Abstract
Aim: The aim of the novel study was to check the efficacy of a locally applied 2%w/w nanoemulgel (NEG) of Ketoprofen (KP) in preventing the periodontitis, and was also checked NEG without KP to ensure the effect of eugenol in NEG as an oil phase.
Design: For experimentally induced periodontitis, sterile silk ligatures (3/0) were placed around the crevices of the first left lower molar teeth of the male Wistar rats. During 8 weeks, all rats were fed with 10%w/v sucrose solution. The experimental assessment was carried out at 11 d after treatment of experimental periodontal disease (EPD) rats by various clinical parameters like gingival index (GI), tooth mobility (TM), alveolar bone loss (ABL), histological analysis, detection of TNF-α, and IL-1β in gingival tissue by ELISA and the roughness were measured by atomic force microscopy (AFM) in tapping modes.
Results: After treatment, comparison studies with EPD were performed. NEG loaded with KP prevents significantly (p < 0.05) various parameters (GI, TM, and ABL), which were responsible for periodontitis. The histopathology of the periodontium showed that Group 3 (NEG loaded with KP) had a more significant reduction in inflammatory cell infiltration, alveolar bones resorption, and cementum (p < 0.05). In the topographical images, significant reduction in roughness of NEG loaded with KP was observed in comparison with EPD without treatment.
Conclusion: The study revealed the great synergistic potential of the combined NEG of an anti-inflammatory drug KP along with eugenol as the oil phase, which have potential antibacterial, analgesic, and anesthetic properties to combat periodontal disease.
Introduction
Periodontitis is one of the most common infections and is a major cause of tooth loss (Liu et al., Citation2012). In the present days, 75% of the adult population of the world experience at least mild periodontal disease (gingivitis), 20–30% exhibit a severe destructive form of chronic periodontitis (Javed & Kohli, Citation2010). Periodontitis is an inflammatory disease, which is characterized as gingival bleeding, destruction of connective tissue tooth attachment, periodontal pocket formation, and alveolar bone resorption (Xiao et al., 2011). The pathogenesis of periodontitis involves the presence of a bacterial plaque that may initiate a local inflammatory reaction against predisposed hosts. The host inflammatory responses are primarily responsible factors for the progression of periodontal disease and for most of the breakdown of dental connective tissues (Ozdemir et al., Citation2012). The host inflammatory responses lead to edema, leukocyte infiltration, and release of inflammatory mediators, causing periodontal pocket formation, connective tissue detachment, and alveolar bone resorption, which ultimately lead to tooth loss (Samejima et al., Citation1990; Botelho et al., Citation2007). The histology of ligature induced EPD Wistar rats at dissimilar part depths were analyzed (Semenoff et al., Citation2008).
Non-steroidal anti-inflammatory drugs (NSAIDs) are considered as a vital pharmacological class of agents. These drugs act as modulators of the host response mechanism, modifying the development of periodontal disease (Pimentel et al., Citation2012). NSAID could suppress the inflammatory and alveolar loss during chronic periodontitis (Goldhaber et al., Citation1973; Lee et al., Citation2004). NSAIDs were shown to reduce alveolar bone loss (ABL) associated with periodontal disease (Miyauchi et al., Citation2004). Various formulations of KP, i.e., 1%w/w cream (Li et al., Citation1996), 1.5% w/w gel (Lawrence et al., Citation1998; Srinivas et al., Citation2011), and KP with fluoride (Bowen et al., Citation2000) were previously used for the treatment of ligature-induced periodontitis.
For the treatment of chronic periodontal diseases, there is a need of combination of anti-bacterial, anti-inflammatory, analgesic, and local anesthetic agents in effective concentration at the site of action with extended residence time and with lesser side effects. Eugenol, the principal chemical component of clove oil obtained from Eugenia aromatica, has long been known for its analgesic, local anesthetic, anti-inflammatory, and anti-bacterial effects (Srivastava et al., Citation2014). Eugenol, a phenolic compound, shows anti-inflammatory activity by inhibiting the enzyme cyclooxygenase-II, analgesic activity due to selective binding at capsaicin receptor, and antibacterial activity against Gram (+) ve and Gram (−) ve microorganisms (Jadhav et al., Citation2004).
We have already described in vitro part of NEG loaded with KP containing eugenol as the oil phase in our previous publication (Srivastava et al., Citation2014). The developed and optimized in situ NEG by using Quality by Design (QbD) approach was satisfactory in terms of rheological properties and pharmaceutical characterization. Significantly lower in vitro toxicity by the HET CAM (Hen's Egg Test Chorioallantoic Membrane) method and nano-size (37.230 ± 0.210 nm) suggests the suitability of NEG loaded with KP for intra-pocket delivery.
The purpose of this novel study was to assess the efficacy of NEG loaded with KP containing eugenol as the oil phase in the treatment of EPD of Wistar rats.
Materials and methods
Animals
Male Wistar rats (Rattus norvegicus) (two months old and weight between 180 and 210 g) were selected for in vivo study. The protocol used for the experiment was approved by the Institutional Ethical Committee of Animal Research, Jamia Hamdard, New Delhi, India (Protocol approval no. 789). All rats were acclimatized for 1 week before the assay and housed under normal laboratory condition, with chow food and water available ad libitum.
Induction of experimental periodontitis and treatment
Twenty-four Wistar rats were used in the current study; out of them, 18 were infected with ligature-induced EPD. The remaining six rats were used in the normal group, i.e., no ligature induction to periodontal diseases. To induce periodontitis, 18 rats (180–210 g) were initially anesthetized with an intramuscular injection (i.m.) of ketamine (90 mg/kg) and xylazine (10 mg/kg) (Branco-de-Almeida et al., 2012). Experimental periodontitis were induced by the placement of a non-absorbable sterile surgical silk ligature 3/0 (Ethicon, Johnson & Johnson LTD., Baddi, Himachal Pradesh, India) around the gingival crevices of the first left lower molar teeth utilizing the procedure used by Györfi et al. (Citation1994) with some modification (Xu & Wei, Citation2006). The ligatures were checked after application, and all loose ligatures were replaced.
During 8 weeks, all EPD rats were fed with 10%w/v sucrose solution. They were divided into four groups containing six animals in each group. Group 1 was the model group, i.e., the rats were subjected to ligature-induced periodontal disease and received no pharmacological treatment; Group 2 rats were received the NEG formulation without KP; Group 3 rats were the NEG loaded with KP formulation of equivalent 10 mg doses of KP (Srivastava et al., 2014) and Group 4 was the control group with no induction of periodontal diseases. After 11th day of treatments, the effects of the different treatment groups were examined on various parameters (Liu et al., Citation2012). Comparison of different clinical parameters liked Gingival index (GI), tooth mobility (TM), and ABL with histological changes may give a better understanding of progressive inflammatory condition in periodontitis.
The current study was also revealed a reduction of pro-inflammatory cytokines such as IL-1β and TNF-α on the gingival tissue of Wistar rats treated with NEG with KP and without KP. The role of IL-1β and TNF-α, on periodontal disease, has been discussed earlier (Lima et al., Citation2004; Botelho et al., Citation2010).
GI and TM
Before sacrificing the rats, the GI was classified according to the inflammation of the gingival. Based on the visual observation, we scored 0 = normal gingival; 1 = mild inflammation, slight edema, minor change in color, and absence of bleeding on probing; 2 = moderate inflammation, edema, glazing, redness, and bleeding on probing; 3 = severe inflammation, extreme redness, presence of ulcer, edema, and severe bleeding. The TM was scored on the basis of mobility of second molar teeth (ligated) as per following scales: 0 = no mobility; 1 = slight mobility; 2 = moderate mobility; 3 = severe mobility (Xu & Wei, Citation2006).
Measurements of ABL
The rats were euthanized on the 11th day of periodontitis induction and had their maxillae excised and fixed in 10% neutral formalin. Both maxillaries halves were defleshed, cleaned, and kept in 1 M NaOH at 25 °C for 1 h to remove all soft tissue debris. In order to identify the cemento-enamel junction, jaws were stained with aqueous Loeffler's methylene blue (1%w/v) (SD fine chemical Limited, Mumbai, India). The horizontal ABL was measured by using the similar method as described by Samejima et al. (Citation1990) and Botelho et al. (2008). Measurements were made along the axis of each root of the first (three roots), second, and third molar teeth (two roots). The total alveolar bone loss was obtained by taking the sum of the recordings from buccal tooth surfaces and subtracting the value of the right maxilla (unlighted control) from the left, in millimetres (Botelho et al., Citation2010). The distance between the crest of the alveolar bone and the cemento-enamel junction was measured using an eyepiece micrometer in a dissecting microscope at 20 × magnifications (Xu & Wei, Citation2006).
Histological analysis
The rats were euthanized under anesthesia. The dental–alveolar segment having soft tissue was fixed in 10% v/v formalin solution and demineralized in 7% nitric acid for 24 h (Botelho et al., Citation2010). These specimens were dehydrated, embedded in paraffin, and sectioned along the molars in a mesiodistal plane for hematoxylin and eosin (HE) staining. Sections of 6 µm thicknesses were evaluated using light microscopy (×40 magnifications). The stained sections were analyzed by the parameters like ABL and inflammatory cell infiltration in gingival tissue. Alveolar bone specimens from Group 4 (no ligature) were also measured to analyze the results from ligature Groups 2 and 3. (Xu & Wei, Citation2006).
TNF-α and IL-1β detection in gingival tissue
The gingival tissue part from the area adjacent the lower left molars (between first and second) of different groups was collected at 11th day after periodontitis induction. The tissue of groups was collected, homogenized, and processed (Safieh-Garabedian et al., Citation1995; Ku et al., Citation2011; de Araújo Júnior et al., Citation2013). The detection of TNF-α and IL-1β concentrations was determined by ELISA (enzyme-linked immune sorbent assay) RayBio® (New Delhi, India) as earlier described by Cunha et al. (1993). Briefly, 96-well micro-titer plates were coated overnight at 4 °C with an antibody against rat TNF-α or IL-β (100 µg/mL). After blocking the plates, the samples and standard at various dilutions were added in duplicate and incubated at 4 °C for 24 h. The plates were washed three times with the buffer. After then 100 μL of biotin antibody anti-rat TNF-α and anti-rat IL-1β were added to the plate wells and incubated for 1 h at 25 ± 2 °C. The plates were further washed three times and added 100 μL of Streptavidin solution and incubated 45 min at 25 ± 2 °C. We further added 100 μL of TMB (3,3′,5,5′-tetramethylbenzidine) one-step substrate reagent to each well and incubated for 30 min. The enzyme reaction was stopped with 50 μL stop solutions (0.2 M sulfuric acid) and the absorbance was measured at 450 nm. The results were reported as mean ± standard error mean for six animals. The least measurable dose of IL-1β and TNF-α were 80 pg/mL and 25 pg/mL, respectively, as per manual.
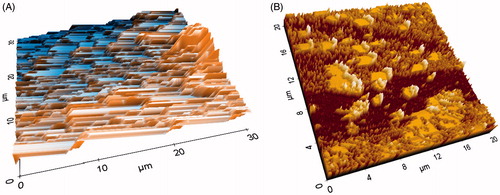
Atomic force microscopy study
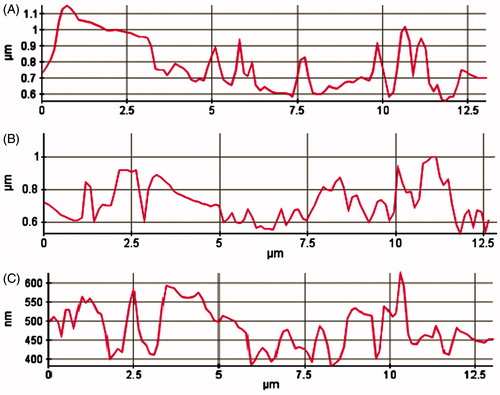
Paraffin-embedded histological sample was taken for atomic force microscopy (AFM) studies. The paraffin was gently removed by slight heating of the slab containing paraffin and the sample was further treated for chemical etching with xylene. The obtained tissue sample was again treated with methanol and then acetone to obtain clean and dried sample for AFM study. Semi-thin sections of 5 µm thickness with the microtome knife were cut, which included the infected areas between the first and second molars. The induction of periodontitis and different treatment groups were assessed using AFM in the tapping mode. The surface morphology of samples of rats' maxillary was scanned and analyzed by using AFM (Park System-XE-70, Suwon, South Korea) equipped with a non-contact cantilever. The instrument was operated in the frequency of 263.4E−3 Hz with silicon cantilever keeping on scan mode, non-contact head mode; XY scan size 30 µm × 30 µm, and constant amplitude at 17.248E−3 µm. The dried sample was analyzed in lateral force and topographic image along with the 3D view was captured using XEP software version 1.7.6 (SPSS Inc.,. Chicago, IL) for surface morphological analysis. Scan controls of AFM were properly adjusted to avoid cantilever tip contact throughout the scanning of the experimental samples. The mean of height and roughness was analyzed by the procedure as described by Botelho et al. (Citation2009), where each study group sample had been diagonally traced, and then topographical differences were observed and compared for each group.
Antibacterial activity
In vitro determination of the antibacterial activity of eugenol gel and NEG loaded with KP containing eugenol as oil phases in same concentration were measured by using the agar diffusion method (the cup plate method). Staphylococcus aureus and Escherichia coli bacteria were used in the study. The culture media for antibacterial assay was nutrient agar media. The actively growing broth cultures of both microbes were prepared, and the turbidity was adjusted to contain approximately 108 CFU/mL. Sterilized molten nutrient agar was poured into sterilized petri dishes and allowed to solidify. The plates were swabbed with the 100 µL culture of the microorganisms. Uniform-sized cups of 6-mm diameter were aseptically punched into the seeded agar medium using a sterilized well bore at a equidistant position. The prepared gel samples were filled into the cylinder cup and incubated at 37 ° ± 0.5 °C for 48 h. The diameter (mm) of the zone of growth inhibition was measured as the diameter (mm). All the tests were carried out in triplicate (n = 3).
Results and discussions
TM and GI
TM in both treatment groups (on the basis of score) was significantly lower compared with Group 1 (p < 0.05); TM in Group 3 was significantly lower than that of Group 2 (p < 0.05) (). TM in Group 1 rats was increased to 3.652 ± 0.115; after treatment, it was reduced to 1.445 ± 0.121 in Group 2 and 0.4360 ± 0.101 in Group 3 with a mean percentage of bone reductions of 60.43% and 88.06%, respectively ().
Table 1. Visual observation from experimental groups in rats (treated/non-treated group).
GI in both treatment groups (based on the score) was significantly lower compared with Group 1 (p < 0.05); GI in Group 3 was significantly lower than that of Group 2 (p < 0.05) (). GI in Group 1 rats was increased to 3.420 ± 0.021; after treatment, it was reduced to 1.356 ± 0.031 in Group 2 and 0.328 ± 0.012 in Group 3 with a mean percentage of bone reductions of 61.22% and 90.40%, respectively ().
Measurement of ABL
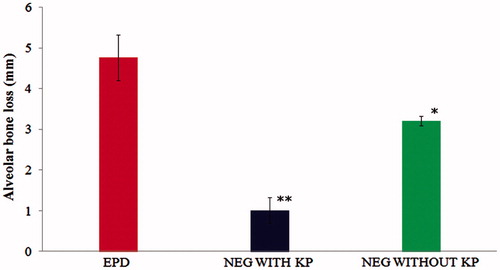
ABL in both the treatment groups was significantly lower compared with Group 1 (p < 0.05); ABL in Group 3 was significantly lower than that of Group 2 (p < 0.05) (). The rats were subjected to treatment for 11 d of EPD with the topical NEG loaded with KP. The ABL (mm) in Group 1 rats was increased to 4.76 ± 0.56 mm; after treatment, it was reduced to 3.21 ± 0.32 mm in Group 2 and 1.01 ± 0.12 mm in Group 3 with a mean percentage of bone reductions of 32.563% and 78.78%, respectively ( and and ).
Figure 1. (A) The group with no ligature (control), i.e., macroscopic aspects of normal maxillae. (B) Macroscopic aspects of the maxillae excised from a non-treated ligature-induced experimental periodontitis animal rats showing severe alveolar bones resorption and root exposure. (C) Macroscopic aspects of the maxillae excised from the group with ligature-induced experimental periodontitis and treated with NEG without KP, showing less significant effect on alveolar bones resorption and root exposure. (D) Macroscopic aspects of the maxillae excised from the group with ligature-induced experimental periodontitis and treated with NEG loaded with KP, showed significant effect on alveolar bone loss.
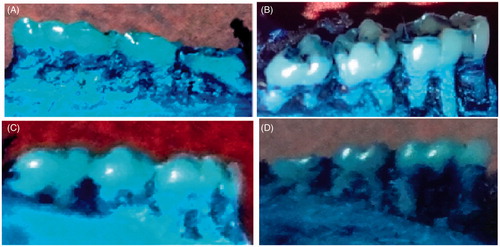
Figure 2. Effect of 2% w/w nanoemulgel of KP and without KP on the alveolar bone loss in experimental periodontal disease (EPD) in rat, comparison with EPD without treatment. Bars represent the mean ± S.D of alveolar bone loss (mm; n = 6). *p < 0.05 was considered less significant difference compared with NEG without KP and non-treated groups; **p<0.05 was considered more significant difference compared with NEG loaded with KP and non treated group. (ANOVA, Turkey–Kramer multiple comparisons test).
Histopathological analysis
Histopathological analysis of the area between the first molar and second molar teeth of rats' different groups was stained by HE revealed healthy alveolar bone and cementum, and no sign of cell infiltration (); in the Group 1, a large number of inflammatory cell infiltration (monocytes infiltration and resorption lacunae with osteoclasts) accompanied by severe cementum and alveolar bone destruction () were shown. After treatment of EPD rats, the histopathology of the periodontium showed that Group 2 had a less significant reduction inflammatory cell infiltration alveolar bones' resorption and cementum (), whereas more significant was observed with Group 3 ().
Figure 3. (X1) The group with no ligature (control). Rats without ligature showed healthy alveolar bone and cementum, and no sign of cell infiltration; (X2) the group with ligature-induced experimental periodontitis. Rats subjected to experimental periodontitis revealed inflammatory cell infiltration accompanied by cementum and alveolar bone destruction. (X3) The group with ligature-induced experimental periodontitis and treated with NEG without KP. Eugenol, as the oil phase, showed less significant effect on inflammatory cell infiltration and alveolar bone loss. (X4) The group with ligature-induced experimental periodontitis and treated with NEG with KP, showed significant effect on inflammatory cell infiltration and alveolar bone loss. D, dentin; PL, periodontal ligament; AB, alveolar bone; G,gingival tissue (hematoxylin and eosin staining, magnification 40×).
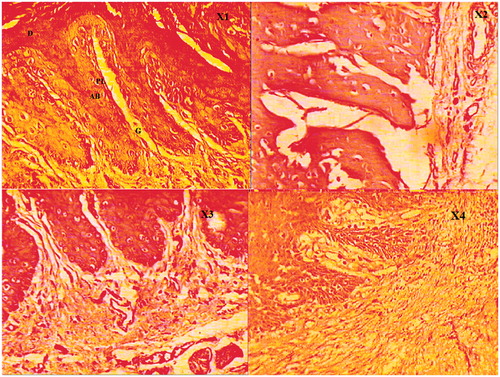
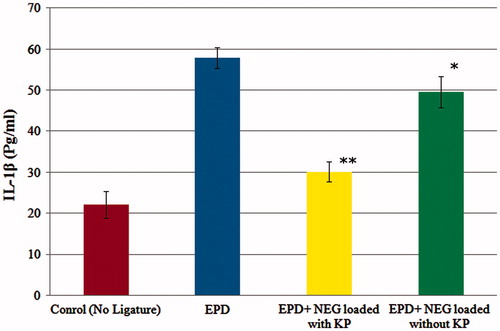
Figure 4. Effect of 2% w/w nanoemulgel of KP and without KP on IL-1β in experimental periodontal disease (EPD) in rat, comparison with EPD without treatment. Bars represent the mean ± S.D of alveolar bone loss (mm; n = 6). *p < 0.05 was considered less significant difference compared with the NEG without KP and non-treated groups; **p<0.05 was considered more significant difference compared with NEG loaded with KP and non treated group. (ANOVA, Turkey–Kramer multiple comparisons test).
TNF-α and IL-1β detection in gingival tissue
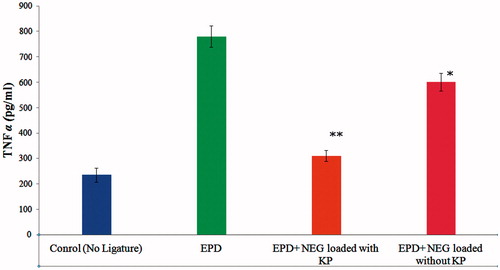
The level of TNF-α and IL-1β in Group 1 rats was increased to 780.21 ± 22.23 and 57.82 ± 2.56 respectively. After treatment of EPD rats, the level of TNF-α and IL-1β showed that Group 2 had a less significant reduction of cytokine levels [TNF-α (601.56 ± 27.21) and IL-1β (49.56 ± 3.78)], respectively, in gingival tissue of Wistar rats subjected to EPD. Whereas the Group 3 was observed more significant reduction of cytokine levels [TNF-α (310.45 ± 35.06) and IL-1β (30.13 ± 2.45)], respectively, as compared with subjected to EPD. The cytokine levels of Group 3 were closed to the value of the control group [TNF-α (235.24 ± 42.12) and IL-1β (22.12 ± 3.21)], suggests that developed KP-loaded NEG formulation effectively treats periodontitis. The reduction in TNF-α 22.89% and 60.21% and reduction in IL-1β 14.28% and 47.89% were observed in Group 2 and Group 3, respectively ( and ).
Figure 5. Effect of 2% w/w nanoemulgel of KP and without KP on TNF-α in experimental periodontal disease (EPD) in rat, comparison with EPD without treatment. Bars represent the mean±S.D of alveolar bone loss (mm); (n = 6). *p < 0.05 was considered less significant difference compared with NEG without KP and non-treated groups; **p<0.05 was considered more significant difference compared with NEG loaded with KP and non treated group. (ANOVA, Turkey–Kramer multiple comparisons test).
AFM studies
The AFM topographical changes in alveolar bone surface roughness of rats subjected to the EPD, and normal groups were clearly observed and assessed surface morphology (). The topographic images of the samples were analyzed by the diagonal method in Group 1 rats were revealed deeper alterations with grooves on its structures (). After treatment, roughness was less significantly reduced in Group 2 (), whereas significant reduction of roughness of alveolar bone surface was achieved with 2%w/w NEG-loaded KP equivalent to 10 mg of dose ().
Antibacterial activity
The zone of growth inhibition of S. aureus and E. coli was studied by the agar-cup diffusion method. The NEG-loaded KP containing eugenol showed more significant (p < 0.05) antibacterial effect on microbes S. aureus and E. coli having the zone of growth inhibition 11.6 ± 0.34 mm and 10.1 ± 0.36 mm, respectively. The zone of growth inhibition of S. aureus and E. coli in eugenol gels was found to be 8.7 ± 0.24 mm and 7.4 ± 0.22 mm, respectively. The NEG-containing eugenol as the oil phase showed more antibacterial activity in comparison with eugenol alone because in NEG, eugenol was in nano-droplet forms, which can be easily fused with the outer membrane of the microbes, and surfactants of the NEG can disrupt the external membrane and kill the microbes (Hamouda et al., Citation1999; Lee et al., 2010).
Conclusion
The cytokine levels of the group treated with NEG loaded with KP were closed to the value of the control group, suggests that developed KP-loaded NEG formulation effectively treats periodontitis. The histopathology of the periodontium showed that Group 3 had a more significant reduction of inflammatory cell infiltration, alveolar bones resorption, and cementum (p < 0.05). The outcomes may be due to arbitrate by its inhibitory effect on the periodontal microbes and its modulator role in the inflammatory mechanism. The study revealed the great synergistic potential of the combined NEG of an anti-inflammatory drug KP along with eugenol as the oil phase, which have potential antibacterial, analgesic, and anesthetic properties to combat periodontal disease and further studies are warranted to clarify its usefulness in clinical situation.
Acknowledgments
The authors are thankful to Council for Scientific and industrial Research (CSIR) New Delhi, India, files number (09/591(0130)/2012), for providing Senior Research Fellowship (SRF). The authors are grateful to Gattefosse (France) for providing the gift samples of oils, surfactants, and cosurfactants. The authors are also thankful to Matrix Laboratories Ltd., Hyderabad, India, for providing the gift sample of drug and Central Instrumental Facility (CIF) Laboratory, Jamia Hamdard, New Delhi, India for carry out the present research work.
Declaration of interest
The authors report that they have no conflicts of interest.
References
- Botelho MA, Rao VS, Carvalho CB, et al. (2007). Lippia sidoides and Myracrodruon urundeuva gel prevents alveolar bone resorption in experimental periodontitis in rats. J Ethnopharmacol 113:471–8
- Botelho MA, Martins JG, Ruela RS, et al. (2009). Protective effect of locally applied carvacrol gel on ligature-induced periodontitis in rats: a tapping mode AFM study. Phytother Res 23:1439–48
- Botelho MA, Martins JG, Ruela RS, et al. (2010). Nanotechnology in ligature-induced periodontitis: protective effect of a doxycycline gel with nanoparticules. J Appl Oral Sci 18:335–42
- Bowen WH, Pearson SK, Jerussi TP. (2000). Influence of (S)-ketoprofen and fluoride on caries in rats. Oral Dis 6:12–4
- Branco-de-Almeida LS, Franco GC, Castro ML, et al. (2012). Fluoxetine inhibits inflammatory response and bone loss in a rat model of ligature-induced periodontitis. J Periodontol 83:664–71
- Cunha FQ, Boukili MA, da Motta JI, et al. (1993). Blockade by fenspiride of endotoxin-induced neutrophil migration in the rat. Eur J Pharmacol 238:47–52
- de Araújo Júnior RF, Souza TO, de Medeiros CA, et al. (2013). Carvedilol decrease IL-1β and TNF-α, inhibits MMP-2, MMP-9, COX-2, and RANKL expression, and up-regulates OPG in a rat model of periodontitis. PLoS One 8:e66391
- Hamouda T, Cao Z, Tonda R, et al. (1999). A novel surfactant nanoemulsion with broad spectrum sporicidal activity against bacillus species. J Infect Dis 180:1939–49
- Jadhav BK, Khandelwal KR, Ketkar AR, Pisal SS. (2004). Formulation and evaluation of mucoadhesive tablets containing eugenol for the treatment of periodontal diseases. Drug Dev Ind Pharm 30:195–203
- Javed S, Kohli K. (2010). Local delivery of minocycline: a therapeutic paradigm in periodontal diseases. Curr Drug Deliv 7:398–406
- Goldhaber P, Rabadjija L, Beyer WR, Kornhauser A. (1973). Bone resorption in tissue culture and its relevance to periodontal disease. J Am Dent Assoc 87:1027–33
- Györfi A, Fazekas A, Suba Z, et al. (1994). Neurogenic component in ligature-induced periodontitis in the rat. J Clin Periodontol 21:601–5
- Ku SK, Cho HR, Sung YS, et al. (2011). Effects of calcium gluconate on experimental periodontitis and alveolar bone loss in rats. Basic Clin Pharmacol Toxicol 108:241–50
- Lawrence HP, Paquette DW, Smith PC. (1998). Pharmacokinetic and safety evaluations of ketoprofen gels in subjects with adult periodontitis. J Dent Res 77:1904–12
- Lee HM, Ciancio SG, Tüter G, et al. (2004). Subantimicrobial dose doxycycline efficacy as a matrix metalloproteinase inhibitor in chronic periodontitis patients is enhanced when combined with a non-steroidal anti-inflammatory drug. J Periodontol 75:453–63
- Li KL, Vogel R, Jeffcoat MK, et al. (1996). The effect of Ketoprofen creams on periodontal disease in rhesus monkeys. J Periodontal Res 31:525–32
- Lima V, Vidal FD, Rocha FA, et al. (2004). Effects of tumour necrosis factor-alpha inhibitors pentoxifylline and thalidomide on alveolar bone loss in short-term experimental disease in rats. J Periodontol 75:162–8
- Liu R, Li N, Liu N, et al. (2012). Effects of systemic ornidazole, systemic and local compound ornidazole and pefloxacin mesylate on experimental periodontitis in rats. Med Sci Monit 18:BR95–102
- Miyauchi M, Hiraoka M, Oka H, et al. (2004). Immuno-localization of COX-1 and COX-2 in the rat molar periodontal tissue after topical application oflipopolysaccharide. Arch Oral Biol 49:739–46
- Ozdemir H, Kara MI, Erciyas K, et al. (2012). Preventive effects of thymoquinone in a rat periodontitis model: a morphometric and histopathological study. J Periodontal Res 47:74–80
- Pimentel SP, Barrella GE, Casarin RC, et al. (2012). Protective effect of topical Cordia verbenacea in a rat periodontitis model: immune-inflammatory, antibacterial and morphometric assays. BMC Complement Altern Med 12:224
- Safieh-Garabedian B, Poole S, Allchorne A, et al. (1995). Contribution of interleukin-1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br J Pharmacol 115:1265–75
- Samejima Y, Ebisu S, Okada H. (1990). Effect of infection with Eikenella corrodens on the progression of ligature-induced periodontitis in rats. J Periodontal Res 25:308–15
- Semenoff TA, Semenoff SA, Bosco AF, et al. (2008). Histometric analysis of ligature-induced periodontitis in rats: a comparison of histological section planes. J Appl Oral Sci 16:251–6
- Srinivas M, Medaiah S, Girish S. (2011). The effect of Ketoprofen in chronic periodontitis: a clinical double-blind study. J Indian Soc Periodontol 15:255–9
- Srivastava M, Kohli K, Ali M. (2014). Formulation development of novel in situ nanoemulgel (NEG) of ketoprofen for the treatment of periodontitis. Drug Deliv Apr 30:1–13. [Epub ahead of print]
- Xiao X, Li Y, Zhang G, et al. (2012). Detection of bacterial diversity in rat's periodontitis model under imitational altitude hypoxia environment. Arch Oral Biol 57:23–9
- Xu Y, Wei WA. (2006). Comparative study of systemic subantimicrobial and topical treatment of minocycline in experimental periodontitis of rats. Arch Oral Biol 51:794–803