Abstract
Most of the newly designed drug molecules are lipophilic in nature and often encounter erratic absorption and low bioavailability after oral administration. Finding ways to enhance the absorption and bioavailability of these lipophilic drugs is one of the major challenges that face pharmaceutical industry nowadays. In view of that, the purpose of this review is to shed some light on a novel particulate self-assembling system named “beads” than can act as a safe carrier for delivering lipophilic drugs. The beads are prepared simply by mixing oils with cyclodextrin (CD) aqueous solution in mild conditions. A unique interaction between oil components and CD molecules occurs to form in situ surface-active complexes which are prerequisites for beads formation. This review mainly focuses on the fundamentals of beads preparation through reviewing present, yet scarce, literature. The key methods used for beads characterization are discussed in details. Also, the potential mechanisms by which beads increase the bioavailability of lipophilic drugs are illustrated. Finally, the related research areas that needs to be addressed in future for optimizing this promising delivery system are briefly outlined.
Introduction
Most of the newly designed potent drug entities are large in size with poor aqueous solubility and high degree of lipophilicity (Porter et al., Citation2007; Shukla et al., Citation2011; Kuentz, Citation2012). These properties in drug molecules, unfortunately, cause unfavorable drug absorption associated with low and variable bioavailability after oral administration (Porter et al., Citation2007; Shukla et al., Citation2011; Kuentz, Citation2012). During the last two decades, lipid-based drug delivery systems have been extensively investigated to cope with these impediments (Mu et al., Citation2013). Broadly, lipid-based drug delivery systems are classified into emulsifying systems or particulate systems. The emulsifying systems represents micro/nanoemulsions (Tiwari & Amiji, Citation2006; Sun et al., Citation2012; Chavhan et al., Citation2013) and self-emulsifying systems (Wei et al., Citation2005; Poullain-Termeau et al., Citation2008; Aburahma et al., Citation2010; Singh et al., Citation2011; Seo et al., Citation2013), while the lipid-based particulate delivery systems includes nanocapsules (Groo et al., Citation2013; Zhao et al., Citation2013), solid lipid nanoparticles (SLNs) (Bondi et al., Citation2003; Hu et al.,Citation2004; Fontana et al., Citation2005; Silva et al., Citation2012; Tran et al., Citation2014) and nanostructured lipid carriers (NLCs) (Jia et al., Citation2010; Zhuang et al., Citation2010; Mandpe & Pokharkar, Citation2013; Wang et al., Citation2013; Shangguan et al., Citation2014). The lipid-based drug delivery systems present a viable mean for enhancing the oral bioavailability of lipophilic drugs using different mechanisms (Hauss, Citation2007; Mu et al., Citation2013). The lipid excipients present in these formulations resemble body components, which help in enhancing in vivo drugs solubility and bioavailability (Hauss, Citation2007; Mu et al., Citation2013). In addition, the digestion process and absorption of lipids in the gastrointestinal tract (GIT) significantly enhances the uptake of the associated drugs into the lymphatic system that pours directly into systemic circulation (Ali Khan et al., Citation2013; Singh et al., Citation2014). Nevertheless, lipid-based formulations suffer from a number of drawbacks that undermine their oral safety and scaling-up suitability. The presence of organic solvents during the manufacturing process of SLNs and NLCs leave toxic residues within the formulations (Bochot et al., Citation2007). Furthermore, their fabrication process sometimes involves melting the lipid phase at high temperature which damages thermolabile or fragile drugs (Bochot et al., Citation2007; Parhi & Suresh, Citation2012). Besides, the large amount of surface-active agents present in the emulsifying systems are associated with adverse side effects (Bochot et al., Citation2007).
Cyclodextrins (CDs) represents a family of cyclic α-(1–4)-linked oligosaccharides composed of α-d-glucopyranose subunits (Loftsson & Brewster, Citation1996; Duchêne et al., Citation2003; Loftsson & Duchêne, Citation2007; Sharma & Baldi, Citation2014). Natural CDs are obtained from enzymatic degradation of starch using amylase excreted by Bacillus macerans to form a mixture of six-, seven- and eight-member cyclic oligosaccharides rings corresponding to α-CD, β-CD and γ-CD, respectively (Duchêne et al., Citation2003). Natural CDs have rather limited aqueous solubility, especially β-CD, due to binding of CD molecules in the crystal state and formation of intra-molecular hydrogen bond within the CD molecule, averting hydrogen bond formation with water molecules (Coleman et al., Citation1992). To improve their aqueous solubility, different synthetic CD derivatives of pharmaceutical interest were manufactured and include the hydroxypropyl derivatives of β-CD and γ-CD (HP-β-CD and HP-γ-CD), the randomly methylated-β-CD (RM-β-CD), and sulfobutylether-β-CD (SB-E-β-CD) (Loftsson & Brewster, Citation1996; Brewster & Loftsson, Citation2007).
CD molecules exhibit a truncated cone outline with a hydrophilic outer surface and a lipophilic central cavity capable of including drug molecules (Challa et al., Citation2005; Loftsson et al., 2004). For this reason, they are considered as interesting agents for solubilization or stabilization of drug molecules within their cavity (Brewster & Loftsson, Citation2007). In aqueous solutions, CDs are capable of self-assembling in form of spheres, disks, or fiber aggregates that further contribute to their solubilizing property (Loftsson et al., Citation2004; Messner et al., Citation2010). Although CDs have been studied since the 1970s (Loftsson & Duchêne, Citation2007) and are currently present in numerous pharmaceutical market products (Loftsson & Duchêne, Citation2007; Kurkov & Loftsson, Citation2013), they are still considered as novel excipients due to their ongoing innovative applications in pharmaceutical industry. In the current era of nanoparticulate delivery systems, CDs are widely employed in formulating nanoparticles (Kanwar et al., Citation2011; Perret et al., Citation2013) and liposomes (Ascenso, Citation2013; Chen et al., Citation2014) to enhance drug solubility, stability and loading. Further, CDs have been explored as a stabilizer for emulsions and multiple emulsions (Duchêne et al., Citation2003; Yu et al., Citation2003; Mathapa & Paunov, Citation2013). Formation of a partial inclusion complex between CDs and certain lipophilic guest molecules can convert them from normal oligosaccharides to surfactant-like molecules with more diverse properties (Messner et al., Citation2010). In this context, the stabilization of emulsions by CDs is credited to the formation of in situ surface-active agents from the interactions between triglycerides (TGs) or fatty acids (FAs) in oil phase with CDs comprising of a hydrophilic CD molecule with the hydrophobic FA residue protruding from it (Duchêne et al., Citation2003).
In, 2007, Bochot et al. and Trichard et al. took advantage of this interesting interaction between CDs and TGs to presented a new self-aggregate particulate system known as “beads” prepared by mixing CDs aqueous solution with certain oils (Bochot et al., Citation2007; Trichard et al., Citation2007, Citation2011). The uniqueness of these novel beads rely on their high oil content (>80%), which enable efficient encapsulation of lipophilic drugs, thus, open new prospects for oral (Trichard et al., Citation2007; Hamoudi et al., Citation2012) and topical drug delivery (Trichard et al., Citation2008a). Furthermore, the preparation process for beads is very simple and involves only continuous external orbital shaking of a mixture of CD aqueous solution and oil at room temperature without the use of organic solvents, cross-linking or surface-active agents (Trichard et al., Citation2011; Hamoudi et al., Citation2013).
In this article, the available literature on the proposed beads was carefully reviewed and presented. A deep insight on the mechanism by which beads are formed is provided with special focus on the interaction between TGs and CDs. In addition, beads characterization parameters, stability and regulatory consideration are explored in-depth. Particular emphasize have been placed on the expected in vivo behavior and mechanisms for drug absorption enhancement by beads after oral administration. Finally, a brief perspective on research areas that needs to be addressed is encompassed with the aim of encouraging further researches as a step towards scaling-up of this potential, yet not widely investigated, drug delivery system.
Components of beads
Simplicity is the foundation of good formulation design (Chang & Chang, Citation2007). It is desirable to minimize the number of ingredients present in a formulation in order to decrease the probability of any chemical or physical interaction between drug and one of the excipients. Not to mention, the associated better economic value that arises when pharmaceutical products are manufactured with less excipients (Chang & Chang, Citation2007). The proposed beads represent a typical example of good formulation design as they are formulated using only two ingredients (oils and CDs) (Hamoudi et al., Citation2011; Trichard et al., Citation2011). Up to date, only natural CDs were investigated to obtain beads. On the other hand, different oils whether vegetable (soybean oil, sweet almond oil, wheat germ oil or borage oil) (Trichard et al., Citation2011), synthetic (Silicon 200® fluid 10, 50 or 100cSt) or mineral (Primol® 352 and Marcol® 82) have been examined to form beads with natural CDs (Trichard et al., Citation2007). depicts the combinations of natural CDs and different oils that were explored to form beads. Among all of the combinations that formed beads, α-CD and soybean oil are the most investigated due to their ability to produce beads with suitable surface characters and high drug load (Trichard et al., Citation2007, Citation2008b; Hamoudi et al., Citation2011, Citation2012).
Table 1. Possibility of forming beads using different oils and natural cyclodextrins combinations.
Beads preparation and drug loading
Preparation and mechanism of beads formation
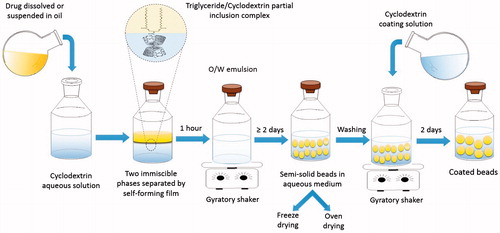
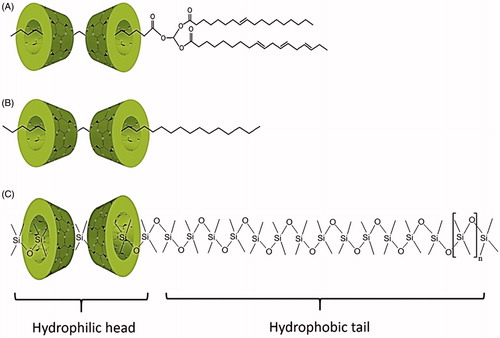
Beads are formed as a result of a unique interaction between CDs and oil components to form surfactant-like molecules with unique physicochemical properties (Bochot et al., Citation2007; Trichard et al., Citation2008b). Schematic presentation of the successive steps involved in beads preparation is presented in . The preparation process involves the addition of oil to CD aqueous solution which results in the formation of two non-miscible phases separated at the oil/water interface by a macroscopically visualized self-forming film (Shimada et al., Citation1992). For α-CD and soybean oil combination, the latter is composed of partial inclusion complexes of α-CD molecules with TGs (Shimada et al., Citation1992; Bochot et al., Citation2007). This partial inclusion complex constitutes a surface-active agent (hydrophilic head and a hydrophobic tail) with amphiphilic property where α-CD containing the FA chain being oriented toward the aqueous phase while the two remaining free FA chains are oriented toward the oily phase (Shimada et al., Citation1992; Duchêne et al., Citation2003). When exposed to gyratory shaking, the film curves and surrounds the oil to form a stable milky O/W emulsion where crystallization and self-aggregation of CD molecules around the oily globules occurs (Shimada et al., Citation1992). Finally, after continuous shaking for several days, semi-solid beads suspended in aqueous medium are formed (Bochot et al., Citation2007).
Hamoudi et al. (Citation2012) attempted to coat the beads described above by a layer of α-CD to slowing down their erosion and prolong the drug release (Hamoudi et al., Citation2012). Coating was achieved by reintroducing the beads, after washing, into aqueous solution containing α-CD followed by continuous shaking for another 48 h. Beads washing is critical before reincorporating α-CD solution in order to remove any residual oil remaining in the dispersion medium, thus, avoid any possible interaction between the coating α-CD molecules and the rest of TGs (Hamoudi et al., Citation2012).
Drug loading
To prepare drug-loaded beads, the active ingredient is either dissolved (isotretinoin, adapalene, progesterone) or dispersed (adapalene, progesterone, indomethacin) within the oil phase (Trichard et al., Citation2007, Citation2008a; Hamoudi et al., Citation2012). Indeed, drug load is highly dependent on drug concentration in the oily phase and increase when the drug incorporated in the oil phase increases (Trichard et al., Citation2008a). However, special consideration must be taken when incorporating lipophilic drugs to prevent possible competition between the added drug and the FA for entering the CD cavity (Yu et al., Citation2001). Drugs that interact with CDs may displace the FA hydrocarbon chain of the TG within CD cavity, and therefore, destroy the surface-active agent formed and prevent the formation of stable emulsion (Yu et al., Citation2001, Citation2003; Duchêne et al., Citation2003). In the same way, drugs which are able to form inclusion complexes with α-CD are not suitable candidates for beads formulation (Yu et al., Citation2001; Duchêne et al., Citation2003). Nevertheless, since all the new potent drug molecules are large in size and α-CD cavity is small in size, there is less chance for them to compete with the TG for CD cavity (Duchêne et al., Citation2003). For that reason, drug loading does not constitute a real limitation in beads formation (Trichard et al., 2011).
Time required to form beads
The time required to prepare beads varied depending on the type of oil and CD used as well as drug load. Compared to α-CD/soybean oil combination that required only 2.5 d to form homogenous beads, both wheat germ and sweet almond oils took 3 d to form beads with α-CD (Trichard et al., Citation2011). On the other hand, Marcol 82/α-CD took 8 d to form beads while Silicon 50cSt/γ-CD formed beads after only 1.5 d (Trichard et al., Citation2008b). Generally speaking, drug loading increased the time required to achieve a monodisperse population of α-CD/soybean beads. The loaded drugs interfere with the interaction between TGs and CDs at the oil/water interface that is essential for bead formation (Bochot et al., Citation2007). Besides, the drug molecules in oil may delay the self-assembling and crystallization of α-CD molecules to form beads matrix (Hamoudi et al., Citation2011). While only 4 d were necessary to prepare beads from progesterone dissolved in soybean oil with α-CD, 10 d were required for progesterone suspended in soybean oil (Hamoudi et al., Citation2011). Isotretinoin-loaded beads along with adapalene-loaded beads from either drug solution or suspension in soybean oil required 7 d to form homogenous beads while indomethacin-loaded ones required 9 d () (Trichard et al., Citation2007, Citation2008a; Hamoudi et al., Citation2011).
Table 2. Characteristics of drug loaded freeze-dried beads formed of soybean oil and α-cyclodextrin.
Drying of beads (freeze drying versus oven drying)
To facilitate beads handling and use for oral administration, beads are dried by either freeze drying for 48 h (Trichard et al., Citation2008b) or oven drying for 6 h (Hamoudi et al., Citation2013). The shape of the beads is not affected by the drying method as they remained spherical, yet, drying changes the beads structural properties and drug release profile (Hamoudi et al., Citation2013). A comparison between oven-dried beads and freeze-dried beads is presented in . Oven-dried beads are generally harder and smaller than freeze-dried beads logically due densification and shrinking of CD matrix during oven drying (Hamoudi et al., Citation2013). Besides, exudation of oil located close to the beads’ surface gives them sticky texture. Contrary to that, the freeze-dried beads are porous and with nearly unchanged diameter due to direct evaporation of ice without passing through the liquid state (Hamoudi et al., Citation2013).
Table 3. Comparison between the characteristics of uncoated oven-dried beads and freeze-dried beads.
Beads structure
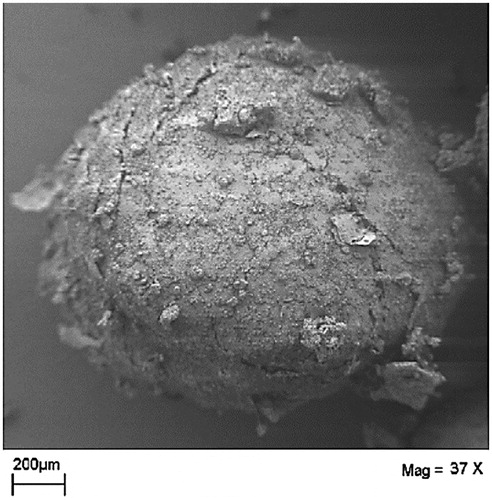
Morphologically, the beads are minispheres composed of partially crystalline CD matrix surrounding micro-domains of oil () (Bochot et al., Citation2007). The diameter of freshly prepared beads using α-CD/soybean oil is approximate 1.6 ± 0.2 mm and the water content is nearly 70% (Bochot et al., Citation2007). After the removal of water from the beads, the oily content in the bead is as high as 80% (w/w) and the CD content around 20% of the weight (Bochot et al., Citation2007).
Figure 2. Scanning electron micrograph of freeze-dried beads (modified from Bochot et al., Citation2007).
Advantages of beads
The preparation process for beads is very simple and only involves continuous external orbital shaking of oil with CD aqueous solution in very mild conditions that are suitable for poorly-stable or thermolabile drug (Trichard et al., Citation2007). This was evident when the poorly stable drug isotretinoin was successfully encapsulated into beads without any degradation or conversion into isomers (Trichard et al., Citation2007). Besides, beads improve the stability of the encapsulated fragile drugs such as retinoids by concealing them in the inner micro-compartments of oil present (Trichard et al., Citation2007).
Further, beads preparation process does not involve any organic solvents, cross-linking polymers or surface-active agents which are beneficial from the safety aspect (Bochot et al., Citation2007). The high oil content (80%) in the beads makes them a promising carrier for encapsulating lipophilic drugs (Trichard et al., Citation2007, Citation2008b; Hamoudi et al., Citation2011, Citation2012). The drug release from the beads can be controlled by coating them with a layer of CD (Hamoudi et al., Citation2012). Besides, different research groups have shown that beads dramatically increase the oral bioavailability of lipophilic drugs in rats (Trichard et al., Citation2007; Hamoudi et al., Citation2012). On the other hand, due to their semi-solid consistency before freeze-drying, beads have great potential for topical and cosmeceuticals applications (Trichard et al., Citation2008a).
Factors affecting beads formation
Studying and adjusting the formulation variables is critical for optimizing the characteristics of a delivery system.
Formulation factors
Type and concentration of CD and oil
Based on the published researches, only certain combinations and ratios of CDs and oils can successfully form beads depending on oil chemical composition and formation of partial inclusion complexes between CD molecules and oil components () (Bochot et al., Citation2007; Trichard et al., Citation2008b, Citation2011). The appropriate fitting and molecular organization of oil components within CD molecules is also crucial to provide strong inclusion complexes with emulsifying property (Duchêne et al., Citation2003; Bochot et al., Citation2007; Trichard et al., Citation2008b). As stated previously, up to date, only natural CDs have been investigated to form beads probably because the synthetic CDs have greater potential for complexation with the drug rather that the TGs along with their high water solubility and limited self-aggregation ability (Loftsson et al., Citation2004; Messner et al., Citation2010).
To define the exact domain for beads formation, Bochot et al. (Citation2007) constructed three ternary phase diagrams using soybean oil, water and natural CDs (α-CD, β-CD or γ-CD solutions). Beads were formed from α-CD solution and soybean oil combinations employing 12–24% (w/w) of soybean oil, 70–82% (w/w) of water and 3–6% (w/w) of α-CD. The optimal formulation that showed homogeneous beads with high fabrication yield was composed of 19.6% of soybean oil, 74.4% of water and 6% of α-CD (Bochot et al., Citation2007). Oppositely, beads were never formed from combinations of soybean oil with β-CD or γ-CD aqueous solutions and only milky mixtures were formed (Bochot et al., Citation2007). Although, β-CD is capable of forming inclusion complexes with TGs that have emulsifying property (Shimada et al., Citation1992), yet beads were not obtained. The low aqueous solubility of β-CD (18.4 ± 0.2 mg/ml) limited the amount of partial inclusion complexes formed that were not sufficient enough for efficient emulsification step (Bochot et al., Citation2007). On the contrary to that, though the aqueous solubility of γ-CD is higher (249.2 ± 0.2 mg/ml) than that of β-CD, beads were not obtained as well from its mixture with soybean oil. The wide cavity of γ-CD (0.80 nm) impeded optimal stable interaction of α-CD with TG (Shimada et al., Citation1991; Yu et al., Citation2001). It is evident that strong interactions occurs when the distance between CD atoms and TG chain are short, which was the reason for successful beads formation with α-CD (inner cavity diameter = 0.50 nm) (Bochot et al., Citation2007). It is worth mentioning that the CD/TG inclusion complex has relatively poor solubility related to the fact that only one FA chain is included in the CD which in fact is a prerequisite for the potential role of the inclusion complex at the water/oil interface of emulsions (Duchêne et al., Citation2003). Trichard et al. (Citation2011) examined whether soybean oil can be replaced by other vegetable oils namely, borage, sweet almond and wheat germ oils, which are usually used in skin care products, for forming beads with α-CD using the same optimum ratio observed for soybean oil (Trichard et al., Citation2011). Sweet almond and wheat germ oils (>99% TGs) formed smaller beads than soybean oil-based ones but within the same time frame and similar fabrication yield (Trichard et al., Citation2011). The presence of trace amounts of diglycerides (DGs), monoglycerides (MGs) or FAs from degradation of TGs in soybean oil probably competed with TG for α-CD molecules (Szente et al., Citation1993; Duchêne et al., Citation2003) resulting in less efficient emulsification step. Certainly, this reduces the number of CD molecules involved in emulsion stabilization. As a result more CD molecules are available for matrix crystallization forming larger beads (Trichard et al., Citation2011).
In contrast to that, borage oil mixed with α-CD aqueous solution did not evolve any beads and only milky fluids were formed (Trichard et al., Citation2011). Borage oil contains high proportion of TG degradation products that compete with TG for the α-CD cavity and the number of TG:α-CD inclusion complexes formed in this case is not sufficient for efficient stabilization of emulsion preventing the crystallization of CD molecules at the interface (Trichard et al., Citation2011).
The presence of oil components with higher affinity for the α-CD cavity than TG can either increase the size of beads or prevent their formation completely (Trichard et al., Citation2011). In this domain, increasing amounts of oleic acid (one of TG degradation products) were added to soybean oil and then the feasibility of producing beads was examined. At 3.0% of oleic acid upwards in soybean oil, beads were never formed and only milky fluids were obtained due to the formation of oleic acid/α-CD inclusion complexes (Trichard et al., Citation2011). With this in mind, for successful beads formation from vegetable oils, it is important to select oils with a high degree of purity and to verify the absence of any TG degradation products or adulterations. Alternatively, Trichard et al. (Citation2008b) investigated the feasibility of forming beads form mineral oils (Primol 352 and Marcol 82) or synthetic oils (Silicon 200 fluid 10, 50 or 100cSt) with natural CDs to give more flexibility in beads formulation (Trichard et al., Citation2008b). The mineral and silicon oils differed in the average molecular weight thus the hydrocarbon or polydimethylsiloxanes chain length, respectively (Hamoudi & Bochot, Citation2014). As presented in , both Primol 352 and Silicon 10cSt did not induce bead formation with any of the natural CDs (α-, β- or γ-CD). Also, Silicon 100 or 50cSt failed to form beads with either α- or β-CD (Trichard et al., Citation2008b).
On the other hand, combinations of Marcol 82 and α-CD solution formed beads that were dispersed in turbid medium and not easily separated. Besides, only few ratios of Silicon 50 and 100cSt with γ-CD/aqueous water formed beads with low oil content limiting their ability to encapsulate large amount of lipophilic drugs (Trichard et al., Citation2008b). Marcol 82 contains linear but also branched hydrocarbon chains, it was suggest that linear part of hydrocarbons chains could enter α-CD molecules whereas the ramified hydrocarbon branch(es) remains outside the cavity (Trichard et al., Citation2008b). In the case of silicone oils, polydimethylsiloxane chains interact strongly with γ-CD to form inclusion complexes (Kumura et al., 2000; Porbeni et al., Citation2001; Marangoci et al., Citation2009). Both types of partial inclusion complexes can stabilize emulsions due to their amphiphilic property where CD molecules entrapping one part of oil components is oriented in the aqueous phase whereas the non-included portion occupies the oily phase (Yu et al., Citation2001, Citation2003; Duchêne et al., Citation2003). Though, the above mentioned oils succeeded in forming beads, yet, vegetable oil-based beads still offer the greatest potential in pharmaceutical field as they were superior in terms of oil content (>80%) and fabrication yield (Bochot et al., Citation2007; Trichard et al., Citation2011).
Fabrication factors
Both temperature and rate of shaking of the mixture of oil and CD aqueous solution influence beads formation. Considering optimum macroscopic properties (high production yield and monodisperse population) of the beads along with the shortest preparation time, temperature of 28 °C and rate of shaking of 200 rpm was reported to be ideal for beads manufacturing (Bochot et al., Citation2007; Trichard et al., Citation2011).
Temperature
The emulsifying step of the vegetable oil using the partial inclusion complex of CD molecules with TGs plays a major role in beads formation. It was reported that the emulsifying properties of natural CD decrease when the temperature increases (Shimada et al., Citation1991). For that reason, soybean oil failed to disperse within the CD aqueous solution and form beads when the temperature was raised to 37 °C (Bochot et al., Citation2007).
Rate of shaking
Along with temperature, the rate of shaking of CD aqueous solution with oil in the gyratory shaker affected bead formation. When 150 rpm was employed, soybean oil and α-CD aqueous solution remained as two immiscible liquids as the rate of shaking was not sufficient to form stable o/w emulsion (Bochot et al., Citation2007).
Characterization of beads
Adequate and appropriate characterization of beads is essential for quality control purposes to ensure that they are prepared with desirable features suitable for administration. The key parameters evaluated and characterization techniques for beads presented in different researches are summarized below.
Scanning electron microscopy
Scanning electron microscopy (SEM) is a useful tool that is widely used for direct observation of particulate systems. It was used for examining the external morphological appearance of the freeze-dried beads () (Bochot et al., Citation2007).
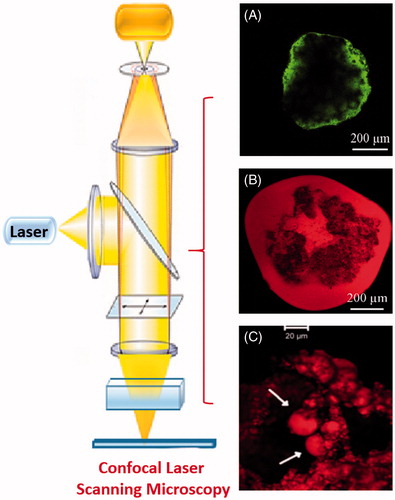
Confocal laser scanning microscopy
Confocal laser scanning microscopy (CLSM) examinations were used to visualize the inner structure of beads in order to confirm the presence of oil domains as well as the formation of a coat layer in case of coated beads (Bochot et al., Citation2007; Hamoudi et al., Citation2012). The confocal micrographs of fresh beads prepared using calcein (hydrophilic probe) showed preferentially concentrated calcein on bead surface whereas those prepared using Nile red (lipophilic probe) showed dye localization inside the inner structure of beads demonstrating the presence of micro-compartments of free oil () (Bochot et al., Citation2007). On the other hand, the CLSM was used to visualize the erosion behavior of Nile red-loaded beads in the simulated gastrointestinal fluids (SGIF) and to demonstrate the release of micro-droplets of oil () (Hamoudi et al., Citation2012).
Figure 3. Confocal micrographs of (A) fresh beads with calcein and (B) fresh beads with Nile red (C) microdroplets of oil released from Nile red-loaded beads (Bochot et al., Citation2007; Hamoudi et al., Citation2011).
Fluorescent microscopy
The physical state of the drug loaded in beads prepared with adapalene either previously solubilized or dispersed in soybean oil was examined using fluorescent microscopy (Trichard et al., Citation2008a). Homogenous distribution of fluorescence was seen in the beads prepared from drug solubilized in oil. However, needle-shaped heterogeneous drug crystals were visualized in beads prepared using adapalene suspension (Trichard et al., Citation2008a). It should be emphasized that the physical state of a lipophilic drug is important for the in vivo performance (Kuentz, Citation2012). If the drug is solubilized in the beads, no dissolution step is needed. This is a crucial advantage for the delivery of lipophilic drugs, but if the drug is dispersed within the delivery system or the solubilization capacity is lost upon aqueous dilution in the GIT. A typical consequence of such precipitation might therefore result in incomplete and erratic drug absorption (Chen, Citation2008).
X-ray diffraction studies
X-ray diffraction studies (XRD) is widely used to assess the degree of crystallinity of a solid from the geometric scattering of radiation obtained from its crystal planes. In addition, XRD is generally used for qualitative identification of CD complex formation (Fernandes et al., Citation2002; Han et al., Citation2014). XRD diffractograms confirmed that the crystalline nature of the beads was retained after freeze drying as the main peaks (q = 0.528, 0.917 and 1.392 Å−1) identified by an arrow in the spectrum of fresh beads (, spectrum 1) were also present in the spectra of freeze-dried beads prepared using either soybean oil or sweet almond oil with α-CD (, spectrum 2; , spectrum 1). Likewise, these distinguished beads were depicted in the spectra of different TG/α-CD inclusion complexes (, spectra 3–5) elucidating the presence of TG/α-CD inclusion complexes within the beads formations.
Figure 4. (Diagram A; Bochot et al., Citation2007) X-ray diffraction spectra of (1) fresh beads, (2) freeze-dried beads, (3) inclusion complex of triolein with α-CD, (4) inclusion complex of trilinolein with α-CD, and (5) Complex of trilinolenin with α-CD. (Diagram B; Trichard et al., Citation2011) X-ray diffraction spectra of (1) sweet almond oil beads, (2) soybean oil beads, (3) soybean oil + oleic acid 0.5%, (4) 2.0%, (5) 5.0%, (6) borage oil formulation, and (7) oleic acid/α-CD inclusion complexes. (Diagram C; Trichard et al., Citation2008b) X-ray diffraction spectra of (1) Soybean oil/α-CD beads, (2) Marcol 82/α-CD beads, (3) α-CD, (5) Silicon 50cSt/γ beads, and (5) γ-CD.
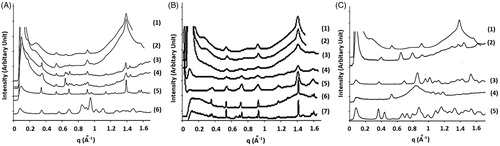
According to Szente et al. (Citation1993) the complex stability depends on the acylation degree and decreases in the order: free FA > MG > DG > TG. It is hypothesized that FAs resulting from TGs degradation competes with TGs for CD cavity. In this domain, oleic acid (one of the TGs degradation products) was added in increasing concentrations to soybean oil and the XRD spectra of the beads prepared using this oils mixture (oleic acid + soybean oil) were examined and compared to that of oleic acid/α-CD inclusion complex (Trichard et al., Citation2011). The addition of small amount of oleic acid (0.5%) evoked changes in the diffraction spectra of soybean beads, though the characteristic peaks (q = 0.528, 0.917 and 1.392 Å−1) were still visible but became narrower as oleic acid concentration increase (, spectra 3–5) (Trichard et al., Citation2011). Besides, new peaks similar to that were present in the spectrum of oleic:α-CD inclusion complex appeared (, spectrum 7) in the beads spectra which indicated that FA displaced TG in the CD cavity distorting beads formation (Trichard et al., Citation2011). Also, the XRD spectrum of borage oil formulations contained characteristic peaks oleic:α-CD inclusion complex (, spectrum 6) indicating that the degradation products in this oil interacted with α-CD instead of the TGs (Trichard et al., Citation2011).
Similar to the diffractograms of beads prepared using vegetable oils (Bochot et al., Citation2007; Trichard et al., Citation2011), (Marcol 82/α-CD and Silicon 50cSt oil/γ-CD beads also showed a crystalline organization but with different packing type () (Trichard et al., Citation2008b).
XRD diffraction pattern can also give ideas about the number of CDS capable of interacting with the oil components as long hydrophobic chains can interact with more than one CD molecules (Duchêne et al., Citation2003; Trichard et al., Citation2008b). The relative peak intensities obtained from the XRD diffractogram of TG:α-CD complex showed that α-CD molecules were ordered in form of dimers in TG:α-CD inclusion complex () (Noltemeyer & Saenger, Citation1980). Likewise, the first inter-planar distance calculated from the diffractograms of beads prepared from Marcol 82/α-CD and Silicon 50cSt oil/γ-CD corresponded to the height of channel type α-CD and γ-CD dimers, respectively, confirming that CDs in these cases as well were arranged in dimers (). (Noltemeyer & Saenger, Citation1980; Porbeni et al., Citation2001; Kawasaki et al., Citation2007).
Bead size
Optical microscope was used to determine the diameter of the freeze-dried beads. For unloaded beads, the diameters of beads prepared using wheat germ oil (0.9 ± 0.3 mm) and sweet almond (0.7 ± 0.3 mm) were smaller than that of soybean oil/α-CD-based beads (1.6 ± 0.2 mm) (Trichard et al., Citation2011). Loading the beads with indomethacin or progesterone, previously dissolved or dispersed in soybean oil, did not affect the beads diameter (Hamoudi et al., Citation2011, Citation2012). On the contrary to that, lipophilic retinoid drugs, adapalene and isotretinoin, caused an increase of the diameter of beads to an average of 2.0 ± 0.2 mm and 2.3 ± 0.2 mm, respectively (Trichard et al., Citation2007, Citation2008a). The diameter of drug-loaded beads in different studies is presented in .
Encapsulation efficiency
Encapsulation efficiency is one of the important parameters to judge the suitability of a drug carrier system as it measures the incorporation and localization of drug within the carrier. Different drugs have been successfully encapsulated into the soybean/oil beads with encapsulating efficiently ranging from 63 ± 2% to 98 ± 1% () (Trichard et al., Citation2007, Citation2008a; Hamoudi et al., Citation2011, Citation2012). The high oil content (>80%) in the beads associated with the lipophilic nature of the investigated drugs (high log p value) positively contributed to the observed efficient entrapment.
Behavior of beads in simulated gastrointestinal fluids
Studying the behavior of a new delivery system in simulated in vivo conditions is essential for predicting their performance and release patterns after oral administration. For that purpose, Hamoudi et al. examined the behavior of uncoated freeze-dried beads, coated beads, and oven-dried beads incubated in simulated gastric fluid (SGF) for 55 min then in different intestinal media, namely control simulated intestinal fluid (SIF) free of sodium taurocholate and lecithin, fasted state simulated intestinal fluid (FaSSIF), and fed state simulated intestinal fluid (FeSSIF) (Hamoudi et al., Citation2011, Citation2012, Citation2013).
In SGF, the beads volume decreased due to progressive dissolution of α-CD molecules from the beads surface (Hamoudi et al., Citation2011). Their volume continued to decrease in the intestinal media according to the following order: FeSSIF > FaSSIF > SIF (Hamoudi et al., Citation2011). The presence of sodium taurocholate in FeSSIF reduced the stability of beads due to its surfactant properties that facilitate the extraction of the oily droplets from the beads (de Smidt et al., Citation1991). Likewise, the coated beads volume decrease in SGIF, though they were more resistant due to the presence of extra α-CD that protected the inner core of the beads (Hamoudi et al., Citation2012). The stability of beads in SGIF are strongly related to the drying procedure. Oven-dried beads were more resistant to fragmentation than freeze-dried beads because the shrinking process during oven drying lead to densification of CD matrix resulting in harder beads (Hamoudi et al., Citation2013). On the other hand, freeze drying increased the porosity of the beads making them more sensitive to fragmentation in SGIF (Hamoudi et al., Citation2013).
In vitro drug release from beads
In vitro drug release studies represent an acceptable tool that provides rough estimation of the in vivo performance of a drug formulation. Drug release from beads is attributed to the erosion of CD hydrophilic matrix allowing the release of oil droplets followed by drug partitioning between the oily droplets and the aqueous release medium (Hamoudi et al., Citation2011, Citation2012). Similar to the behavior of beads in simulated conditions, the rate of drug release from the beads depend on the beads drying process (Hamoudi et al., Citation2013), presence of coat layer on beads surface (Hamoudi et al., Citation2012), and nature of release media (Hamoudi et al., Citation2011). Indomethacin release from the oven-dried beads was always slower than that from freeze-dried beads in different release media. This was attributed to the less porosity of oven-dried beads compared to freeze-dried beads (Hamoudi et al., Citation2013). Further coating of beads with a layer of α-CD decreased the release of indomethacin compared to the uncoated ones. The α-CD coat behave as a barrier for drug release in early incubation time and protect the inner core of the bead (Hamoudi et al., Citation2012). On the other side, faster drug release from beads was continuously observed in FeSSIF and to a lesser extent in FaSSIF compared to those seen in control SIF. The presence of sodium taurocholate in FeSSIF accelerated the extraction of oil droplets from the beads and thereby enhanced beads fragmentation (Hamoudi et al., Citation2011). Also, the surfactant property of sodium taurocholate improves the drug solubility causing an increase in the amount of the drug released in the release media (de Smidt et al., Citation1991).
In vivo performance of beads
Oral administration of beads
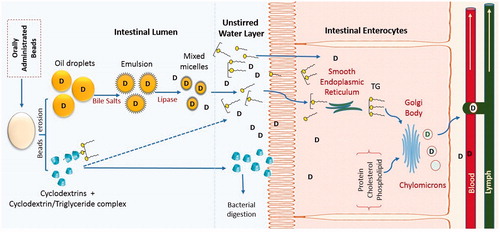
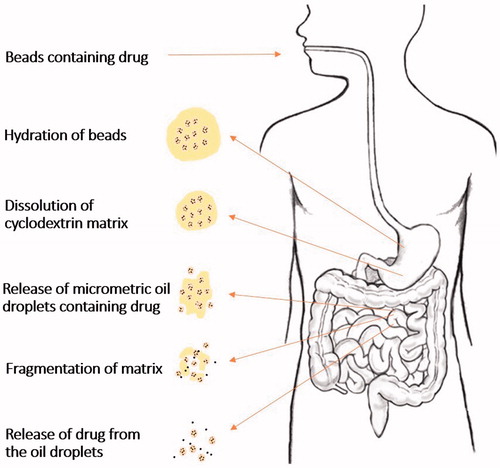
The importance of performing in vivo studies is irrefutable due to the fact that unsatisfactory in vivo results sometimes follow promising theoretical predictions and in vitro experimental data. The main target of formulating new oral drug delivery systems is to achieve better in vivo performance compared to the available formulations. Schematic presentation of the potential behavior of beads after oral administration is presented in . The beads are expected to improve drug absorption through different ways (). After beads disintegration in biological fluids, the micrometric drug containing oil globules within the beads matrix are released conferring high surface area. The rate of lipolysis depends on the surface area at the oil–water interface as pancreatic lipase hydrolyzes the TG molecules at the oil/water to MGs and FAs (Porter et al., Citation2007). Further, administration of exogenous lipids simulates the secretion of bile salts, phospholipids, cholesterol (Porter et al., Citation2007; Kuentz, Citation2012). The latter collectively contribute to the formation of solubilizing colloidal species (micelles, mixed micelles, multilamellar and unilamellar vesicles) that decrease the partition coefficient of drugs and play a crucial role in maintaining them in solubilized form within the GI environment (Hauss, Citation2007; Porter et al., Citation2007; Kuentz, Citation2012; Mu et al., Citation2013). An additional barrier to the effective uptake of drugs from the intestinal lumen into enterocytes is diffusion across the unstirred water layer, which separates the bulk fluid phase in the intestine lumen from the brush border membrane of enterocytes (Thomson et al., Citation1993; Nordskog et al., Citation2001). Solubilization of lipophilic drugs with FAs and acids MGs in the colloidal structures and the presence of CD molecules, however, can greatly enhance the permeation and transport of drug molecules across the unstirred water layer, thereby enhancing drug absorption (Kurkov & Loftsson, Citation2013). The enhancement of drug bioavailability may also result from the increase in intestinal lymphatic absorption as reported for other lipid-based systems (Nordskog et al., Citation2001; Ali Khan et al., 2013; Singh et al., Citation2014).
Figure 6. Potential behavior of oil-cyclodextrin beads after oral administration (Based on Hamoudi et al., Citation2011).
Although oral delivery of drugs using beads is an attractive approach, two studies have been carried in vivo to investigate this administration route using rats as an animal model (Trichard et al., Citation2007; Hamoudi et al., Citation2012). After oral administration of isotretinoin beads to rats, the absolute bioavailability of isotretinoin was enhanced two-fold compared to that of an oily solution with similar lipid composition. This indicates that isotretinoin dose could be decreased in beads to reach the required plasma drug concentration (Trichard et al., Citation2007). Using the same animal model, the in vivo performance of indomethacin from coated beads, non-coated beads, and dry emulsion formulated with α-CD and soybean oil was superior to that of the market formulation (Indocid®) under both fasted and fed conditions. Also, the bioavailability of indomethacin was higher in fed rats than in fasted ones however the formulation was due to the alteration in luminal environment of the small intestine evoked by lipids administration, which induces pancreatic lipases and bile salts secretions (Kossena et al., Citation2004). Compared to uncoated beads, coated beads act as a sustained release carrier due to protective effect imposed by α-CD layer surrounding the beads making them more resistant to erosion (Hamoudi et al., Citation2012).
Topical applications of beads
The human skin exhibits an excellent barrier function that limits the penetration of therapeutic agents into the body (Brown et al., Citation2006). Lipid-based formulations are widely used for topical drug delivery because they are capable of enhanced drug permeation along with controlling drug release (Gupta et al., Citation2013; Pando et al., Citation2013). Using tape stripping procedure and fluorescent microscopic observation, adapalene release and penetration from α-CD/soybean oil beads into the stratum corneum was confirmed to be comparable to the market formulations (Differin® gel or cream) despite of the difference between the excipients used in the three formulations. The beads decreased the trans-epidermal water loss from the skin indicating an efficient occlusive effect (Trichard et al., Citation2008a).
Skin tolerance test is a prerequisite for efficient topical drug delivery systems to confirm the absence of associated side effects and also prove its suitability for clinical use. Trichard et al., (Citation2008a) assessed the tolerance of the unloaded beads on seven human volunteers in comparison to alcoholic gel as an irritant reference. Interestingly, after 72 h of continuous application, the beads were well tolerated due to the absence of clinical reactions (erythema/desquamation) on the skin. (Trichard et al., Citation2008a).
Stability studies
Processing stability
The integrity of either the drug or excipients is affected by the different processing variables (high temperature, pressure and other additives) they encounter during formulation production. Maintaining the stability of drug and excipient during formulation processes is crucial for introducing drug formulations into the pharmaceutical market (Melveger & Huynh-Ba, Citation2009). There are extensively researched products that could not enter the market due to poor drug or excipient stability during manufacturing (Kalasz & Antal, Citation2006).
Since beads formulation process is conducted at mild conditions, the acid value (measures the amount of free acid resulting from TG degradation) of soybean oil extracted from the prepared beads remains inferior to the detection limit of the method used (<0.2 mg KOH/g of soybean oil) which indicates that the integrity of soybean oil was retained during formulation (Bochot et al., Citation2007).
Storage stability
The quality, safety and efficacy of a new formulation should be maintained throughout the shelf-life storage. For that purpose, stability testing is an important requirement for regulatory approval of any drug formulation (Melveger & Huynh-Ba, Citation2009). In this context, the stability of freeze- and oven-dried beads was investigated using long term (25 ± 2 °C for 12 months) and accelerated storage studies (40 ± 2 °C for 6 months) in closed and open vials (Hamoudi et al., Citation2013). At the end of the stability study, yellow coloration was observed on beads stored in open vials due to water uptake by hydrophilic α-CD. Regardless of the storage conditions, oven-dried beads showed more pronounced yellow coloration relative to freeze-dried beads due to the exudation of oily droplets to the surface. Oven-dried beads stored in either closed or open vials had greater tendency to agglomerate than freeze-dried beads (Hamoudi et al., Citation2013).
FT-IR spectroscopy was used to examine the oils degradation resulting from autoxidation (Ismail et al., Citation1993; Van de Voort et al., Citation1994). The characteristic degradation band (carbonyl stretching band (C=O) at around 1710 cm−1) was absent in the spectra of freeze- and oven-dried beads stored for 6 months at 75% RH/40 °C confirming the oil stability (Hamoudi et al., Citation2013).
The free FA and peroxide values determined for oil extracted from the stored freeze- and oven-dried beads revealed that only minor hydrolysis of TGs occurred with moderate increase in peroxide values, especially for oven-dried beads compared to freeze-dried beads, though they remain close to or lower than those recommended limits (Hamoudi et al., Citation2013).
Drug stability
A substantial advantage of encapsulating drugs into beads is the improvement in the sensitivity of fragile drugs. The stability of the fragile drug isotretinoin was examined in beads after light exposure according to ICH Guidelines for photostability testing are compared to the stability of isotretinoin in soybean oil solution (Trichard et al., Citation2007). The degradation half-time of isotretinoin was significantly longer when it was formulated in beads compared to a soybean oil solution. The protection insured by α-CD/oil beads for isotretinoin does not involve fitting the drug molecule within the α-CD cavity (Muñoz Botella et al., Citation1996; Yap et al., Citation2005) but due to the localization of isotretinoin within the micro-compartments of oil present in their inner structure of beads (Trichard et al., Citation2007).
Digestion of beads components
The beads are composed of oil and CD. After oral administration, the in vivo digestion of CD depends on its nature where γ-CD is susceptible to digestion by salivary and pancreatic α-amylase whereas α-CD and β-CD are stable toward α-amylase enzyme and both are predominantly digested by bacteria in the colon (Irie & Uekama, Citation1997; Stella & He, Citation2008). On the other hand, the digestion of the TGs in soybean oil occurs mainly in the intestinal lumen where TGs are initially emulsified by bile prior to digestion by pancreatic lipase into free FA, glycerol, and MG and some DG (Carlier et al., Citation1991). The latter products of soybean digestion are subsequently incorporated in different colloidal structures for absorption. In the enterocyte, the FA are re-esterified to TG and packaged into chylomicrons prior to entering the lymphatic circulation (Mu & Høy, Citation2001).
Regulatory aspects of excipients used in beads
Unlike drugs, excipients do not have a separate regulatory status but are reviewed within a new drug application containing a given excipient (Kalasz & Antal, Citation2006). Acceptance by authorities is often based on previous usage in marketed pharmaceutical products or in food industry (Kurkov & Loftsson, Citation2013). Compared to the other oils used in preparing beads, only the regulatory status of soybean oil will be presented as it is the most investigated oil for beads formation (Trichard et al., Citation2007, Citation2008a; Hamoudi et al., Citation2011, Citation2012). According to European Pharmacopeia Commission(Citation2004), soybean oil is a clear, pale yellow oil obtained from seeds of Glycine soja Sieb. and Zucc. and Glycine max (L.) Merr. (G. hispida (Moench) Maxim by extraction followed by refining. Soybean oil is comprised of different FA in form of glycerides linoleic acid 50–57%; linolenic acid 5–10%; oleic acid 17–26%; palmitic acid 9–13%; and stearic acid 3–6% (Cable, Citation2009). Within the pharmaceutical market, soybean oil is used in intramuscular injections as a drug vehicle or as an energy source in emulsions used in parenteral nutrition regimens (Tian et al., Citation2013). Also, it is present in some oral dosage forms like tablets and capsules (). Further, soybean oil is consumed as an edible oil within food industry (Karasulu et al., Citation2011). Generally, soybean oil is regarded as an essentially non-toxic and non-irritant material. It is cited in both the FDA Inactive Ingredients Database and in the Canadian List of Acceptable Non-medicinal Ingredients (Cable, Citation2009). One particular aspect that is important to note is that some individuals may be allergic to soybean derivatives (Cable, Citation2009) though the highly refined soybean oil, which was used in preparing beads, is not labeled as an allergen (Crevel et al., Citation2000).
Table 4. Soybean-containing marketed pharmaceutical products.
Regarding natural CDs, they are well tolerated due to lack of absorption from the GIT (Irie & Uekama, Citation1997; Arima et al., Citation2011). γ-CD is included in the FDA’s list of Inactive Pharmaceutical Ingredients. γ-CD, β-CD and α-CD have been included in the GRAS list of the FDA. (Hincal et al., Citation2011; Kurkov & Loftsson, Citation2013). The Joint FAO/WHO Committee of Food Additives (JECFA) has a recommended Acceptable Daily Intake (ADI) of 5 mg/kg/d for βCD in food products. However, no ADI was defined for α-CD and γ-CD due to their favorable toxicological profile (Kurkov & Loftsson, Citation2013). Furthermore, γ-CD, β-CD and α-CD are found in different marketed pharmaceutical products ().
Table 5. Natural cyclodextrin-containing marketed pharmaceutical products.
Critical opinion
The hereby review presents a promising self-assembling lipid-based system named “beads” prepared using safe components, natural CDs and oils, and capable of encapsulating high drug load (Trichard et al., Citation2007, Citation2008a; Hamoudi et al., Citation2011, Citation2012). Although, the list of natural and semi-synthetic oils that are abundant and suitable for oral administration is fairly long (Strickley, Citation2004). The suitability of these oils have not yet been explored in producing beads with different properties, drug-loading ability and stability profiles. As, up to date, most of the research efforts have been almost exclusive for beads prepared using the combination of α-CD and soybean oil (Trichard et al., Citation2007, Citation2008a; Hamoudi et al., Citation2011, Citation2012). One of the key issues that need to be considered when choosing other oils is ensuring that the oil has high degree of purity and free from adulteration or oil degradation products that may compete for the inner cavity of CD and prevent the formation of stable and sufficient partial inclusion complex essential for beads formation (Trichard et al., Citation2011).
Still, in order to fully realize the potential of beads as a safe drug carrier, more fundamental research prompting deeper understanding of mechanism behind the formation of the inclusion complexes between CD and oily components are required.
Although not tackled by the presented researches, different options exist for beads scaling-up and producing the final on-market dosage form. Aspects such as the anticipated automated filling process into capsules, the compatibility of beads with capsule shell material (Chen et al., Citation2010) or tableting beads with the aid of cautioning agents (Vergote et al., Citation2002) must be taken into account by researchers.
Further, the in vivo performance of the beads is complicated as the oil present may be subjected to different lipolytic enzymes in the GIT. A critical aspect for development of safe and effective delivery systems is establishing reliable in vitro in vivo correlations (IVIVC). In view of that, a clear understanding of the lipolysis profile and in vivo performance of the beads is essential to guide formulation design strategies. Though, the release testing of the beads were performed in biorelevant dissolution testing using SIGF (Hamoudi et al., Citation2011, Citation2012, Citation2013), there is still a need for investigating the drug releases from beads in in vitro digestion models that effectively mimic the physiological conditions to be able to predict the dynamic changes the beads would encounter in vivo by taking digestion into account (Fatouros & Mullertz, Citation2008; Larsen et al., Citation2011). This would play a role in adequately modifying the formulation design in early stage of development and minimize the risk of in vivo therapeutic failure.
Despite of the fact that beads represent a promising drug delivery system for lipophilic drugs, so far, all the work has been conducted only in laboratory setting (Trichard et al., Citation2008b, Citation2011) with two pharmacokinetic studies for the drug-loaded beads conducted in rats (Trichard et al., Citation2007; Hamoudi et al., Citation2012). Although, the latter may still serve as a model for the human situation, nevertheless, care is needed when rats are used as animal models (Zhang et al., Citation2012). The amounts of lipid formulation studied are often comparatively high for this species and physiologically the rat is known for continuous bile secretions (Porter et al., Citation2007; Kuentz, Citation2012). To reflect the human situation better in terms of gastric transit profile and biliary secretion patterns, dogs or pigs are considered better animal models in this instant (Kuentz, Citation2012). It is expected that the growing shifting towards safer drug delivery platforms that does not include hazardous solvents will open additional functional roles for beads in drug delivery systems.
Conclusion
The current review presents an interesting lipid-based self-assembling multiparticulate carrier composed of safe materials, oil and CD, which are well tolerated and prepared using mild process. These beads are formed of partial crystalline CD matrix surrounding micro-domains of oil that favor high drug loading and improves fragile drugs stability. After oral administration, beads were able to increase the bioavailability of lipophilic drug in animal model. Yet, till date, studies concerning beads are still limited and focus only on soybean oil and α-CDs beads. Researches that examine the feasibility of using different oils and CDs to produce different beads systems suitable for encapsulating wider range of drugs are recommended. In addition, supplementary studies for better understanding of the in vivo behavior of beads in suitable animal models are definitely needed to exploit the broad applications of this promising lipid-based formulations.
Declaration of interest
The author reports no declarations of interest. The author alone is responsible for the content and writing of this article. This review did not receive grant from any funding agency.
References
- Aburahma MH, El-Laithy HM, Hamza YE. (2010). Oral bioavailability enhancement of vinpocetine using self-microemulsifying drug delivery system containing long chain triglycerides: preparation and in vitro/in vivo evaluation. Clin Res Regul Aff 27:97–107
- Ali Khan A, Mudassir J, Mohtar N, Darwis Y. (2013). Advanced drug delivery to the lymphatic system: lipid-based nanoformulations. Int J Nanomed 8:2733–44
- Arima H, Motoyama K, Irie T. (2011). Recent findings on safety profiles of cyclodextrins, cyclodextrin conjugates, and polypseudorotaxanes. In: Bilensoy E, ed. Cyclodextrins in pharmaceutics, cosmetics, and biomedicine: current and future industrial applications. Hoboken (NJ): Wiley, 91–122
- Ascenso A, Cruz M, Euletério C, et al. (2013). Novel tretinoin formulations: a drug-in-cyclodextrin-in-liposome approach. J Liposome Res 23:211–19
- Bochot A, Trichard L, Le Bas G, et al. (2007). α-cyclodextrin/oil beads: an innovative self-assembling system. Int J Pharm 339:121–9
- Brewster ME, Loftsson T. (2007). Cyclodextrins as pharmaceutical solubilizers. Adv Drug Deliv Rev 59:645–66
- Brown MB, Martin GP, Jones SA, Akomeah FK. (2006). Dermal and transdermal drug delivery systems: current and future prospects. Drug Deliv 13:175–87
- Bondì ML, Fontana G, Carlisi B, Giammona G. (2003). Preparation and characterization of solid lipid nanoparticles containing cloricromene. Drug Deliv 10:245–50
- Cable CG. (2009). Soybean oil. In: Rowe RC, Sheskey PJ, Owen SC, eds. Handbook of pharmaceutical excipients, 6th ed. Washington (DC): American Pharmaceutical Association, 682–5
- Carlier H, Bernard A, Caselli C. (1991). Digestion and absorption of polyunsaturated fatty acids. Reprod Nutr Dev 31:475–500
- Challa R, Ahuja A, Ali J, Khar RK. (2005). Cyclodextrins in drug delivery: an updated review. AAPS PharmSciTech 6:E329–57
- Chang D, Chang R. (2007). Review of current issues in pharmaceutical excipients. Pharmaceut Technol. Available from: http://pharmtech.findpharma.com/pharmtech/article/articleDetail.jsp?id=423551&pageID=1&sk=&date [last accessed 29 Jun 2014]
- Chavhan SS, Petkar KC, Sawant KK. (2013). Simvastatin nanoemulsion for improved oral delivery: design, characterisation, in vitro and in vivo studies. J Microencapsul 30:771–9
- Chen FJ, Etzler FM, Ubben J, et al. (2010). Effects of lipophilic components on the compatibility of lipid-based formulations with hard gelatin capsules. J Pharm Sci 99:128–41
- Chen J, Lu WL, Gu W, et al. (2014). Drug-in-cyclodextrin-in-liposomes: a promising delivery system for hydrophobic drugs. Expert Opin Drug Deliv 11:565–77
- Chen ML. (2008). Lipid excipients and delivery systems for pharmaceutical development: a regulatory perspective. Adv Drug Deliv Rev 60:768–77
- Coleman AW, Nicolis I, Keller N, Dalbiez JP. (1992). Aggregation of cyclodextrins: an explanation of the abnormal solubility of b-cyclodextrin. J Incl Phenom Macrocycl Chem 13:139–43
- Crevel RW, Kerkhoff MA, Koning MM. (2000). Allergenicity of refined vegetable oils. Food Chem Toxicol 38:385–93
- de Smidt JH, Offringa JC, Crommelin DJ. (1991). Dissolution rate of griseofulvin in bile salt solutions. J Pharm Sci 80:399–401
- Duchêne D, Bochot A, Yu SC, et al. (2003). Cyclodextrins and emulsions. Int J Pharm 266:85–90
- European Pharmacopoeia Commission. (2004). European Pharmacopoeia, 5th ed. Strasbourg Cedex, France: Council of Europe
- Fatouros DG, Mullertz A. (2008). In vitro lipid digestion models in design of drug delivery systems for enhancing oral bioavailability. Expert Opin Drug Metab Toxicol 4:65–76
- Fernandes CM, Teresa Vieira M, Veiga FJ. (2002). Physicochemical characterization and in vitro dissolution behavior of nicardipine-cyclodextrins inclusion compounds. Eur J Pharm Sci 15:79–88
- Fontana G, Maniscalco L, Schillaci D, et al. (2005). Solid lipid nanoparticles containing tamoxifen characterization and in vitro antitumoral activity. Drug Deliv 12:385–92
- Groo AC, Saulnier P, Gimel JC, et al. (2013). Fate of paclitaxel lipid nanocapsules in intestinal mucus in view of their oral delivery. Int J Nanomed 8:4291–302
- Gupta M, Tiwari S, Vyas SP. (2013). Influence of various lipid core on characteristics of SLNs designed for topical delivery of fluconazole against cutaneous candidiasis. Pharm Dev Technol 18:550–9
- Hamoudi M, Fattal E, Gueutin C, et al. (2011). Beads made of cyclodextrin and oil for the oral delivery of lipophilic drugs: in vitro studies in simulated gastro-intestinal fluids. Int J Pharm 416:507–14
- Hamoudi MC, Bochot A. (2014). Oil-cyclodextrin based beads for oral delivery of poorly-soluble drugs. Curr Top Med Chem 14:510–17
- Hamoudi MC, Bourasset F, Domergue-Dupont V, et al. (2012). Formulations based on alpha cyclodextrin and soybean oil: an approach to modulate the oral release of lipophilic drugs. J Contr Rel 161:861–7
- Hamoudi MC, Saunier J, Gueutin C, et al. (2013). Beads made of α-cyclodextrin and soybean oil: the drying method influences bead properties and drug release. Drug Dev Ind Pharm 39:1306–14
- Han B, Yang B, Yang X, et al. (2014). Host-guest inclusion system of norathyriol with β-cyclodextrin and its derivatives: preparation, characterization, and anticancer activity. J Biosci Bioeng 117:775–9
- Hauss DJ. (2007). Oral lipid-based formulations. Adv Drug Deliv Rev 59:667–76
- Hincal AA, Eroğlu H, Bilensoy E. (2011). Regulatory status of cyclodextrins in pharmaceutical products. In: Bilensoy E, ed. Cyclodextrins in pharmaceutics, cosmetic, and biomedicine: current and future industrial applications. Hoboken (NJ): John Wiley and Sons, 123–30
- Hu L, Tang X, Cui F. (2004). Solid lipid nanoparticles (SLNs) to improve oral bioavailability of poorly soluble drugs. J Pharm Pharmacol 56:1527–35
- Karasulu HY, Karasulu E, Büyükhelvacıgil M, et al. (2011). Soybean oil: production process, benefits and uses in pharmaceutical dosage form, soybean and health. In: El-Shemy H, ed. InTech, Chapter 13; 283–310. Available from: http://www.intechopen.com/books/soybean-and-health/soybean-oil-productionprocess-benefits-and-uses-in-pharmaceutical-dosage-form
- Irie T, Uekama K. (1997). Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J Pharm Sci 86:147–62
- Ismail AA, Van de Voort FR, Emo G, Sedman J. (1993). Rapid quantitative determination of free fatty acids in fats and oils by fourier transform infrared spectroscopy. J Am Oil Chem Soc 70:335–41
- Jia LJ, Zhang DR, Li ZY, et al. (2010). Preparation and characterization of silybin-loaded nanostructured lipid carriers. Drug Deliv 17:11–18
- Kalasz H, Antal I. (2006). Drug excipients. Curr Med Chem 13:2535–63
- Kanwar JR, Long BM, Kanwar RK. (2011). The use of cyclodextrins nanoparticles for oral delivery. Curr Med Chem 18:2079–85
- Kawasaki J, Satou D, Takagaki T, et al. (2007). Structural features of inclusion complexes of ɣ-cyclodextrin with various polymers. Polymer 48:1127–38
- Kossena GA, Charman WN, Boyd BJ, et al. (2004). Probing drug solubilization patterns in the gastrointestinal tract after administration of lipid-based delivery systems: a phase diagram approach. J Pharm Sci 93:332–48
- Kuentz M. (2012). Lipid-based formulations for oral delivery of lipophilic drugs. Drug Discov Today Technol 9:e171–4
- Kumura H, Okada M, Kawaguchi Y, Harada A. (2000). Complex formation between poly(dimethylsiloxane) and cyclodextrins: new pseudo-polyrotaxanes containing inorganic polymers. Macromolecules 33:4297–8
- Kurkov SV, Loftsson T. (2013). Cyclodextrins. Int J Pharm 453:167–80
- Larsen AT, Sassene P, Müllertz A. (2011). In vitro lipolysis models as a tool for the characterization of oral lipid and surfactant based drug delivery systems. Int J Pharm 417:245–55
- Loftsson T, Brewster ME. (1996). Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J Pharm Sci 85:1017–25
- Loftsson T, Duchêne D. (2007). Cyclodextrins and their pharmaceutical applications. Int J Pharm 329:1–11
- Loftsson T, Másson M, Brewster ME. (2004). Self-association of cyclodextrins and cyclodextrin complexes. J Pharm Sci 93:1091–9
- Mandpe L, Pokharkar V. (2013). Quality by design approach to understand the process of optimization of iloperidone nanostructured lipid carriers for oral bioavailability enhancement. Pharm Dev Technol 2013:1–10. [Epub ahead of print]
- Marangoci N, Farcas A, Pinteala M, et al. (2009). Synthesis, morphology, and thermal behavior of polyrotaxanes composed of γ-cyclodextrin and polydimethylsiloxanes. J Incl Phenom Macrocycl Chem 63:355–64
- Mathapa BG, Paunov VN. (2013). Cyclodextrin stabilised emulsions and cyclodextrinosomes. Phys Chem Chem Phys 15:17903–14
- Melveger AJ, Huynh-Ba K. (2009). Critical regulatory requirements for a stability program, chapter 2. In: Kim Huynh-Ba, ed. Handbook of stability testing in pharmaceutical development. New York, NY: Springer, 9–19
- Messner M, Kurkov SV, Jansook P, Loftsson T. (2010). Self-assembled cyclodextrin aggregates and nanoparticles. Int J Pharm 387:199–208
- Mu H, Holm R, Müllertz A. (2013). Lipid-based formulations for oral administration of poorly water-soluble drugs. Int J Pharm 453:215–24
- Mu H, Høy CE. (2001). Intestinal absorption of specific structured triacylglycerols. J Lipid Res 42:792–8
- Muñoz Botella S, Martìn MA, del Castillo B, et al. (1996). Analytical applications of retinoid-cyclodextrin inclusion complexes. 1. Characterization of a retinal-beta-cyclodextrin complex. J Pharm Biomed Anal 14:909–15
- Noltemeyer M, Saenger W. (1980). Structural chemistry of linear α-cyclodextrin-polyiodide complexes. X-ray crystal structures of (α-cyclodextrin)2. LiI3. I2. 8H2O and (α-cyclodextrin)2. Cd0. 5. I5. 27H2O. Models for the blue amylose–iodine complex. J Am Chem Soc 102:2710–22
- Nordskog BK, Phan CT, Nutting DF, Tso P. (2001). An examination of the factors affecting intestinal lymphatic transport of dietary lipids. Adv Drug Deliv Rev 50:21–44
- Pando D, Caddeo C, Manconi M, et al. (2013). Nanodesign of olein vesicles for the topical delivery of the antioxidant resveratrol. J Pharm Pharmacol 65:1158–67
- Parhi R, Suresh P. (2012). Preparation and characterization of solid lipid nanoparticles-a review. Curr Drug Discov Technol 9:2–16
- Perret F, Duffour M, Chevalier Y, Parrot-Lopez H. (2013). Design, synthesis, and in vitro evaluation of new amphiphilic cyclodextrin-based nanoparticles for the incorporation and controlled release of acyclovir. Eur J Pharm Biopharm 83:25–32
- Porbeni FE, Edeki EM, Shin ID, Tonelli AE. (2001). Formation and characterization of the inclusion complexes between poly(dimethylsiloxane) and polyacrylonitrile and ɣ-cyclodextrin. Polymer 42:6907–12
- Porter CJ, Trevaskis NL, Charman WN. (2007). Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat Rev Drug Discov 6:231–48
- Poullain-Termeau S, Crauste-Manciet S, Brossard D, et al. (2008). Effect of oil-in-water submicron emulsion surface charge on oral absorption of a poorly water-soluble drug in rats. Drug Deliv 15:503–14
- Seo YG, Kim DH, Ramasamy T, et al. (2013). Development of docetaxel-loaded solid self-nanoemulsifying drug delivery system (SNEDDS) for enhanced chemotherapeutic effect. Int J Pharm 452:412–20
- Shangguan M, Lu Y, Qi J, et al. (2014). Binary lipids-based nanostructured lipid carriers for improved oral bioavailability of silymarin. J Biomater Appl 28:887–96
- Sharma N, Baldi A. (2014). Exploring versatile applications of cyclodextrins: an overview. Drug Deliv 2014:1–19. [Epub ahead of print]
- Shimada K, Kawano K, Ihsii J, Nakamura T. (1992). Structure of inclusion complexes of cyclodextrins with triglyceride at vegetable oil/water interface. J Food Sci 57:655–6
- Shimada K, Ohe Y, Ohguni T, et al. (1991). Emulsifying properties of alpha-, beta- and gamma-cyclodextrins. Nippon Shokuhin Kogyo Gakkaishi 38:16–20
- Shukla D, Chakraborty S, Singh S, Mishra B. (2011). Lipid-based oral multiparticulate formulations - advantages, technological advances and industrial applications. Expert Opin Drug Deliv 8:207–24
- Silva AC, Kumar A, Wild W, et al. (2012). Long-term stability, biocompatibility and oral delivery potential of risperidone-loaded solid lipid nanoparticles. Int J Pharm 436:798–805
- Singh B, Khurana L, Bandyopadhyay S, et al. (2011). Development of optimized self-nano-emulsifying drug delivery systems (SNEDDS) of carvedilol with enhanced bioavailability potential. Drug Deliv 18:599–612
- Singh I, Swami R, Khan W, Sistla R. (2014). Lymphatic system: a prospective area for advanced targeting of particulate drug carriers. Expert Opin Drug Deliv 11:211–29
- Stella VJ, He Q. (2008). Cyclodextrins. Toxicol Pathol 36:30–42
- Strickley RG. (2004). Solubilizing excipients in oral and injectable formulations. Pharm Res 21:201–30
- Sun H, Liu K, Liu W, et al. (2012). Development and characterization of a novel nanoemulsion drug-delivery system for potential application in oral delivery of protein drugs. Int J Nanomed 7:5529–43
- Szente L, Szejtli J, Szeman J, Kato L. (1993). Fatty-acid cyclodextrin complexes: properties and applications. J Inclus Phenom Mol 16:339–54
- Thomson AB, Schoeller C, Keelan M, et al. (1993). Lipid absorption: passing through the unstirred layers, brush-border membrane, and beyond. Can J Physiol Pharmacol 71:531–55
- Tian H, Yao X, Zeng R, et al. (2013). Safety and efficacy of a new parenteral lipid emulsion (SMOF) for surgical patients: a systematic review and meta-analysis of randomized controlled trials. Nutr Rev 71:815–21
- Tiwari SB, Amiji MM. (2006). Improved oral delivery of paclitaxel following administration in nanoemulsion formulations. J Nanosci Nanotechnol 6:3215–21
- Tran TH, Ramasamy T, Cho HJ, et al. (2014). Formulation and optimization of raloxifene-loaded solid lipid nanoparticles to enhance oral bioavailability. J Nanosci Nanotechnol 14:4820–31
- Trichard L, Chaminade P, Grossiord JL, et al. (2011). Beads made of alpha-cyclodextrin and vegetable oils: oil composition and physicochemical properties influence bead feasibility and properties. J Drug Deliv Sci Tec 21:189–94
- Trichard L, Delgado-Charro MB, Guy RH, et al. (2008a). Novel beads made of alpha-cyclodextrin and oil for topical delivery of a lipophilic drug. Pharm Res 25:435–40
- Trichard L, Fattal E, Besnard M, Bochot A. (2007). α-cyclodextrin/oil beads as a new carrier for improving the oral bioavailability of lipophilic drugs. J Control Release 122:47–53
- Trichard L, Fattal E, Le Bas G, et al. (2008b). Formulation and characterisation of beads prepared from natural cyclodextrins and vegetable, mineral or synthetic oils. Int J Pharm 354:88–94
- Van de Voort FR, Ismail AA, Sedman J, Emo G. (1994). Monitoring the oxidation of edible oils by Fourier transform infrared spectroscopy. J Am Oil Chem Soc 71:243–53
- Vergote GJ, Kiekens F, Vervaet C, Remon JP. (2002). Wax beads as cushioning agents during the compression of coated diltiazem pellets. Eur J Pharm Sci 17:145–51
- Wang Q, Cheng H, Zhou K, et al. (2013). Nanostructured lipid carriers as a delivery system of biochanin A. Drug Deliv 20:331–7
- Wei L, Sun P, Nie S, Pan W. (2005). Preparation and evaluation of SEDDS and SMEDDS containing carvedilol. Drug Dev Ind Pharm 31:785–94
- Yap KL, Liu X, Thenmozhiyal JC, Ho PC. (2005). Characterization of the 13-cis-retinoic acid/cyclodextrin inclusion complexes by phase solubility, photostability, physicochemical and computational analysis. Eur J Pharm Sci 25:49–56
- Yu SC, Bochot A, Bas GL, et al. (2001). Characteristics of o/w emulsions containing lipophilic molecules with cyclodextrins as emulsifiers. STP Pharm Sci 11:385–91
- Yu SC, Bochot A, Bas GL, et al. (2003). Effect of camphor/cyclodextrin complexation on the stability of O/W/O multiple emulsions. Int J Pharm 261:1–8
- Zhang D, Luob G, Ding X, Lu C. (2012). Preclinical experimental models of drug metabolism and disposition in drug discovery and development. Acta Pharmaceutica Sinica B 2:549–61
- Zhao YQ, Wang LP, Ma C, et al. (2013). Preparation and characterization of tetrandrine-phospholipid complex loaded lipid nanocapsules as potential oral carriers. Int J Nanomed 8:4169–81
- Zhuang CY, Li N, Wang M, et al. (2010). Preparation and characterization of vinpocetine loaded nanostructured lipid carriers (NLC) for improved oral bioavailability. Int J Pharm 394:179–85