Abstract
Spironolactone (SL) is a poorly water-soluble drug. Being poorly soluble affects its dissolution rate which in turn affects its oral bioavailability. This work aimed to prepare freeze-dried SL-Soluplus®/polyvinyl alcohol (PVA) oral thin film in an attempt to enhance the drug solubility on one hand and at the same time prepare a solid dosage form convenient for the pediatric use. SL-Soluplus®/PVA films were prepared using polyethylene glycol 400 (PEG 400) as a plasticizer applying the solvent-casting technique. The prepared films were evaluated for their thickness, tensile strength, and in vitro dissolution studies. Box–Behnken design (17 runs) was applied to optimize the effects of the formulation variables on the film properties. The optimized film formulation was freeze-dried after casting so as to enhance the drug dissolution. Moreover, the optimized freeze-dried film was re-characterized in vitro and evaluated in vivo in human volunteers to investigate its palatability and satisfaction. The results showed that the optimized formulation composed of 10% polymer concentration containing Soluplus®:PVA (0.33:0.66) and plasticized with 30% PEG 400 possessed the highest desirability value (0.836). Freeze-drying of the optimized formulation succeeded to improve SL in vitro dissolution due to the preparation of a more porous film compared to the non-freeze-dried one. In vivo evaluation of the optimized freeze-dried film showed high satisfaction among the participating volunteers concerning the ease of administration and sensation thereafter, where all the film specimens dissolved without the need for water and no film residues remained in the mouth following film dissolution. In conclusion, freeze-dried Soluplus®/PVA-based oral thin film proved to be a successful carrier for the oral delivery of insoluble drugs like SL for pediatrics.
Introduction
Hypertension is a serious problem from which several patients suffer. It is more prevalent in adults; nevertheless, recently, hypertension and its complications are being seen with increasing incidence in children. The cause of hypertension in children is frequently due to renovascular, renal parenchymal disease which may be related to the epidemic of pediatric obesity (Kiessling & Chisti, Citation2009). Being one of the most prevalent diseases among children which has significant morbidity and mortality (Seikaly, Citation2007); hence, the need for antihypertensive dosage forms for children has greatly increased.
Several examples of antihypertensive drugs are present in the market which are used mainly for the treatment of adults but infrequently for the treatment of the pediatric population. Spironolactone (SL) is an example of these drugs. For several decades, SL has been used as an antagonist for the aldosterone receptors of the kidney’s epithelial cells and, was also used for the treatment of hyperaldosteronism. Besides, it was sporadically considered as a potassium-sparing diuretic (Doggrell & Brown, Citation2001). The first report indicating the use of SL in both infants and children was in 1964, when the administered drug succeeded in producing moderate diuresis in three infants (Walker & Cumming, Citation1964). SL has been previously recommended for the management of hypertension in children aged between 1 and 17 years. The treatment is initially started with a dose of 1 mg/kg/day with a maximum of 3.3 mg/kg/day up to 100 mg/day (National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents, Citation2004).
Even though previously prescribed in children, the literature lacks the presence of SL solid dosage forms for the treatment of pediatrics. Orally dissolving film is one of the favored solid dosage forms for children. Examples of marketed orally dissolving films for pediatric use include Triaminic® and multivitamins (Corniello, Citation2006). However, orally dissolving films loaded with antihypertensive drugs have not been much studied in the pediatric population. Accordingly, attempts for preparing SL-loaded orally thin films for the pediatric treatment were carried out in this study.
Soluplus® (BASF SE, Ludwigshafen, Germany) (polyvinyl caprolactam–polyvinyl acetate–polyethylene glycol graft copolymer) was used in the preparation of the oral thin film. Soluplus® is a new polymer with amphiphilic properties. It differs from other solubilizers like Cremophor® and Tween® by possessing dual acting properties of being a matrix former and an active solubilizer by micellization (Nagy et al., Citation2012). This polymer is usually intended for the use in the aqueous instant-release film coatings in the preparation and formulation of food supplement products. Being a strong solubilizer and a matrix former, therefore, Soluplus® was used to enhance the solubility of the poorly soluble drug SL (Nagy et al., Citation2012) and to form the film in a one-step preparation.
Hence, this study aimed to prepare different SL-loaded Soluplus® based orally dissolving films using solvent-casting technique. Besides, different concentrations of polyvinyl alcohol (PVA) were added to the film to enhance its mechanical properties and to prepare films with optimized properties. A Box–Behnken experimental design was used to evaluate the influence of formulation parameters including polymer concentration and ratio, as well as the concentration of plasticizer used on the film’s mechanical properties like thickness, tensile strength (TS), and in vitro dissolution properties. The film with optimized properties was further freeze-dried to prepare a porous film with enhanced properties. This freeze-dried film was re-characterized in vitro, in addition, the palatability and in vivo disintegration time of the selected film was assessed in healthy volunteers.
Materials and methods
Spironolactone was purchased from EIPICO, Cairo, Egypt. The graft copolymer polyvinyl caprolactam–polyvinyl acetate–polyethylene glycol under trade name Soluplus® was kindly gifted from BASF SE, Ludwigshafen, Germany. PVA (molecular weight 22 000), peach flavor, and polyethylene glycol 400 (PEG 400) were obtained from Sigma-Aldrich Corporation (St Louis, MO). Potassium dihydrogen phosphate, disodium hydrogen phosphate, sodium chloride, and potassium chloride were purchased from Riedel-de Haën, Sigma-Aldrich, GmbH, Seelze, Germany. All other reagents were of analytical grade and used as received. All water used was deionized and distilled water.
Preparation of SL oral thin films
SL oral thin films were prepared by solvent-casting technique as previously described by Abruzzo et al. (Citation2012) but with slight modifications. The aqueous dispersion was formed by dissolving PVA in 10 mL distilled water with continuous stirring using a magnetic stirrer with a rotation speed of 125 rpm, and then the Soluplus® was sprinkled portion wise to avoid clumping over the PVA dispersion. PEG 400 was then added as a plasticizer to the polymer dispersion. Finally, a specified amount of SL (50 mg) was added to the polymer dispersion. The aqueous dispersion was kept under magnetic stirring for 1 h at room temperature. Before film casting, the aqueous dispersion was set aside for at least 12 h of rest to ensure complete removal of the entrapped air bubbles. To cast the film, the aqueous dispersion was poured into a dry clean silicone dish (10 cm in diameter) and then was dried at 50 °C in an oven for 4 h. After complete drying, the films were peeled off carefully from the silicone dishes, wrapped in aluminum foil and stored in glass containers at room temperature until further investigations.
Preparation of SL oral thin films applying statistical design
Box–Behnken design was used to evaluate and optimize the effects of the formulation variables on the film properties using Design-Expert® software (version 8.0; Stat-Ease, Inc., Minneapolis, MN). In the applied design, three factors within 17 runs were studied. The design parameters and the experimental design are given in . The independent factors were the Soluplus® fraction in the film (X1), the total polymer concentration (X2), and the concentration of plasticizer (PEG 400) (X3), calculated as percentage (w/w) of the total polymer used. Besides, the dependent variables were selected to be the film thickness (Y1), the TS of the film (Y2), the percentage drug dissolved after 5 min (Y3), and the percentage drug dissolved after 15 min (Y4). The composition of the prepared formulations is collected in .
Table 1. Design parameters and experimental conditions for Box–Behnken design.
Table 2. Experimental runs, independent variables, and measured responses of the Box–Behnken experimental design.
Characterization of the prepared SL oral thin films
Film thickness measurements
Film thickness was measured in five different positions (center and four corners) using Vernier caliper micrometer (Shanghai Measuring and Cutting Tools Limited Company, Shanghai, China). The calculated values were the average of five measurements.
Tensile strength
TS is measured using Texture analyzer (Chatillon force measurement apparatus; Chatillon Inc., Greensboro, NC). Test samples, each of 1 × 3 cm, were investigated for its TS by placing it between two clamps in the apparatus, the upper one is fixed and the lower is movable. TS was calculated using the following equation (Byun et al., Citation2012):
(1)
where, F is the maximum load applied on the film measured in newtons (N) and A is the original cross-sectional area measured in centimeter squared (cm2). The calculated values were the average of five measurements.
Determination of moisture uptake
The weight gain method was used previously adopted by Li et al. (Citation2003) was applied to investigate the moisture uptake behavior of the tested films. Films were carefully cut into 2 × 2 cm square strips (4 cm2), and then the cut strips were accurately weighed in pre-weighed Petri dishes. The Petri dishes containing the film strips were then stored in desiccators at constant relative humidity of 75% (prepared using saturated solution of sodium chloride) at room temperature (Li et al., Citation2003). After 2 weeks the dishes were weighed and the percentage moisture uptake was calculated using the following equation (Li et al., Citation2003):
(2)
Where, Wo is the initial weight of the film and Wt is the weight of film at time t. The experiment was conducted three times and the results were statistically analyzed applying one-way analysis of variance (ANOVA) using the SPSS 19.0 software (SPSS Inc., Chicago, IL). Differences were considered to be significant when p values <0.05.
Drug content determination
Prior to the in vitro dissolution studies, the drug content in the films was investigated. To determine the drug content, the films were cut into small pieces each of 3 cm2. These small pieces represented five different regions (center and four corners) within the film. Each piece was added to 10 mL ethanol and was allowed to dissolve overnight using a magnetic stirrer with a rotation speed of 125 rpm. Finally, the solutions were filtered through Millipore® filter: Durapore® membrane, pore size 0.22 μm; Millex GV, Millipore Corporation (Millipore, Vimodron, Italy) (0.22 μm), properly diluted using ethanol and then the ultraviolet absorbance of the filtrates were measured at 240 nm using UV Spectrophotometer (UV-1601/PC, Shimadzu Corporation, Kyoto, Japan) against ethanol as a blank. The experiment was repeated in triplicates.
In vitro dissolution study
The in vitro dissolution of SL from the investigated films (cut into pieces equivalent to 12.5 mg drug) was conducted in 900 ml simulated saliva fluid (SSF) of pH 6.8 at a temperature of 37 ± 0.5 °C using a USP Dissolution Tester, Apparatus II (rotating paddle) (Pharma Test, Hainburg, Germany) at a rotation speed of 100 rpm. Five milliliters aliquots of the dissolution medium were withdrawn at pre-determined time intervals of 5, 10, 15, 20, 30, 45, and 60 min and were replaced with the same volumes of fresh medium. The withdrawn aliquots were filtered through Millipore® filter (0.22 μm) and their SL content was determined using UV Spectrophotometer at λmax 240 nm using SSF as a blank.
Preparation of freeze-dried SL oral thin film
Solvent-casting technique used for the preparation of SL films was also adapted to prepare freeze-dried SL films of the optimized film formulation. Accurately, 1% peach flavor was incorporated into the optimized film formulation in order to enhance the oral palatability and improve the taste of the film. In order to prepare the freeze-dried film, the optimized film previously prepared was initially frozen at −20 °C and then freeze-dried for 24 h using a Novalyphe-NL 500 Freeze-dryer (Savant Instruments, Halprook, NY). The pressure of the condenser chamber was kept at 7 × 10−2 mbar and the temperature was at −50 °C.
Characterization of freeze-dried SL oral thin films
TS and in vitro dissolution studies were repeated for the freeze-dried film of the optimized formulation to investigate the effect of the freeze-drying process on the properties of the optimized film formulation. The surface pH of the freeze-dried optimized oral thin film was determined in order to investigate the possibility of any side effect in vivo. As an acidic or alkaline pH may cause irritation of the oral mucosa, it was determined to keep the surface pH as close to neutral as possible. Oral film was slightly wet with the help of water. The pH was measured by bringing the electrode in contact with the surface of the oral film (Kunte & Tandale, Citation2011).
Determination of the saturated solubility of SL in the optimized freeze-dried SL film
The effect of the formulation on the saturated solubility of SL was investigated according to the solubility method previously discussed by Higuchi & Connors (Citation1965). Briefly, two films of the optimized freeze-dried formula (equivalent to 100 mg SL) were cut into small pieces and were added to 10 mL distilled water in glass vials. For comparison, excess amounts of SL powder were added to 10 mL of distilled water in another glass vials. The obtained suspensions were agitated in a water bath for 3 days (Oscillating thermostatically controlled shaker; GallenKamp, Loughborough, England) at 30 ± 0.5 °C to attain equilibrium. Consequently, the vials contain drug suspensions were centrifuged at 5000 rpm for 30 min using an ultracentrifuge (Model 8880; Centurion Scientific Ltd., W. Sussex, UK) to separate the precipitated SL. Then, aliquots from the supernatant were withdrawn and filtered through a cellulose filter (Millipore® filter 0.22 μm) and their SL content were assayed spectrophotometrically after appropriate dilution against distilled water as blank at λmax = 240 nm. Each experiment was conducted three times and the results were expressed as mean values (milligram/milliliter) ± standard deviation (SD). The obtained data were analyzed statistically applying one-way ANOVA using SPSS 19.0 software (SPSS Inc., Chicago, IL). Differences were considered to be significant when p values <0.05.
Powder X-ray diffraction
X-ray diffraction (XRD) patterns of the optimized freeze-dried SL film besides its individual components were determined using X-ray diffractometer (XD-610; Shimadzu Corporation, Kyoto, Japan). The investigated samples were prepared by irradiation with Ni-filtered Cu Kα radiation, at 45 kV and 40 mA. The scanning rate employed was 2°/min over a diffraction angle (2θ) range of 3–70°.
Image analysis optical microscopy of the optimized freeze-dried SL film
The surface properties of the freeze-dried film of the optimized formula as well as the optimized film formula before the freeze-drying process were investigated using optical microscope (Leica Imaging Systems Ltd, Cambridge, UK). The film was cut into small pieces which were placed onto a microscopy slides which were then mounted on the optical microscope. The images of the film were taken through the fitted camera.
Evaluation of the optimized freeze-dried oral thin film formulation in human volunteers
The optimized formulation were evaluated in 12 healthy human volunteers (eight females and four males, aged between 25 and 40 years) for their in vivo disintegration time in the oral cavity. In vivo disintegration test in the oral cavity is stated in brief in the literature (Morita et al., Citation2002). The prepared film previously cut into strips each of (2 × 3 cm2) were randomly administered to the volunteers. Prior to the test, all volunteers were asked to clean their mouth using distilled water. Subsequently, all volunteers were asked to place the film strip on the tongue and were allowed to move the film strip with their tongues in order to create a gentle tumbling action but without biting on it. On the other hand, the volunteers were restricted with respect to swallowing the saliva during the test. The mean time required for the complete disintegration of each film in the oral cavity was recorded. The obtained results were expressed as mean values (n = 12) ± SD. Besides, the in vivo disintegration time the optimized oral thin film formulation was evaluated in the human volunteers for their palatability, ease of administration without water and sensation after film dissolution. These parameters were evaluated based on a scoring system that was scaled from 1 to 3, where 1 and 3 correspond to highest satisfaction and dissatisfaction, in that order. Sensation of the oral films was evaluated considering residues of the film left in the mouth after administration. The study protocol was approved by the Research Ethics Committee of Faculty of Pharmacy, Cairo University, Egypt and complied with the principles of the Declaration of Helsinki. All subjects were completely informed about the study and signed a written consent form before the administration of the film strips.
Results and discussion
Preparation of SL oral thin films
Solvent-casting technique has been the mainstay manufacturing approach for the marketed oral thin films which was endorsed to the ease of the manufacture and the low system setup cost (Dixit & Puthli, Citation2009; Hoffmann et al., Citation2011; Kianfar et al., Citation2012). Nevertheless, it is not that easy, some important key elements must be available for the success of the technique. These key parameters include three important perquisites which are: the use of polymers which are soluble in the used solvent, the formed polymer solution must contain adequate solid content, and the obtained film must be homogenous and easily removed from the casting support (Siemann, Citation2005). In our casting studies, we selected Soluplus® as the film polymer which fulfilled all the required perquisites except the ease of being removed from the used silicone dishes due to the brittle characters of the formed films. That is why, different concentrations of PVA were added to Soluplus® to form different polymer blends in an attempt to enhance the film’s mechanical properties. Being a film intended for the pediatric use and since there are regulatory constraints governing solvents used for the infants and children, therefore, water was selected as a solvent during the film manufacturing technique (Morales & McConville, Citation2011). The formulated SL oral thin films were transparent, flexible, and showed no blooming, indicating complete solubilization of SL within the film formulation.
Analysis of the statistical design
Design of experiments (DOE) is a useful tool for reducing the number of experiments needed for improving formulations and help researchers in understanding relationships between studied factors and responses of interest (Hao et al., Citation2011). Several design methods are available for DOE and the choice of method depends on the objective of the study. When the objective is to screen for significant factors that affect responses, full factorial or fractional factorial designs are preferred. Three-level factorial designs, such as Box–Behnken designs are more sophisticated design methods that are being used for optimizing formulation optimization. This is because these design methods provide suitable mathematical models; for example, the quadratic model in order to predict the responses being studied. Box–Behnken designs are suitable for exploring quadratic response surfaces and constructs according to a second-order polynomial model, thus assisting in the optimization of a formulation with a small number of experimental runs (Zidan et al., Citation2007). Due to these advantages, Box–Behnken designs have been recently applied toward the optimization of different types of formulations.
The independent variables and responses were related using polynomial equation with statistical analysis through Design-Expert® software. All the responses (Y1–Y4) showed a non-linear relationship with regard to the factors investigated (X1–X3). This was the case because at least one coefficient with more than one factor was presented, which indicated the presence of interaction effects. As shown in , the approximation of response values of Y1, Y2, Y3, and Y4 based on the quadratic model was the most suitable (R2 = 0.9221, R2 = 0.9243, R2 = 0.9197, and R2 = 0.9182, respectively). The values of the coefficients of independent variables X1–X3 are related to the effect of these variables on the responses. The larger coefficient means the independent variable has more potent influence on the response. Coefficients with more than one-factor term represent the interaction terms and coefficients with higher order terms indicate the quadratic nature of the relationship. A positive sign indicates a synergistic effect while a negative sign represents an antagonistic effect (Huang et al., Citation2004; Shamma et al., Citation2011).
Table 3. Regression results of the measured responses (coded values).
The preliminary studies provided a setting of the levels for each formulation variable. The non-linear computer generated quadratic model is given as:
where, Y is the measured response associated with each factor level combination, b0 is an intercept, b1–b33 are regression coefficients computed from the observed experimental values of Y, and X1, X2, and X3 are the coded levels of independent variables. The terms X1X2 and
(i = 1, 2, or 3) represent the interaction and quadratic terms, respectively. The independent variables studied were the total polymer concentration (X1), concentration of Soluplus® in the film (X2), and concentration of plasticizer (X3).
Film thickness measurements
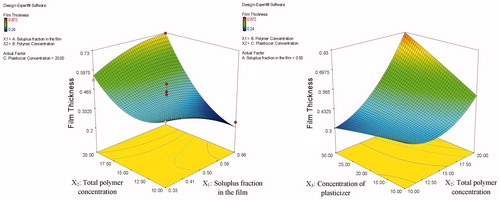
The mean film thickness (Y1) of the investigated films ranged from 0.24 to 0.87 mm. Statistical analysis of the film thickness measurements showed that increasing the total polymer concentration (X2) resulted in a significant increase in the film thickness (p = 0.0003). This could be attributed to the higher binding capacity of the higher polymer concentration. On the other hand, Soluplus® fraction in the film (X1) and the concentration of plasticizer (PEG 400) (X3) possessed a non-significant effect (p = 0.4171 and p = 0.0860, respectively) on the film thickness. Besides, the interactions (X1X2 and X2X3) have also positive synergistic effects in increasing the film thickness where the obtained p values were 0.0199 and 0.0173, respectively. represents the response surface plot showing the effect of the three investigated factors (X1, X2, and X3) on the film thickness. As presented in the figures, it is clear that at low polymer concentration (X2), the film thickness (Y1) decreased enormously but was practically not affected by the increase in both the plasticizer concentration and the Soluplus® fraction in the film. On the other hand, at high polymer concentration (X2), the film thickness (Y1) increased manifestly with increasing both the plasticizer concentration and the Soluplus® fraction in the film.
Tensile strength
TS is defined as the maximum stress that a film can withstand while being pulled or stretched before breaking. Hence, it is important that the prepared film must contain the optimal polymeric blends to afford adequate TS to endure necessary handling. Nevertheless, it must possess certain flexibility to guarantee patient compliance (Lim & Hoag, Citation2013).
The obtained TS values for the investigated films ranged from 1.68 to14 N/cm2. Statistical analysis revealed that both Soluplus® fraction in the film (X1) and the concentration of PEG 400 (X3) significantly affected the TS of the tested films, in which the obtained p values were 0.0050 and 0.0011, respectively. As presented in , it is clear that increasing both Soluplus® fraction in the film and the concentration of PEG 400 resulted in decreasing TS of the investigated films. Also from and , it could be observed that increasing the plasticizer concentration decreased films’ TS more efficiently. Moreover, a positive synergistic effect is also seen by the quadratic nature of the responses and
, where the obtained p values were found to be 0.0200 and 0.0012, respectively. Conversely, the total polymer concentration (X2) possessed a non-significant (p = 0.8436) effect on the TS of the investigated films.
Figure 2. Response surface plot presenting the effect of the Soluplus® fraction in the film (X1) and the concentration of PEG 400 (X3) on the film TS.
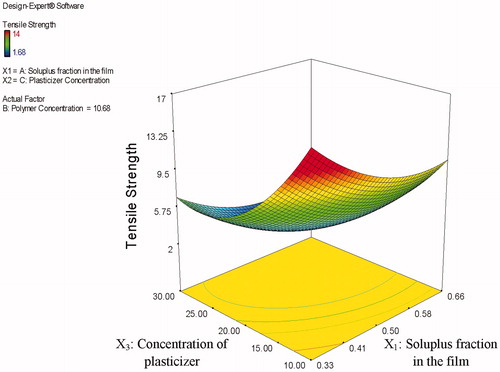
This observed decrease in the TS of the investigated films with increasing plasticizer (PEG 400) concentration could be attributed to imparting more flexibility to the films. This obtained flexibility was endorsed to the interference of the PEG 400 molecules with the bonding of the polymer. This obtained interference reduces the bonding strength and allows for greater mobility of the polymer molecules, hence increases the polymer flexibility on one hand and decreases the TS on the other hand. Similar results were obtained by Adel & El Kasabgy (Citation2013) who found that the use of triethyl citrate as a plasticizer increased the flexibility of the prepared ethylcellulose films. Rabek et al. (Citation2014) also found that increasing triethyl citrate concentration decreased the TS of the investigated films prepared using cellulose acetate phthalate and Pluronic F-127 combined together (70:30 w/w). These results are also in accordance with the results obtained by ElMeshad & El Hagrasy (Citation2011) in their study on the preparation of mosapride orodispersible films. Their results demonstrated that using 15% plasticizer resulted in significantly higher film stiffness as can be seen from the higher TS values compared to films containing 20 and 25% plasticizer.
Determination of moisture uptake
The moisture uptake study was conducted at a relatively high relative humidity of 75% to reflect the effect of the uncontrolled conditions on the prepared films. From the obtained data (data not shown), it was manifest that the moisture uptake of all the investigated films was <2% (w/w). This indicates good mechanical stability of the film upon storage.
Determination of drug content
The mean % SL content was found to be >95% from all the prepared oral thin film formulations (data not shown).
In vitro dissolution study
Aside from mechanical properties, the drug release profile is equally important. A good formulation has full drug release. illustrates the in vitro dissolution profiles of SL from the prepared films in SSF (pH = 6.8) as well as the dissolution behavior of SL plain powder. All SL-loaded oral thin films achieved almost complete dissolution after 1 h compared to only 39.69% dissolved for the plain drug. These results indicate that the process used to prepare the films greatly enhanced the extent and rate of dissolution of SL from the prepared SL-loaded oral thin films.
Figure 3. In vitro dissolution profile of SL from the investigated films in comparison to SL powder in SSF (pH 6.8) for 1 h.
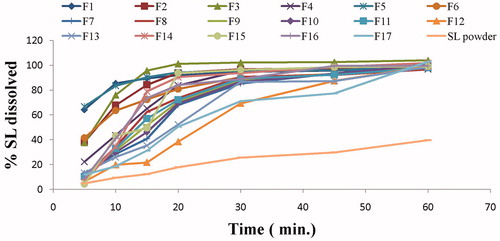
The cumulative percentage of drug released after 5 (Q5min) and 15 min (Q15min) were employed as the comparison criteria between different films. The values of Q5min and Q15min are represented in . As shown in the table, the values of Q5min varied between 4.20 and 66.89% while the values of Q15min ranged between 21.80 and 95.70%.
Regarding the response Y3 and as shown in , factors X1, X2, and X3 have a negative antagonistic effect on the response Y3. Results show that factors with the most influence on SL dissolution are, in descending order, are X1 then X2 and X3. In other words, by increasing the Soluplus® fraction in the film (X1), and the total polymer concentration (X2) we obtained a significant effect in retarding the dissolution of SL from the film (p = 0.0002 and p = 0.0003, respectively). The same effect, yet somehow smaller, was obtained by increasing the concentration of plasticizer (X3).
The presented interaction (X1X2) has also positive synergistic effects in retarding the drug dissolution (Y3) from the film (p = 0.0200). represents the response surface plot showing the effect of the Soluplus® fraction in the film (X1), and the total polymer concentration (X2) on the percentage SL dissolved after 5 min (a) and after 15 min (b). The highest percentage SL dissolved after 5 min was obtained at low levels of both Soluplus® fraction in the film (X1) and the total polymer concentration (X2). This could be attributed to the higher binding capacity at higher polymer concentration. Similar results were obtained by Low et al. (Citation2013) who found that the higher Soulplus®-containing films exhibited slower release rates. This could be due to the amphiphilic nature of Soulplus® which may have impeded the penetration of dissolution media (Low et al., Citation2013).
Figure 4. Response surface plots showing the effect of Soluplus® fraction in the film (X1), total polymer concentration (X2) on the percentage drug dissolved after 5 min (a) and after 15 min (b).
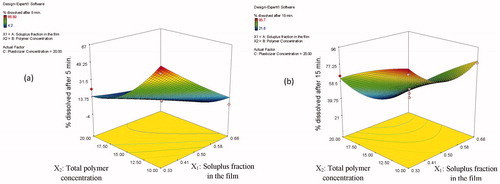
On the contrary, positive synergistic effects are seen by the quadratic nature of responses, which were especially high in the and
terms (p = 0.0091 and p = 0.0349, respectively).
Concerning the response (Y4) and as seen in , again it was found that X1 and X2 have a negative, i.e. antagonistic effect on the response Y2 (p = 0.0044 and p < 0.0001, respectively). The most important are X2 then X1. In other words, by increasing the total polymer concentration (X2) we obtained a remarkable effect in decreasing the percentage drug dissolved from the film after 15 min. The same effect, yet somehow smaller, was obtained by increasing the Soluplus® fraction in the film (X1). A positive synergistic effect is also seen by the quadratic nature of this response () (p = 0.0041), which was especially high. The concentration of plasticizer (X3) has a positive, i.e. synergistic effect on the response Y2 (although this effect appeared to be non-significant (p = 0.3013).
Optimization of SL oral thin films
The aim of the optimization of pharmaceutical dosage formulations is generally to determine the levels of variables from which a robust product with optimum characteristics may be produced.
SL oral thin film formulation was optimized for the responses Y1, Y2, Y3, and Y4. The optimum values of these responses were to minimize film thickness and TS in addition to maximize the percentage drug dissolved after 5 and 15 min. The optimum values of the variables were obtained by graphical and numerical analyses using the Design-Expert® software and based on the criterion of desirability (Basalious et al., Citation2010; Shamma & Elsayed, Citation2013). The optimized film formulation composed of 10% polymer concentration containing Soluplus®:PVA (0.33:0.66), and plasticized with 30% PEG 400 had the highest desirability value (0.836). The optimized film formulation with the predicted levels of formulation variables were prepared to confirm the validity of the optimization procedure. The composition, predicted and observed responses of the optimized film formulation are presented in . Results show that the observed values of the prepared optimized film formulation were mostly similar with predicted values. Based on these results, it can be concluded that optimized SL orally thin film provides a promising one-step manufacturing formulation without any other mixing or formulation steps; therefore, it was selected for further investigations.
Table 4. Composition, predicted, and observed responses of the optimized SL-loaded oral thin film formulation.
Preparation of freeze-dried SL oral thin film
Freeze-drying technique was employed to remove the remaining water from the optimized film formula after the oven dry in an attempt to enhance the physicochemical properties of the film.
Characterization of freeze-dried SL oral thin films
TS and surface pH
The TS test was repeated for the optimized SL oral film after the freeze-drying process to investigate the effect of the freeze-drying process on the mechanical properties of the prepared film. The TS value for the freeze-dried optimized SL film was found to be 6.32 N/cm2. Statistical analysis revealed no significant difference between the TS values before and after the freeze-drying process. Surface pH of the freeze-dried optimized SL film was found to be 7.6 which is close to the neutral pH. This indicates that the film has a low potential to irritate the sublingual mucosa, and hence, more acceptable by the patients.
In vitro dissolution study
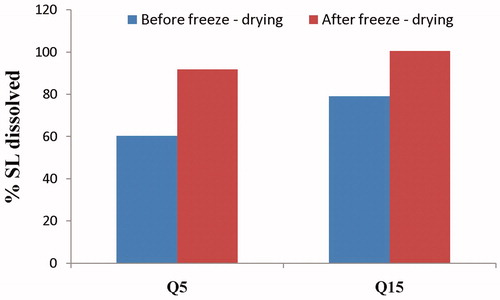
The effect of freeze-drying on the % SL dissolved after 5 (Q5) and 15 min (Q15) from the optimized film formulation is shown in . The cumulative percentage of drug dissolved after 5 (Q5min) and 15 min (Q15min) were 79.18 and 99.50%, respectively. Hence, it can be concluded that an enhanced SL dissolution was obtained after the freeze-drying process in comparison to the formulation before the freeze-drying process. This could be attributed to the more porous nature of the freeze-dried film (Caparino et al., Citation2012).
Determination of the saturated solubility of SL in the optimized freeze-dried SL film
SL saturated solubility in distilled water was determined for the plain drug powder and for the optimized freeze-dried SL film. After calculation, it was evident that the optimized freeze-dried SL film formulation improved drug’s saturated solubility in distilled water by 160-fold, where the obtained saturated solubility values of SL were 9.720 ± 0.67 and 0.060 ± 0.002 mg/mL for the optimized freeze-dried film formula and the plain drug powder, in that order. From the previous results, it is clear that the optimized freeze-dried film formula succeeded in improving drug’ solubility. This might be attributed to the use of Soluplus® which possess an amphiphilic chemical structure with large number of hydroxyl groups which make it an excellent solubilizer for poorly soluble drugs like SL in aqueous media (Thakral et al., Citation2012). In addition, the use of PVA as a water-soluble polymer possessed a positive synergistic effect in enhancing the drug’s saturated solubility. Similar results were previously stated by Sikarra et al. (Citation2012) in a study on improving the solubility of different poorly water-soluble drugs. This was also in accordance with Thakral et al. (Citation2012) who found that the solubility of camptothecin increased by ∼40 times in the presence of Soluplus®.
Image analysis optical microscopy of the optimized freeze-dried SL film
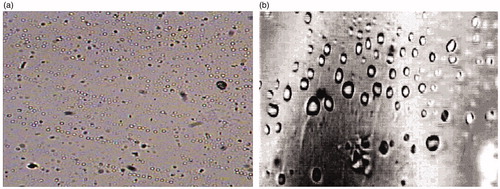
presents the microscopic images of the optimized SL film formula before and after the freeze-drying process. In case of the freeze-dried film formula, it is clear that the film pieces were more porous and also the pores were larger in size compared to those in the film pieces before the freeze-drying process. Similar results were obtained by El-Mahrouk et al. (Citation2009) and Ciper & Bodmeier (Citation2006) who found that freeze-dried hard gelatin capsule shells were more porous than the conventional capsule shells. This could be attributed to the removal of residual moisture content of the film during the freeze-drying process. This explains the faster dissolution of the film following the freeze-drying process.
Powder X-ray diffraction
Powder X-ray diffraction (XRD) has been previously used to investigate the molecular structure of oral films (Cilurzo et al., Citation2008). The diffractograms of pure SL, the optimized freeze-dried SL film formula and its corresponding physical mixture are displayed in . From the figure, it is evident that the XRD patterns of SL showed many sharp peaks at 9.21, 16.05, 16.67, and 17.31 (2θ) indicating its high crystalline character. But the characteristic peak with maximum peak intensity at 9.21, 16.67, and 17.31 (2θ) were used for comparison and studying the effect of the formulation on the crystalline nature of the drug.
Figure 7. XRD of pure SL, the optimized freeze-dried SL film formula and its corresponding physical mixture.
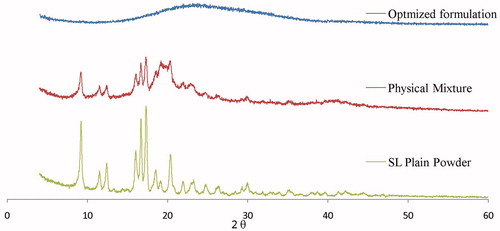
Also from , it is clear that the physical mixture of the optimized film formulation displayed similar diffuse patterns as pure SL. On the other hand, the diffractogram of the optimized freeze-dried SL film formulation lacked the presence of the numerous distinctive peaks of SL indicating the entirely amorphous nature of SL in the prepared film formulation. Crystallinity was determined by comparing some representative peak heights in the diffraction patterns of the physical mixture and the film formulation with those of SL powder. The relationship used to calculate the relative degree of crystallinity (RDC) was:
Where, Isample is the peak height of the sample under investigation and Idrug is the peak height at the same angle for the drug (Shoukri et al., Citation2009; Ahmed et al., Citation2013). Pure drug peak at 17.31 (2θ) was used for calculating the RDC. The calculated RDC values were 0.58 and 0.15 for the physical mixture and the optimized film formulation, respectively. It was previously reported (Schubert & Muller-Goymann, Citation2005), that the presence of surfactants in the formulation affects the signal intensities only (degree of crystallinity), but not the signal position. Consequently, in our work the use of water-soluble surfactants like Soluplus® and PVA in the prepared film formulation resulted in disrupting the crystalline nature and/or lowering the crystal lattice energy of the drug which in turn would improve the aqueous solubility of the drug. The crystalline nature of the drug can be disrupted by solid-state dispersion of the drug into water-soluble carrier molecules which replace the drug molecule in the crystal lattice. This results in a partial or total loss of crystallinity, resulting in a significant increase in solubility. Similar results were previously reported in several researches (Guns et al., Citation2011; Linn et al., Citation2012; Thakral et al., Citation2012).
Evaluation of the optimized freeze-dried oral thin film formulation in human volunteers
The optimized freeze-dried SL oral thin film dissolved in the oral cavity within 1.28 ± 0.05 min after administration as reported by the volunteers. All the involved volunteers were pleased with the palatability, of the investigated film formula; hence, it was given a score of 1 (very satisfied). Also, the volunteers expressed a high degree of satisfaction (score 1) concerning the ease of administration and sensation thereafter, where all the film specimens dissolved without the need for water and no film residues remained in the mouth following film dissolution. This was accredited to the rapid hydration of the film due to its thin and porous nature.
Conclusion
In the present study, freeze-dried SL oral thin film with enhanced physicochemical properties was successfully prepared using solvent-casting technique. Different ratios of Soluplus® and PVA were used as film forming materials on one hand and on the other hand as solubilizing agents. In order to improve the film mechanical properties PEG 400 was added as a plasticizer. The film formulation composed of 10% polymer concentration containing Soluplus®:PVA (0.33:0.66), and plasticized with 30% PEG 400 possessed the minimal film thickness and TS in addition to maximum percentage drug release after 5 and 15 min. That’s why; it was selected as the optimized film formula to be prepared for freeze-drying. The freeze-dried film formula succeeded in enhancing the dissolution properties of the film due to the formation of a more porous film. In vivo evaluation of the optimized freeze-dried SL oral thin film showed high satisfaction as expressed with the involved volunteers. In conclusion, freeze-dried Soluplus®/PVA-based oral thin film proved to be a good carrier for the delivery of insoluble drugs like SL to be successfully administered for pediatrics.
Declaration of interest
The authors report no declaration of interest.
References
- Abruzzo A, Federica B, Teresa C, et al. (2012). Mucoadhesive chitosan/gelatin films for buccal delivery of propranolol hydrochloride. Carbohyd Polym 87:581–8
- Adel S, Elkasabgy NA. (2013). Design of innovated lipid-based floating beads loaded with an antispasmodic drug: in-vitro and in-vivo evaluation. J Liposome Res in press
- Ahmed IS, Shamma RN, Shoukri RA. (2013). Development and optimization of lyophilized orally disintegrating tablets using factorial design. Pharm Dev Technol 18:935–43
- Basalious EB, Shawky N, Badr-Eldin SM. (2010). SNEDDS containing bioenhancers for improvement of dissolution and oral absorption of lacidipine. I: development and optimization. Int J Pharm 391:203–11
- Byun Y, Ward A, Whiteside S. (2012). Formation and characterization of shellac-hydroxypropyl methylcellulose composite films. Food Hydrocolloid 27:364–70
- Caparino OA, Tang J, Nindo CI, et al. (2012). Effect of drying methods on the physical properties and microstructures of mango (Philippine ‘Carabao’ var.) powder. J Food Eng 111:135–48
- Cilurzo F, Cupone IE, Minghetti P, et al. (2008). Fast dissolving films made of maltodextrins. Eur J Pharm Biopharm 70:895–900
- Ciper M, Bodmeier R. (2006). Modified conventional hard gelatin capsules as fast disintegrating dosage form in the oral cavity. Eur J Pharm Biopharm 62:178–84
- Corniello CM. (2006). Quick dissolve strips: from concept to commercialization. Drug Delivery Technology 6:68–71
- Dixit RP, Puthli SP. (2009). Oral strip technology: overview and future potential. J Control Release 139:94–107
- Doggrell SA, Brown L. (2001). The spironolactone renaissance. Expert Opin Investig Drugs 10:943–54
- El-Mahrouk G, Aboul-Einien MH, Elkasabgy NA. (2009). Formulation and evaluation of meloxicam orally dispersible capsules. Asian J Pharm Sci 4:8–22
- ElMeshad AN, El Hagrasy AS. (2011). Characterization and optimization of orodispersible mosapride film formulations. AAPS PharmSciTech 12:1384–92
- Guns S, Dereymaker A, Kayaert P, et al. (2011). Comparison between hot-melt extrusion and spray-drying for manufacturing solid dispersions of the graft copolymer of ethylene glycol and vinylalcohol. Pharm Res 28:673–82
- Hao J, Fang X, Zhou Y, et al. (2011). Development and optimization of solid lipid nanoparticle formulation for ophthalmic delivery of chloramphenicol using a Box-Behnken design. Int J Nanomedicine 6:683–92
- Higuchi T, Connors KA. (1965). Phase solubility techniques in “Advances in Analytical Chemistry and Instrumentation”. In: Reilly CN, ed. Phase solubility techniques. : Willey Interscience, 117–212
- Hoffmann EM, Breitenbach A, Breitkreutz J. (2011). Advances in orodispersible films for drug delivery. Expert Opin Drug Deliv 8:299–316
- Huang YB, Tsai YH, Yang WC, et al. (2004). Once-daily propranolol extended-release tablet dosage form: formulation design and in vitro/in vivo investigation. Eur J Pharm Biopharm 58:607–14
- Kianfar F, Chowdhry BZ, Antonijevic MD, Boateng JS. (2012). Novel films for drug delivery via the buccal mucosa using model soluble and insoluble drugs. Drug Dev Ind Pharm 38:1207–20
- Kiessling SG, Chisti A. (2009). Management of pediatric hypertension. Therapy 6:51–63
- Kunte S, Tandale P. (2011). Fast dissolving strips: a novel approach for the delivery of verapamil. J Pharm Bioallied Sci 2:325–8
- Li Y, Sanzgiri YD, Chen Y. (2003). A study on moisture isotherms of formulations: the use of polynomial equations to predict the moisture isotherms of tablet products. AAPS PharmSciTech 4:461–8
- Lim H, Hoag SW. (2013). Plasticizer effects on physical-mechanical properties of solvent cast Soluplus® films. AAPS PharmSciTech 14:903–10
- Linn M, Collnot EM, Djuric D, et al. (2012). Soluplus® as an effective absorption enhancer of poorly soluble drugs in vitro and in vivo. Eur J Pharm Sci 45:336–43
- Low AQ, Parmentier J, Khong YM, et al. (2013). Effect of type and ratio of solubilising polymer on characteristics of hot-melt extruded orodispersible films. Int J Pharm 455:138–47
- Morales JO, McConville JT. (2011). Manufacture and characterization of mucoadhesive buccal films. Eur J Pharm Biopharm 77:187–99
- Morita Y, Tsushima Y, Yasui M, et al. (2002). Evaluation of the disintegration time of rapidly disintegrating tablets via a novel method utilizing a CCD camera. Chem Pharm Bull 50:1181–6
- Nagy ZK, Balogh A, Vajna B, et al. (2012). Comparison of electrospun and extruded Soluplus®-based solid dosage forms of improved dissolution. J Pharm Sci 101:322–32
- Rabek CL, Van Stelle R, Dziubla TD, Puleo DA. (2014). The effect of plasticizers on the erosion and mechanical properties of polymeric films. J Biomater Appl 28:779–89
- Schubert MA, Muller-Goymann CC. (2005). Characterisation of surface-modified solid lipid nanoparticles (SLN): influence of lecithin and nonionic emulsifier. Eur J Pharm Biopharm 61:77–86
- Seikaly MG. (2007). Hypertension in children: an update on treatment strategies. Curr Opin Pediatr 19:170–7
- Shamma RN, Basalious EB, Shoukri RA. (2011). Development and optimization of a multiple-unit controlled release formulation of a freely water soluble drug for once-daily administration. Int J Pharm 405:102–12
- Shamma RN, Elsayed I. (2013). Transfersomal lyophilized gel of buspirone HCl: formulation, evaluation and statistical optimization. J Liposome Res 23:244–54
- Shoukri RA, Ahmed IS, Shamma RN. (2009). In vitro and in vivo evaluation of nimesulide lyophilized orally disintegrating tablets. Eur J Pharm Biopharm 73:162–71
- Siemann U. (2005). Solvent cast technology – a versatile toolfor thin film production. Progr Colloid Polym Sci 130:1–14
- Sikarra D, Shukla V, Kharia A, Chatterjee DP. (2012). Techniques for solubility enhancement of poorly soluble drugs. J Med Pharm Allied Sci 1:1–22
- Thakral NK, Ray AR, Bar-Shalom D, et al. (2012). Soluplus–solubilized citrated camptothecin–a potential drug delivery strategy in colon cancer. AAPS PharmSciTech 13:59–66
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. (2004). The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114:555–76
- Walker RD, Cumming GR. (1964). Response of the infant kidney to diuretic drugs. Can Med Assoc J 91:1149–53
- Zidan AS, Sammour OA, Hammad MA, et al. (2007). Quality by design: understanding the formulation variables of a cyclosporine A self-nanoemulsified drug delivery systems by Box-Behnken design and desirability function. Int J Pharm 332:55–63