Abstract
Today, ∼74% of drugs are taken orally and are not found to be as effective as desired. To improve such characteristics, transdermal drug delivery was brought to existence. This delivery system is capable of transporting the drug or macromolecules painlessly through skin into the blood circulation at fixed rate. Topical administration of therapeutic agents offers many advantages over conventional oral and invasive techniques of drug delivery. Several important advantages of transdermal drug delivery are prevention from hepatic first pass metabolism, enhancement of therapeutic efficiency and maintenance of steady plasma level of the drug. Human skin surface, as a site of drug application for both local and systemic effects, is the most eligible candidate available. New controlled transdermal drug delivery systems (TDDS) technologies (electrically-based, structure-based and velocity-based) have been developed and commercialized for the transdermal delivery of troublesome drugs. This review article covers most of the new active transport technologies involved in enhancing the transdermal permeation via effective drug delivery system.
Introduction
Transdermal delivery system is capable of transporting the drug through skin into the blood circulation at fixed rate. Transdermal route gives an alternative to oral and i.v. delivery. Benefits of local delivery are properly documented which includes targeted delivery, lower systemic exposure and lower toxicity than oral medications. This system is also helpfull in the treatment of hair loss, neuropathic pain, acne, genital herpes, migraine, headaches and sexual dysfunction (Singh et al., Citation2014a). It comprises list of advantages over conventional routes such as:
Transdermal delivery avoids the stomach environment where the drug can be degraded (Singh et al., Citation2012b).
Transdermal delivery avoids the first pass effect where active drug molecules can be converted to inactive molecules or even to molecules responsible for side effects (Singh et al., Citation2012a).
Provides steady plasma levels.
Easy to use and non-invasive.
Drug input can be stopped at any point after removal of the patch from the site (Singh et al., Citation2014c).
Increases compliance and reduces medical costs.
Improves bioavailability.
Best route for pediatrics patients.
Suitable route for unconsious or vomiting patient.
Lesser chances of overdose and easy detection of drug (Durand et al., Citation2012).
Human skin: penetration barrier
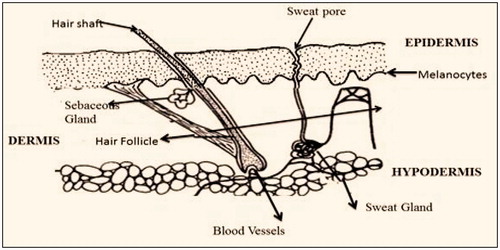
Human skin covers the largest part of the body. The primary function of the skin is to maintain the body hydration (Fukushima et al., Citation2011). demonstrates cross-sectional area of skin. Skin are comprised of three layers.
Stratum corneum
The exposed layer of the skin (also termed as horny layer) which is approxmateily 10 mm thick. It has barrier property due to presence of 79–90% of protein and 5–15% of lipids. The multilayered epidermis varies in thickness which mainly depends upon cell thickness and layers of epidermis. represents the reginoal variations (thickness, water permeability and diffusivity of water) in drug permeability through epidermis (Patel & Baria, Citation2011).
Table 1. Regional variations in drug permeability of stratum corneum.
Dermis
Thickness of this layer is ∼3–5 mm. It mainly consist of connective tissues, mandatory in regulation of body temperature, oxygen, nutrients to the skin and removing toxic products (Singh et al., Citation2014b).
Hypodermis
It holds the fat tissues and acts as a support memberane for both epidermial and dermal layer of skin. It has its own importance in transdermal drug delivery. Drug shall penetrate through all three layers to reach systemic circulation (Sun et al., Citation2014).
Drug penetration pathway
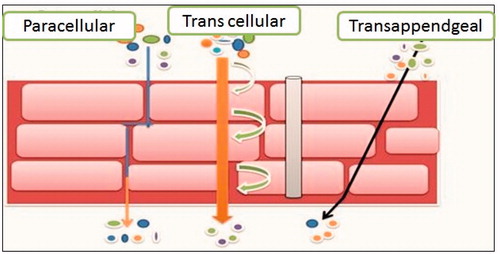
Drug can be penetrated by three pathways such as transcellular route, paracellular lipid route and transappendgeal route ().
Transcellular Route: Moeity passes through both keratinocytes and lipids (straight path to the dermis) (Ghaffarian & Muro, Citation2013).
Paracellular Route: The most common penetration pathway of drug molecules. In this pathway, drug remains in lipid moeity and stay around keratin (easy for lipid soluble drug rather than proteins) (Parnami et al., Citation2013).
Transappendgeal Route: It makes continues channel for drug permeation but it hindered easily due to presence of hair follicles and sweat ducts (Sharma et al., Citation2011).
Factors affecting transdermal drug delivery
Physiochemical properties of active moiety (Dhote et al., Citation2012; Park et al., Citation2012)
Partition coefficient
Drug possess both water and lipid solubility. Ideal partition coefficient for intermediate trasdermal delivery is log K 1–3. For highly lipophillic drug (log k > 3), intracellular route is favourable, whereas for hydrophillic drugs (log k < 1), it is permeated via transcellular route.
Molecular size
Molecular size of the drug is inversaly proportional to transdermal flux. The ideal molecular size of drug molecule for transdermal delivery is ≤400.
Solubility/melting point
Most organic solutes have high melting point and low solubility at normal temperature and pressure. Lipophillic drug permeates faster than hydrophillic substances, but it should also have aqueous solubility as needed in most of topical formulations.
Ionization
Unionized drug permeates the skin as according to pH-Partition hypothesis.
Diffusion coefficient
Penetration of drug depends on diffusion coefficient of drug. At a constant temperature, the diffusion coefficient of drug mainly depends on properties of drug, diffusion medium and their interaction.
Physiochemical properties of the drug delivery system (Rehman & Zulfakar, Citation2014; Schoellhammer et al., Citation2014)
Release characteristics
Drug release mechanism mainly depends on drug molecules which are dissolved or suspended in the delivery system and on interfacial partition coefficient or pH of the drug from delivery system to the skin tissue. If the drug is easily released from the delivery system, the rate of transdermal permeation will be higher.
Composition of drug delivery system
Composition may not affect release properties but may affect its permeability functionality. For example, methyl salicylate is more lippophilic than parent acid, i.e. salicylic acid, and its percutaneous absorption is high when applied to skin in a lipoidal vehicle.
Enhancement of transdermal permeation
Majority of drugs will not permeate into skin for theraputic use. Some enhancers are used for syergistic action without showings its properties (e.g. dimethyl sulphoxide, acetone, propylene glycol and tetradihydrofuryl alcohol).
Physiological properties (Jones & Moss, Citation2010; Subedi et al., Citation2010)
Skin barrier properties in the neonate and young infant
The skin surface of the newborn is slightly hydrophobic, relatively dry and rough when compared to that of older infants. Stratum corneum hydration stabilizes by the age of 3 months.
Skin barrier properties in aged skin
There are some changes in the physiology of aged skin (>65 years). The moisture content of human skin decreases with age. There is a destruction of the epidermal junction and consequently, the area available for transmission into the dermis is diminished.
Race
Racial differences between black and white skins have shown some anatomical and physiological functions of the skin. In black skin, there is increased intracellular permeation due to higher lipid content and higher electrical skin resistance levels when compared to whites, but this difference is not detected in stripped skin.
Skin temperature
The human body maintains a temperature of ∼32–37 °C across the skin. Hence, increase in temperature leads to increase in diffusion through the tissue (Vinod et al., Citation2010).
Various issues with transdermal and topical delivery system
Not very helpful for gedriatric patients because skin undergoes various deformations and alterations (lipids and protein composition changes) (Kaur et al., Citation2014c).
Irritant dermatitis is the most common reaction reported with many transdermal delivery systems. It is an inflammatory reaction which is non-allergic and their skin may be more fragile or react more easily (Brown & Langer, Citation1988; Fowler et al., Citation2009).
Allergic contact dermitis with intense erythema, intense itching, redness and blistering are common. The reaction tends to magnify for a few days after the transdermal delivery system is removed (Kaur et al., Citation2014b).
Causes allergic contact urticaria (ACU) is type I hypersensitivity reaction, with IgE-mediated inflammatory response to a specific allergen (Mackay et al., Citation2001).
Products which contain vasodilators (nitroglycerin and nicotine) or pressure-sensitive adhesives can cause a transient reactive hyperemia (Murphy & Carmichael, Citation2000).
Transdermal delivery system that has a metallic component (usually aluminum) in their waterproof layer can cause burns if they absorb heat. Products are not always labeled with a warning if they contain metals (Elmoslemany et al., Citation2012).
Strategies applied for permeation enhancement
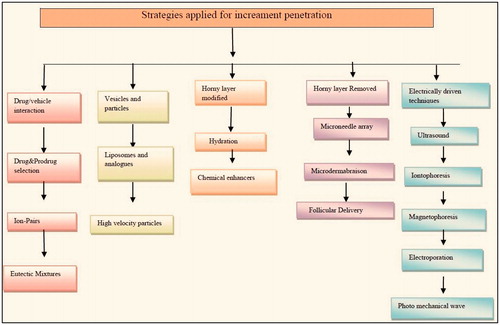
Drawback of transdermal delivery is permeation of active moeity through skin. So, various studies are done for enhancing its permeability percutaneously. depicts various strategies for increament of pentration. They act by three mechanisms:
By altering physicochemical properties of stratum corneum.
By changing hydrating property of stratum corneum.
By altering structure of lipids and protein in intercellular channel via carrier mechanism (Csizmazia et al., Citation2012).
Drug/vehicle interaction
Drug and prodrug selection
Active ingredient to be used should be judiciously chosen on the bases of pharmacological or physiochemical properties.
Ideal properties for drug selection are:
Less than 600 Da is prefered when diffusion coefficient is high.
Good solubility in oil and water.
High and adequate partition coefficient (1–3).
Should carry melting point <200°F.
It should not metabolize in skin (Kataria et al., Citation2014; Kaur et al., Citation2014a).
Ion-pair
Though active moeity, which can penetrate into skin, is a charged molecule, it cannot be permeate. However, lipophilic ion pair technique can enhance epidermal penetration. In this technique, oppositely charged species forms pair and neutralize the charge as well as dissociates in viable aqueous epiderms and realesing parent molecule (Goyal et al., Citation2013b). The coacervate permeates in skin as it diffuses or dissociates and behaves like ion pair (Goyal et al., Citation2013a). Valenta et al. determine the significance of ion pairing on the permeation of lignocaine. Diffusion studies were performed through polydimethylsiloxane (PDMS) at different pH values 4.0, 6.0, 7.0 and 8.0. The steady state lignocaine flux was increased up to 2.45-fold using different counter ions. The highest flux was measured from lignocaine morpholinopropane sulfonate (L-mps) (Valenta et al., Citation2000). Riserdronate is an inhibitor of osteoclastic activity used for treating various disorders of bones. It is highly ionized and very acidic which leads its low permeating properties into skin. Found out ion-paired solution with l-arginine which were tested in vitro on hairless mouse. The result showed that flux was increased, i.e. 14.13 ± 5.49 µg/cm2 to 36 times (475.18 ± 94.19 g/cm2 and 511.21 ± 106.52 g/cm2) (Nam et al., Citation2011).
Eutectic mixtures
Solubility is a factor which is mostly affected by melting point of a substance (directly affects skin permeation). The lower the melting point, more will be solubility in lipids of skin (Garg & Goyal, Citation2014c). For lowering the higher melting point, we make eutectic mixtures (a mixture of two components which at a certain ratio due to which crystalline phase is inhibited) so that melting point of two components is less than the single component (Fiala et al., Citation2010). For example, ibuprofen with terpenes, menthol with methyl nicotine, propranolol with fatty acids and lignocaine with menthol. Analgesics such as NSAIDs have gastrointestinal side effects and topical dosage forms of these drugs are mainly preferred, especially for local pains. Meloxicam is one of NSAIDs with no topical form in the market. Mohammadi-Samani et al. (Citation2014) aimed to design a formulation of meloxicam for transdermal delivery using thymol as the penetration enhancer. In vitro permeation studies showed that 5.5 ratio (Melocxicam:Thymol) in guinea pig have maximum flux, i.e. 22.06 µg/cm2 (Mohammadi-Samani et al., Citation2014).
Horny layer modification
Hydration
Water is the best way for permeating both hydrophillic and hydrophobic drugs. In normal conditions, 10–20% concentration is present in stratum corneum. Hydration of epidermis is an important aspect for permeation enhancement. In this technique, water-soaked skin can absorb a generous amount of water in a short span of time. Natural Mosturizing Factor (NMF) acts as mediator for hygroscopic property of corneum. It mainly consist of free amino acids and their salts. Skin hydration causes swelling of kertins and effecting lipid packing as well as interupting polar and non-polar routes (Haftek et al., Citation1998). explains hydration affects on skin permeability via various pharmaceutical systems.
Table 2. Effects of hydration on skin permeability.
Chemical enhancer
Chemical enhancers help in permeation across the skin by disruption of the highly ordered structure of stratum corenum lipid, interaction with intercellular protein or improve partition of the drug into stratum corneum (Garg et al., Citation2013). Chemical enhancers should contain the following properties:
It should be non-toxic and non-allergen.
It should have rapid working activity and the duration should be predictable (Garg et al., Citation2012d).
It should be unidirectional.
Its compatibility with both excipients and drugs.
Its properties according to drugs and cosmetic act (Erdal et al., Citation2014).
Classification of percutaneous chemical enhancers
The classification of percutaneous enhancers is frequently based on the chemical class to which the compounds belong. shows the principal classes of percutaneous enhancers.
Table 3. Enlisting various chemical enhancer on the bases of their structure.
Horny layer removed
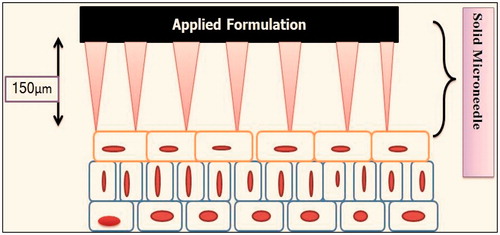
Microneedle array
Microneedles are devices which act as both hypodermic needles and transdermal patch. It consists of drug pool and some protrusion termed as microneedles which helps in drug permeation across without reaching nerve (). These needles are 200–750 microns in length and are composed of groups called arrays which contains 150–650 microneedles/cm2 and have diameter of tip 25 µm interfacial area of 490 μm2 with an insertion force required is 0.058 N. Materials which makes these arrays are silicon, metal, sugar and plastics (Duan et al., Citation2011). These can be of four kinds: (i) Poke with patch approach (excruciating into epidermal layer and applying patch locally), (ii) Coat and poke approach (needles incorporated with drug, release of pharmacological active moeity takes place by dissolution), (iii) Biodegradable Microneedle (insertion of drug incorporated polymeric microneedle which is biodegradable) and (iv) Hollow Microneedle (puncturing epidermal surface with needles with a hollow bore) (Garg et al., Citation2011d). Various formulation under clinical trials. Good technique for large molecular weight drugs, e.g. insulin, hormones, calcitonin and various peptides and proteins (Donnelly et al., Citation2014). shows various microneedle projects at different stages of clinical trials.
Table 4. Microneedles projects under clinical trials.
Recent marketed product of ALZA Corporation named Macro flux® estimated to be ∼50–200 μm and therefore are not believed to reach the nerve endings. Kim et al. (Citation2012) enhanced hydrophillic peptides such as oxytocin, tetrapeptide-3, hexapeptide with pretreatment with microneedle array within porcine ear skin and achieved a positive correlation with weight of peptides (Kim et al., Citation2012).
Stratum corneum removed
Microdermabrasion is introduced as a method to increase skin permeability by selectively removing the stratum corneum. The depth of slash depends on patient’s requirement. This technique is boon to large molecular weight drugs like peptides, insulin and vaccines. In this method, complete removal of epidermis is not employed. Skountzou et al. observed transcutaneous immunization using topical delivery of influenza vaccine. The outer skin barrier can be overcome through the use of mild chemical and/or physical treatments, including ethanol–water hydration and stripping, which allows large vaccine molecules or even particulate antigens to gain access to the skin’s immune cells (Skountzou & Kang, Citation2009). Microdermabrasion can be achieved via three promising ways.
Microdermaabrasion with histological imaging
Basically, this technique is applied for beauty treatment for removing scars and wrinkles. Aluminium oxide crystals (pressure 30–35 kPa), aluminium oxide diamond, magnesium oxide and sodium bicarbonate are also used for this purpose (Garg & Goyal, Citation2014a).
Dye penetration
Dyes should be approved by FDA and Cosmetic act. These techniques are maily used for tatoo removal or permanent make up (Garg et al., Citation2012c).
Skin electrical resistance
An average specific resistance (1750 ± 1340 kΩ cm2) is required for stratum corneum removal (Garg et al., Citation2014).
Follicular delivery
Therapeutically, active drug can be penetrated through sweat glands and hair follicles (rich invascularization) (Terminal Hair Follicles 172 ± 70 µm, Sebaceous Follicles 86 ± 37 µm).
These hydrophillic as well high molecular weight moieties can act as storage for min. upto 10 days. This technique is best suited for topical preparations (Garg et al., Citation2011c).
Electrically driven technique
Ultrasound
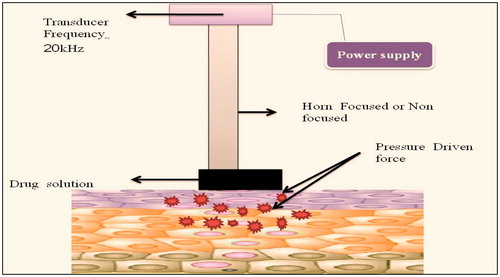
It is also termed as phonophoresis or sonphoresis. In this enhancement technique, permeation is increased via ultrasonic waves which means frequency is >20 kHz which is shown in . Mechanism involves either of the two ways: (a) application of sound waves to the skin increases to fluidity of lipids and increases permeation via transcellular pathway or (b) formation of bubbles which generates pores which even allows large molecular weight drugs such as protein or vaccines. Trainers prefer this method to permeate dexamethasone, ketoprofn or lidocaine in patients. But this techinique has some limitations, i.e. formation of attenuation which is due to the fact that sound waves transforms to heat energy (Tiwari et al., Citation2004). The additional advantages, limitations and applications that the ultrasound technique comprises are summarized in and , respectively.
Table 5. Advantages and limitations of ultrasound techniques.
Table 6. Research application of ultrasound techniques.
Iontophoresis
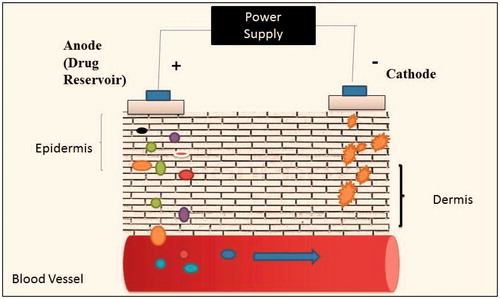
It is defined as permeation of ionized drug through electrical impulses of 0.5 mA/cm by either galvanic or voltaic cell (). It contains cathode and anode which attracts positively charged ion and negatively charged ions, respectively (Garg & Goyal, Citation2012). Its mechanism strictly follows Faraday’s law.
D = IT/(IZI)F, where D is the drug to be permeated, I is the current (amperes), IZI is the valance and F is the Faraday’s constant (coloumb/Mol). According to this equation, drug drawn into skin is directly proportional to current applied. pH of skin plays an important role, higher the pH of the skin more will be the permeability in the skin. Iontophoresis shows the action in either way such as electromagnation, in which charged ions is attracted to oppositively charged electrode or electroosmosis in which non-ionic substance permeates with solvent (Dhote et al., Citation2012). Hashim et al. (Citation2010) studied topical application of nuclear factor-kappa B (NF-kappa B) decoy in the treatment of inflammation and atopic dermatitis. The results suggest that iontophoresis is a useful and promising enhancement technique for transdermal delivery of NF-kappa B decoy ODN (Hashim et al., Citation2010). Gomez et al. (Citation2012) studied topical delivery of calcium and phosphate for treatment of osteoporesis. The results showed an increase in bone density by restoration of calcium and phosphate in bone (Gomez et al., Citation2012). discusses various advantages and limitations of iontophoresis technique.
Table 7. Advantage and limitations of iontoporesis technique.
Types of iontoporesis
Direct iontoporesis
Anions are permeated through passive diffusion.
Reverse iontoporesis
It allows the movement of neutral and positive ions into cathode and negative ions into anode. Future expectation for delivery of large molecular weight drugs like calcitonin, inulin, gonadotropin releasing hormone, parathyroid hormone,vassopresin and insulin can be actively deliverd by this mechanism (Gagandeep et al., Citation2014). At present, apomorphine is used for treatment of idiopathic Parkinson’s disease in many volunteers and it has good future in diagnosis of cystic fibrosis (Garg et al., Citation2011b).
Magnetophoresis
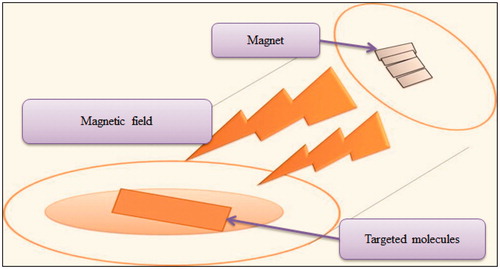
This enhancement technique involves applying magnetic field around the solute that penetrates into the skin. For example, benzoic acid is a diamagnetic substance and when the magnetic field is applied around, it increases diffusion properties, as shown in . The major limitation associated with this technique is that, it might change properties of stratum corneum (Garg, Citation2014).
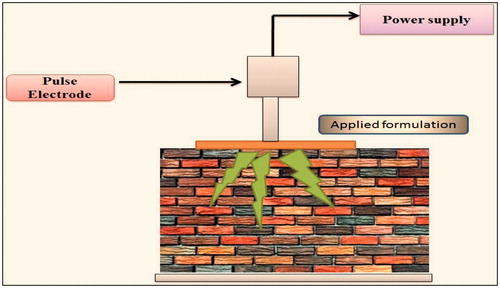
Electroporation
This application involves short electrical pulses of DC voltage >100 V for milliseconds (). This technique works in either of the two ways: first is pore formation in lipid bilayers, corneocytes but small-charged molecules cannot pass via this route and the second is applying high voltage which automatically creates aqueous pores through epidermis which are helpful in permeation across five to six lipid membranes (Arora et al., Citation2012). Eriksson et al. (Citation2013) studied that DNA vaccine (coding for prostate specific antigen to treating prostate cancer) can be given transdermally with the help of electroporation technique. Patients with good biochemical relapse of prostate cancer were observed (Eriksson et al., Citation2013). It has positive applications in delivery of proteins, oligonucleotides, small molecules, heparin, insulin, dextran and vitamin C. Electroporation are of two types.
Reversible
Electric pulses cause short-term increment in permeation enhancement and cell survives. This approach has its application in biotechnology and medicine.
Irreversible
Electric pulses lead membrane permeation which leads to cell death or necrosis or apoptotic. This approach has its application in food industry and sterlization.
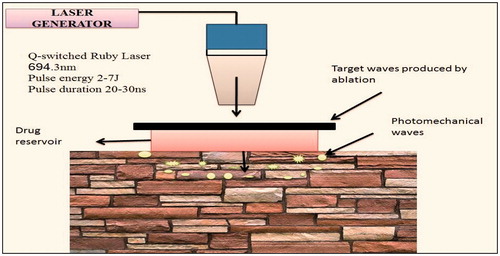
Photomechanical wave
It is also termed as laser-generated stress pressure wave, which is generated by incision of the targeted substance polystyrene. It has no specific mechanism of action, but it changes the lacnuar system in the epidermis as shown in . This technique is capable in delivering of macromolecules (40 000 Da). It is used in dermatalogical techniques such as skin rejuvenation and acne scar’s healing (Lee et al., Citation1999).
High-velocity particles
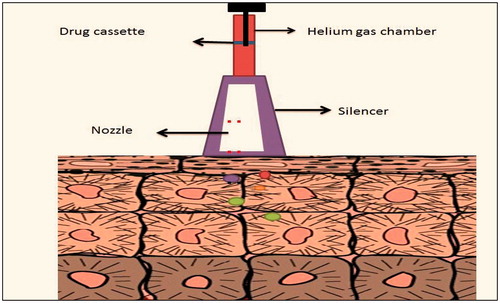
Powderject device
It consist of propulsion of solid drug into skin by gas (helium) as medium with a speed of 600–900 m/s (). This technique is painless and non-invasive. This system ruptures the epidermal layer which is reversible in nature. Postive outcomes of this technique are delivery of testosterone, lidocaine hydrochloride and macromolecules such as calcitonin and insulin (Degano et al., Citation1999).
Needle-free injections
This method consist of non-invasive technique which is boon over conventional dosage form. Some of its injectors are as follows:
Intrajet: It uses nitrogen propelled gas. Patient breaks up the tip and presurized gas forces the liquid drug into generated small pores.
Implajet: It pushes a fine needle into the skin with opens channels and drug permeates immediatly.
Jet-syringe: This is capable of delivering 0.5 ml dose into the skin. It is best for short therapies.
Ilject: It is capable of delivering drug upto 0.1–1 ml drug and it is needle-free therapy.
Miniject: This velocity injector uses polycarbonate syringe which can deliver drug subcutaneously as well as intramusculary.
Cross-ject: This needle-free device uses gas source to propelled drug into subcutaneous tissue with the help of polycarbonate nozzel (Garg, Citation2012).
Nanocarrier systems
Many techniques have been aimed to disrupt intercellular lipids in an attempt to enhance drug transport across the intact skin. One of the most convenient methods is the use of vesicle formulations as skin delivery systems. summarizes various researches carried on novel drug delivery carriers. The rationale behind using vesicles in dermal and transdermal drug delivery is as follows:
Act as drug carriers into or across the skin (Garg & Goyal, Citation2014b).
Act as penetration enhancers for the penetration by altering the intercellular lipids in skin layer (Garg et al., Citation2012b).
Serve as a depot for sustained release (Garg et al., Citation2012a).
Serve as a rate limiting membrane barrier for the modulation of systemic absorption, hence providing a controlled transdermal delivery system (Garg et al., Citation2011a). represents comparison between different transdermal drug delivery systems based on various pharamceutical aspects.
Table 8. Proposed mechanism of drug absorption through different drug delivery carriers.
Table 9. Comparison between different transdermal drug delivery systems on the basis of various pharamceutical aspects.
Marketed products of transdermal patches
Various marketed transdermal patches successfully run in the market. represents the various transdermal patches products along with their applications.
Table 10. Marketed products of transdermal patches.
Conclusions
Skin permeation enhancement technology is a rapidly emerging field which would expressively increase the number of drugs which is suitable for transdermal drug delivery. Transdermal drug administration route offers many benefits over oral administration of drugs and has stimulated research to find ways to overcome the barrier function of the skin by use of various enhancers’ approaches. The exploration for the ideal skin penetration enhancer has been the emphasis of significant research effort over a number of eras. Many potent enhancers have been revealed, but in most of the cases, their effects are associated with toxicity. In recent years, better understanding of the nature of the stratum corneum barrier, interaction of enhancers with the stratum corneum and the development of structure activity relationships for enhancers will aid in optimal characteristics and minimal toxicity. In future, penetration enhancers will play a major role in developing effective transdermal products.
Acknowledgements
Authors Dr. Amit K. Goyal and Dr. Goutam Rath are thankful to the Department of Biotechnology (DBT), New Delhi, India, for providing financial assistance to carry out research.
Declaration of interest
The authors report no conflict of interest.
References
- Arora N, Singh K, Garg T. (2012). Areas of nanomedicine applications. Int J Univ Pharm Life Sci 2:216–27
- Barker DJ, Bercovicz D, Servilio LC, et al. (2013). Rat ultrasonic vocalizations demonstrate that the motivation to contextually reinstate cocaine-seeking behavior does not necessarily involve a hedonic response. Addict Biol. [Epub ahead of print]. doi: 10.1111/adb.12044
- Basnet P, Hussain H, Tho I, Skalko-Basnet N. (2012). Liposomal delivery system enhances anti-inflammatory properties of curcumin. J Pharm Sci 101:598–609
- Bei D, Meng J, Youan BB. (2010). Engineering nanomedicines for improved melanoma therapy: progress and promises. Nanomedicine (Lond) 5:1385–99
- Brown L, Langer R. (1988). Transdermal delivery of drugs. Annu Rev Med 39:221–9
- Cao J, Guo LH, Wan B, Wei Y. (2011). In vitro fluorescence displacement investigation of thyroxine transport disruption by bisphenol A. J Environ Sci (China) 23:315–21
- Chen M, Gupta V, Anselmo AC, et al. (2014). Topical delivery of hyaluronic acid into skin using SPACE-peptide carriers. J Control Release 173:67–74
- Csizmazia E, Berko S, Maroda M, et al. (2012). [Modelling of pecutaneous drug permeation and investigation of penetration enhancer effect]. Acta Pharm Hung 82:15–22
- Degano P, Schneider J, Hannan CM, et al. (1999). Gene gun intradermal DNA immunization followed by boosting with modified vaccinia virus Ankara: enhanced CD8+ T cell immunogenicity and protective efficacy in the influenza and malaria models. Vaccine 18:623–32
- Desai PR, Marepally S, Patel AR, et al. (2013). Topical delivery of anti-TNFalpha siRNA and capsaicin via novel lipid-polymer hybrid nanoparticles efficiently inhibits skin inflammation in vivo. J Control Release 170:51–63
- Dhote V, Bhatnagar P, Mishra PK, et al. (2012). Iontophoresis: a potential emergence of a transdermal drug delivery system. Sci Pharm 80:1–28
- Donnelly RF, Morrow DI, McCrudden MT, et al. (2014). Hydrogel-forming and dissolving microneedles for enhanced delivery of photosensitizers and precursors. Photochem Photobiol 90:641–7
- Duan D, Moeckly C, Gysbers J, et al. (2011). Enhanced delivery of topically-applied formulations following skin pre-treatment with a hand-applied, plastic microneedle array. Curr Drug Deliv 8:557–65
- Dubey V, Mishra D, Jain NK. (2007). Melatonin loaded ethanolic liposomes: physicochemical characterization and enhanced transdermal delivery. Eur J Pharm Biopharm 67:398–405
- Dudelzak J, Hussain M, Phelps RG, et al. (2008). Evaluation of histologic and electron microscopic changes after novel treatment using combined microdermabrasion and ultrasound-induced phonophoresis of human skin. J Cosmet Laser Ther 10:187–92
- Durand C, Alhammad A, Willett KC. (2012). Practical considerations for optimal transdermal drug delivery. Am J Health Syst Pharm 69:116–24
- El-Kamel AH, Al-Fagih IM, Alsarra IA. (2008). Effect of sonophoresis and chemical enhancers on testosterone transdermal delivery from solid lipid microparticles: an in vitro study. Curr Drug Deliv 5:20–6
- Elmoslemany RM, Abdallah OY, El-Khordagui LK, Khalafallah NM. (2012). Propylene glycol liposomes as a topical delivery system for miconazole nitrate: comparison with conventional liposomes. AAPS PharmSciTech 13:723–31
- Erdal MS, Pekoz AY, Aksu B, Araman A. (2014). Impacts of chemical enhancers on skin permeation and deposition of terbinafine. Pharm Dev Technol 19:565–70
- Eriksson F, Totterman T, Maltais AK, et al. (2013). DNA vaccine coding for the rhesus prostate specific antigen delivered by intradermal electroporation in patients with relapsed prostate cancer. Vaccine 31:3843–8
- Fang JY, Hong CT, Chiu WT, Wang YY. (2001). Effect of liposomes and niosomes on skin permeation of enoxacin. Int J Pharm 219:61–72
- Fiala S, Jones SA, Brown MB. (2010). A fundamental investigation into the effects of eutectic formation on transmembrane transport. Int J Pharm 393:68–73
- Foldvari M, Badea I, Kumar P, et al. (2011). Biphasic vesicles for topical delivery of interferon alpha in human volunteers and treatment of patients with human papillomavirus infections. Curr Drug Deliv 8:307–19
- Fowler JF, Warshaw EM, Squires L. (2009). Methylphenidate patch-test protocol and irritancy threshold determination in healthy adult subjects. Dermatitis 20:271–4
- Fukushima K, Ise A, Morita H, et al. (2011). Two-layered dissolving microneedles for percutaneous delivery of peptide/protein drugs in rats. Pharm Res 28:7–21
- Gagandeep G, Garg T, Malik B, et al. (2014). Development and characterization of nano-fiber patch for the treatment of glaucoma. Eur J Pharm Sci 53:10–16
- Garg T. (2012). An evolutionary approaches in development of needle free injection technologies. Int J Pharm Pharm Sci 4:590–6
- Garg T. (2014). Current nanotechnological approaches for an effective delivery of bio-active drug molecules in the treatment of acne. Artif Cells Nanomed Biotechnol 1–8 . [Epub ahead of print]
- Garg T, Bilandi A, Kapoor B. (2011a). Organogels: advanced and novel drug delivery system. Int Res J Pharm 2:15–21
- Garg T, Singh O, Arora S. (2011b). Opportunities and growth of conduct clinical trials in India. Int J Pharm Sci Rev Res 8:152–60
- Garg T, Singh O, Arora S, Murthy R. (2011c). Dendrimer – a novel scaffold for drug delivery. Int J Pharm Sci Rev Res 7:211–20
- Garg T, Singh O, Arora S, Murthy R. (2011d). Patented microencapsulation techniques and its application. J Pharm Res 4:2097–102
- Garg T, Bilandi A, Kapoor B. (2012a). Evolutionary approaches for drug and gene delivery by micro and nano magnetic carriers. Indo Global Res J Pharm Sci 2:182–9
- Garg T, Chanana A, Sharma G. (2012b). Pulsatile drug delivery systems: pulsincap system. IOSR J Pharm 2:338–9
- Garg T, Goyal AK, Arora S, Murthy R. (2012c). Development, optimization & evaluation of porous chitosan scaffold formulation of gliclazide for the treatment of type-2 diabetes mellitus. Drug Deliv Lett 2:251–61
- Garg T, Singh O, Arora S, Murthy R. (2012d). Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst 29:1–63
- Garg T, Goyal AK. (2012). Iontophoresis: drug delivery system by applying an electrical potential across the skin. Drug Deliv Lett 2:270–80
- Garg T, Goyal AK. (2014a). Biomaterial-based scaffolds – current status and future directions. Expert Opin Drug Deliv 11:767–89
- Garg T, Goyal AK. (2014b). Liposomes: targeted and controlled delivery system. Drug Deliv Lett 4:62–71
- Garg T, Goyal AK. (2014c). Medicated chewing gum: patient compliance oral drug delivery system. Drug Deliv Lett 4:72–8
- Garg T, Rath G, Goyal AK. (2014). Comprehensive review on additives of topical dosage forms for drug delivery. Drug Deliv. [Epub ahead of print]
- Garg T, Singh S, Goyal AK. (2013). Stimuli-sensitive hydrogels: an excellent carrier for drug and cell delivery. Crit Rev Ther Drug Carrier Syst 30:369–409
- Ghaffarian R, Muro S. (2013). Models and methods to evaluate transport of drug delivery systems across cellular barriers. J Vis Exp (80):e50638
- Gomez I, Szabo A, Pap L Jr, et al. (2012). In vivo calcium and phosphate iontophoresis for the topical treatment of osteoporosis. Phys Ther 92:289–97
- Goyal AK, Rath G, Garg T. (2013a). Nanotechnological approaches for genetic immunization. DNA RNA Nanobiotechnol Med Diagn Treat Dis 67–120
- Goyal G, Garg T, Malik B, et al. (2013b). Development and characterization of niosomal gel for topical delivery of benzoyl peroxide. Drug Deliv. [Epub ahead of print]. doi:10.3109/10717544.2013.855277
- Haftek M, Teillon MH, Schmitt D. (1998). Stratum corneum, corneodesmosomes and ex vivo percutaneous penetration. Microsc Res Tech 43:242–9
- Ham AS, Cost MR, Sassi AB, et al. (2009). Targeted delivery of PSC-RANTES for HIV-1 prevention using biodegradable nanoparticles. Pharm Res 26:502–11
- Han G, Tar M, Kuppam DS, et al. (2010). Nanoparticles as a novel delivery vehicle for therapeutics targeting erectile dysfunction. J Sex Med 7:224–33
- Hashim II, Motoyama K, Abd-Elgawad AE, et al. (2010). Potential use of iontophoresis for transdermal delivery of NF-kappaB decoy oligonucleotides. Int J Pharm 393:127–34
- Hou J, Zuo G, Shen G, et al. (2009). Hollow sodium tungsten bronze (Na0.15WO3) nanospheres: preparation, characterization, and their adsorption properties. Nanoscale Res Lett 4:1241–6
- Jain S, Tiwary AK, Sapra B, Jain NK. (2007). Formulation and evaluation of ethosomes for transdermal delivery of lamivudine. AAPS PharmSciTech 8:E111
- Jones DS, Moss GP. (2010). Themed issue: recent advances in transdermal drug delivery. J Pharm Pharmacol 62:669–70
- Kataria K, Sharma A, Garg T, et al. (2014). Novel technology to improve drug loading in polymeric nanofibers. Drug Deliv Lett 4:79–86
- Kaur M, Garg T, Rath G, Goyal AK. (2014a). Current nanotechnological strategies for effective delivery of bioactive drug molecules in the treatment of tuberculosis. Crit Rev Ther Drug Carrier Syst 31:49–88
- Kaur M, Malik B, Garg T, et al. (2014b). Development and characterization of guar gum nanoparticles for oral immunization against tuberculosis. Drug Deliv. [Epub ahead of print]. doi: 10.3109/10717544.2014.894594
- Kaur R, Garg T, Malik B, et al. (2014c). Development and characterization of spray-dried porous nanoaggregates for pulmonary delivery of anti-tubercular drugs. Drug Deliv 1–6
- Kim J, Jang JH, Lee JH, et al. (2012). Enhanced topical delivery of small hydrophilic or lipophilic active agents and epidermal growth factor by fractional radiofrequency microporation. Pharm Res 29:2017–29
- Kim ST, Lee KM, Park HJ, et al. (2009). Topical delivery of interleukin-13 antisense oligonucleotides with cationic elastic liposome for the treatment of atopic dermatitis. J Gene Med 11:26–37
- Lee S, Kollias N, McAuliffe DJ, et al. (1999). Topical drug delivery in humans with a single photomechanical wave. Pharm Res 16:1717–21
- Li N, Peng LH, Chen X, et al. (2014). Antigen-loaded nanocarriers enhance the migration of stimulated Langerhans cells to draining lymph nodes and induce effective transcutaneous immunization. Nanomedicine 10:215–23
- Liu H, Wang Y, Han F, et al. (2007). Gelatin-stabilised microemulsion-based organogels facilitates percutaneous penetration of Cyclosporin A in vitro and dermal pharmacokinetics in vivo. J Pharm Sci 96:3000–9
- Luis J, Park EJ, Meyer RJ, Smith NB. (2007). Rectangular cymbal arrays for improved ultrasonic transdermal insulin delivery. J Acoust Soc Am 122:2022–30
- Mackay IR, Rosen FS, Kay A. (2001). Allergy and allergic diseases. N Engl J Med 344:30–7
- Mahor S, Rawat A, Dubey PK, et al. (2007). Cationic transfersomes based topical genetic vaccine against hepatitis B. Int J Pharm 340:13–19
- Manosroi A, Khanrin P, Lohcharoenkal W, et al. (2010). Transdermal absorption enhancement through rat skin of gallidermin loaded in niosomes. Int J Pharm 392:304–10
- Mohammadi-Samani S, Yousefi G, Mohammadi F, Ahmadi F. (2014). Meloxicam transdermal delivery: effect of eutectic point on the rate and extent of skin permeation. Iran J Basic Med Sci 17:112–18
- Montanari J, Vera M, Mensi E, et al. (2013). Nanoberries for topical delivery of antioxidants. J Cosmet Sci 64:469–81
- Murphy M, Carmichael AJ. (2000). Transdermal drug delivery systems and skin sensitivity reactions. Am J Clin Dermatol 1:361–8
- Nam SH, Xu YJ, Nam H, et al. (2011). Ion pairs of risedronate for transdermal delivery and enhanced permeation rate on hairless mouse skin. Int J Pharm 419:114–20
- Onishi H, Machida Y, Koyama K. (2007). Preparation and in vitro characteristics of lactoferrin-loaded chitosan microparticles. Drug Dev Ind Pharm 33:641–7
- Park CW, Son DD, Kim JY, et al. (2012). Investigation of formulation factors affecting in vitro and in vivo characteristics of a galantamine transdermal system. Int J Pharm 436:32–40
- Parnami N, Garg T, Rath G, Goyal AK. (2013). Development and characterization of nanocarriers for topical treatment of psoriasis by using combination therapy. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. doi: 10.3109/21691401.2013.837474
- Patel RP, Baria AH. (2011). Formulation and evaluation considerations of transdermal drug delivery system. Int J Pharm Res 3:1–9
- Rehman K, Zulfakar MH. (2014). Recent advances in gel technologies for topical and transdermal drug delivery. Drug Dev Ind Pharm 40:433–40
- Romero EL, Morilla MJ. (2013). Highly deformable and highly fluid vesicles as potential drug delivery systems: theoretical and practical considerations. Int J Nanomed 8:3171–86
- Schoellhammer CM, Blankschtein D, Langer R. (2014). Skin permeabilization for transdermal drug delivery: recent advances and future prospects. Expert Opin Drug Deliv 11:393–407
- Sharma N, Agarwal G, Rana A, et al. (2011). A review: transdermal drug delivery system: a tool for novel drug delivery system. Int J Drug Dev Res 3:70–84
- Singh D, Pradhan M, Nag M, Singh MR. (2014a). Vesicular system: versatile carrier for transdermal delivery of bioactives. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. doi: 10.3109/21691401.2014.883401
- Singh H, Sharma R, Joshi M, et al. (2014b). Transmucosal delivery of docetaxel by mucoadhesive polymeric nanofibers. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. doi: 10.3109/21691401.2014.885442
- Singh O, Garg T, Rath G, Goyal AK. (2014c). Microbicides for the treatment of sexually transmitted hiv infections. J Pharm 1–18
- Singh K, Arora N, Garg T. (2012a). RFID: a trustable security tool in pharmaceutical industry. Am J Pharm Tech Res 2:113–27
- Singh K, Arora N, Garg T. (2012b). Superbug: antimicrobial resistance due to NDM-1. Int J Inst Pharm Life Sci 2:58–66
- Skountzou I, Kang SM. (2009). Transcutaneous immunization with influenza vaccines. Curr Top Microbiol Immunol 333:347–68
- Subedi RK, Oh SY, Chun MK, Choi HK. (2010). Recent advances in transdermal drug delivery. Arch Pharm Res 33:339–51
- Sun R, Celli A, Crumrine D, et al. (2014). Functional epidermal permeability barrier in human epidermal equivalents. Tissue Eng Part C Methods. [Epub ahead of print]
- Tiwari SB, Pai RM, Udupa N. (2004). Influence of ultrasound on the percutaneous absorption of ketorolac tromethamine in vitro across rat skin. Drug Deliv 11:47–51
- Valenta C, Siman U, Kratzel M, Hadgraft J. (2000). The dermal delivery of lignocaine: influence of ion pairing. Int J Pharm 197:77–85
- Vinod K, Sarvani P, Banji D, Teja B. (2010). Transdermal drug delivery system-over coming challenges of popular drug delivery system. Int J Pharm World Res 3:49–53