, swelling and hydration, in vitro drug release was studied. Based on these studies, film F7D was selected for ex vivo permeation across porcine cheek mucosa. The steady state flux of prednisolone across the buccal mucosa was found to be 105.33 ± 32.07 µg/cm2/h. A comparative pharmacokinetic study of prepared film (F7D) and oral suspension of prednisolone was conducted. In vivo data of buccal film show greater bioavailability (AUC0–α: 24.26 ± 4.06 µg.h/ml versus 10.65 ± 2.15 µg.h/ml) and higher Cmax (2.70 ± 0.38 µg/ml versus 2.29 ± 0.32 µg/ml) value when compared to oral suspension. The data observed from this study highlight the feasibility of the buccal route as a viable option for delivery of prednisolone.
Introduction
Prednisolone is widely used in the treatment of various disorders viz inflammation, allergies, ulcerative colitis, psoriasis, skin diseases, etc. owing to its high therapeutic potential. Furthermore, the therapeutic application of this drug is more prominent in chronic inflammatory diseases like asthma, rheumatic arthritis, inflammatory bowel disease, etc. Typically, prednisolone is a synthetic corticosteroid with predominant glucocorticoid activity. The anti-inflammatory effect of prednisolone is primarily due to its potential to inhibit the production of prostaglandins and leukotrienes. Local delivery of prednisolone is preferred in topical disorders wherein the topical formulations provide and maintain higher drug concentration at the site of administration. However, in systemic therapy, the dose of prednisolone is generally individualized depending on the nature and severity of the disease so as to obtain optimum therapeutic effectiveness. Currently, oral therapy is considered as the preferred route of administration of prednisolone, possibly on account of a higher patient compliance. However, research suggests that this drug exhibits variation in bioavailability with change in dose and also possess short biological half-life (∼ 2–4 h) following oral administration (Disanto & Desante, Citation1975; Bergrem et al., Citation1983). Considering the severity of acute cases of inflammatory diseases, it is likely that a dosage form, which provides rapid and prolonged delivery of prednisolone, could be more effective than the conventional dosage forms. Review of literature indicates that few approaches have been attempted to enhance the therapeutic efficiency of prednisolone in treating inflammatory diseases. For example, Metselaar et al. (Citation2003) have demonstrated the potential of liposomes in enhancing the therapeutic efficiency of prednisolone in animal models of rheumatic arthritis. In another attempt, Lobatto et al. (Citation2010) have prepared long circulating multifunctional, multimodal liposomes of prednisolone for the treatment of atherosclerotic plaque inflammation, which in turn enhanced the efficacy and reduce the dose. Alternatively, controlled, delayed and extended release dosage forms of prednisolone were also developed to enhance the therapeutic efficiency for the successful treatment of various inflammatory diseases (Park et al., Citation2002; Mohammadi-Samani et al., Citation2005; Di Colo et al., Citation2006). Despite all these efforts, successful therapy of prednisolone in treating inflammatory diseases remains elusive. In this context, we hypothesize that the buccal drug delivery route could be a promising approach for the delivery of prednisolone.
Drug delivery through the buccal route offers rapid and direct delivery into the systemic circulation, bypassing any degradation in the gastrointestinal tract and the first-pass metabolism in the liver. This increases the bioavailability of the drug as well as provides a steady state plasma drug level, which in turn increases the therapeutic efficiency. In addition, this route is easily accessible and is more permeable than the skin (Jones et al., Citation2013). Drug delivery via the buccal route largely takes place by passive diffusion across the lipid membranes through the para-cellular as well as trans-cellular pathways, which provide opportunity for transport of both hydrophilic as well as lipophilic drugs (Patel et al., Citation2011). Moreover, this drug delivery route is also preferred for targeted, controlled and sustained release of drug molecules. Considering the significance of the buccal route, the objective of this study was to assess the feasibility of delivering prednisolone through the oral mucosa by preparing buccoadhesive films. Formulation development was aimed on developing buccoadhesive films, which could provide quicker onset of action, prolonged drug release and improved bioavailability. The prepared films were characterized and the in vitro and in vivo evaluation of dosage form was performed. Furthermore, the effect of different polymers on the mucoadhesion time of buccal films and factors influencing drug release from the film were studied.
Materials and methods
Materials
Hydroxy propyl methyl cellulose (HPMC) K100, Carbopol 940, Eudragit® NE 40 D, sodium carboxymethyl cellulose, ethyl cellulose and polyvinylpyrrolidone (PVP) K-30 were obtained as ex-gratis from Ind Swift Ltd., Parwanoo, HP, India. Prednisolone was obtained as a gift sample from Ind Swift Laboratories Ltd., Jawarpur, Punjab, India. Sodium lauryl sulfate (SLS) and propylene glycol (PG) were purchased from S.D Fine chemicals, Mumbai, India. All other chemicals/reagents used in the study were of analytical grade.
Formulation of oral buccoadhesive films
Placebo polymeric films were prepared using varying amounts of HPMC K100, Carbopol 940 and Eudragit® NE 40D (). Placebo films with desirable physicomechanical properties were selected for drug loading. The formulation codes and the composition of drug-loaded films are listed in . Formulation code of drug-loaded films has been suffixed with letter D. For the preparation of films, weighed amount of Carbopol was added to water under constant stirring to obtain a polymeric dispersion. HPMC was separately dispersed in required volume of ethanol premixed with PG. Carbopol polymeric dispersion was added to HPMC dispersion under constant stirring. Whereever required, Eudragit® NE 40 D was added to the polymer mix and stirred well to form a uniform blend. In case of HPMC–Eudragit® films, HPMC solution was prepared in ethanol–water mix containing PG, and Eudragit® was added to the above dispersion under constant stirring. The prepared blend was casted on a petri dish, and the content of the petri dish was allowed to dry in an oven maintained at 40 °C for 35–40 h. In case of drug-loaded films, prednisolone was dissolved in minimum quantity of ethanol and mixed to the polymer blend. The drug–polymer blend was sonicated to remove entrapped air and casted as above onto a petri dish and dried. Drug-loaded films were prepared to contain 2 mg/cm2 of drug. Dried films were wrapped in butter paper and stored in a vacuum desiccator until used. In order to ensure unidirectional drug release during the in vivo studies, a backing membrane of ethyl cellulose was adhered to the film. Backing membrane was casted by pouring 5% w/v solution of ethyl cellulose (plasticized using 2% v/v of dibutyl phthalate) onto a petri dish (Satishbabu & Srinivasan, Citation2008). This layer was allowed to dry similar to as explained for films. The dried membrane was pasted to the drug-loaded film formulation making use of a 5% w/v/solution of PVP as a binder.
Table 1. Composition of different polymeric films.
Table 2. Composition of drug-loaded films.
Physical characterization of oral films
The prepared films were evaluated for physical characteristics, i.e. color (visual inspection), transparency (against white light), softness (as per feel), peelability (removal of film after formation and drying) and homogeneity (visually against white light). Content uniformity of the films was tested by cutting the films of uniform size (1 × 1 cm2), weighing each segment individually and estimating its drug content. Each portion of the cut film was dissolved in 10 ml of phosphate buffer saline (PBS; pH 6.8) by shaking for 24 h on a mechanical shaker (Adhikari et al., Citation2010). The solution was filtered through a Whatman filter paper and suitably diluted. The concentration in different samples was calculated making use of absorbance at a λmax of 246 nm.
The pH of the prepared film was determined by cutting film (size 1 cm2) and allowing it to swell in 5 ml of distilled water for 15 min. The swollen film was taken out, drained and the pH of the film was measured using a flat surface electrode (Orion 4 Star Benchtop, Thermo Fischer Scientific Inc., Waltham, MA). Thickness of the films was measured at five different locations using calibrated screw gauge (Mitutoyo, Kawasaki, Japan) (Perioli et al., Citation2004).
Mucoadhesion studies
Two different parameters were observed in this part of the study. First one was the mucoadhesive time or retention time (measured in min or h) and the second was the mucoadhesive strength (measured in g/cm2). The mucoadhesive time of the film determines the time for which the film remains in contact with the mucosal membrane without eroding or dissolving. As the mucoadhesive time of film increases, there is an increase in the time available for the drug to permeate across the mucosa. This may also increase the permeability of certain high-molecular-weight drugs (Kamath & Park, Citation1994). The second parameter of mucoadhesion is the mucoadhesive strength. This determines the force with which the film sticks to the buccal mucosa.
In vivo mucoadhesion time
This study was approved by Human Ethical Committee of Maharishi Markendeshwar University, Mullana, Ambala, India. Written consent was taken from healthy human volunteers (n = 6) to carry out the study. Placebo films of size 1 × 1 cm2 were cut and pasted to the upper cheek pouch of the volunteers by applying a gentle force for 10 s. The volunteers were advised not to take any food or water during the study as this may cause abrasion of the film ends. The films were monitored for irritation, residence time in the human buccal cavity, discomfort and palatability (Perioli et al., Citation2004; Yehia et al., Citation2009).
Ex vivo mucoadhesion/retention time
The ex vivo mucoadhesion/retention time of the oral buccoadhesive films was determined using porcine cheek mucosa. Porcine cheek pouch of size 2 × 2 cm2 was cut and pasted on the inner side of the beaker using double-sided adhesive tape. The film of size 1 × 2 cm2 was cut, and its surface was made wet using a drop of PBS. Films were pasted on the surface of the porcine pouch by applying a gentle force for 10 s. PBS (500 ml), maintained at 37 ± 1 °C, was poured into the beaker and stirred at 150 rpm to simulate buccal conditions (Perioli et al., Citation2004). All the experiments were performed in triplicate.
Ex vivo mucoadhesive strength
A TA-XTi, texture analyzer, (Stable Micro Systems Ltd., Surrey, UK) was used to measure the mucoadhesive strength of the prepared films. Mucoadhesive strength was measured using porcine cheek mucosa as substrate. The cheek pouch was checked for integrity and placed on the stationary platform. A piece of film (size; 1 × 2 cm2) to be tested was cut and attached to the movable probe of the texture analyzer using double-sided adhesive tape (Giovino et al., Citation2013). The assembly containing the porcine cheek mucosa was filled with 2 ml of the buffer solution to keep the mucosa wet during the contact period. The movable probe of the texture analyzer was lowered until it made contact with the mucosa. The contact time between the cheek mucosa and film was 20 s. Measurements were obtained using the following parameters: pre-test speed: 0.5 mm/s; test/post-test speed: 0.5 mm/s; and applied force: 1 N. The mucoadhesive strength was measured as the maximum force generated during probe return. Results were obtained in triplicate for each film and were expressed as mean ± SD.
Swelling studies
The swelling behavior of the film helps to predict drug release from film. Swelling characteristics of films depends upon the nature and the type of polymer incorporated in the film. This also helps to predict drug release from the film sample (Boateng et al., Citation2009). Swelling studies were carried out making use of percent hydration.
Percent hydration
Swelling behavior of films was determined by placing a 1 × 1 cm2 of the film on a weighed stainless steel mesh. Collective weight of the mesh and film was noted. The mesh was then immersed in sufficient volume of PBS. The film samples were withdrawn from the solution at predetermined time intervals, wiped using tissue paper and reweighed. Percentage hydration of the films was determined using the following relation (Ritthidej et al., Citation2002):
where W2 = weight of film at particular time interval and W1 = initial weight of the film.
In vitro drug release studies
Standard paddle apparatus USPXXIV Type II (paddle type) (Electro Lab TDC 50, Mumbai, India) was used to carry out the drug release studies (Okamoto et al., Citation2001; Perioli et al., Citation2004). The film of size 1 × 2 cm2 was cut and pasted onto the inner side of the dissolution beaker using double-sided adhesive tape. The pre-warmed dissolution medium maintained at 37 ± 1 °C was poured into the beaker, and the paddle was rotated at 50 rpm. During the study, the temperature of the dissolution medium was maintained at 37 ± 1 °C. The drug release study was carried out for a period of 8 h. The samples were analyzed using double beam UV Visible spectrophotometer at a λmax of 246 nm.
Drug release from backing membrane
In order to evaluate the barrier property of the backing membrane, drug release study across the backing membrane was carried out using Franz diffusion cell. The film was punched using biopsy punch and clamped between the donor and receptor compartments of the diffusion cell maintained at 37 ± 1 °C. The donor compartment (1 ml) and the receptor cell was filled with of PBS (5 ml). Samples were withdrawn at specific time intervals from the donor compartment analyzed using high-performance liquid chromatography (HPLC).
Ex vivo drug permeation studies
Ex vivo drug permeation studies were carried out using standard two chambered Franz diffusion cell (Nair et al., Citation2013). The film was punched using biopsy punch (1 cm2) and weighed. Porcine cheek pouch was used as the permeation barrier for the drug-loaded films. The receptor cell was filled with 5 ml of PBS (pH 6.8) containing 0.5% SLS. Film sample was applied on the buccal mucosal membrane with an exposed area of ∼ 1 cm2. This was clamped between the donor and receptor compartments of the diffusion cell (Okamoto et al., Citation2001). The jacket of the receptor cell was maintained at 37 ± 1 °C. Samples were withdrawn at specific time intervals from the receptor compartment and suitably diluted. The samples were analyzed using HPLC system (Cyberlab, Millbury, MA, LC P100) consisting of a Symmetry C-18 analytical column (Waters, Milford, MA, 4.6 × 250 mm, Shiseido, 5 µm) with a detector LC UV-100. Mobile phase consisted of methanol and 0.5% formic acid (60:40, v/v). Elution was performed isocratically at a flow rate of 1 ml/min, and the column effluent was monitored at 254 nm.
In vivo study
The experimental procedures were approved by the Animal ethical committee of Maharishi Markandeshwar University. Eighteen male Sprague–Dawley rats were used in the study. Rats were divided in three groups of six each. Group I rats were kept as control. Group II rats were given prednisolone oral suspension equivalent to 2 mg dose. Group III rats were anesthetized by intraperitoneal injection of phenobarbital sodium at a dose of 30 mg/kg, with an additional dose given periodically to maintain general anesthesia. A film of size equivalent to ∼ 2 mg dose of prednisolone was cut into two halves and applied bilaterally to the inner cheeks of group III rats (Nishimura et al., Citation2009; Shimoda et al., Citation2009). Blood specimens were taken (0.25 ml) from retero-orbital plexus at 1, 2, 4, 6, 8 and 12 h after drug administration. Equal amount of acetonitrile was added to each collected blood sample to precipitate plasma proteins and were centrifuged at 8000 rpm for 15 min. The supernatant was collected and analyzed using HPLC.
Data analysis
The cumulative amount of drugs permeated per unit buccal surface area was plotted against time, and the slope of the linear portion of the plot was estimated as the steady-state flux (Nair et al., Citation2009). The data were tested by one-way analysis of variance and t-test using GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA) to test the effects of various treatments. p Value less than 0.05 was considered statistically significant. The data points provided in the graph are an average of six trials. The error bars represent the standard deviation.
Results and discussion
Physical characterization
Drug-loaded films of all the polymeric compositions were found to be colorless, translucent, soft, peelable, dry, homogeneous and tack free. No stain or spot was found on the prepared films. It was noticed that the films comprising Eudragit® NE 40D were more flexible (groups I and III) when compared to films prepared without Eudragit (group II). All the prepared films were found to be tack free when pressed between layers of paper for one minute. Moreover, all the prepared films exhibited uniform thickness and were homogenous when inspected visually.
Content uniformity is an important quality control parameter for films, and it should be within 85%–110% (Dixit & Puthli, Citation2009). The assessment of drug content (per cm2 area) in the prepared film indicated uniform distribution of prednisolone within the film when assessed at three different regions. Furthermore, it was also noticed that the drug content in the prepared films of various groups were comparable (range of 97.5%–99.5%) suggesting that the polymer content and polymer type did not influence the uniformity of drug under the current experimental condition ().
Table 3. Physical characteristics and mucoadhesive behavior of drug-loaded/placebo films.
Acidic or alkaline pH may cause irritation to the buccal mucosa and influence the degree of hydration of polymers. Hence, it is preferable to keep the surface pH of the formulations as near to buccal or salivary pH as possible. The prepared films exhibited a pH range of 6–7, which is comparable to the buccal pH and is not likely to cause any irritation. Furthermore, the prepared films did not show any substantial difference in the thickness within the group; however, marginal difference in the thickness was observed between the groups ().
Mucoadhesion studies
The buccal films prepared in this study utilized three different polymers, i.e. HPMC K-100, Carbopol and Eudragit® NE 40 D based on their mucoadhesive/film forming/controlled release properties (Nair et al., Citation2007; Morales & McConville, Citation2011; Kumria et al., Citation2013). In order to understand the role of each polymer on film characteristics, the prepared films were grouped into three categories depending upon the polymer composition (). Group I films (F1–F4) were prepared making use of different proportions of all the three polymers mentioned above. Group II films (F5–F8) were prepared making use of HPMC K-100 and Carbopol, while group III films (F9–F12) consisted of HPMC K-100 and Eudragit® NE 40D.
Mucoadhesion time
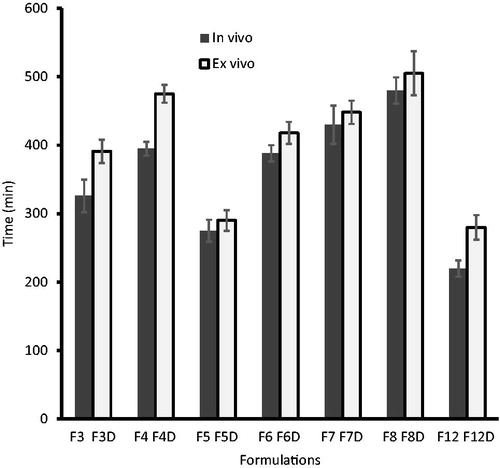
In vivo mucoadhesive studies were carried out in humans making use of placebo films. In group I, the observed retention time of films F1 (175 ± 5 min) and F2 (202 ± 6 min) were relatively low when compared to films F3 (326 ± 24 min) and F4 (395 ± 10 min) suggesting that an increase in HPMC content enhances the mucoadhesive time. However, the values observed also signify that a change in the quantity of Eudragit® did not significantly affect the retention time of the prepared films. Retention time of films in group II [F5 (275 ± 16 min), F6 (388 ± 12 min), F7 (430 ± 28 min) and F8 (480 ± 19 min)], indicate that the films prepared by using HPMC and Carbopol (without Eudragit) possess greater mucoadhesive retention than the corresponding group I and group III films. On the other hand, the retention time was found to be moderately low in films of group III [F9 (120 ± 11 min), F10 (142 ± 16 min), F11 (189 ± 12 min) and F12 (220 ± 17 min)]. Films exhibiting retention time of less than 210 min (3.5 h) were not selected for further investigation.
Retention time of the film is dependent upon the dissolution of the film. A film with slower dissolution showed a higher retention time/mucoadhesion time. Ex vivo mucoadhesion time results were found to correlate well with the in vivo mucoadhesion time results observed above (). summarizes the ex vivo mucoadhesion time data observed using drug loaded films. It is evident from that the films in groups I and II showed a higher mucoadhesion time. Highest retention time was observed in films of F8D film of group II with a value of 505 ± 32 min. The possible explanation for these observation could be that in the case of group I films, the presence of hydrophobic polymer (Eudragit) reduces the dissolution of the film, while in group II films, the high viscosity Carbopol retards the dissolution of the film. The slow dissolution of the film in turn increases the retention time of the film. From , it is apparent that the retention time in the ex vivo study was marginally higher than the in vivo data, regardless of the formulation.
Mucoadhesive strength
Adequate mucoadhesion is a prerequisite for optimal performance of buccal films because the formulation with low adhesion may generally cause spitting or ingestion of the film. The mucoadhesion strength observed for the prepared films revealed that group II films possess the greatest mucoadhesive strength when compared to the other films prepared in this study (). The data suggest that the mucoadhesive strength of films was largely dependent upon the amount of Carbopol present in the film. Films containing higher amount of Carbopol showed higher mucoadhesion (F5D and F8D). However, the decrease in the quantity of Carbopol from 100 mg (F5D) to 75 mg (F6D) and then to 50 mg (F7D), proportionately reduced the mucoadhesive strength of the film (). Drug-loaded films of group I showed a relatively lower mucoadhesive strength (∼ 49.21 g/cm2 and ∼ 35.43 g/cm2 in the case of F3D and F4D formulation, respectively). Lowest mucoadhesive strength was recorded for group III film (∼ 32.90 g/cm2 in the case of F12D) prepared without Carbopol.
The results observed in this study are consistent with results reported by Wong et al. (Citation1999) wherein they reported that the bio-adhesiveness increases with a corresponding increase in the content of hydrophilic polymer, while presence of Eudragit was found to lower the bioadhesion. The plausible explanation could be that the group II films showed greater bioadhesive strength contributable to the presence of hydrophilic polymers present in the film (HPMC and Carbopol). Although group I films also contained these two hydrophilic polymers, the presence of Eudragit® in the film contributed to lower the bioadhesive strength of the film. Group III films exhibited lowest mucoadhesive strength probably due to (i) absence of Carbopol and (ii) presence of hydrophobic polymer (Eudragit®) in the film and were excluded from further studies.
Swelling studies
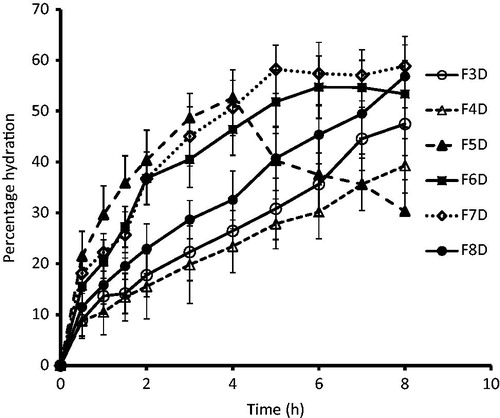
Swelling is a pre-requisite for bioadhesion. Adhesion occurs shortly after the beginning of swelling but initially the bond formed is weak. Bioadhesion increases with the degree of hydration until a point where hydration leads to an abrupt drop in adhesive strength due to disentanglement at the polymer tissue interface. The rate and extent of film hydration and swelling affects the film adhesion and consequently the drug release from the film. The percent hydration profile of the films is depicted in . Group I films (F3D and F4D) showed a lower percentage hydration amounting to ∼ 50% and ∼ 40%, respectively, at the end of the study period (8 h). This low percentage hydration may be attributable to the presence of hydrophobic polymer (Eudragit®) in the film. Among the group II formulations, film prepared using lower total polymer content (F5D) showed rapid hydration for initial 4 h (∼ 52%) and thereafter hydration reduced abruptly, signifying erosion of film. In films F6D and F7D, the hydration was found to be ∼ 55–60% in 6–7 h. Film prepared using highest polymer content (F8D) showed slow and steady hydration throughout the study period (). These results signify the importance of polymer content on hydration properties of the films. Hydration pattern is of importance in case of mucoadhesive formulations as excessive hydration of the film may cause formation of loose mass, which may detach from the mucosal membrane.
In vitro dissolution profile of prednisolone films
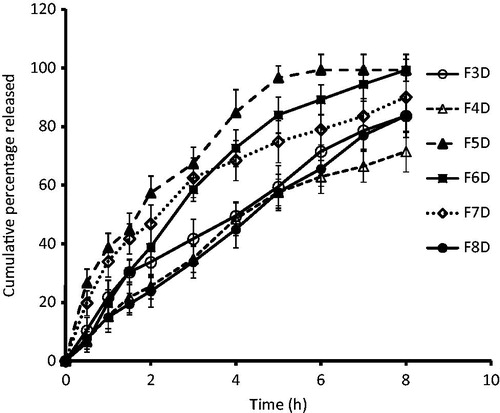
In vitro drug release profiles of the films are presented in . Group I films (F3D and F4D) prepared using a combination of HPMC, Carbopol and Eudragit could sustain drug release over an extended period of time. Among the group I formulations, F3D film showed marginally higher drug release as compared to F4D films (71% of drug was released in 8 h). Drug release from F4D films was found to be first order (R2 = 0.995) (). The retarded release can be explained considering the fact that as the concentration of Eudragit® in the film is increased, drug release is retarded. These results are in agreement with our earlier studies where we observed that the presence of Eudragit® was found to control drug release from the films (Kumria et al., Citation2013, Citation2014). Group II film, F5D, showed ∼ 96% of drug release in the initial 5 h. Increasing the content of polymers, i.e. HPMC or Carbopol within the group retarded drug release, i.e. in film F7D (drug release was ∼ 75% in 5 h, p < 0.0001). Keeping the content of HPMC same as in F7D and increasing the content of Carbopol, i.e. in film F8D, further retarded drug release ∼ 57% drug release in 5 h). The release of drug from F8D films followed Higuchian pattern, and the main mechanism of drug release was found to be erosion and diffusion. The retardation in drug release seen in group II films upon increasing the hydrophilic polymer content is likely due to the excessive swelling of the polymers, which in turn produces a thicker gel barrier for diffusion of the drug thereby increasing the diffusion path length (Wong et al., Citation1999; Semalty et al., Citation2008; Meher et al., Citation2013).
Drug release from backing membrane
The possible drug release from the backing membrane was carried out for a period of 12 h, and the samples were regularly withdrawn and analyzed. However, no detectable amount of prednisolone was measured in the donor during the study indicating the potential of the backing membrane to prevent the drug release though the backing membrane.
Ex vivo permeation studies
Based on the promising results observed in the ex vivo mucoadhesion time (448 ± 17 min), swelling studies, ex vivo mucoadhesion strength (138.91 ± 9.54 g/cm2) and in vitro drug release (∼ 90% of drug release in 8 h), the film F7D was selected for ex vivo studies. The permeation studies were carried out in a Franz diffusion cell by placing the film (size 1 × 1 cm2) over the porcine cheek pouch. The drug transported across the membrane was measured periodically. A plot of cumulative amount of drug permeated against time (for a period of 12 h) exhibited a typical permeation profile substantiating the potential of buccal delivery of prednisolone. It was observed that the permeation rate of prednisolone was relatively constant throughout the study period with a steady state flux of 105.33 ± 32.07 µg/cm2/h. Indeed, prednisolone was detected in the receiver from the initial hour (69.98 ± 11.94 µg/cm2) itself indicating rapid permeation through the buccal membrane without any lag time. The amount of drug transported across the buccal membrane progresses with increase in duration and the cumulative amount at various time periods was found to be ∼ 158.38 µg/cm2 (2 h), ∼ 381.49 µg/cm2 (4 h), ∼ 634.68 µg/cm2 (6 h), ∼838.59 µg/cm2 (8 h) and ∼ 1053.04 µg/cm2 (10 h). Furthermore, the cumulative amount of prednisolone permeated at the end of the study period (12 h) was 1178.32 ± 122.74 µg/cm2, which represents ∼ 60% of the drug content in the film. The data observed in this study substantiate the potential of the prepared film to deliver a significant amount of prednisolone through the buccal mucosa.
In vivo studies
Buccal film formulation (F7D) with ethyl cellulose-backing membrane was cut into two halves (0.5 cm × 1 cm) and applied bilaterally to the cheek of Sprague–Dawley rats. A similar dose of prednisolone (∼ 2 mg) in the form of oral suspension was used as control. The pharmacokinetic parameters such as peak plasma concentration (Cmax), time for peak plasma concentration (Tmax) and the area under the plasma concentration time curve (AUC0–α) were determined. compares the plasma level profiles of prednisolone following administration of buccoadhesive film F7D and oral prednisolone suspension. It was apparent from that the absorption of prednisolone was rapid and was not influenced by the dosage form, under the current experimental condition. However, the profile indicates that the application of buccal film prolonged the prednisolone delivery and increases the duration of absorption, which in turn enhanced the absorption of prednisolone in the body (measured from plasma concentration level) when compared to oral suspension (control) (). The observed pharmacokinetic parameters of prednisolone following administration of buccal film and oral suspension are recorded in . It was found that the values of Cmax were comparable in both the delivery routes. However, the Tmax value was found to be significantly increased following buccal delivery (4 h) when compared to oral suspension (for control, it was 2 h). The overall mean value of AUC0–∞ by buccal route (24.26 ± 4.06 µg h/ml) was found to be ∼ 2 folds higher as compared to oral suspension (). The results were extremely significant as indicated by the p value, which was found to be less than 0.0001, demonstrating improved bioavailability of prednisolone by buccal films. This noticeable enhancement in AUC values (in the case of buccal films) signifies increased rate and extent of prednisolone absorption (relative bioavailability ∼ 200%) from the buccal films compared to the control oral suspension. Thus, the developed sustained release buccal film can be a good alternative for administering prednisolone.
Figure 4. Plasma prednisolone profiles obtained after administration of prednisolone buccal film and control prednisolone suspension in Sprague–Dawley rats. Data were expressed as means ± SD (n = 6).
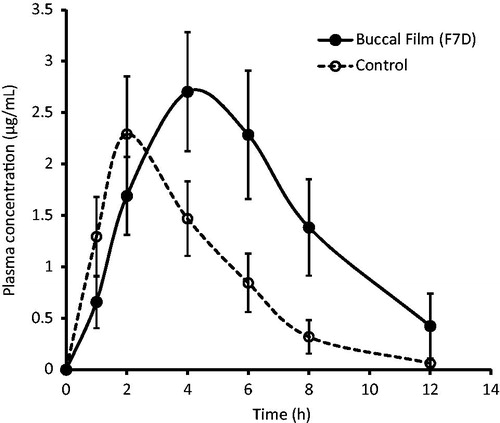
Table 4. Comparison of pharmacokinetic parameters of prednisolone buccal film and Prednisolone oral suspension (control) in Sprague–Dawley rats.
Conclusion
Mucoadhesive films for transmucosal buccal delivery of prednisolone were designed. Films were prepared making use of a combination of mucoadhesive polymers, i.e. hydroxyl propyl methyl cellulose, Carbopol with Eudragit® NE 40 D. Placebo films exhibited adequate mucoadhesive strength, time and possessing good mechanical strength were selected for drug loading. Physicochemical tests were performed on drug-loaded films. Drug-loaded films with adequate buccoadhesive time and strength were selected for further study. The data observed indicate that the polymeric content of the film can be varied to tailor drug release from the film. In this study, both Eudragit and Carbopol were found to control drug release from the film. Ex vivo studies indicate that the HPMC–Carbopol film (F7D) showed good permeation across buccal mucosa. In vivo drug release studies carried out in rats showed a higher Cmax and an increased AUC values (in the case of buccal films) signifying an increased rate and extent of prednisolone absorption from the buccal films as compared to the oral suspension. It can be concluded from this study that buccal films provide a better alternative to oral delivery of prednisolone especially for handling acute situations like asthma attacks, acute bronchitis and other such situations where the steroidal drugs may be lifesaving medications. Patients with compromised lung function may also be benefitted in acute situations where medical facilities for parenteral administration may not be immediately available.
Declaration of interest
The authors report no conflict of interest.
References
- Adhikari SN, Nayak BS, Nayak AK, Mohanty B. (2010). Formulation and evaluation of buccal patches for delivery of atenolol. AAPS PharmSciTech 11:1038–44
- Bergrem H, Grøttum P, Rugstad HE. (1983). Pharmacokinetics and protein binding of prednisolone after oral and intravenous administration. Eur J ClinPharmacol 24:415–19
- Boateng JS, Matthews KH, Auffret AD, et al. (2009). In vitro drug release studies of polymeric freeze-dried wafers and solvent-cast films using paracetamol as a model soluble drug. Int J Pharm 378:66–72
- Di Colo G, Baggiani A, Zambito Y, et al. (2006). A new hydrogel for the extended and complete prednisolone release in the GI tract. Int J Pharm 310:154–61
- Disanto AR, Desante KA. (1975). Bioavailability and pharmacokinetics of prednisone in humans. J Pharm Sci 64:109–12
- Dixit RP, Puthli SP. (2009). Oral strip technology: overview and future potential. J Control Release 139:94–107
- Giovino C, Ayensu I, Tetteh J, Boateng JS. (2013). An integrated buccal system combining chitosan films impregnated with peptide loaded PEG-b_PLA nanoparticles. Colloids Surf B Biointerfaces 112:9–15
- Jones E, Ojewole E, Pillay V, et al. (2013). Monolayeredmultipolymeric buccal films with drug and polymers of opposing solubilities for ARV therapy: physico-mechanical evaluation and molecular mechanics modelling. Int J Pharm 455:197–212
- Kamath KR, Park K. (1994). Surface modification of polymeric biomaterials by albumin grafting using h-irradiation. J Appl Biomater 5:163–73
- Kumria R, Gupta V, Bansal S, et al. (2013). Oral buccoadhesive films of ondansetron: development and evaluation. Int J Pharm Investig 3:112–8
- Kumria R, Nair AB, Al-Dhubiab BE. (2014). Loratidine buccal films for allergic rhinitis: development and evaluation. Drug Dev Ind Pharm 40:625–31
- Lobatto ME, Fayad ZA, Silvera S, et al. (2010). Multimodal clinical imaging to longitudinally assess a nanomedical anti-inflammatory treatment in experimental atherosclerosis. Mol Pharm 7:2020–9
- Meher JG, Tarai M, Yadav NP, et al. (2013). Development and characterization of cellulose-polymethacrylate mucoadhesive film for buccal delivery of carvedilol. Carbohydr Polym 96:172–80
- Metselaar JM, Wauben MH, Wagenaar-Hilbers JP, et al. (2003). Complete remission of experimental arthritis by joint targeting of glucocorticoids with long-circulating liposomes. Arthritis Rheum 48:2059–66
- Mohammadi-Samani S, Bahri-Najafi R, Yousefi G. (2005). Formulation and in vitro evaluation of prednisolone buccoadhesive tablets. Farmaco 60:339–44
- Morales JO, McConville JT. (2011). Manufacture and characterization of mucoadhesive buccal films. Eur J Pharm Biopharm 77:187–99
- Nair A, Gupta R, Vasanti S. (2007). In vitro controlled release of alfuzosin hydrochloride using HPMC-based matrix tablets and its comparison with marketed product. Pharm Dev Technol 12:621–5
- Nair A, Reddy C, Jacob S. (2009). Delivery of a classical antihypertensive agent through the skin by chemical enhancers and iontophoresis. Skin Res Technol 15:187–94
- Nair AB, Kumria R, Harsha S, et al. (2013). In vitro techniques to evaluate buccal films. J Control Release 166:10–21
- Nishimura M, Matsuura K, Tsukioka T, et al. (2009). In vitro and in vivo characteristics of prochlorperazine oral disintegrating film. Int J Pharm 368:98–102
- Okamoto H, Taguchi H, Iida K, Danjo K. (2001). Development of polymer film dosage forms of lidocaine for buccal administration. I. Penetration rate and release rate. J Control Release 77:253–60
- Park HS, Lee JY, Cho SH, et al. (2002). Colon delivery of prednisolone based on chitosan coated polysaccharide tablets. Arch Pharm Res 25:964–8
- Patel VF, Liu F, Brown MB. (2011). Advances in oral transmucosal drug delivery. J Control Release 153:106–16
- Perioli L, Ambrogi V, Angelici F, et al. (2004). Development of mucoadhesive patches for buccal administration of ibuprofen. J Control Release 99:73–82
- Ritthidej GC, Phaechamud T, Koizumi T. (2002). Moist heat treatment on physicochemical change of chitosan salt films. Int J Pharm 232:11–22
- Satishbabu BK, Srinivasan BP. (2008). Preparation and evaluation of bucoadhesive films of atenolol. Indian J Pharm Sci 70:175–9
- Semalty M, Semalty A, Kumar G. (2008). Formulation and characterization of mucoadhesive buccal films of glipizide. Indian J Pharm Sci 70:43–8
- Shimoda H, Taniguchi K, Nishimura M, et al. (2009). Preparation of a fast dissolving oral thin film containing dexamethasone: a possible application to antiemesis during cancer chemotherapy. Eur J Pharm Biopharm 73:361–5
- Wong CF, Yuen KH, Peh KK. (1999). Formulation and evaluation of controlled release Eudragit buccal patches. Int J Pharm 178:11–22
- Yehia SA, El-Gazayerly ON, Basalious EB. (2009). Fluconazole mucoadhesive buccal films: in vitro/in vivo performance. Curr Drug Deliv 6:17–27