 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Poor peroral therapeutic efficiency of selegiline is primarily due to the extensive hepatic metabolism and hence the need for an alternative route of administration. The present study is based on evaluation of a buccal film which is impregnated with selegiline nanospheres to enhance the systemic bioavailability. Selegiline-loaded nanospheres prepared using poly(lactide-co-glycolide) was embedded into buccal films (F1–F4) with varying polymer composition [hydroxypropyl methylcellulose and eudragit]. The developed films were evaluated for their physicomechanical properties, hydration, mucoadhesive strength, in vitro drug release and ex vivo permeation in order to identify the ideal system suitable for further development. In vivo studies were carried out on rabbits to assess the comparative pharmacokinetics profile of the selected buccal film with oral solution. Preliminary studies indicated that the prepared films exhibited excellent physical properties, adequate mucoadhesive strength and moderate hydration. In vitro drug release data of the buccal films (F1, F2 and F3) showed distinct profiles. Permeation studies indicated higher steady-state flux from film F3 (p < 0.0001) when compared to film F2. In-vivo results of film (F3) demonstrated significant increase in absorption (p < 0.0001), Cmax (∼1.6-fold), Tmax, AUC0–α (∼3-fold, p < 0.0001) and improved bioavailability, when compared to control. This study concludes that the buccal delivery of selegiline using the developed buccal film (F3) would be a promising alternative approach for the treatment of Parkinson's disease.
Introduction
Parkinson's disease is a chronic progressive neurodegenerative disorder characterized by motor complications which impair the quality of life of the patient and cause severe disability (Chen, Citation2010). This disease is reported to affect over 10 million people worldwide irrespective of race and gender, although the prevalence is much higher in people over the age of 60 years (Prakash & Tan, Citation2010). There are reports which indicate that this disorder is increasing rapidly and the size of population affected by this disease is likely to double by 2030 (Dorsey et al., Citation2007). Existing treatment for Parkinson's disease include oral, parenteral as well as neurosurgical procedures depending upon the stage and severity of disease (Marjama-Lyons & Koller, Citation2000). A wide variety of drugs from various classes are available for the treatment of this complex neurological disorder (Connolly & Lang, Citation2014). Despite the availability of a variety of drugs for Parkinsonism, effective relief of the features associated with the disease remains elusive (Onofrj et al., Citation2008). A long-term therapy with most of the agents leads to several complications and non-motor manifestations (Brichta et al., Citation2013).
Selegiline is an irreversible and selective inhibitor of cerebral monoamine oxidase type B. It has proven safety, efficacy and is considered as a first-line therapy in early stages of Parkinson's disease (Needham & Worth, Citation2012). Significantly, the conventional oral therapy of this drug has shown poor systemic bioavailability (∼10%) on account of extensive hepatic metabolism and degradation in the gastrointestinal tract (Aldhubiab, Citation2013). Treatment with higher doses of selegiline causes adverse events such as acute hypertensive reactions in patients due to cheese effect and requires dietary restrictions of tyramine-containing food (Wimbiscus et al., Citation2010). Dysphagia is yet another common problem in patients with Parkinson's disease which limits the oral drug administration (Baijens & Speyer, Citation2009). These limitations restrict the usage of oral selegiline therapy and amplify the need for an alternative route of administration. Attempts have been made to develop new delivery systems to overcome the issues associated with oral therapy of selegiline. Few studies were carried out to develop transdermal delivery systems of this drug which could enhance the systemic delivery as well as prevent the first-pass effect (Chen et al., Citation2011; Fang et al., Citation2009). These studies suggest that transdermal route could be a positive approach for the effective delivery of selegiline, but issues such as skin reactions and high inter/intra-variability in drug absorption have limited their clinical relevance (Feiger et al., Citation2006; Frampton & Plosker, Citation2007). Concurrently, freeze dried, rapidly disintegrating oral tablets of selegiline have also been developed to deliver the drug through buccal route into the systemic circulation (Clarke et al., Citation2003; Tetrud & Koller, Citation2004). The major disadvantage of such formulation is the very short residence time for the dosage form to transport the drug through the buccal mucosa (Clarke et al., Citation2003). In this context, we hypothesize that a delivery system which can prolong the residence time in the oral cavity could be the most promising and effective approach for the successful delivery of selegiline through the buccal epithelium.
Buccal route has been in focus in the last two decades and captivated the drug delivery scientists as it offers several advantages over oral delivery (Jaipal et al., Citation2014; Nerkar & Gattani, 2011). This drug delivery route, by virtue of its ability to circumvent the degradation in the gastrointestinal tract and bypass the first-pass hepatic metabolism, is considered to be the ideal (Sudhakar et al., Citation2006). The buccal mucosa is a relatively permeable membrane which is a promising site for effective non-invasive delivery of actives. This alternative site offers rapid and direct delivery into the systemic circulation, easy accessibility, higher patient compliance, besides being capable of enhancing bioavailability (Palem et al., Citation2011). Among the various buccal dosage forms, buccal film is the most advanced and highly preferred drug delivery system on account of easy adherence to buccal mucosa, better adaptation to the mucosal surface and prolonged retention (Castán et al., Citation2014; Lv et al., Citation2014). It was also reported that the buccal delivery of selegiline is likely to enhance the absorption as well as reduce the formation of metabolites when compared to conventional therapy (Nyholm, Citation2006). Thus, the buccal delivery of selegiline seems to be advantageous and promising approach, which can surmount the issues that limit the successful delivery of this drug. It is significant to note that this drug delivery system can reduce metabolite formation of selegiline, eliminate the need for a tyramine restricted diet and could be easily administered to patients with dysphagia (Nyholm, Citation2006). Indeed, a buccal delivery system which can provide continuous steady-state plasma concentration of selegiline could be more beneficial in the therapeutic management of motor and non-motor complications in Parkinson's patients (Jenner et al., Citation2011; Senek & Nyholm, Citation2014). Thus, the objective of this study is to develop an alternative drug delivery system for selegiline which can adhere to the buccal mucosa, provide long residence time, prolong the drug release and enhance the bioavailability. The physiochemical properties of selegiline (molecular weight 187, pKa 7.5 and log p 2.8) indicate that this drug has favorable characters for greater permeation through the buccal mucosa. However, some reports suggest that the release of drug could be controlled by loading the drug into a carrier and impregnating into the film (El-Mahrouk et al., Citation2014; Giovino et al., Citation2012). Accordingly, the selegiline-loaded nanospheres were prepared using poly(lactide-co-glycolide) (PLGA) and were embedded into the buccal films. Additionally, the prepared films were characterized, evaluated in vitro and assessed for the in vivo pharmacokinetics in rabbit model.
Material and methods
Selegiline hydrochloride (98% purity), polyvinyl alcohol, poly(d,l-lactide-co-glycolide) (50:50, molecular weight: 7000–17 000), polyethylene glycol (PEG)-400, methanol, dichloromethane, ethyl alcohol and triethanolamine were purchased from Sigma Aldrich (St. Louis, MO). Carbopol 971P, ethyl cellulose and hydroxypropyl methylcellulose (HPMC) K15 were received ex gratis sample from Ind-Swift Ltd., Parwanoo, Himachal Pradesh, India. Eudragit RS 100 (Evonik Röhm GmbH, Darmstadt, Germany), dibutyl phthalate (Loba Chemie Pvt Ltd, Mumbai, Maharashtra, India) and polyvinylpyrrolidone K30 (PVP K30) (Loba Chemie Pvt Ltd, Mumbai, Maharashtra, India) were purchased commercially.
Analytical method
The high-performance liquid chromatography (HPLC) system (LC-10ATVP; Shimadzu Corporation, Tokyo, Japan) consisted of a Symmetry C18 analytical column (4.6 × 150 mm) with a detector LC UV-100 was used to determine the amount of selegiline in various samples. Chromatographic separation of selegiline was performed with a mobile phase of methanol and water (70:30). Elution was performed isocratically at 25 °C at a flow rate of 1.2 mL/min. Injection volume was 25 µL, and the column effluent was monitored at 205 nm. The method was validated by determination of linearity, precision and accuracy.
Preparation of nanospheres
Selegiline-loaded PLGA nanospheres were prepared by double emulsion-solvent evaporation method reported earlier with minor modifications (Kocbek et al., Citation2007). Briefly, PLGA (100 mg) was added to dichloromethane (6 mL) and stirred to obtain a clear solution. Selegiline hydrochloride (1 mL, 2.5, 5, 7.5 or 10 mg/mL) in water was added slowly to the polymeric solution under high-speed stirring and sonicated to provide primary emulsion. After emulsification (∼2 min), the primary emulsion was immediately added to PVA solution (2% w/v, 25 mL; optimized from concentrations ranging from 0.5 to 2.5% w/v) and was homogenized at high speed for 2 min. The residual solvent in the multiple emulsions was removed by stirring for 12 h at room temperature. The resulting dispersion was centrifuged (20 000 rpm, 20 min) and the supernatant was decanted. The nanospheres prepared were washed (three times) with deionized water to remove the surface residue and freeze dried for 24 h. The supernatant separated was analyzed for drug content to determine the entrapment efficiency.
Particle size characterization
Measurement of particle size, particle size distribution and Zeta potential of prepared nanospheres were carried out using Zetasizer (Nano-ZS; Malvern Instruments, Westborough, MA). The freeze-dried nanospheres were suspended in deionized water prior to measurement. Each sample was then vortexed for 1 min before sampling to reduce the aggregation between the nanospheres and particle sizes (volume weighted mean diameter) were measured.
Formulation of buccoadhesive films-containing selegiline-loaded nanospheres
The compositions of various buccal films are summarized in . The buccal films were prepared by adding the required quantity of eudragit in ethyl alcohol (75% v/v) under constant stirring using magnetic stirrer. Separately, weighed amount of HPMC K15 was soaked (12 h) in ultrapure water containing PEG-400 (1.5% w/v) and stirred with a mechanical stirrer. Similarly, carbopol 971P was dispersed in water with gentle stirring. Both aqueous dispersions were mixed. To this, eudragit dispersion was added under stirring to form a homogeneous mixture. The required amount of nanospheres (equivalent to 1 mg selegiline/cm2) were incorporated in the above mixture and mixed well to obtain uniform dispersion. Further, the dispersion was sonicated in an ultrasonicator to remove the entrapped air bubbles. Accurately measured volume of the dispersion was then casted on custom-made glass mold (area 6 cm2) and covered with inverted glass funnel and allowed controlled evaporation of the solvent at room temperature for 24 h.
Table 1. Composition of the prepared buccal films.
Backing membrane was prepared by mixing ethyl cellulose dispersion (5% w/v) with dibutyl phthalate (20% v/v as plasticizer) and casted on a custom-made glass mold and allowed to dry at room temperature for 24 h. The dried membrane was attached to the mucoadhesive film using PVP K30 solution (5% w/v). The prepared films adhered to backing membrane were then cut into 1 cm2 sizes, wrapped and stored in desiccator until used.
Characterization of buccal films
The prepared films were evaluated for their physical characteristics, in essence color, transparency, softness, peelability and homogeneity. Thickness of the films was measured at five different locations using digital screw gauge. The pH of the films was determined by cutting the film (size 1 cm2) and allowing it to swell in 5 ml of distilled water for 30 min. The swollen film was taken out, drained and the pH of the film was determined using a flat surface electrode (Orion 4 Star Benchtop; Thermo Fischer Scientific Inc., Waltham, MA).
Drug content
To determine the drug content of buccal films, these were punched from different regions using biopsy punch (1 cm2). These films were soaked in ethanol–water mixture (40:60, 50 mL) and stirred using vortex mixer. The extraction period was selected (36 h) as the next sampling point (40 h) did not improve the extraction of selegiline. The solution was centrifuged at 10 000 rpm (5 min) and the supernatant was collected and filtered (0.2 μm). The drug content was determined by HPLC and represented in percentage.
Percent swelling
The swelling properties of prepared films were measured by evaluating the percentage hydration. Film (1 cm2 area) was weighed (W1), placed on a stainless steel mesh and the mesh was immersed into the phosphate-buffered saline (10 mL). These were then incubated at 37 ± 1 °C. The film samples were removed from the solution at regular intervals; wiped using tissue paper and weighed (W2). Percentage hydration of the films was determined using the equation (Nair et al., Citation2013):
Drug release
The in vitro release of selegiline from the buccal films was determined by paddle over disc method using USP XXIV type II apparatus (TDC-50; Electro Lab Pvt Ltd, Mumbai, India). Film of size 2 × 1 cm2 was cut and pasted on a glass slide using double-sided adhesive tape and immersed in simulated saliva (pH 6.2, 900 mL) such that the drug release occurs from the side exposed to dissolution medium. The medium was maintained at 37 ± 1 °C and the paddle was rotated at 50 rpm (Perioli et al., Citation2004). Samples (1 mL) were withdrawn at predetermined time intervals and replaced with fresh media to compensate the loss of sample withdrawn. Further, samples were filtered through syringe filter and analyzed by HPLC.
Mucoadhesive strength
Ex vivo mucoadhesive strength of the prepared buccal films was determined using TA.XT2 texture analyzer (Stable Micro Systems Ltd, Surrey, UK) equipped with a 5-kg load cell. Mucoadhesive strength was measured using rabbit cheek pouch as biological substrate. A piece of film (size 1 cm2) to be tested was cut and attached to the probe of the texture analyzer using cyanoacrylate adhesive (Castán et al., Citation2014). The rabbit epithelium mucosa was mounted on the stationary platform. The assembly containing the rabbit cheek mucosa was filled with 2 mL of the buffer solution to keep the mucosa wet during the contact period. The movable probe of the texture analyzer was lowered until it made contact with the mucosa. The contact time between the cheek mucosa and film was 1 min. Measurements were obtained using the following parameters: pre-test speed: 0.5 mm/s; test/post-test speed: 0.5 mm/s and applied force: 1 N. The mucoadhesive strength was measured as the maximum force required to detach the film from the mucosal surface.
Ex vivo permeation studies
Permeation studies of the buccal films were carried out using rabbit buccal mucosa as permeation barrier. The buccal mucosa was separated from the buccal cavity and the connective tissue was separated using scalpel and scissors. The membrane was mounted on a Franz diffusion cell with the active diffusion area of 0.64 cm2 exposed to both the donor and receiver compartments. Selegiline nanospheres-loaded film was punched using biopsy punch (0.6 cm2) and was placed on the donor compartment, while the simulated saliva (5 mL, maintained at 37 ± 1 °C) was added to the receptor compartment (5 mL). The receiver compartment was stirred at 600 rpm with the help of a 3-mm magnetic stir bar. Samples were withdrawn at regular interval of time and analyzed by HPLC.
Surface morphology
Scanning electron microscopy (SEM) was used to examine the shape and surface characteristics of selected buccal film. The samples were mounted using silver electrical tape and coated (SCD005 Baltek Sputter Coater) with gold layer in neutral environment under reduced pressure. Samples were viewed under different magnification using Jeol SEM [457 V; Japan Electron Optics Laboratory (JEOL), Tokyo, Japan] and photographed.
In vivo study
In vivo experiments were carried out on white male rabbits (2.5–3 kg) which were maintained on a 12-h/12-h light/dark cycle in an animal facility with unlimited access to food and water (IAEC/SSP/13/PR-006). Rabbits used in the study were fasted overnight with free access to water. The animals were anaesthetized for 3–4 h by intramuscular injection of ketamine (40 mg/kg) and xylazine (5 mg/kg). Buccal film (1 cm2) was wetted (∼30 µL of water) and applied to the buccal mucosa of the rabbits while the control animals received oral solution of selegiline equivalent to 1 mg dose (1 mL). The buccal film was removed after 4 h. The blood samples were collected (∼500 µL) from the marginal ear vein at predetermined time intervals (1–12 h) using 25 gauge 1 inch needle. Plasma (500 µL) samples were mixed with equal volume of acetonitrile and centrifuged at 10 000 rpm for 10 min. Supernatant was filtered and injected into HPLC. The sample withdrawn at time zero served as the baseline value.
Data analysis
The cumulative amount of drugs permeated per unit buccal surface area was plotted against time and the slope of the linear portion of the plot was estimated as the steady-state flux (Anroop et al., Citation2009). The data were tested by one-way analysis of variance (ANOVA) and unpaired t-test using GraphPad prism 5 (GraphPad Software Inc., San Diego, CA) to test the effects of various treatments. p Value < 0.05 was considered as the level of significance. The data points provided in the graph is the mean of six trials and the error bars represents the standard deviation (SD), unless otherwise specified.
Results and discussion
Selegiline-loaded polymeric nanospheres were prepared by double emulsion-solvent evaporation method using a biocompatible and biodegradable polymer, PLGA. In this study, we have selected this polymer primarily due to its potential in providing controlled drug release (Dalpiaz et al., Citation2014). The drug entrapment potential of polymers is primarily influenced by the partition coefficient of the drug and its solubility in the particular polymer, which in turn is related to its molecular weight, chemical nature and the possible drug–polymer interaction. Preliminary studies were carried out to determine the effect of drug concentration (1–10 mg/mL) on the encapsulation efficiency of PLGA nanospheres. These studies revealed that PLGA nanospheres showed a moderately high entrapment efficiency (65–78%) and drug loading and these values were found to increase slightly with an increase in drug content. Nanospheres with highest amount of drug loading were selected for further studies. Selected formulation was characterized for particle size, size distribution and zeta potential using Zetasizer (Malvern Instruments). Polymeric nanospheres were found to be in the sub-nano size range (150–500 nm) with an average mean particle size of 300 nm. Further, the mean zeta potential and polydispersity index of the prepared nanospheres was found to be −28.2 mV and 0.14, respectively.
In the next phase, the buccoadhesive films loaded with selegiline nanospheres were formulated. The compositions of the prepared films are summarized in . The buccoadhesive films were prepared making use of two mucoadhesive polymers (HPMC K15 and carbopol 971P) and a water insoluble film forming polymer (Eudragit RS 100). The selection of polymers for the preparation of buccal films in the current study is based on the literature as well as from our earlier reports wherein these polymers contributed significantly in developing controlled release buccoadhesive films (Kumria et al., Citation2014a; Nair et al., Citation2007; Perioli et al., Citation2004). In the current study, four buccal films were developed (F1, F2, F3 and F4) to optimize selegiline release by varying the amount of hydrophilic polymer (HPMC K15, 1–4% w/v) and hydrophobic polymer (Eudragit RS 100, 1–7.5% w/v). However, the amount of carbopol 971P (2% w/v) and PEG-400 (2% w/v; plasticizer) was fixed based on the preliminary studies for mucoadhesion (data not shown). The amount of selegiline (incorporated as nanoparticles) in the film was equivalent to ∼1 mg/cm2 area. A backing membrane was attached to the prepared films as described above. The prepared buccal films were found to be colorless, translucent, soft, peelable, dry, homogenous and tack free.
Measurement of buccal film thickness is necessary to determine the accuracy of dose in the film as well as bioadhesion (Nair et al., Citation2013). It is evident from that the prepared buccal films are thin (0.28–0.33 mm) and suitable for buccal application without causing any discomfort to patient. Further, the films possessed uniform thickness, demonstrating no significant difference within the batches or between the batches. In addition, the pH of the buccal films was measured in order to ensure that the prepared films possess optimum pH and do not cause any damage to oral mucosal membrane. Indeed, the prepared films showed pH of 6.5–7, which is near to the buccal pH, indicating that the formulations is not likely to cause any irritation following buccal application.
Table 2. Comparison of thickness, pH and drug content of different buccal films.
Measurement of drug content is of utmost importance to ensure presence as well as uniformity of therapeutic agent in the prepared formulations. This was determined within the film at three different regions as well as between the films by punching exactly 1 cm2 area of the buccal films. Amount of selegiline present in the buccal films in 1 cm2 was determined and the observed percentage drug content in different formulations is depicted in . The percentage drug content was high in all the prepared buccal films (∼ 94–96%) and the low values of SD and coefficients of variation (2.84–4.37%) proved the uniformity of drug content. This observation also suggests that the polymer content, polymer type or method of preparation did not influence the content uniformity in the current experimental condition.
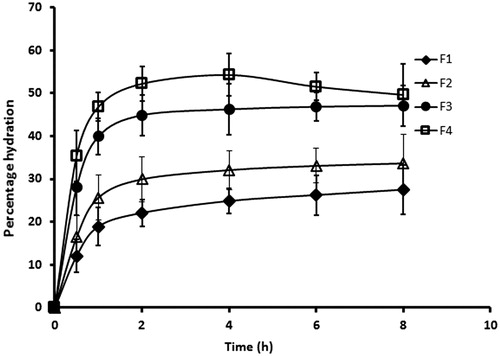
Assessing the swelling capacity of buccal film is essential to get insight into the bioadhesive property and drug release rate which is dependent on the film structure and nature of the polymer matrix used (Nair et al., Citation2013). It is likely that once the film is applied on buccal mucosa, water molecules diffuse into the polymer, hydrate the matrix and the film swell which in turn facilitates diffusion of drug from the films. Meanwhile, as the matrix uptakes water, the bonding and adhesion of the film to the membrane also begins and progresses. Thereafter, it reaches a point where the polymers get disentangled and leads to decrease in the binding/adhesion (Nair et al., Citation2013). In the current study, swelling properties of the prepared films were measured for a period of 8 h and the observed percentage hydration are depicted in . It was evident from the profile that the rate of hydration was rapid in the initial period (∼2 h) when compared to the later phase (2–8 h), irrespective of the formulation. Indeed, the percentage hydration observed at the initial point (0.5 h) was relatively high for all the films [F1 (12.06 ± 3.76%), F2 (16.42 ± 5.09%), F3 (28.06 ± 6.47%) and F4 (35.35 ± 5.91%)] indicating the potential of the film to provide bioadhesion as well as drug release. Further, it can also be seen that the percentage hydration improved (F1 < F2 < F3 < F4) with an increase in hydrophilic polymer content (HPMC) and decreases with increase in hydrophobic polymer (eudragit). However, in the case of film F4, the percentage hydration reduces slightly after 4 h, which is due to the erosion of polymer from the film. For the films F1, F2 and F3, the swelling profile reveals that the percentage hydration in later phase (2–8 h) were comparable, pointing to the fact that equilibrium has been achieved (). In addition, the overall percentage hydration achieved by the films F1, F2 and F3 at the end of the study period (8 h) was 27.53 ± 5.67%, 33.64 ± 6.81% and 47.10 ± 4.72%, respectively. This moderate hydration observed in these films is likely to provide adequate bioadhesion and controlled release of selegiline, which also substantiate our selection of polymers in the current study. This observation has significant importance as over hydration may lead to distortion of polymer molecule and/or erosion of the polymer matrix. As the film F4 shows the possible erosion, this formulation was excluded from the further studies.
Figure 1. The percentage hydration pattern of the prepared buccal films for a period of 8 h determined using 1 × 1 cm2 of the film. The value represents average of six trials ± SD.
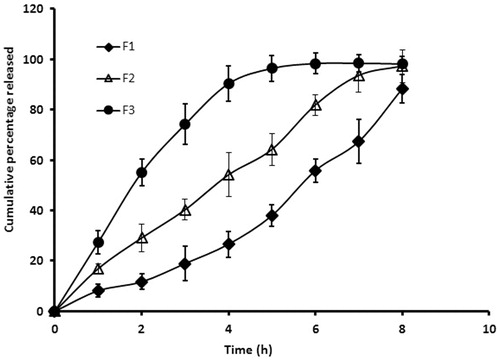
In the next phase of the study, we assessed the release of drug from the selected films (F1, F2 and F3) which is essential for permeation of drug molecules into and through the buccal epithelium. compares the cumulative percentage of selegiline released from the nanospheres-loaded buccal films at different time intervals. It is apparent from the that the profiles of all the three films were distinct. In the case of film F1, the drug release was comparatively slow during initial period (∼12% in 2 h) and this was followed by a steady increase in drug release in the later phase (2–8 h), although the drug release was incomplete (∼88% released in 8 h). However, film F2 showed relatively higher release rate in the initial period and was comparatively steady in the later phase. In contrast, the film F3 showed biphasic release pattern with rapid drug release in the initial phase (∼90% in 4 h) followed by a slow release (4–8 h). The initial burst release observed could be significant as this is likely to contribute in achieving the required therapeutic level rapidly by providing loading dose. Separately, the percentage of drug released from formulations F1, F2 and F3 at the end of 4 h was found to be 26.61 ± 5.14%, 54.17 ± 8.79% and 90.33 ± 7.13%, respectively indicating a significant difference (p < 0.001) in the release rate (). This substantial difference in the release rate observed in the films could be directly related to the polymer composition in the current experimental condition. This study demonstrates that an increase in hydrophilic polymer (HPMC) content or decrease in hydrophobic polymer (eudragit) in films could lead to increase in the drug release. As the drug release was significantly slow and low in buccal films F1, it was excluded from the further studies.
Figure 2. Comparison of the cumulative percentage of selegiline released from different buccal films at various time intervals. In vitro drug release study was carried out by placing the film (2 × 1 cm2) in USP apparatus (paddle over disc) using simulated saliva as dissolution medium. The value represents average of six trials ± SD.
Mucoadhesive studies of buccal films are considered as one of the critical and important parameter to be determined in order to ensure the successful delivery of drug molecules following buccal application. The measurement of mucoadhesive strength provides the quantification of the degree of binding of polymer in the adhesive system to the buccal epithelia which would offer an insight into the retention of the prepared films at the site of application (Nair et al., Citation2013). The mucoadhesive strength of the selected buccal films (F2 and F3) were determined with a texture analyzer using rabbit buccal mucosa as biological substrate. The peak adhesive force measured for the film F2 and F3 were found to be 6.35 ± 0.44 and 7.62 ± 0.58 N, respectively. The data generated here signify good mucoadhesion of both the films (F2 and F3) which suggests that these films possess adequate mucoadhesion, long residence time and endure the movement of the buccal cavity. However, it was also observed that the peak adhesive force measured in films (F2 and F3) were significant (p = 0.0016), suggesting that the film composition have influenced the mucoadhesive strength. This could be explained by the fact that the films F2 and F3 were prepared using two mucoadhesive polymers (HPMC and carbopol) and one film forming polymer (eudragit) (). However, the composition of these two films (F2 and F3) differs with respect to the concentration of HPMC and eudragit, while the carbopol content was similar (2% w/w). Thus, the plausible explanation for a greater mucoadhesive strength observed in the case of F3 films is likely due to the higher HPMC content (3% w/w) when compared to film F2 (HPMC content was 2% w/w). These findings are consistent with the earlier reports where it was observed that an increase in HPMC content enhances the mucoadhesive strength (Kumria et al., Citation2014b).
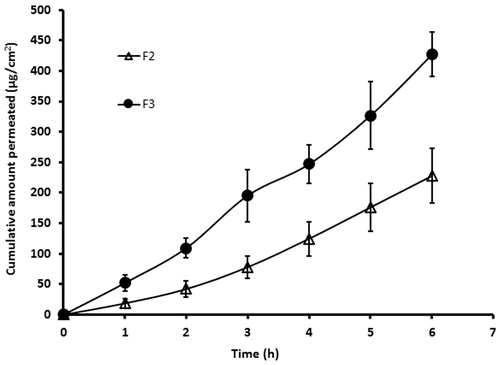
It is well known that the ex vivo permeation studies using biological membranes can be used to predict the absorption kinetics of drug following in vivo application. In general, the transport of drug molecules into and through the membrane is primarily influenced by the physicochemical properties of the drug molecule as well as the physiological property of the membrane. In the current study, we assessed the ex vivo permeation potential of selegiline by placing the selected buccal films (F2 and F3) over rabbit buccal mucosa, which is considered to be a good model for human buccal membrane (Nair et al., Citation2013). The amount of drugs transported through the buccal mucosa and reaching the receptor fluid was assessed and is depicted in . It is apparent from that films (F2 and F3) exhibited typical permeation profile, substantiating the potential of these films to deliver selegiline through buccal mucosa. On the other hand, the rate of drug permeation was found to be significantly higher with film F3 and the steady-state flux values for film F2 and F3 were found to be 38.60 ± 4.82 µg/cm2/h and 70.25 ± 7.35 µg/cm2/h (p < 0.0001), respectively. The cumulative amount of selegiline permeated through the buccal epithelial tissue from films F2 and F3 at the end of 6 h was found to be 227.75 ± 45.22 and 426.72 ± 36.54 µg/cm2, respectively. Indeed, the drug was detected in receiver fluid in the first sampling period itself (1 h) in both the cases (film F2 and F3) indicating the rapid permeation potential of selegiline into and through the buccal membrane. Based on the higher steady-state flux values, the film F3 was selected for further investigation.
Figure 3. Comparison of ex vivo permeation profile of selegiline from the buccal films (F2 and F3) using rabbit buccal mucosa for a period of 6 h in Franz diffusion cell. The value represents average of six trials ± SD.
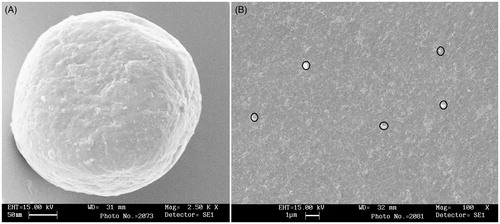
SEM was utilized to produce high-resolution three-dimensional images which can provide topographical and morphological features of the prepared nanospheres and buccal film (F3). It is apparent from that the prepared nanospheres were spherical in shape and possess smooth surface with a continuous wall and no apparent cracks, which is vital to provide lesser permeability to gases, better protection and core retention. The SEM images of buccal film (F3) also indicate that the film surface is smooth, uniform and non-porous. Further, it is also evident that the nanospheres were uniformly embedded in the prepared film (). This observation indicates that the prepared buccal film possess all the morphological characteristics (smooth, uniform, non-porous and nanospheres are uniformly embedded) required for a buccal film.
Figure 4. SEM image of selegiline-loaded nanospheres (A) and buccal film F3 (B) impregnated with nanospheres.
In vivo studies are designed to provide a better insight into the real-time performance of the prepared buccoadhesive films as the drug absorption is influenced by several intrinsic factors (Nair et al., Citation2013). Thus in the final phase of the study, we assessed the pharmacokinetics of selegiline following buccal application of the selected film (F3) and compared the bioavailability parameters with oral delivery of selegiline solution. The studies were carried out in rabbit model, which is the primary choice among the animal models (Jay et al., Citation2002; Rana & Murthy, Citation2013). The dose of selegiline administered (1 mg) by buccal route and oral solution was fixed in order to assess the relative bioavailability. The pharmacokinetic parameters, namely, area under the plasma concentration time curve from time 0 to ∞ (AUC0–α), peak plasma drug concentration (Cmax) and time to reach maximum drug concentration (Tmax) were calculated using non-compartmental pharmacokinetic model. AUC0–α was calculated using the trapezoid rule and Cmax, Tmax were determined from the plasma drug concentration curves. compares the plasma level profiles of selegiline following buccal application of the nanospheres-loaded buccal film (F3) and the control solution by oral route. It is evident from that the pharmacokinetic profiles of selegiline following buccal application were different from the control solution, suggesting that the delivery of selegiline is influenced by the route of administration. In both the cases, the drug was present in the systemic circulation at the initial sampling point (0.5 h) after administration but was statistically significant [∼45.66 and 74.33 ng/mL (p < 0.001) of selegiline with buccal film and control, respectively]. Further, in the case of buccal film it can be seen that the duration of absorption was significantly prolonged (up to 4 h) which in turn significantly enhanced the absorption of selegiline into the systemic circulation (measured from plasma concentration level) when compared to oral solution (). The greater amount of selegiline observed in the absorptive phase following buccal application suggests that the drug is continuously released from the film and is rapidly transported through the buccal epithelium without much hindrance from the barrier, probably due to the high partition coefficient of selegiline (log p = 2.8). However, the drug plasma concentration was found to reduce rapidly after the buccal film was removed (4 h) in the post-absorptive phase, signifying short biological half-life of selegiline. In the case of control formulation, the drug absorption was relatively rapid (peak plasma drug concentration was achieved in 1 h) and then declined rapidly suggesting rapid metabolism or quick distribution of drug into tissue compartments (). The measured pharmacokinetic parameters of selegiline following administration of buccal film and oral solution are summarized in . It was found that the buccal administration of selegiline significantly increased the values of Cmax, Tmax and AUC0–α, compared to control. The maximum amount of selegiline in the plasma (Cmax) after buccal administration of film was found to be ∼1.6-fold higher than control (). This data signifies that the amount of selegiline reaching the systemic circulation following buccal administration was significantly greater (p < 0.0001) than oral delivery. Further, the Tmax value was found to increase following buccal application (4 h) when compared to oral solution (1 h) suggesting that the buccal administration of the developed film prolonged the delivery and is likely to reduce the frequency of selegiline therapy. Indeed, these data substantiate the objective of buccal delivery of selegiline by impregnating the drug-loaded nanospheres into the buccal films. In addition, significant enhancement in the AUC0–α was also observed (∼3-fold, p < 0.0001) in the case of buccal administration, when compared to control (). This significant enhancement in AUC values (in the case of buccal film) demonstrate improved bioavailability (relative bioavailability ∼350%) from the prepared buccal film (F3) when compared to control. The improvement in bioavailability of selegiline is probably because the application of buccal film could directly transport the drug into the systemic circulation and overcome extensive metabolism by the liver, although the oral delivery could not prevail over the first-pass effect.
Figure 5. Comparison of the plasma profiles of selegiline following buccal application (4 h) of the nanospheres-loaded buccal film (F3) and the control solution by oral route in rabbits. The value represents average of six trials ± SD.
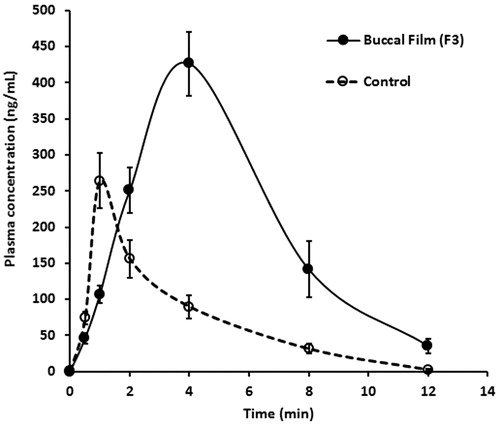
Table 3. Mean pharmacokinetic parameters of selegiline in plasma following buccal application (4 h) of film F3 (1 mg) and oral solution (control) in rabbits.
Conclusion
A systematic study was carried out with the objective of developing an alternative dosage form for the effective delivery of selegiline. Drug-loaded nanospheres were embedded into the buccal film and evaluated both in vitro and in vivo. The data demonstrated at various phases of the study indicates that the selected film exhibited excellent characteristics such as good physical properties, adequate bioadhesion and controlled drug release in addition to its objective of delivering a higher amount of selegiline through the buccal mucosa. Further, the results of in vivo studies substantiated the in vitro and ex vivo data and suggested that the prepared buccal film has the potential to prolong the retention, provide controlled release and enhance the bioavailability of selegiline. Indeed, this drug delivery approach is likely to overcome the major issues related to the oral therapy of selegiline and provide therapeutic value to patients with Parkinson's disease.
Declaration of interest
The authors report no conflicts of interest. The authors thank the Deanship of Scientific Research, King Faisal University for funding this research project (#140103).
References
- Aldhubiab BE. (2013). Formulation and in vitro evaluation of gelatin nanospheres for the oral delivery of selegiline. Curr Nanosci 9:21–5
- Anroop B, Ghosh B, Parcha V, Khanam J. (2009). Transdermal delivery of atenolol: effect of prodrugs and iontophoresis. Curr Drug Deliv 6:280–90
- Baijens LW, Speyer R. (2009). Effects of therapy for dysphagia in Parkinson's disease: systematic review. Dysphagia 24:91–102
- Brichta L, Greengard P, Flajolet M. (2013). Advances in the pharmacological treatment of Parkinson's disease: targeting neurotransmitter systems. Trends Neurosci 36:543–54
- Castán H, Ruiz MA, Clares B, Morales ME. (2014). Design, development and characterization of buccal bioadhesive films of Doxepin for treatment of odontalgia. Drug Deliv [Epub ahead of print 27 March 2014]
- Chen JJ. (2010). Parkinson's disease: health-related quality of life, economic cost, and implications of early treatment. Am J Manag Care 16(Suppl. Implications):S87–93
- Chen CC, Fang CL, Al-Suwayeh SA, et al. (2011). Transdermal delivery of selegiline from alginate-Pluronic composite thermogels. Int J Pharm 415:119–28
- Clarke A, Brewer F, Johnson ES, et al. (2003). A new formulation of selegiline: improved bioavailability and selectivity for MAO-B inhibition. J Neural Transm 110:1241–55
- Connolly BS, Lang AE. (2014). Pharmacological treatment of Parkinson disease: a review. JAMA 311:1670–83
- Dalpiaz A, Contado C, Mari L, et al. (2014). Development and characterization of PLGA nanoparticles as delivery systems of a prodrug of zidovudine obtained by its conjugation with ursodeoxycholic acid. Drug Deliv 21:221–32
- Dorsey ER, Constantinescu R, Thompson JP, et al. (2007). Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 68:384–6
- El-Mahrouk GM, El-Gazayerly ON, Aboelwafa AA, Taha MS. (2014). Chitosan lactate wafer as a platform for the buccal delivery of tizanidine HCl: in vitro and in vivo performance. Int J Pharm 467:100–12
- Fang JY, Hung CF, Chi CH, Chen CC. (2009). Transdermal permeation of selegiline from hydrogel-membrane drug delivery systems. Int J Pharm 380:33–9
- Feiger AD, Rickels K, Rynn MA, et al. (2006). Selegiline transdermal system for the treatment of major depressive disorder: an 8-week, double-blind, placebo-controlled, flexible-dose titration trial. J Clin Psychiatry 67:1354–61
- Frampton JE, Plosker GL. (2007). Selegiline transdermal system in the treatment of major depressive disorder. Drugs 67:257–65
- Giovino C, Ayensu I, Tetteh J, Boateng JS. (2012). Development and characterisation of chitosan films impregnated with insulin loaded PEG-b-PLA nanoparticles (NPs): a potential approach for buccal delivery of macromolecules. Int J Pharm 428:143–51
- Jaipal A, Pandey MM, Charde SY, et al. (2014). Controlled release effervescent buccal discs of buspirone hydrochloride: in vitro and in vivo evaluation studies. Drug Deliv 1–7 [Epub ahead of print 3 June 2014]
- Jay S, Fountain W, Cui Z, Mumper RJ. (2002). Transmucosal delivery of testosterone in rabbits using novel bi-layer mucoadhesive wax-film composite disks. J Pharm Sci 91:2016–25
- Jenner P, McCreary AC, Scheller DK. (2011). Continuous drug delivery in early- and late-stage Parkinson's disease as a strategy for avoiding dyskinesia induction and expression. J Neural Transm 118:1691–702
- Kocbek P Obermajer N, Cegnar M, et al. (2007). Targeting cancer cells using PLGA nanoparticles surface modified with monoclonal antibody. J Control Release 120:18–26
- Kumria R, Nair AB, Al-Dhubiab BE. (2014a). Loratidine buccal films for allergic rhinitis: development and evaluation. Drug Dev Ind Pharm 40:625–31
- Kumria R, Nair AB, Goomber G, Gupta S. (2014b). Buccal films of prednisolone with enhanced bioavailability. Drug Deliv 1–8 [Epub ahead of print 3 June 2014]
- Lv Q, Shen C, Li X, et al. (2014). Mucoadhesive buccal films containing phospholipid-bile salts-mixed micelles as an effective carrier for Cucurbitacin B delivery. Drug Deliv [Epub ahead of print 27 January 2014]
- Marjama-Lyons J, Koller W. (2000). Tremor-predominant Parkinson's disease. Approaches to treatment. Drugs Aging 16:273–8
- Nair A, Gupta R, Vasanti S. (2007). In vitro controlled release of alfuzosin hydrochloride using HPMC-based matrix tablets and its comparison with marketed product. Pharm Dev Technol 12:621–5
- Nair AB, Kumria R, Harsha S, et al. (2013). In vitro techniques to evaluate buccal films. J Control Release 166:10–21
- Needham E, Worth P. (2012). Parkinson's disease: a guide to pharmacological management. Prescriber 23:21–31
- Nerkar PP, Gattani S. (2011). In vivo, in vitro evaluation of linseed mucilage based buccal mucoadhesive microspheres of venlafaxine. Drug Deliv 18:111–21
- Nyholm D. (2006). Pharmacokinetic optimisation in the treatment of Parkinson's disease: an update. Clin Pharmacokinet 45:109–36
- Onofrj M, Bonanni L, Thomas A. (2008). An expert opinion on safinamide in Parkinson's disease. Expert Opin Investig Drugs 17:1115–25
- Palem CR, Gannu R, Yamsani SK, et al. (2011). Development of bioadhesive buccal tablets for felodipine and pioglitazone in combined dosage form: in vitro, ex vivo, and in vivo characterization. Drug Deliv 18:344–52
- Perioli L, Ambrogi V, Angelici F, et al. (2004). Development of mucoadhesive patches for buccal administration of ibuprofen. J Control Release 99:73–82
- Prakash KM, Tan EK. (2010). Development of Parkinson's disease biomarkers. Expert Rev Neurother 10:1811–25
- Rana P, Murthy RS. (2013). Formulation and evaluation of mucoadhesive buccal films impregnated with carvedilol nanosuspension: a potential approach for delivery of drugs having high first-pass metabolism. Drug Deliv 20:224–35
- Senek M, Nyholm D. (2014). Continuous drug delivery in Parkinson's disease. CNS Drugs 8:19–27
- Sudhakar Y, Kuotsu K, Bandyopadhyay AK. (2006). Buccal bioadhesive drug delivery-a promising option for orally less efficient drugs. J Control Release 114:15–40
- Tetrud JW, Koller WC. (2004). A novel formulation of selegiline for the treatment of Parkinson's disease. Neurology 63:S2–6
- Wimbiscus M, Kostenko O, Malone D. (2010). MAO inhibitors: risks, benefits, and lore. Cleve Clin J Med 77:859–82
