Abstract
Vaginal candidiasis or vulvovaginal candidiasis (VC) is a common mucosal infection of vagina, mainly caused by Candida species. The major symptoms of VC are dyspareunia, pruritis, itching, soreness, vagina as well as vulvar erythema and edema. Most common risk factors that lead to the imbalance in the vaginal micro biota are the use of antibiotics, pregnancy, diabetes mellitus, immuno suppression as in AIDS or HIV patients, frequent sexual intercourse, spermicide and intra-uterine devices and vaginal douching. Various anti-fungal drugs are available for effective treatment of VC. Different conventional vaginal formulations (creams, gels, suppositories, powder, ointment, etc.) for VC are available today but have limited efficacy because of lesser residence time on vaginal epithelium due to self-cleansing action of vagina. So to overcome this problem, an extended and intimate contact with vaginal mucosa is desired; which can be accomplished by utilizing mucoadhesive polymers. Mucoadhesive polymers have an excellent binding capacity to mucosal tissues for considerable period of time. This unique property of these polymers significantly enhances retention time of different formulations on mucosal tissues. Currently, various novel formulations such as liposomes, nano- and microparticles, micro-emulsions, bio-adhesive gel and tablets are used to control and treat VC. In this review, we focused on current status of vaginal candidiasis, conventional and nanotechnology inspired formulation approaches.
Introduction
Vaginal candidiasis (VC) often referred to as vulvovaginal candidiasis, is a common mucosal infection of vagina, mainly caused by Candida species (Alexander et al., Citation2004) and alleged to be the second most prevalent mucosal infection after bacterial vaginosis. It is a far-flung infectious disease affecting about 75% of women of reproductive age (Song et al., Citation2004). In the United States alone, annually 13 millions of cases of VC are observed which further results in 10 million gynecologic office visits (Francois et al., Citation2003). In 2002 in United States, women spend over half a billion dollars on the medication for the treatment of VC, and about half of this amount was spend on over the counter medicines (Jyotsana et al., Citation2010). This is despite the fact that most of the women may wrongly diagnose VC as bacterial vaginosis (De Blaey & Polderman, Citation1980). The major symptoms of VC are dyspareunia, pruritis, itching, soreness, signs of vagina and vulvar erythema and edema (Lee, Citation1990; Francois et al., Citation2003). Candida species, especially Candida albicans is responsible for VC. It is a dimorphic commensal organism that domiciliation on skin, mucosa and gastrointestinal tract of 30–50% of normal healthy individual. Candida albicans is not a pathogen, but when local or systemic defense mechanism of the host got afflicted, Candida spp. can induce oropharyngeal, esophageal or VC (Woolfson et al., Citation2000). Under normal healthy conditions, lactobacillus in vagina produces lactic acid, which act as buffer and maintains the pH of vagina in the range 4–5 (acidic) and bacteriocins and hydrogen peroxide (H2O2), which resist the overgrowth of pathogenic microbes. In certain ill conditions, when this balance gets disturbed, there occurs excessive overgrowth of Candida sp. and diminution or depletion Lactobacillus spp. Following the overgrowth, there are two crucial elements responsible for the developments of VC are vaginal epithelium colonization and transformation of asymptomatic (saprophytic phase) to symptomatic (pathogenichyphal phase). Most common risk factors that lead to the imbalance in the vaginal micro biota are the use of antibiotics, pregnancy, diabetes mellitus, immuno suppression as in AIDS or HIV patients, frequent sexual intercourse, vaginal douching, spermicide and intra-uterine devices (Gagandeep et al., Citation2014). Most commonly used drugs for VC are Fluconazole, Clotrimazole, Metronidazole, Miconazole, Econazole, Ticonazole, Voriconazole, and Isoconazole. In pharmaceutical literature, vagina is described as slightly S-shaped fibro muscular, collapsible tubular organ of approximately 6–10 cm length that extends from cervix of the uterus to the vestibule of the external genitalia (Washington et al., Citation2000; Woolfson et al., Citation2000) and has two main functions: (1) Serves as receptacle for penis during sexual intercourse and carries sperm to the uterus and fallopian tubes. (2) As a birth canal for the passage of the baby during labor. The vagina comprises of three different cell layers: epithelial layer (superficial layer), lamina propria or tunica, muscular coat (D Amati et al., Citation2003). Overall of 10–15 layer cell turnover is expected in the time period of 7 d (Sjöberg et al., Citation1988). A brief description of vaginal anatomy and physiology is presented in .
Table 1. Anatomy and physiology of vagina.
Pathophysiology of vaginal candidiasis
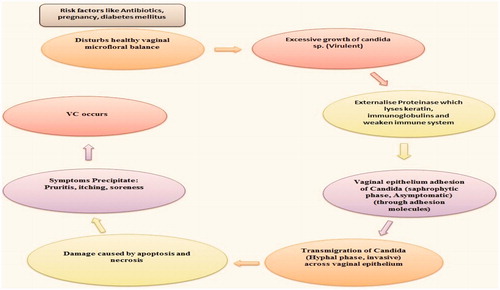
A normal healthy micro floral balance of Lactobacillus sp. and Candida sp. exists in vagina. When this balance gets disturbed due to certain risk factors, there occurs excessive growth of Candida sp. (Virulent). This is followed by cascade of reactions that finally leads to damage to vaginal epithelium and then symptoms get precipitated and VC occurs ().
Adhesion to vaginal epithelia
The initial and critical step toward fungal infections is the adhesion of Candida to epithelial cells. Candida albicans interacts by colonization and proliferation on epithelial cells followed by invasion, dissemination and damage. Cell wall components of Candida play a key role in adhesion process. Different cell wall protein adhesion candidates are:
The Als (agglutinin-like sequence) family: Till date 8 ALS genes have been recognized ALS 1–ALS 7 and ALS 9, that are involved in adhesion. N terminus of ALS protein is involved in ligand binding (Loza et al., Citation2004; Rauceo et al., Citation2006; Liu & Filler, Citation2011). Study of ALS-deleted mutants have variable effect on adhesion like expression of C. albicans ALS1 or ALS5 genes in non-adhesive, ALS4 deletion decreases C. albicans adherence to endothelial cell.
Hypha-associated genes: Hyphal wall protein (Hwp 1), major protein on hyphal cell wall. Its N-terminal domain serves as a substrate for epithelium transglutaminases. Thus, a strong covalent linking occurs between Hwp and epithelium proteins. Eap1, and Int1, also involved in adhesion but their binding ligands are unknown.
Integrin αm β2-like adhesins: Different ligands, including iC3b, fibrinogen, factor X, urokinase receptor, CD14, CD23, CD54 (ICAM-1), CD102 (ICAM-2), CD242 (ICAM-4), heparin, haptoglobin, kininogen, and various microbial proteins (Haas & Plow, Citation1994). Out of these molecules, only ICAM-1 and -2 are widely expressed on endothelial cells.
Integrin αv β3 and αvβ5-like adhesins: αv β3 like adhesion has been shown to bind to vitronectin (Spreghini et al., Citation1999; Santoni et al., Citation2001), but other ligands for αv β3 include CD31 (PECAM-1), Fibronectin, fibrinogen, thrombospondin, von Willebrand factor, and RGD sequence peptides (Haas & Plow, Citation1994). CD31 is expressed by endothelial cells and could act as a direct ligand for Candida adhesion αvβ5 ---- vitronectin, RGD sequence peptides but lack epithelium specific binding ligand (Jouault et al., Citation2006).
Transmigration vaginal epithelium
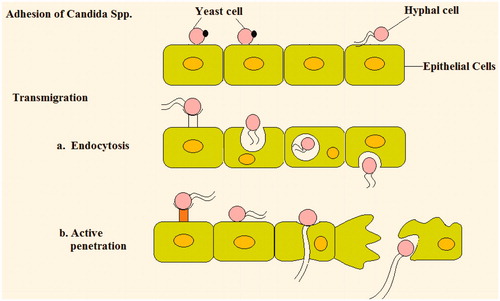
After adhesion, next step is the migration across vaginal epithelium. Different mechanism through which Candida migrates is:
Induced endocytosis: Two Candida invasions, ALS 3 and SSA 1 (SSA 1 is a member of the heat Shock protein (HSP) 70 family that is expressed on the cell surface). Present in hyphal cell wall and induce endocytosis. These binds to E- cadherin, then tyrosine phosphorylation occurs that leads to microfilament rearrangement and then leads to pseudopod formation and subsequent engulfment into the cell through clathrin mediated actin-dependent mechanism.
Active penetration: Fungi must be viable and changes to hyphal form during or after the penetration. Sap enzymes primarily contribute to active penetration. Sap (Secreted aspartic proteinases) 5 degrade E- cadherin of epithelial cell and violate integrity of vaginal epithelium, thereby enabling hyphal penetration into epithelial cells.
Damage: Once Candida goes across vaginal epithelium, it cusses severe damage by apoptosis and necrosis. However, exact mechanism behind this is yet to be revealed.
Table 2. Ligand specific adhesion and invasions involved in adhesion and transmigration across vaginal epithelium.
Prevalence of vaginal candidiasis
VC has a wide geographical distribution all over the world (). On the basis of various research papers it can be concluded that C. albicans is the most dominant and prevalent sp. in females with VC as it has an excellent binding capacity for mucus membrane. Non-albicans species are contributing to VC for a far lesser extent. VC with positive samples was observed to occur on higher levels on USA and UK while other countries had significant lesser number of positive samples. Among all positive samples, occurrence of C. albicans sp. was much higher than non-albicans sp. Among non-albicans sp., C. glabrata was the most common fungi found in subject's vagina. This vast variation was observed in females which are illiterate, high school education, married, having diabetes mellitus (Alli et al., Citation2011; Faraji et al., Citation2012), frequent sexual intercourse, oral contraceptives, spermicides (Alli et al., Citation2011), etc.
Table 3. Worldwide distribution of various Candida sp. responsible for vaginal candidiasis.
A concerning trend was observed in prevalence of Candida sp. in different age groups. With increasing age, a drastic fall in albicans sp. was observed; however, there is a significant increase in distribution of non-albicans sp. among which C. glabrata was the dominant one. Increase in number of C. glabrata was also observed in elderly diabetic patients (Vermitsky et al., Citation2008). This can be justified, as continuous use of azole agents leads to development of resistance in C. glabrata, which is characterized by higher colonization of C. glabrata in vaginal epithelium as compared to C. albicans. Thus for effective treatment of VC, proper microscopic identification of virulent sp. should be done. Non-azole anti-fungal drugs like Boric acid and Flucytosine can be used for non-albicans species (Ogunshe et al., Citation2008; Vermitsky et al., Citation2008; Abruquah, Citation2012).
Factor affecting vaginal drug absorption (Stewart-Tull, Citation1964; Hussain & Ahsan, Citation2005; Mathiowitz et al., Citation2013)
Like other mucosal routes, drug administrated via vaginal route is absorbed by three major ways: (1) transcellularly; mediated via concentration-dependent gradient (2) paracellularly; through tight junctions present in between the cells (3) vesicular or receptor-mediated transport as remarked by Ilium and Richardson. Absorption of drug from vagina follows two main steps: drug dissolution in vaginal lumen and membrane penetration. So any factor influencing physiology of vagina and formulation aspects like drug dissolution and membrane transport will potentially alter the absorption profile of drug from vaginal drug delivery systems (Garg et al., Citation2014a). Different vaginal physiological factors that influence drug absorption in vaginal cavity are discussed in .
Table 4. Factor affecting vaginal drug absorption.
Available therapies for vaginal candidiasis
Various anti-fungal drugs are available for effective treatment of VC. The treatment is initiated in symptomatic women because they have 80% of Candida colonization (Syed & Braverman, Citation2004). The endeavor of the treatment is to prevent over-growth of Candida that precipitates symptoms. Almost 3–7 d are sufficient for effective results. Formulation other than oral, are usually administered at night to prevent any leakage or removal from vagina. There is no evidence available favoring any specific formulation or any particular azole agent. However in case of severe infection, oral preparations may not provide symptomatic relief. In that case low-potency steroids as topical formulations should be used (Cejtin & Mason, Citation2000). Different oral and topical therapies available for VC are given in .
Table 5. Different available therapies for VC (Faro, Citation1994; Carr et al., Citation1998; Watson & Pirotta, Citation2011; Newson, Citation2013).
While these therapies are quite effective, but still associated with number of limitations like side effects, drug interaction, contraindication, etc., as presented in .
Table 6. Drug associated limitations for anti-fungal therapy.
Conventional topical intravaginal delivery systems
Creams and gels
Creams and gels as intravaginal delivery systems are used to deliver contraceptives and anti-bacterial agents (Garg & Goyal, Citation2014a). However these systems are messy in use, uncomfortable and because of non-uniformity and leakage, exact dose can never be provided. The worthy properties of vaginal creams and gels are acceptability; feasibility and non-toxic, non-irritant nature towards vaginal mucosa. Vaginal creams of metronidazole and clindamycin are found to be as efficacious as the orally administered drugs for treatment of bacterial vaginosis (Mcgregor et al., Citation1998). Oxytocin, dinoprostone and misoprostol used for cervical ripening and labor induction, can be administered in gel form. Shetty et al. studied efficacy of dinoprostone vaginal gel against oral tablet for induction of labor and observed significant difference there. Several researchers are comparing efficacy of vaginal gel with oral products for misoprostone, and the results obtained leads to conflicting outcome. Hall et al. reported that orally given misoprostone is far more safe and effective in labor induction against when it is vaginally administered. However, Shetty et al. concluded that among vaginally and orally administered misoprostone, vaginal delivery was the most effective. Vaccines can also be delivered intravaginal in the form of gel.
Pesseries and suppositories
A variety of vaginal medications are available in form of pesseries and suppositories. They are designed in such a way to melt in vaginal cavity and release active medicament in controlled manner. Suppositories are used for localized delivery of drugs like anti-septic, anti-fungal and contraceptives. Primarily, they are used to deliver drugs like dehydroepiandrosterone Sulphate (Yamashita et al., Citation1991) for cervical ripening, prior to birth; miconazole for VC (Vukovich et al., Citation1977; Abrams & Weintraub, Citation1983) and progesterone for hormonal replacement therapy. Different techniques for preparation of suppositories are hand-molding, pour-molding or by automatic machine (Brannon-Peppas, Citation1993; Hussain & Ahsan, Citation2005) where drug is dispersed in suppository base, e g. cocoa butter. Pesseries are known to deliver prostaglandin E2 (PGE2) for cervical ripening and labor induction. A semi-crystalline hydrogel of cross linked polyethylene oxide swells in saturated solution of PGE2 to give final product, pesseries.
Vaginal tablets, powder and ointment
Vaginal tablets contain same components as that of conventional oral tablets like binders, disintegrants and other excipients. These are advantageous over the other dosage form as have ease of manufacture and insertion. Usually deliver prostaglandins and anti-fungal drugs like Itraconazole, Clotrimazole, etc. Highly hydrophobic drugs are not suitable candidates for vaginal tablets as they have poor absorption. However, use of penetration enhancers like surfactants, bile salts can overcome this problem. Sometimes mucoadhesive polymers can be incorporated to enhance vaginal residence time. Polystyrene sulfonate (PSS) when formulated as vaginal tablet, have higher anti-microbial effect against HIV and HSV and is neither cytotoxic nor it inhibit vaginal flora (Kast et al., Citation2002). Vaginal powder is prepared by dissolving Hydroxypropyl cellulose in water with continuous heating. This mixture is then slightly cooled and bisphosphonate was added. This final mixture was then lyophilized. Vaginal ointment comprises of an aqueous phase and oil phase. Drug was dissolved in the aqueous phase and the oil phase was incorporated into it with mixing (Kaur et al., Citation2014).
Vaginal ring
Vaginal rings are circular device inserted in the vagina to achieve controlled release of the active medicament. These are approximately 5 cm in diameter and have 4–5 mm of cross-sectional diameter. These are generally polymeric rings in which the drug is homogeneously dispersed. These offer several advantages: user controlled, deliver drug continuously and do not interfere with coitus. From the surface of the ring, drug release at faster rate as compared to the inner layer of ring. This may provide an initial burst release of the drug followed by sustained release for several days. In order to achieve constant release, two types of system are developed for vaginal rings: sandwich and reservoir type. In sandwich type, a narrow layer of drug is placed between non-medicated central core and non-medicated outer band. In reservoir type, central core having the drug is encapsulated with drug-free polymer layer (Garg & Goyal, Citation2012). Commonly used polymers are poly (dimethylsiloxane) or silicone devices. Moreover in the recent years, elastomeric polymer like ethylene vinyl acetate and styrene are extensively used, as it have increased flexibility, improved optical properties, greater adhesion and increased impact and punch resistance (Novak et al., Citation2003). Vaginal rings are most commonly employed for hormonal replacement therapy and contraceptives delivery. To deliver contraceptive, rings are placed in vagina for 21 d followed by 1 week ring free for menstrual cycle to take place. NuvaRing is a common example of vaginal ring available in U.S market to deliver contraceptives. It is transparent, flexible ring containing etonogestrel and ethinyl estradiol and releases 120 mg/d of former and 15 mg/d of later one over a period of 3-week. Femring and Estring are employed for the hormonal replacement therapy. Dapivirine also known as TMC 120 is given in the form of ring acting as potent microbicide against transmission of STIs and HIV. Plastic rings are sometimes used to hold and support suppositories in position in vagina.
Limitations of conventional vaginal formulations
These conventional vaginal delivery systems are somewhat effective; however, they still offer several disadvantages which we need to encounter in order to deliver anti-fungal therapy in an efficacious way. Disadvantages associated are:
Leakage and messiness as in case of creams and gel
Uncomfortable
Efficacy is quite low as gels may not provide an exact dose because of non-uniformity and leakage
Low retention to the vaginal epithelium
Poor patient compliance
Frequent administration of drug is required
Prolonged duration of therapy
Low bioavailability
Drug release pattern is inappropriate (Parnami et al., Citation2013; Singh et al., Citation2014).
Novel approaches in vaginal formulations
Different conventional vaginal formulations for VC are available today but have limited efficacy because of lesser residence time on vaginal epithelium due to self-cleansing action of vagina. This leads to frequent administration of the formulation, which ultimately cause inconvenience to the user. So to overcome this problem, an extended and intimate contact with vaginal mucosa is desired; which can be accomplished by utilizing mucoadhesive polymers. Mucoadhesive polymers have an excellent binding capacity to mucosal tissues for considerable period of time (Kataria et al., Citation2014). This unique property of these polymers significantly enhances retention time of different formulations on mucosal tissues. Thus, controlled release can be fruitfully achieved and in turn frequent administration of dosage forms is prevented. Several bio-adhesive polymers are available like polycarbophil, hydroxypropylcellulose, polyacrylic acid, chitosan, carbopol, etc.
Vaginal bio-adhesive tablets
Method for preparation of vaginal bio-adhesive tablets is similar to those of normal tablet; however, they differ in composition of excipients as the former have a single or combination of bio-adhesive polymer and the latter one is devoid of it. In case of evaluation of these tablets, additional parameters are included like swelling index, bio-adhesion time and bio-adhesive strength. The very first bio-adhesive tablet prepared was of Bleomycin, antibiotic; containing polymers like hydroxy propyl cellulose (HPC) and poly acrylic acid (PAA) or Carbopol-934. It was observed that with increasing amount of HPC, in-vitro release rate increases and increment in concentration of PAA, water absorption property rises. 5-Flurouracil and Carbaquinone, potent anti-cancer drugs were also formulated in the tablet form (Brannon-Peppas, Citation1993). Various anti-fungal agents are formulated in form of bio-adhesive tablets are mentioned in .
Table 7. Vaginal bio-adhesive tablets loaded with anti-fungal agent.
Vaginal liposome
Liposomes are the spherical vesicle, characterized on their lipid composition, size, number of lamellae, and inner/outer phases (Garg & Goyal, Citation2014b). Because of biocompatibility, stability and structural versatility, these have been extensively used for different therapies (Goyal et al., Citation2013). For having high stability with good mucoadhesive strength, positively charged vesicles are preferred over negative ones; as mucus membrane is negatively charged (Garg et al., Citation2014b). Before 1990, liposomes were used for parenteral and skin delivery but later on there was a drastic shift in their use in vaginal drug delivery. Jain et al. utilized liposomes for vaginal delivery of progesterone (Citation1997). Foldvari et al. developed interferon alpha liposomes for treating genital papilloma virus infections. Pavelić et al. developed Lecithin liposomes of Clotrimazole, metronidazole and chloramphenicol for treating fungal infections; then tested for in-vitro stability in pre- and post- menopausal environment, as well as for in-situ stability in cow vaginal mucosa. In order to enhance stability, better release characteristics and overall applicability of these drugs, author incorporated these liposomes in bio-adhesive carbopol hydrogels. In-vitro release testing performed in vaginal fluid stimulant ensured controlled release of all three drugs (Pavelić et al., Citation1999). Ning et al. also reported controlled release of Clotrimazole from proliposomes for vaginal therapy. Poorly soluble anti-fungal drug, Amphotericin B was successfully administered in vagina when formulated as thermo-sensitive gel of poloxamers 407 and 188 having drug-loaded cationic liposomes (Kang et al., Citation2010). Curcumin, a well-known anti-oxidant and anti-inflammatory agent when formulated in form of liposomal gel against vaginal inflammation; overall anti-inflammatory activity was significantly enhanced as revealed by in-vitro studies (Basnet et al., Citation2012). Liposomal preparations loaded with anti-fungal agents are represented in .
Table 8. Anti-fungal agents formulated as vaginal liposomes.
Vaginal micro-emulsions
In recent times, micro emulsion serves as an efficient candidate for vaginal delivery of proteins, peptides and anti-fungal drugs, because of their long term stability, ease of preparation and high solubilization capacity. Micro-emulsion based vaginal gel system has been efficiently used to deliver different anti-fungal drugs as given in .
Table 9. Micro-emulsion based vaginal gel loaded with anti-fungal agents.
Vaginal bio-adhesive suppositories
Another novel approach towards successful vaginal delivery is the concept of bio-adhesive suppositories. To deliver anti-miotic agent, Clotrimazole into vagina, suppositories of semi-synthetic solid triglycerides were prepared having bio-adhesive polymers viz. polycarbophil, hydroxypropylmethylcellulose and hyaluronic sodium salt. The author reported that these polymer increased residence time of suppositories in vagina by modifying adhesion force, liquefaction time and permanence of drug at expected site without any adverse effect. The developed formulation showed controlled release profile.
Vaginal bio-adhesive gel
A marketed bio-adhesive gel, Replens R of polycarbophil; used to lubricate and to retain moisture of vagina. The formulation maintains healthy acidic pH in vagina and remains over there for 3–4 d (Lee et al., Citation1996; Hwang et al., Citation1977). Another bio-adhesive gel named Prochieve TM is used in hormonal replacement therapy. Later on, concept of hydrogel was introduced which provides excellent controlled release profile of most drugs. Hydrogels can be defined as a three-dimensional, cross-linked hydrophilic polymeric network which can absorb significant amount of water (Singh et al., Citation2010). These are insoluble in water because of cross-linked structure and can imbibe water up to 10–20 times of its molecular weight and become swollen (Kim et al., Citation1992; Peppas et al., Citation2000). Hydrogel swells under the influence of different stimuli-like temp, magnetic field, sound, electric field, etc. and then drug releases from swelled hydrogel in controlled manner (Garg et al., Citation2013). Different anti-fungal drugs formulated in form of bioadhesive gel are given in .
Table 10. Bio-adhesive gel loaded with different anti-fungal agents.
Vaginal micro particles (microspheres, microcapsules)
In the recent time, micro particles systems have also employed for designing vaginal delivery system. With addition of mucoadhesive polymers, these systems were made bio-adhesive so as to gain intimate prolonged contact with vaginal mucosa for controlled drug delivery (Garg et al., Citation2012). Ketoconazole was formulated as bio-adhesive microcapsules and incorporated in tablet for vaginal delivery. Dissolution studies of these microcapsules attest sustained release of the drug. Different anti-fungal drugs formulated in form of microparticles are given in .
Table 11. Different anti-fungal vaginal micro particle formulations.
Cyclodextrin in vaginal therapy
Cyclodextrin complexation was primarily used to increase bioavailability of poorly soluble drugs by incorporating them in cyclodextrin complexes (Patel & Rajesh). However, several studies advocate the use of Cyclodextrin for improving vaginal drug delivery. Hydroxypropyl-β-cyclodextrin formulation having Itraconazole was found to have good drug solubility and excellent mucoadhesive property. Vaginal cream having Itraconazole complexed with β-cyclodextrin was well tolerated and remained in vagina for several days. Hydroxypropyl-β-cyclodextrin complex was reported to increase solubility of Amphotericin B; when both were formulated as thermo-sensitive, pH-sensitive gel, controlled release was successfully achieved. Cyclodextrin complexes were also successfully employed in anti-viral therapy to deliver anti-HIV agents (Yang et al., Citation2008). Chang Yun et al (Citation2002) fabricated Clotrimazole-loaded cyclodextrin complex by using combination of poloxamers (P) 407, 188, and polycarbophil (PC). The results showed that controlled release of drug was achieved and exhibit excellent in-vivo anti-fungal activity in female rats (Yun Chang et al., Citation2002).
Other novel approaches against VC
With continuous use of anti-fungal agents for VC, subsequent failure of therapy was observed. This is due to development of resistant by Candida sp., so further prolongation of the therapy will be ineffective. In spite of that, a new concept of genetically engineered antibody was introduced, which successfully encounter this limitation (Garg et al., Citation2011). Different vaccines having modified antigens are introduced that produce C. albicans specific antibodies that either have fungicidal activity or inhibit adhesion of Candida to epithelial cells as described in . Different techniques employed for generating monoclonal antibodies are Hybridoma cell production, Recombinant antibody engineering technique, complementary-determining region (CDR) engraftment, Cambridge Antibody Technology (CAT) (De St Groth & Scheidegger, Citation1980), etc. Vaccine consists of diseases causing micro-organism either in dead or partly killed form which will stimulate immune system to recognize it as foreign microbe and act against it by producing antibodies. Antibodies are Y-shaped protein produced by plasma cells and utilized by immune system to recognize and neutralize foreign particles like bacteria or fungi. Monoclonal antibodies are the mono-specific antibodies produced from single parent immune cell by different techniques like hybridoma, recombinant technique, etc. Idiotypic is a shared characteristic of immunoglobulin or T cell receptors (TCR). Idiotypic describes distinctive sequence and region that makes any immunoglobing/TCR unique from others of the same type which is its variable region. Variable region has a specific amino-acid sequence that determines its antigen binding affinity and therefore the idiotope of the molecule. IgG or T cell receptor having shared idiotope is the same idiotype (Miller et al., Citation1982). The main component of these vaccines can be whole Candida cell or its cell extract, antibodies, idiotypes, glycoconjugate and its subunits, human peptide, antigen-pulsed cell, etc. Method for production these different antigens are discussed below
Human Peptide Hepcidin 20: Human liver derived Hepticin-20 has a significant anti-fungal activity and is either purchased or extracted from human source. This peptide release β-glycosidase that cleaves β-Glycoside linking in fungal cell wall.
Candida surface mannan complex: Candida surface mannan was obtained by extracting yeast cell with β-mercaptoethanol and then encapsulated in multilamillar liposomescomposed of phosphatidylcholine and cholesterol in ratio 3.2:1. After immunization antibodies specific to β 1, 2-mannotriose (cell wall component of Candida) is produced, and exerts their anti-fungal activity (Han et al., Citation1998). This mannan extract may complexed with Bovine serum albumin, and immunization leads to production of IgG and IgM antibodies that degrade Candida cell wall mannan (mannoprotein; Han et al., Citation1999).
Dendritic cells pulsed with fungal RNA: Candida cells were ruptured through repeated thawing and freezing on liquid nitrogen. Hot extraction buffer (a 1:1 mixture of phenol and 0.1 M LiCl, 100 mM Tris-HCl (pH 8), 10 mM EDTA, and 1% SDS at 80 °C) and then a mixture ((24:1, v/v) of chloroform and isoamyl alcohol) was added to the cells. Followed by centrifugation at 10 000 rpm at 4 °C and water phase was mixed with equal volume of 4M lithium chloride. This mixture was again centrifuged at 10 000 at 4 °C and the RNA gets precipitated. Obtained RNA pellet was dissolved in water and precipitated using sodium acetate and ethanol at −20 °C. Dendritic cells (DCs) were either extracted from bone-marrow of spleen. Spleen cells were subjected to overnight plastic adherence to remove macrophages, then reacted with 100 µl of anti-mouse CD11c mAbs (against CD11c present on macrophage surface) conjugated with Micro Beads followed by magnetic separation. Bone marrow DCs, were obtained from femur of mice and seeded for 6 d in six-well plates in 3 ml IMDM (Iscove's Modified Dulbecco's Medium) with 10% FCS (fetal calf serum), 50 mM 2-ME(2-mercaptoethanol), 50 µg/ml gentamicin sulfate, 2000 U/ml GM-CSF (granulocyte-macrophage colony-stimulating factor), and 1 × 103 U/ml IL-4. On day 3, non-adherent cells were replaced with mixture of GM-CSF and interleukin-4. On day 6, DCs were isolated from non-adherent cells and incubated at 37 °C for 3 h. RNA (25 µg in 250 µl Opti-MEM medium) and DOTAP (50 µg in 250 µl Opti-MEM medium (minimum essential media)) was mixed in 12 × 75-mm polystyrene tubes and then 2 ml of it was added to DC and incubated for 37 °C for 24 h. IL-4 Immunization leads to activation of T-helper 1 thus initiating immune response against Candida by releasing cytokines IL-4,IL-6,IL-10,IL-12 P70 (Bacci et al., Citation2002).
Human domain antibodies: A complex procedure was adopted to yield Human domain antibodies against virulent traits of Candida (De Bernardis et al., Citation2007). In brief, the author uses genetically modified Antibody variable domains (domain antibodies [DAbs]) that have individual heavy-chain (VH) or k-chain (Vk) variable domains and lacks the Fc region. From Phage expression libraries, Human DAbs against 65-kDa mannoprotein (MP65) or the secretory aspartyl proteinase (SAP)–2 of C. albicans (mono-specific DAbs) or against both fungal antigens (heterodimeric, bispecific DAbs) were generated. A significant inhibition of fungal adherence (mediated by MP65 and SAP)–2) and complete clearance of vaginal infection of fungus was observed using both mono- and bi-specific DAbs in rat vagina.
B-glucan-conjugate vaccine for VC: Donatella et al. formulated β-glucan-conjugate in human compatible MF59 adjuvant and anti-fungal activity was assessed in murine model (Pietrella et al., Citation2010). The infection was monitored using genetically engineered, luminescent C. albicans strain and then Cfu was measured. The mice were immunized with this conjugate and then a prominent fall in Cfu of C. albicans was observed. This anti-fungal activity was due to production of serum and vaginal anti-β-glucan IgG antibodies. This antibody recognizes octa-β- 1, 3-glucan epitope which is present in hyphal cell wall protein that mediates fungal adhesion and invasion (Torosantucci et al., Citation2009). Then in-vivo imaging techniques confirm excellent anti-fungal activity. Antonella et al. reported good protection against VC in mice by formulating β-glucan (preparation from the brown alga Laminaria digitata) conjugate with diphtheria toxoid CRM197 (carrier protein). This conjugate produces anti-β-glucan IgG antibodies which provide protection against Candida sp. (Torosantucci et al., Citation2005).
Recombinant ALS vaccine for VC: Ibrahim et al. developed vaccine of recombinant N-terminus of Als 1p (rAls 1p) for protection of mice against disseminated and mucosal candidiasis. The vaccine enhances cell-mediated immunity rather than humoral and improves survival of mice during candidiasis (Ibrahim et al., Citation2005). Latter on they formulated another vaccine of recombinant N-teminus of Als 3p (rAls 3p) against disseminated and mucosal Candidiasis. The vaccine proved to as effective as rAls 1p in disseminated Candidiasis and more effective in Mucosal (vaginal) Candidiasis (Spellberg et al., Citation2006).
Candida albicans mannan extract–protein conjugates: Vaccine having C. albicans Mannan (fungal cell wall constituent) extract- Bovine serum albumin conjugate was assessed for its anti-fungal activity in mice. The vaccine was administered intraperitoneal (i.p) followed by i.v. administration of viable Candida spp. Mice developed both IgG and IgM antibodies specific for the cell surface of Candida yeast cells and exerts its anti-fungal activity (Han et al., Citation1999). represents the recently developed vaccines against VC.
Table 12. Recently developed vaccines against VC.
Conclusion
Although much of research work has been done to deliver anti-fungal drugs safely and effectively for VC, various conventional dosage forms are available like creams, gel, suppositories, etc. But have numerous limitations like systemic side-effects, lesser residence time, etc. To overcome these limitations, a novel concept of bio-adhesive formulations was introduced. While this delivery system successfully encountered most of the disadvantages of conventional dosage forms, but there continuous use has led to significant resistance in Candida against azole agents. However now a day, vaccines are employed for anti-fungal therapy and they have been proved to be a good and potential alternative for VC. But this delivery system still needs to be exploited, in order to develop a novel, ideal, effective delivery system against all Candida spp. and to protect and maintain integrity of epithelial cells.
Declaration of interest
The authors declare no conflicts of interests. The authors alone are responsible for the content and writing of this article.
Acknowledgement
Authors Amit K Goyal (under IYBA scheme; BT/01/IYBA/2009 dated 24/05/2010) thankful to Department of Biotechnology (DBT), New Delhi, India.
References
- Abrams L, Weintraub H. (1983). Disposition of radioactivity following intravaginal administration of 3H-miconazole nitrate. Am J Obstetr Gynecol 147:970–1
- Abruquah H. (2012). Prevalence and antifungal susceptibility of Candida species isolated from women attending a gynaecological clinic in Kumasi, Ghana. J Sci Technol (Ghana) 32:39–45
- Ahmad FJ, Alam MA, Khan ZI, et al. (2008). Development and in vitro evaluation of an acid buffering bioadhesive vaginal gel for mixed vaginal infections. Acta pharmaceutica 58:407–19
- Alexander NJ, Baker E, Kaptein M, et al. (2004). Why consider vaginal drug administration? Fertil Steril 82:1–12
- Alli J, Okonko I, Odu N, et al. (2011). Detection and prevalence of Candida isolates among patients in Ibadan, Southwestern Nigeria. J Microbiol Biotechnol Res 1(3):176–184
- Ameen DW. (2011). Development and in vitro evaluation of bioadhesive vaginal tablet using econazole nitrate as a model drug. Iraqi J Pharmaceut Sci 57:57–65
- Bacci A, Montagnoli C, Perruccio K, et al. (2002). Dendritic cells pulsed with fungal RNA induce protective immunity to Candida albicans in hematopoietic transplantation. J Immunol 168:2904–13
- Bachhav YG, Patravale VB. (2009). Microemulsion-based vaginal gel of clotrimazole: formulation, in vitro evaluation, and stability studies. AAPS PharmSciTech 10:476–81
- Basnet P, Hussain H, Tho I, et al. (2012). Liposomal delivery system enhances anti-inflammatory properties of curcumin. J Pharmaceut Sci 101:598–609
- Bhalekar MR, Pokharkar V, Madgulkar A, et al. (2009). Preparation and evaluation of miconazole nitrate-loaded solid lipid nanoparticles for topical delivery. AAPS PharmSciTech 10:289–96
- Bhat S, Shivakumar H. (2010a). Bioadhesive controlled release clotrimazole vaginal tablets. Trop J Pharmaceut Res 9(4): 339–46
- Bhat SR, Shivakumar HG. (2010b). Formulation development and evaluation of thermosensitive gel for vaginal drug delivery. Latin Am J Pharm 29(7): 1093–9
- Bhowmik BB, Nayak BS, Chatterjee A. (2009). Formulation development and characterization of metronidazole microencapsulated bioadhesive vaginal gel. Int J Pharm Pharm Sci 1:240–57
- Bilensoy E, Rouf MA, Vural I, et al. (2006). Mucoadhesive, thermosensitive, prolonged-release vaginal gel for clotrimazole: β-cyclodextrin complex. AAPS PharmSciTech 7:E54–60
- Brannon-Peppas L. (1993). Novel vaginal drug release applications. Adv Drug Deliv Rev 11:169–77
- Cárdenas-Freytag L, Cheng E, Mayeux P, et al. (1999). Effectiveness of a vaccine composed of heat-killed Candida albicans and a novel mucosal adjuvant, LT (R192G), against systemic candidiasis. Infect Immun 67:826–33
- Carr PL, Felsenstein D, Friedman RH. (1998). Evaluation and management of vaginitis. J Gen Intern Med 13:335–46
- Cejtin HE, Mason ED. (2000). A guide to the diagnosis and treatment of vaginitis and cervicitis. Hosp Phys 53–63
- Choudhury A, Das S, Kar M. (2011). A review on novelty and potentiality of vaginal drug delivery. Int J PharmTech Res 3:1033–44
- D Amati G, Di Gioia C, Pannunzi LP, et al. (2003). Functional anatomy of the human vagina. J Endocrinol Investig 26:92–6
- Dangi AA, Sheth NR, Patel H, et al. (2011). Formulation and evaluation of once daily mucoadhesive vaginal tablet of clotrimazole using natural and synthetic polymers. Asian J. Pharm. Health Sci 1:176–82
- De Bernardis F, Amacker M, Arancia S, et al. (2012). A virosomal vaccine against candidal vaginitis: immunogenicity, efficacy and safety profile in animal models. Vaccine 30:4490–8
- De Bernardis F, Boccanera M, Adriani D, et al. (2002). Intravaginal and intranasal immunizations are equally effective in inducing vaginal antibodies and conferring protection against vaginal candidiasis. Infect Immun 70:2725–9
- De Bernardis F, Liu H, O'Mahony R, et al. (2007). Human domain antibodies against virulence traits of Candida albicans inhibit fungus adherence to vaginal epithelium and protect against experimental vaginal candidiasis. J Infect Dis 195:149–57
- De Blaey C, Polderman J. (1980). Rationales in the design of rectal and vaginal delivery forms of drugs. Drug Design 9:237–66
- De St Groth SF, Scheidegger D. (1980). Production of monoclonal antibodies: strategy and tactics. J Immunol Meth 35:1–21
- Del Gaudio G, Lombardi L, Maisetta G, et al. (2013). Antifungal activity of the noncytotoxic human peptide hepcidin 20 against fluconazole-resistant Candida glabrata in human vaginal fluid. Antimicrob Agents Chemotherap 57:4314–21
- Dhanaraj A, Jaya Raja Kumar, Jayachandran K, et al. (2013). Development and in vitro/in vivo evalution of tripolymers based clotrimazole in situ gel for oral thrush. Journal of pharmaceutical science and technology 3(10):985–95
- Dias LB, Melhem MDSC, Szeszs MW, et al. (2011). Vulvovaginal candidiasis in Mato Grosso, Brazil: pregnancy status, causative species and drugs tests. Brazil J Microbiol 42:1300–7
- El-Din S, Reynolds M, Ashbee H, et al. (2001). An investigation into the pathogenesis of vulvo-vaginal candidosis. Sex Trans Infect 77:179–83
- Ernest J. (1992). Topical antifungal agents. Obstetr Gynecol Clin N Am 19:587–607
- Faraji R, Rahimi MA, Assarehzadegan M. (2012). Prevalence of vaginal candidiasis infection in women referred to Kermanshah hygienic centers, Iran in 2010. Life Sci J 6:2273–8
- Faro S. (1994). Systemic vs. topical therapy for the treatment of vulvovaginal candidiasis. Infect Dis Obstetr Gynecol 1:202–8
- Fong I. (1992). The value of chronic suppressive therapy with itraconazole versus clotrimazole in women with recurrent vaginal candidiasis. Genitourinary Med 68:374–7
- Francois M, Snoeckx E, Putteman P, et al. (2003). A mucoadhesive, cyclodextrin-based vaginal cream formulation of itraconazole. Aaps Pharmsci 5:50–4
- Gagandeep GT, Malik B, Rath G, Goyal AK. (2014). Development and characterization of nano-fiber patch for the treatment of glaucoma. Eur J Pharm Sci 53:10–6
- Garg T, Bilandi A, Kapoor B. (2011). Scaffold: tissue engineering and regenerative medicine. Int Res J Pharm 2:37–42
- Garg T, Goyal AK. (2012). Iontophoresis: drug delivery system by applying an electrical potential across the skin. Drug Deliv Lett 2:270–80
- Garg T, Goyal AK. (2014a). Biomaterial-based scaffolds - current status and future directions. Expert Opin Drug Deliv 11:767–89
- Garg T, Goyal AK. (2014b). Liposomes: targeted and controlled delivery system. Drug Deliv Lett 4:62–71
- Garg T, Rath G, Goyal AK. (2014a). Comprehensive review on additives of topical dosage forms for drug delivery. Drug Deliv
- Garg T, Rath G, Goyal AK. (2014b). Novel technology to improve drug loading in polymeric nanofibers. Drug Deliv Lett 4:79–86
- Garg T, Singh O, Arora S, Murthy R. (2012). Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst 29:1–63
- Garg T, Singh S, Goyal AK. (2013). Stimuli-sensitive hydrogels: an excellent carrier for drug and cell delivery. Crit Rev Ther Drug Carrier Syst 30:369–409
- Gearhart MO. (1994). Worsening of liver function with fluconazole and review of azole antifungal hepatotoxicity. Ann Pharmacother 28:1177–81
- Goyal G, Garg T, Malik B, et al. (2013). Development and characterization of niosomal gel for topical delivery of benzoyl peroxide. Drug Deliv
- Gupta NV, Natasha S, Getyala A, Bhat RS. (2013). Bioadhesive vaginal tablets containing spray dried microspheres loaded with clotrimazole for treatment of vaginal Candidiasis. Acta Pharmaceutica 63:359–72
- Haas TA, Plow EF. (1994). Integrin-ligarid interactions: a year in review. Curr Opin Cell Biol 6:656–62
- Han Y, Morrison RP, Cutler JE. (1998). A vaccine and monoclonal antibodies that enhance mouse resistance to Candida albicans vaginal infection. Infect Immun 66:5771–6
- Han Y, Ulrich MA, Cutler JE. (1999). Candida albicans mannan extract—protein conjugates induce a protective immune response against experimental candidiasis. J Infect Dis 179:1477–84
- Hani U, Shivakumar H. (2013). Development of miconazole nitrate thermosensitive bioadhesive vaignal gel for vaginal candidiasis. American Journal of Advanced Drug Delivery 3:358–368
- Hani U, Shivakumar H, Gowrav M. (2014). Formulation design and evaluation of a novel vaginal delivery system of clotrimazole. International Journal of Pharmaceutical Science and Research 5(1):220–27
- Hay RJ. (1993). Risk/benefit ratio of modern antifungal therapy: focus on hepatic reactions. J Am Acad Dermatol 29:S50–4
- Hussain A, Ahsan F. (2005). The vagina as a route for systemic drug delivery. J Control Rel 103:301–13
- Hwang S, Owada E, Suhardja L, et al. (1977). Systems approach to vaginal delivery of drugs IV: methodology for determination of membrane surface pH. J Pharmaceut Sci 66:778–81
- Hwang S, Owada E, Yotsuyanagi T, et al. (1976). Systems approach to vaginal delivery of drugs II: in situ vaginal absorption of unbranched aliphatic alcohols. J Pharmaceut Sci 65:1574–78
- Ibrahim AS, Spellberg BJ, Avenissian V, et al. (2005). Vaccination with recombinant N-terminal domain of Als1p improves survival during murine disseminated candidiasis by enhancing cell-mediated, not humoral, immunity. Infect Immun 73:999–1005
- Jain SK, Singh R, Sahu B. (1997). Development of a liposome based contraceptive system for intravaginal administration of progesterone. Drug Dev Indust Pharm 23:827–30
- Jindal N, Gill P, Aggarwal A. (2007). An epidemiological study of vulvovaginal candidiasis in women of childbearing age. Ind J Med Microbiol 25:175
- Johnson T, Greer I, Kelly R, Calder A. (1992). The effect of pH on release of PGE2 from vaginal and endocervical preparations for induction of labour: an in-vitro study. BJOG: An Int J Obstetr & Gynaecol 99:877–80
- Jouault T, El Abed-El Behi M, Martínez-Esparza M, et al. (2006). Specific recognition of Candida albicans by macrophages requires galectin-3 to discriminate Saccharomyces cerevisiae and needs association with TLR2 for signaling. J Immunol 177:4679–87
- Jyotsana M, Sagar B, Mahesh D. (2010). Mucosal drug delivery system. IJRAP 1:63–7
- Kang J-W, Davaa E, Kim Y-T, Park J-S. (2010). A new vaginal delivery system of amphotericin B: a dispersion of cationic liposomes in a thermosensitive gel. J Drug Target 18:637–44
- Karavana SY, Rençber S, Şenyiğit ZA, Baloğlu E. (2012). A new in-situ gel formulation of itraconazole for vaginal administration. Pharmacol Pharm 3(4):417–26
- Kast CE, Valenta C, Leopold M, Bernkop-Schnürch A. (2002). Design and in vitro evaluation of a novel bioadhesive vaginal drug delivery system for clotrimazole. J Control Rel 81:347–54
- Kataria K, Garg T, Goyal AK, Rath G. (2014). Novel technology to improve drug loading in polymeric nanofibers. Drug Deliv Lett 4:79–86
- Katz DF, Dunmire EN. (1993). Cervical mucus: problems and opportunities for drug delivery via the vagina and cervix. Adv Drug Deliv Rev 11:385–401
- Kaur M, Garg T, Rath G, Goyal AK. (2014). Current nanotechnological strategies for effective delivery of bioactive drug molecules in the treatment of tuberculosis. Crit Rev Ther Drug Carrier Syst 31:49–88
- Khan F, Baqai R. (2010). In vitro antifungal sensitivity of fluconazole, clotrimazole and nystatin against vaginal candidiasis in females of childbearing age. J Ayub Med Coll Abbottabad 22:197–200
- Kim SW, Bae YH, Okano T. (1992). Hydrogels: swelling, drug loading, and release. Pharmaceut Res 9:283–90
- Lee CH, Anderson M, Chien YW. (1996). Characterization of in-vitro spermicidal activity of chelating agent against human sperm. J Pharmaceut Sci 85:649–54
- Lee V. (1990). Peptide and protein drug delivery. Boca Raton, FL: CRC Press
- Liu Y, Filler SG. (2011). Candida albicans Als3, a multifunctional adhesin and invasin. Eukaryotic Cell 10:168–73
- Loza L, Fu Y, Ibrahim AS, et al. (2004). Functional analysis of the Candida albicans ALS1 gene product. Yeast 21:473–82
- Masters WH, Johnson VE. (1966). Human sexual response. Boston (MA): Little, Brown & Co
- Mathiowitz E, Chickering III DE, Lehr C-M. (2013). Bioadhesive drug delivery systems: fundamentals, novel approaches, and development. Boca Raton, FL: CRC Press
- Mcgregor JA, Ismail M, Mccormack WM. (1998). A pilot study of metronidazole vaginal gel versus oral metronidazole for the treatment of Trichomonas vaginalis vaginitis. Sex Trans Dis 25:176–9
- Miller RA, Maloney DG, Warnke R, Levy R. (1982). Treatment of B-cell lymphoma with monoclonal anti-idiotype antibody. New Engl J Med 306:517–22
- Newson L. (2013). Management of vulvovaginal infections in primary care part 1: Candidiasis. Br J Fam Med 1:3
- Ning M, Guo Y, Pan H, et al. (2005). Preparation, in vitro and in vivo evaluation of liposomal/niosomal gel delivery systems for clotrimazole. Drug Dev Industr Pharm 31:375–83
- Novak A, De La Loge C, Abetz L, Van Der Meulen E. (2003). The combined contraceptive vaginal ring, NuvaRing®: an international study of user acceptability. Contraception 67:187–94
- Ogunshe AA, Lawal OA, Iheakanwa CI. (2008). Effects of Simulated Preparations of Plants used in Nigerian Traditional Medicine on Candida spp. Associated with Vaginal Candidiasis. Ethnobot Res Appl 6:373–83
- Owen DH, Dunmire EN, Plenys AM, Katz DF. (1999). Factors influencing nonoxynol-9 permeation and bioactivity in cervical mucus. J Control Rel 60:23–34
- Paavonen J. (1982). Physiology and ecology of the vagina. Scandi J Infect Dis. Suppl 40:31–5
- Pachl J, Svoboda P, Jacobs F, et al. (2006). A randomized, blinded, multicenter trial of lipid-associated amphotericin B alone versus in combination with an antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin Infect Dis 42:1404–13
- Parnami N, Garg T, Rath G, Goyal AK. (2013). Development and characterization of nanocarriers for topical treatment of psoriasis by using combination therapy. Artif Cells Nanomed Biotechnol
- Patel A, Patel J. (2012). Mucoadhesive microemulsion based prolonged release vaginal gel for anti-fungal drug. Am J PharmTech Res 2:649–61
- Patel A, Patel K, Patel J. (2012). Design, development and in vitro evaluation of sertaconazole mucoadhesive vaginal tablet. Der Pharmacia Lettre 4:418–27
- Patel GM, Patel AP. (2010). A novel effervescent bioadhesive vaginal tablet of ketoconazole: formulation and invitro evaluation. Int J PharmTech Res 2(1):656
- Patel N, Rajesh K. (2014). Ophthalmic in situ gel. Pharmagene 1:29–33
- Pavelić Ž, Škalko-basnet N, Jalšenjak I. (1999). Liposomes containing drugs for treatment of vaginal infections. Eur J Pharmaceut Sci 8:345–51
- Pavelić Ž, Škalko-Basnet N, Schubert R. (2001). Liposomal gels for vaginal drug delivery. Int J Pharmaceut 219:139–49
- Peppas N, Bures P, Leobandung W, Ichikawa H. (2000). Hydrogels in pharmaceutical formulations. Eur J Pharmaceut Biopharmaceut 50:27–46
- Pietrella D, Rachini A, Torosantucci A, et al. (2010). A β-glucan-conjugate vaccine and anti-β-glucan antibodies are effective against murine vaginal candidiasis as assessed by a novel in vivo imaging technique. Vaccine 28:1717–25
- Pirotta MV, Garland SM. (2006). Genital Candida species detected in samples from women in Melbourne, Australia, before and after treatment with antibiotics. J Clin Microbiol 44:3213–17
- Polonelli L, Seguy N, Conti S, et al. (1997). Monoclonal yeast killer toxin-like candidacidal anti-idiotypic antibodies. Clin Diagnos Lab Immunol 4:142–6
- Rauceo JM, De Armond R, Otoo H, et al. (2006). Threonine-rich repeats increase fibronectin binding in the Candida albicans adhesin Als5p. Eukaryotic Cell 5:1664–73
- Richter SS, Galask RP, Messer SA, et al. (2005). Antifungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. J Clin Microbiol 43:2155–62
- Robinson JR, Bologna WJ. (1994). Vaginal and reproductive system treatments using a bioadhesive polymer. J Control Rel 28:87–94
- Sandini S, La Valle R, De Bernardis F, et al. (2007). The 65 kDa mannoprotein gene of Candida albicans encodes a putative β-glucanase adhesin required for hyphal morphogenesis and experimental pathogenicity. Cell Microbiol 9:1223–38
- Santoni G, Spreghini E, Lucciarini R, et al. (2001). Involvement of αvβ3 integrin-like receptor and glycosaminoglycans in Candida albicans germ tube adhesion to vitronectin and to a human endothelial cell line. Microbial Pathogen 31:159–72
- Shafik SA, Hassan YM, Eldin AK. (2007). Vaginal candidiasis: incidence, etiology and pathogenesis. JASMR 2:115–27
- Sharma G, Jain S, Tiwari A, Kaur G. (2006). Once daily bioadhesive vaginal clotrimazole tablets: design and evaluation. Acta Pharmaceutica-Zagreb- 56:337
- Singh A, Sharma PK, Garg VK, Garg G. (2010). Hydrogels: a review. Int J Pharmaceut Sci Rev Res 4(2): 97–105
- Singh H, Sharma R, Joshi M, et al. (2014). Transmucosal delivery of Docetaxel by mucoadhesive polymeric nanofibers. Artif Cells Nanomed Biotechnol
- Sjöberg I, Cajander S, Rylander E. (1988). Morphometric characteristics of the vaginal epithelium during the menstrual cycle. Gynecol Obstetr Invest 26:136–44
- Sobel JD, Brooker D, Stein GE, et al. (1995). Single oral dose fluconazole compared with conventional clotrimazole topical therapy of Candida vaginitis. Am J Obstetr Gynecol 172:1263–8
- Sobel JD, Wiesenfeld HC, Martens M, et al. (2004). Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med 351:876–83
- Song Y, Wang Y, Thakur R, et al. (2004). Mucosal drug delivery: membranes, methodologies, and applications. Crit Rev™ Therapeut Drug Carrier Syst 21(3): 195–256
- Spellberg BJ, Ibrahim AS, Avanesian V, et al. (2006). Efficacy of the anti-Candida rAls3p-N or rAls1p-N vaccines against disseminated and mucosal candidiasis. J Infect Dis 194:256–60
- Spreghini E, Gismondi A, Piccoli M, Santoni G. (1999). Evidence for αvβ3 and αvβ5 integrin-like vitronectin (VN) receptors in Candida albicans and their involvement in yeast cell adhesion to VN. J Infect Dis 180:156–66
- Stein GE, Gurwith D, Mummaw N, Gurwith M. (1986). Single-dose tioconazole compared with 3-day clotrimazole treatment in vulvovaginal candidiasis. Antimicrob Agents Chemother 29:969–71
- Stein GE, Mummaw N. (1993). Placebo-controlled trial of itraconazole for treatment of acute vaginal candidiasis. Antimicrob Agents Chemother 37:89–92
- Stewart-Tull D. (1964). Evidence that vaginal lactobacilli do not ferment glycogen. Am J Obstetr Gynecol 88:676
- Syed TS, Braverman PK. (2004). Vaginitis in adolescents. Adolesc Med Clin 15:235–51
- Torosantucci A, Bromuro C, Chiani P, et al. (2005). A novel glyco-conjugate vaccine against fungal pathogens. J Exp Med 202:597–606
- Torosantucci A, Chiani P, Bromuro C, et al. (2009). Protection by anti-β-glucan antibodies is associated with restricted β-1, 3 glucan binding specificity and inhibition of fungal growth and adherence. PLoS One 4:e5392
- Vanic Z, Hafner A, Bego M, Škalko-basnet N. (2013). Characterization of various deformable liposomes with metronidazole. Drug Dev Indus Pharm 39:481–8
- Vermesh M, Fossum GT, Kletzky OA. (1988). Vaginal bromocriptine: pharmacology and effect on serum prolactin in normal women. Obstetr Gynecol 72:693–8
- Vermitsky J-P, Self MJ, Chadwick SG, et al. (2008). Survey of vaginal-flora Candida species isolates from women of different age groups by use of species-specific PCR detection. J Clin Microbiol 46:1501–3
- Vukovich R, Heald A, Darragh A. Vaginal absorption of 2 imidazole antifungal agents, econazole and miconazole. Clinical pharmacology & therapeutics. St Louis (MO): Westline Industrial DR, Mosby-Year Book INC. 11830;1977:121–21
- Wang L, Tang X. (2008). A novel ketoconazole bioadhesive effervescent tablet for vaginal delivery: design, in vitro and ‘in vivo’evaluation. Int J Pharmaceut 350:181–7
- Washington N, Washington C, Wilson C. (2000). Physiological pharmaceutics: barriers to drug absorption. Boca Raton, FL: CRC Press
- Watson C, Pirotta M. (2011). Recurrent vulvovaginal candidiasis: current management. Austral Fam Phys 40:149
- Woolfson AD, Malcolm RK, Gallagher R. (2000). Drug delivery by the intravaginal route. Crit Rev™ Therapeut Drug Carrier Syst 17(5): 509–55
- Yamashita A, Oshima S, Matsuo K, et al. (1991). [Pharmacological studies of intravaginally applied dehydroepiandrosterone sulfate (DHA-S)]. Nihon yakurigaku zasshi. Folia Pharmacologica Japonica 98:31–9
- Yang H, Parniak MA, Isaacs CE, et al. (2008). Characterization of cyclodextrin inclusion complexes of the anti-HIV non-nucleoside reverse transcriptase inhibitor UC781. AAPS J 10:606–13
- Yellanki SK, Goranti S, Deb SK. (2010). Development of metronidazole intravaginal gel for the treatment of bacterial vaginosis: effect of mucoadhesive natural polymers on the release of metronidazole. Int J PharmTech Res 2(3): 1746–50
- Yun Chang J, Oh Y-K, Soo Kong H, et al. (2002). Prolonged antifungal effects of clotrimazole-containing mucoadhesive thermosensitive gels on vaginitis. J Control Rel 82:39–50