Abstract
Objective: Drug loading into nanocarriers is used to facilitate drug delivery to target cells and organs. We have previously reported a change in cellular localization of epirubicin after loading to poly(butyl cyanoacrylate) (PBCA) nanoparticles. We aimed to further investigate the altered cellular localization and cellular responses to the described drug formulation.
Materials and methods: HeLa cells were treated with epirubicin-loaded PBCA nanoparticles prepared by the pre-polymerization method. A systematic study was performed to evaluate the formulation cytotoxicity. Cellular localization and uptake of the formulation as well as cellular response to the treatment were evaluated.
Results: Our studies revealed decreased cytotoxicity of the nanoparticle-formulated epirubicin compared to the free drug as well as a noticeable change in the drug’s intracellular localization. Epirubicin-loaded nanoparticles were internalized via endocytosis, accumulated inside endosomal vesicles and induced a two-fold stronger pro-apoptotic signal when compared to the free drug. The level of the tumor suppressor protein p53 in HeLa cells increased significantly upon treatment with free epirubicin, but remained relatively unchanged when cells were treated with equivalent dose of nanoparticle-loaded drug, suggesting a possible shift from p53-dependent DNA/RNA intercalation-based induction of cytotoxicity by free epirubicin to a caspase 3-induced cell death by the epirubicin-loaded PBCA formulation.
Introduction
Cervical cancer is among the leading health concerns, considered by the World Health Organization the second most frequent cancer in women around the world (World Health Organization, Citation2013). Currently, treatment of cervical cancer involves surgery, radiation therapy and platinum-based combination chemotherapy (Kamura & Ushijima, Citation2013), although anthracyclines, such as epirubicin, have also been evaluated as components of chemotherapeutic regimes (Calero et al., Citation1991; Ferrandina et al., Citation2012). Unfortunately, current chemotherapy suffers a major disadvantage—the inability to selectively deliver the drugs to their targets—the tumor cells. The inefficient drug delivery and severe side effects (gastrointestinal toxicity, myelosuppression, etc.) remain the major limiting factors for achieving sufficient therapeutic efficacy. Thus, researchers are forced to look for novel compounds, fine-tune existing treatment protocols of combinations of radio- and chemotherapies or investigate the utilization of drug carriers that would possibly enhance targeted delivery of chemotherapeutics and therefore decrease the negative side effects of the drugs.
Targeted drug delivery by drug nanocarrier systems has formed a trend in cancer research in recent years (Yordanov, Citation2013). Various nanocarrier systems (such as liposomes, micelles and nanoparticles) show promising results as carriers, some already with practical application, though all of them are still shaded in obscurity and require further research to elucidate the basis of their qualities (Paudel et al., Citation2008; Mao et al., Citation2012; De Smet et al., Citation2013; Tzeng & Green, Citation2013; Uskokovic, Citation2013; Vats & Pathak, Citation2013). Synthetic nanospheres or nanocapsules made of biocompatible and biodegradable polymers represent a share of highly promising nanocarriers. They present an opportunity for precise control of their physicochemical properties by manipulating their composition and the molecular mass distribution of the polymers (Vasir & Labhasetwar, Citation2007; Faraji & Wipf, Citation2009; Mahajan et al., Citation2011). The main mechanism responsible for accumulation of nanoparticle drug carriers in solid tumors is the so-called enhanced permeability and retention (EPR) effect, based on the existence of fenestrations in the tumor vasculature through which nanoparticles can enter the tumor interstitium and become entrapped there because of their low diffusion rate and the lack of effective lymphatic drainage (Fang et al., Citation2010; Torchilin, Citation2010). On the other hand, an important issue to be addressed in cancer chemotherapy is that cytotoxic drugs and/or nanocarriers should not lead to significant necrosis of tumor cells. Massive lysis of tumor cells is characterized as tumor lysis syndrome, considered a medical emergency and may have devastating effects on the patient (Davidson et al., Citation2004). Therefore, it is desirable that cytotoxic drugs/nanocarriers induce predominantly apoptotic cell death in cancer cells.
Apoptosis is a process of programmed cell death, observed with all multicellular organisms. It is a tightly controlled process, characterized with specific morphological and biochemical changes in cells. A canonical pathway regulating apoptosis includes the activation of series of proteases known as caspases (Riedl & Shi, Citation2004) with different caspases acting as initiators or effectors of apoptosis. Caspase-3 induces apoptosis in damaged or transformed cells, as well as in cells that have already fulfilled their biological function. Pro-caspase-3 could be activated both by intrinsic and extrinsic events, leading to partial proteolysis of this inactive protein thus producing its active form, caspase-3, and activating downstream events leading to apoptosis (Riedl & Shi, Citation2004). The tumor suppressor protein p53 has a major role in regulation of the cell cycle (Schwartz & Rotter, Citation1998) and repair of damaged DNA (Chumakov, Citation2007). Activation of p53 leads to cell cycle arrest between the G1 and S-phases, allowing for the reparative mechanisms of the cell to take action. If the DNA damage is too severe to be repaired, p53 acts to induce apoptosis (Amaral et al., Citation2010) by inducing the expression of numerous genes like BAX, PIG3, PUMA, Apaf-1, etc., associated with apoptosis (Fridman & Lowe, Citation2003). Unlike necrosis, apoptosis produces cell fragments that are engulfed by phagocytic cells and do not cause damage to the organism (Edinger & Thompson, Citation2004; Fink & Cookson, Citation2005; Chaabane et al., Citation2012).
Butyl cyanoacrylate is a main component of medical cyanoacrylate glues (Oowaki et al., Citation2000) and previous reports have demonstrated that poly(butyl cyanoacrylate) (PBCA) nanoparticles are biocompatible, biodegradable and could be used as drug delivery systems in cancer chemotherapy (Vauthier et al., Citation2007; Graf et al., Citation2009; Nicolas & Couvreur, Citation2009; Lince et al., Citation2011, Yordanov, Citation2012), delivery of antibiotics (Pinto-Alphandary et al., Citation2000; Fontana et al., Citation2001; Kisich et al., Citation2007), antiviral agents (Bender et al., Citation1996; Von Briesen et al., Citation2000) and anti-inflammatory (NSAIDs) drugs (Miyazaki et al., Citation2003) for drug delivery through the blood–brain barrier (Ramge et al., Citation2000; Azarmi et al., Citation2006; Weiss et al., Citation2008), to the eyes (Zimmer et al., Citation1994), etc. Furthermore recent clinical trials with drug-loaded PBCA nanoparticles have demonstrated some improvement of chemotherapy in patients with hepatocellular carcinoma (Zhou et al., Citation2009). Epirubicin is a less cardiotoxic derivative of doxorubicin, the most popular anthracycline drug, possessing a differently oriented hydroxyl group at the 4′-carbon atom of the sugar (Plosker & Faulds, Citation1993). Like all anthracyclines, the mechanism of action of epirubicin is still not precisely clear even though those drugs are widely used (Minotti et al., Citation2004). Several possible mechanisms of action exist, including intercalation into DNA, leading to inhibited synthesis of macromolecules, with highest cytotoxic effect of epirubicin established during the S and G2-phases of the cell cycle (Cersosimo & Hong, Citation1986). Another widely considered mechanism is the generation of free radicals, leading to DNA damage or lipid peroxidation. Further possible mechanisms include direct membrane effects and induction of apoptosis in response to topoisomerase II inhibition (Gewirtz, Citation1999).
In this article, we report studies on the cellular response of cervical carcinoma (HeLa) cells to treatment with PBCA nanoparticles loaded with epirubicin. Previous studies have demonstrated that epirubicin loaded via the pre-polymerization method to PBCA nanoparticles exhibits increased cytotoxic effect on lung cancer cells (A549) and a specific change in subcellular localization of the nanoparticle-bound drug (Yordanov et al., Citation2013). Therefore, it was interesting to further investigate whether the observed differences were cell line specific, what are the mechanisms by which the drug-free and epirubicin-loaded PBCA nanoparticles are internalized by cancer cells and to determine whether these nanoparticles induce cell death via apoptosis or necrosis. Such knowledge would be crucial for the in vivo application of the epirubicin–PBCA formulation and would shed new light on the mechanisms of anti-proliferative effects of nanoparticles on cancer cells and improve our understanding of nanoparticle–cell interactions.
Materials and methods
Materials and reagents
Epirubicin hydrochloride (EPI HCl, produced by Synbias Pharma (Actavis), Schaffhausen, Switzerland) was a gift from Actavis. The monomer (BCA) was from Special Polymers Ltd. (Bulgaria). Pluronic F68 (F68; molecular weight: ∼8350 Da; known also as poloxamer 188), hydrochloric acid (37%; molecular biology grade), sodium hydroxide (>98%, pellets, anhydrous) and phosphate-buffered saline (PBS, pH 7.4) were from Sigma-Aldrich (St Louis, MO). Distilled water was used in all experiments.
Preparation of PBCA and EPI-PBCA nanoparticles
PBCA and EPI-PBCA nanoparticles used in these experiments were prepared using the pre-polymerization approach (Yordanov et al., Citation2013). Briefly, BCA monomer (120 µl) was pre-polymerized for 30 min upon magnetic stirring in aqueous solution (9 ml), which contained F68 (60 mg) and HCl (0.01 M). Then, solution (1 ml) of EPI HCl (13.0 mg) in 0.01 M HCl was added and the polymerization continued for additional 2.5 h. The reaction mixture was then neutralized with 0.1 M NaOH (1 ml) and the pH was fixed at 7.4 by addition of 10× PBS (1 ml). The dispersion was stirred overnight at room temperature and kept at 4 °C for a week and periodically dispersed by sonication to allow drug sorption into nanoparticles. The obtained dispersions were filtered through porous glass filter and stored at 4 °C. Drug-free nanoparticles (PBCA) were similarly prepared but without addition of drug. Excess of surfactant and unloaded drug were removed by centrifugation and washing of nanoparticles before performing subsequent experiments.
Characterization of nanoparticles
Nanoparticles were observed using a Scanning Electron Microscope (SEM) JSM-5510 (Jeol Ltd., Tokyo, Japan), operated at 10 kV of acceleration voltage. The nanoparticles were purified from excess of surfactant by repeated centrifugation and washing with distilled water, deposited on a glass substrate, dried and covered with a thin gold film (using JFC-1200 Fine Coater, Jeol Ltd.). Particle size distributions were measured with a dynamic light scattering (DLS) system Zetasizer Nano ZS (Malvern Instruments, Worcestershire, UK) at 25 °C. The ζ-potentials were measured by electrophoretic light scattering (ELS) in diluted PBS (pH 7.4; total ionic strength of 16.5 mM and conductivity 2.0 mS/cm) in the presence of 0.5 mg/ml surfactant (Pluronic F68). Drug-loading efficiency was defined as the amount of epirubicin loaded in PBCA particles divided by the total amount of drug used to prepare the formulation. It was determined by centrifugation (relative centrifugal force: 20 000 g for 30 min) of the nanoparticle dispersion and spectrophotometric measurement of the amount of unentrapped drug in the supernatant at 480 nm.
Cell cultures
Human cervical carcinoma (HeLa) cells were acquired from the Bulgarian National Bank for Industrial Microorganisms and Cell Cultures (NBIMCC). Cells were cultured in humidified atmosphere (5% CO2 at 37 °C) in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and 100 U/ml penicillin–streptomycin.
MTT analysis
Cytotoxicity of the selected epirubicin formulations was determined using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) dye assay. All formulations were stored at 4 °C and used within 3 days after preparation. Control experiments were carried out using growth culture medium only (serving as non-toxic control). Cells were harvested in a logarithmic growth phase and seeded on 24-well flat-bottomed plates at a density of 1.0 × 105 cells/well. After culturing for 24 h, medium was replaced with medium containing 2 μg/ml free EPI, EPI − PBCA, EPI + PBCA and PBCA (with the following ratio of drug to carrier: EPI:PBCA = 0.7 mg/ml:5 mg/ml) and cells were incubated for 2 h at 37 °C. After the incubation cells were washed twice with PBS to eliminate any remaining drug, 450 μl of the culture medium and 50 μl MTT stock solution (5 mg/ml in PBS, pH 7.4) were then added to each well and cells were further incubated at 37 °C for 3 h to allow the formation of formazan crystals. The medium was then removed and the formazan crystals solubilized with 500 μl 10% SDS (sodium dodecyl sulfate) with 0.001 M HCl for 12 h. The amount of formazan crystals formed was determined by measuring the optical density at 570 nm by spectrophotometer (Epoch; Biotek Instruments Inc., Winooski, VT).
Cellular uptake of epirubicin
HeLa cells (1.0 × 105 cells/well) were cultivated for 24 h to allow cell attachment. The medium was replaced by medium-containing free EPI, EPI − PBCA, EPI + PBCA and PBCA, respectively and cells were incubated for 2 h at 37 °C. Total drug concentrations of 2 μg/ml were used with the following ratio of drug and carrier EPI:PBCA = 0.7 mg/ml:5 mg/ml. After incubation, medium was removed and cells were thoroughly washed three times with PBS to remove any excessive drug that was not taken up by the cells. The cellular uptake was visualized by fluorescence microscope (Nikon Eclipse TS100, DXM camera; Nikon Instruments Inc., Amsterdam, Netherlands) and the emitted signal was detected at 575 nm. Images were processed using the ImageJ software (NIH, Bethesda, MD).
Endosome staining with acridine orange
We preformed an acridine orange staining to visualize endosomes, using a previously described method (Matteoni & Kreis, Citation1987). Briefly, cells were seeded at a concentration of 1.0 × 105 cells/well and cultivated for 24 h to allow cell attachment and were then treated with 14 µg/ml PBCA (concentration corresponding to the concentration of the carrier in the EPI-PBCA formulation) for 2 h. Cells were then washed and stained with acridine orange (5 µg/ml) for 5 min. After a thorough wash cells were visualized on an epifluorescence microscope (Nikon Eclipse TS100; Nikon Instruments Inc.) using a filter cube with excitation filter at 450–490 nm and emission filter at 520 nm.
Sample preparation, SDS–polyacrylamide gel electrophoresis and immunoblotting
Cells were seeded at concentration of 1.0 × 105 cells/well and cultivated for 24 h to allow cell attachment. Medium was then replaced by medium-containing free EPI, EPI − PBCA, EPI + PBCA and PBCA with the following ratio of drug and carrier EPI:PBCA = 0.7 mg/ml:5 mg/ml. After 6 h of incubation, cells were lysed using radioimmunoprecipitation assay (RIPA) buffer [50 mM HEPES, pH 7.5, 150 mM NaCl, 10% glycerol, 1.5 mM MgCl2, 1 mM EGTA, 1 mM sodium vanadate, 10 mM sodium pyrophosphate, 100 mM NaF, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, protease inhibitor mixture (Boehringer, Ingelheim, Germany) and 1 mM phenylmethylsulfonyl fluoride]. Protein samples were mixed with equal volume of 2× Laemmli SDS–polyacrylamide gel electrophoresis sample buffer and resolved on 9% acrylamide gels. After electrotransfer to nitrocellulose membranes, the filters were blocked with 5% non-fat dry milk in TBST (150 mM NaCl, 50 mM Tris–HCl, 0.1% Tween-20, pH 7.4) and probed with primary antibodies followed by the appropriate secondary horseradish peroxidase-conjugated antibodies. Antibodies used were anti-tubulin (Santa Cruz Biotechnology, Dallas, TX), anti-cleaved caspase-3 Asp175 (Cell Signaling, Danvers, MA) and anti-p53 (Transduction Laboratories, San Jose, CA). Immunoblots were visualized using the enhanced chemiluminescence (ECL) system (Santa Cruz Biotechnology) and Kodak X-Ray Film (Kodak, Rochester, NY). Results were quantified using the ImageJ software.
Transmission electron microscopy
HeLa cells were seeded at concentration of 1.0 × 105 cells/well and cultivated for 24 h to allow cell attachment. The medium was replaced by medium-containing free EPI, EPI − PBCA and PBCA with a ratio of EPI to PBCA concentrations being 0.7 mg/ml to 5 mg/ml. After 6 h of incubation, medium was removed, cells were thoroughly washed with PBS and fixed in glutaraldehyde (2.5%) for 30 min at room temperature. Samples were post-fixed in 2% OsO4 for 20 min, dehydrated in ascending alcohol series and contrasted in uranyl acetate (0.5%). Samples were then embedded in Durcopan (Fluca, Buchs, Switzerland) resin following manufacturer’s instructions and cut on ultra microtome to produce 50–100 nm sections. Samples were observed on a transmission electron microscope (TEM) JEM-1200EX (Jeol Ltd.) at acceleration voltage of 80 kV.
Statistical analysis
Each experiment was conducted at least in triplicate and values were expressed as mean ± SEM. Comparison between the differences of means was performed by one-way analysis of variance with the Tukey’s or Bonferroni test. Data with p < 0.05 are considered significant.
Results
Physicochemical properties of PBCA and EPI-PBCA nanoparticles
The obtained nanoparticles were of spherical shape and monomodal size distribution with average size of 180–200 nm, as evident from the SEM observation (). The DLS measurements showed slightly larger average sizes (240 nm; PDI = 0.1). Zeta potential measurements in PBS buffer showed negative zeta potential (−3.5 mV) for the PBCA nanoparticles and positive (10 mV) for the EPI-PBCA nanoparticles. This effect was attributed to the strong adsorption of the cationic drug molecules on the nanoparticle surface. The drug loading efficiency was found to be 70%. Studies of the drug release kinetics in PBS buffer showed that the drug was quite strongly associated with the nanoparticles and only <10% of the loaded epirubicin was released within 24 h (release curve not shown).
EPI and EPI-PBCA reveal high cytotoxicity to HeLa cells in vitro
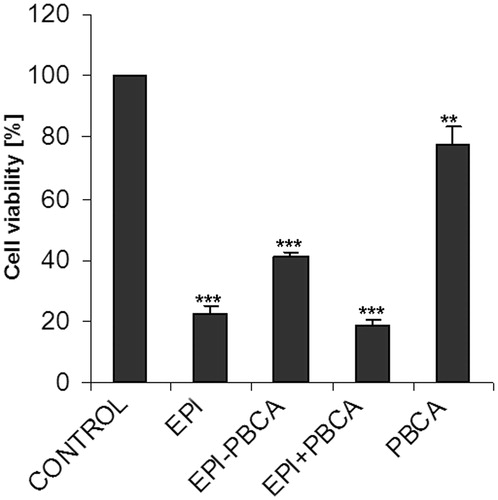
The MTT assay revealed that the effect of epirubicin alone (EPI), as well as the physical mixture of drug and unloaded nanoparticles (EPI + PBCA), was stronger than epirubicin loaded in nanoparticles (EPI − PBCA). Treatment with PBCA nanoparticles alone (with concentration of 14 µg/ml equivalent to the concentration of PBCA in the EPI-PBCA formulation) resulted in ∼20% cytotoxicity compared to control ().
Cellular uptake of EPI-PBCA is higher than free EPI alone and results in cytoplasmic localization
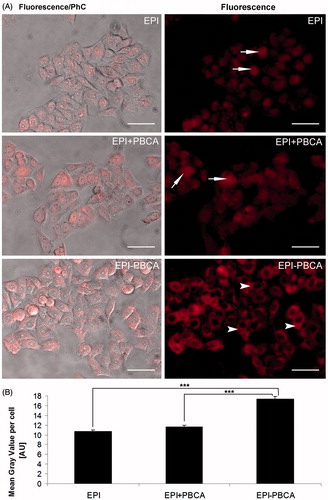
To further investigate the cytotoxicity of the drug formulation, we performed a cell uptake test. Since epirubicin is a naturally fluorescent compound, cells were treated with the respective formulations for 2 h and visualized on an epifluorescence microscope to determine a relative amount of epirubicin entering the tumor cells. As expected all forms of epirubicin were taken up by the cells (), interestingly though the fluorescent signal of the EPI − PBCA formulation was stronger, presumably meaning that more epirubicin has entered the cells and was predominantly localized in the cytoplasm (arrowheads), whereas unbound EPI and the physical mixture of drug and drug-free nanoparticles (EPI + PBCA) showed a predominant nuclear localization (arrows). Similar results have been previously observed in lung cancer cells (A549) (Yordanov et al., Citation2013) and suggest that loaded nanocarriers facilitate the entrance of epirubicin in the cell but at the same time hinder its immediate translocation to the nucleus.
Figure 3. Uptake of epirubicin formulations by HeLa cells. (A) Representative images of epirubicin treated cells. Left column represents phase contrast (PhC) images overlaid with the fluorescent signal. Right column shows the fluorescent images only. Arrows indicate predominant nuclear localization, arrowheads indicate predominant cytoplasmic localization. Scale bar: 50 µm. (B) Uptake of epirubicin formulations by HeLa cells. Graph indicates the average mean gray value per cell, measured with ImageJ software. ***p < 0.001.
HeLa cells internalize PBCA and EPI-PBCA nanoparticles through endocytosis
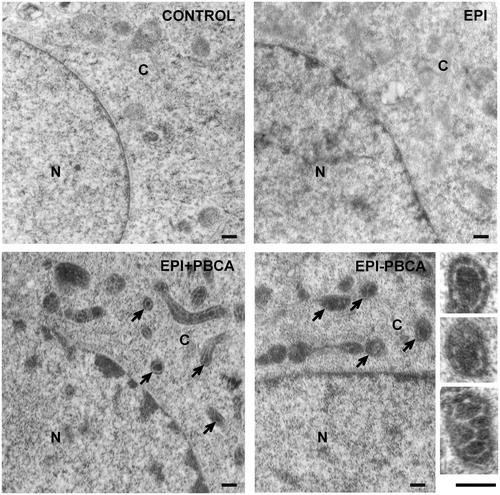
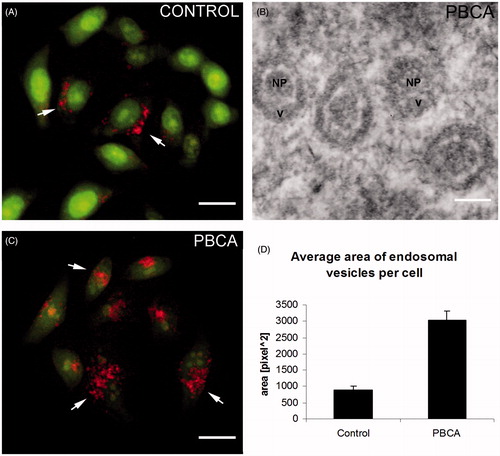
It was important also to determine the mechanism by which the EPI-PBCA nanoparticles were internalized by cells due to the ∼50% increase of the amount of epirubicin taken up by cells, shown in the previous experiment (“Cellular uptake of EPI − PBCA is higher than free EPI alone and results in cytoplasmic localization” section). Therefore, we performed an acridine orange staining to visualize the endosomes and lysosomes formed via endocytosis. We performed the experiment only with empty PBCA nanoparticles since there is a spectral overlap between the emission spectra of epirubicin and acridine orange. The results showed an increase of the endosomes and lysosomes after treatment with PBCA nanoparticles (). To confirm these data in relation to the EPI − PBCA formulation, we used TEM and observed membrane inclusions in the samples treated with EPI + PBCA and EPI-PBCA nanoparticles, but not in the samples treated with free EPI alone or control cells () The TEM observations confirmed that the drug-loaded (EPI-PBCA) nanoparticles were also internalized by endocytosis and were found inside endosomal vesicles.
Figure 4. Acridine orange staining for endosomal and lysosomal vesicles. (A) Fluorescent images of control and PBCA-treated HeLa cells. Arrows indicate red/orange stained endosomal/lysosomal vesicles. Scale bar: 20 µm. (B) Graph represents average area of endosomal vesicles per cell, measured with ImageJ software. (C) Representative TEM image shows PBCA nanoparticles engulfed in endosomes. Scale bar: 100 nm. Labels: NP, nanoparticle; v, vesicle. (D) Graph represents average area of endosomal vesicles per cell in fluorescent images of PBCA-treated cells, measured with ImageJ software.
PBCA and EPI-PBCA nanoparticles induce apoptosis in HeLa cells in vitro
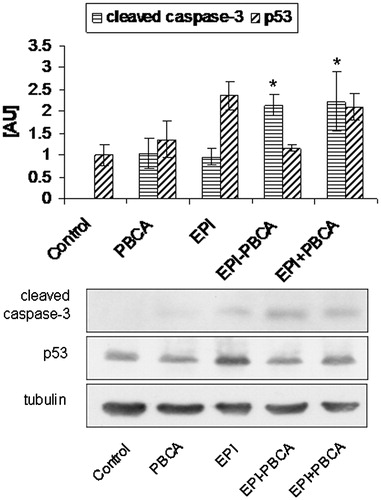
To determine whether the PBCA and EPI-PBCA nanoparticles could induce apoptosis in the cervical cancer cells, we examined two key proteins, known as major regulators of the apoptotic pathway—activated caspase-3 and p53. To detect the early phase of apoptosis, total cell lysates were prepared on the 6th hour post-treatment. Western blot analyses revealed a significant, two-fold increase of the active form of caspase-3 after treatment with EPI-PBCA () compared to free EPI alone. An interesting observation is that free EPI and drug-free PBCA nanoparticles induced the same level of activation of caspase-3. Western blot analyses revealed an increased level of p53 with both treatments, though highest increase was observed after treatment with free EPI alone.
Discussion
At the current stage of development of nanomedicine, research is still focused on novel materials that exhibit some desired effects and could be potentially utilized in clinical practice. However, investigations of the mechanisms underlying those effects are rarely being performed. Details of these mechanisms revealed by in vitro examination of cellular signaling and behavior would extend our knowledge about the properties of nanomedicines and how cells react to them. This information would allow further modifications and improvement of nanoparticle drug delivery systems until eventually a highly controllable, precisely targeted and non-toxic carriers are developed. With the current article, we aimed to investigate the cellular response mechanisms to a PBCA nanoparticle formulation of the anthracycline drug epirubicin and to shed new light on the nature of nanoparticle–cell interactions.
The MTT analysis revealed that all tested epirubicin formulations exhibit cytotoxic effect on HeLa cells, with no significant difference between free EPI and the physical mixture of epirubicin and drug-free PBCA nanoparticles. Interestingly, the epirubicin-loaded (EPI-PBCA) nanoparticles showed lower cytotoxicity than the free EPI (). This was not an unexpected result probably caused by the relatively strong association between the drug and the nanoparticles. Besides similar decrease of cytotoxicity of doxorubicin loaded to poly(styrene-co-maleic acid) (PSMA) micelles compared to the free drug has also been previously described (Greish et al., Citation2004). Furthermore the authors reported a significant decrease of the systemic drug toxicity in mice and much higher antitumor efficacy in vivo of the nanoformulation compared to the free drug. Similar results have also been obtained with nanoformulations of other anticancer drugs: pirarubicin (Greish et al., Citation2005), tanespimycin (Larson et al., Citation2011), daunorubicin (Simeonova et al., Citation2009), etc. In all these cases, the obtained decrease of the drug cytotoxicity after association with nanocarriers has been explained with the long-term retention of significant quantities of drug bound to nanoparticles in the cellular environment. On the other hand, an increased cytotoxicity of drug-loaded nanoparticles to lung carcinoma (A549) cells compared to the free drug has been previously reported (Yordanov et al., Citation2013), indicating a possible cell line specific response and the need for careful evaluation of the treatment protocol. Increased cytotoxicity of other anthracyclines (such as doxorubicin) after entrapment in PBCA nanoparticles has also been previously reported (Juan, Citation2005; De Juan et al., Citation2006; Wohlfart et al., Citation2011), but the mechanisms behind the alteration of the drugs activity remain unclear. However, when cytotoxicity studies from different reports are compared, one should take into account the differences in formulation compositions. For example, it has been demonstrated that surfactant stabilizers, such as poloxamers and polysorbates, could strongly influence the cytotoxicity profile of similar nanoparticles (Yordanov et al., Citation2011). The presence of unloaded drug in the formulation may also affect the cytotoxicity and could mask any differences in the intracellular localization of the drug when observed by fluorescent microscopy [e.g. the unloaded drug in the EPI-PBCA will localize rapidly in the nucleus, similar to EPI from the free drug formulation, as observed in our previous studies with EPI-PBCA prepared by the nanoprecipitation approach (Yordanov et al., Citation2011)]. One should keep in mind though that the lower cytotoxicity of nanoformulated drugs can potentially be advantageous in vivo by minimizing systemic toxicity, while allowing time for accumulation in tumor to occur via the EPR effect. Indeed, pharmacokinetic analysis have revealed that PSMA-formulated doxorubicin possessed increased half-life in blood plasma and concentration in tumor was 13-fold higher than free drug in mice 24 h after intravenous administration, which explains its higher antitumor efficacy in vivo despite the lower cytotoxicity in vitro (Greish et al., Citation2004). The results from the present investigation and the data from the discussed studies show that nanoscale formulations of the used chemotherapeutic drugs often display altered cytotoxicity and/or bioavailability, suggesting that nanocarriers should not be considered only transport vehicles, but also as biologically active substances, that could affect the drug distribution and its properties.
Epirubicin is a naturally fluorescent compound thus allowing us to conveniently monitor the amount of drug entering the cells. The uptake experiment revealed a stronger fluorescent signal in the EPI − PBCA treated cells, likely indicating higher amounts of epirubicin present in the cells. However, the quantitative determination of epirubicin by measuring the fluorescence intensity should be interpreted cautiously, since the fluorescence quantum yield of epirubicin may depend on different factors, such as the local dielectric constant of the surrounding medium and the presence of various compounds that may act as fluorescence quenchers in the different subcellular compartments. An interesting observation we made was the localization of the fluorescent signal. We observed a predominant nuclear localization of the free epirubicin and the unbound epirubicin mixed with drug-free nanoparticles (EPI + PBCA), but a predominant cytoplasmic localization of the EPI-PBCA. These results were similar to previous observations (Yordanov et al., Citation2013), indicating that the observed effect is not cell line specific. The EPI − PBCA formulation probably releases the bound epirubicin too slowly so the whole amount of the drug is not released until the selected time point (24 h) and therefore could not reach the nucleus, where it should act as a DNA-intercalating agent, blocking DNA and RNA synthesis. Based on the cytotoxicity results, we hypothesized that entrapment of epirubicin in PBCA nanoparticles and the slow drug release lead to a change of the dominant mechanism, by which epirubicin induces cell death. Previous research has already shown that epirubicin, a member of the anthracyclines drug family, acts through a number of mechanisms affecting the DNA molecule—intercalating into the DNA, DNA cross-linking, DNA binding and alkylation, inhibition of topoisomerase II and/or interference with DNA-strand unwinding and separation as well as generation of free radicals and direct membrane effects (Cersosimo & Hong, Citation1986; Grankvist & Henriksson, Citation1987; Gewirtz, Citation1999; Minotti et al., Citation2004). Therefore, hypothetically, epirubicin-loaded nanoparticles could be trapped in endocytotic vesicles, releasing a small amount of epirubicin that enters the nucleus, but preventing the major amount of epirubicin from further localization in the nucleus and thus affecting cellular DNA in any of the aforementioned ways, yet the trapped epirubicin could probably still act through generation of free radicals responsible for the observed cell death. Most likely, the slower drug release also increases the time it influences the tumor cells due to the inability of the cells to excrete the drug, thus allowing the epirubicin in the cells to exert its activity for longer periods in either of the possible mechanisms.
We performed the acridine orange staining to determine whether there is a change in the amount of endosomes after treatment with nanoparticles. The results showed almost a three-fold increase of the area of endocytic vesicles per cell, indicating that nanoparticles are indeed taken up via endocytosis. To confirm these data, we performed a TEM analysis and observed membrane vesicles containing nanoparticles, localized exclusively in the cytoplasm, but not inside the nucleus (). The size of the particles found within these vesicles is comparable or even smaller (∼150 nm) than the average size of nanoparticles determined from the SEM observation (∼200 nm). The smaller size of the nanoparticles in the endosomal vesicles could be a result of few reasons: (i) the thickness of slices for the TEM was 50–100 nm and therefore, it is possible to slice through the nanoparticles at various distances from the particle center resulting in particle pieces which are always smaller than the original particle; (ii) nanoparticles could be partially degraded after their endosomal entrapment due to the action of lysosomal enzymes and (iii) smaller nanoparticles (∼150 nm in diameter) could be preferentially endocytosed by cells than larger particles or it could be a combination of the above suggested factors. Entrapment of the nanoparticles in endosomal vesicles, the slower drug release kinetics and diminished nuclear localization of epirubicin suggests that the epirubicin loaded on nanocarriers is taken up by cells and shows reduced action as intercalating agent, but acts via another mechanism. Based on literature data, we speculate that the most probable mechanism is probably through generation of free radicals, since all other mechanisms require nuclear localization of epirubicin. This shift of the mechanism of action though could possibly lead to a different outcome for the cell, especially considering the mechanisms of cell death.
Currently the types of cell death are being revised and broadened as new data is being acquired (Kroemer et al., Citation2009; Ouyang et al., Citation2012). Nevertheless, necrosis is still indicated to result in cytoplasmic and organelle swelling along with rupture of the plasma membrane and is still considered a non-physiological, but pathological event. Apoptosis on the other hand is a tightly controlled process leading to cell death of old or irreparably damaged cells and results in cellular rounding, membrane blebbing and nuclear fragmentation, and is often associated with caspase activation. To determine whether the EPI − PBCA formulation, with supposedly altered mechanism of action of epirubicin, induces apoptosis, we examined the level of activation of the protease caspase-3—a key checkpoint enzyme in the canonical apoptotic pathway (Riedl & Shi, Citation2004). Interestingly, the nanoparticle formulation led to a stronger, almost two-fold, activation of caspase-3 compared to free EPI alone. At the same time the expression of p53 was strongly induced by free EPI but not by the EPI − PBCA formulation. These results suggest that conventional use of epirubicin leads to drug penetration in the nucleus, where it blocks DNA and RNA synthesis, while at the same time activates the cellular reparative mechanisms. It is known that p53 regulates the expression of numerous genes involved in DNA repair or cell cycle arrest as well as induction of apoptosis if irreparable damage has occurred (Fridman & Lowe, Citation2003). The use of the selected drug concentration was apparently high enough and cells could not repair the inflicted damage and entered apoptosis. The nanoparticle-loaded epirubicin though appears to act in a different manner—inducing stronger apoptotic signal (higher levels of activated caspase-3), without activation of the cellular reparative mechanisms (no significant increase of p53 level compared to untreated control was observed). Probably, the observed effect is due to the inability of epirubicin to enter the nucleus in the amount free epirubicin does, but is also not excreted from the cells, thus possibly acts through some of the other mechanisms described. On the other hand, the physical mixture of PBCA nanoparticles and epirubicin (EPI + PBCA) was internalized via endosomal vesicles as well () and also led to increased p53 level, indicating activated cellular reparative mechanisms, but also induced strong apoptotic signal, as measured by the caspase-3 activation. This could probably be attributed to a cumulative effect of both drug-free PBCA nanoparticles and free epirubicin on caspase-3 activation, but at the same time epirubicin is not hindered from locating to the nucleus, blocking DNA and RNA synthesis and activating p53 and the cellular reparative mechanisms.
However, the exact causes for the observed differences remain to be elucidated in future studies. Nevertheless, our results clearly demonstrate that loading of epirubicin to PBCA nanoparticles does not affect the anticancer properties of epirubicin, but the cellular response to the drug, indicating that the properties of the carrier systems should be taken in consideration for optimal drug delivery and treatment outcome.
Conclusion
PBCA is biocompatible and biodegradable material, already established as a suitable material for nanoparticle drug delivery. This article provides some new insights about the mechanism of interaction between epirubicin-loaded PBCA nanoparticles and cervical carcinoma cells. We showed that both free epirubicin and epirubicin-loaded nanoparticles induce apoptosis, but exhibit different cellular localization, induce different cell responses and probably act by different mechanisms. Therefore, we hope that these data may shed new light on the mechanisms of anti-proliferative effects of nanoparticles in cancer cells and may help in future design of nanomedicines with improved anticancer efficacy.
Acknowledgements
TEM observations were performed with the technical help of Dipl. Eng. Ivan Hristov Chavdarov from the Institute of Experimental Morphology, Pathology and Anthropology (IEMPAM-BAS). The authors are also thankful to Mr Nikola Dimitrov and Dr Yasen Atanasov from Sofia University for the assistance with SEM and DLS measurements, respectively.
Declaration of interest
This research was financially supported by the Bulgarian National Science Fund (Project No. DMU-03/111).
References
- Amaral JD, Xavier JM, Steer CJ, Rodrigues CM. (2010). The role of p53 in apoptosis. Discov Med 9:145–52
- Azarmi S, Tao X, Chen H, et al. (2006). Formulation and cytotoxicity of doxorubicin nanoparticles carried by dry powder aerosol particles. Int J Pharm 319:155–61
- Bender AR, von Briesen H, Kreuter J, et al. (1996). Efficiency of nanoparticles as a carrier system for antiviral agents in human immunodeficiency virus-infected human monocytes/macrophages in vitro. Antimicrob Agents Chemother 40:1467–71
- Calero F, Rodriguez-Escudero F, Jimeno J, et al. (1991). Single agent epirubicin in squamous cell cervical cancer. A phase II trial. Acta Oncol 30:325–7
- Cersosimo RJ, Hong WK. (1986). Epirubicin: a review of the pharmacology, clinical activity, and adverse effects of an adriamycin analogue. J Clin Oncol 4:425–39
- Chaabane W, User SD, El-Gazzah M, et al. (2012). Autophagy, apoptosis, mitoptosis and necrosis: interdependence between those pathways and effects on cancer. Arch Immunol Ther Exp 61:43–58
- Chumakov PM. (2007). Versatile functions of p53 protein in multicellular organisms. Biochemistry 72:1399–421
- Davidson MB, Thakkar S, Hix JK, et al. (2004). Pathophysiology, clinical consequences, and treatment of tumor lysis syndrome. Am J Med 116:546–54
- De Juan BS, von Briesen H, Gelperina SE, Kreuter J. (2006). Cytotoxicity of doxorubicin bound to poly(butyl cyanoacrylate) nanoparticles in rat glioma cell lines using different assays. J Drug Target 14:614–22
- De Smet L, Ceelen W, Remon JP, Vervaet C. (2013). Optimization of drug delivery systems for intraperitoneal therapy to extend the residence time of the chemotherapeutic agent. Sci World J 2013:720858. doi:10.1155/2013/720858
- Edinger AL, Thompson CB. (2004). Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol 16:663–9
- Fang J, Nakamura H, Maeda H. (2010). The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev 63:136–51
- Faraji AH, Wipf P. (2009). Nanoparticles in cellular drug delivery. Bioorg Med Chem 17:2950–62
- Ferrandina G, Distefano MG, De Vincenzo R, et al. (2012). Paclitaxel, epirubicin, and cisplatin (TEP) regimen as neoadjuvant treatment in locally advanced cervical cancer: long-term results. Gynecol Oncol 128:518–23
- Fink SL, Cookson BT. (2005). Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun 73:1907–16
- Fontana G, Licciardi M, Mansueto S, et al. (2001). Amoxicillin-loaded polyethylcyanoacrylate nanoparticles: influence of PEG coating on the particle size, drug release rate and phagocytic uptake. Biomaterials 22:2857–65
- Fridman JS, Lowe SW. (2003). Control of apoptosis by p53. Oncogene 22:9030–40
- Gewirtz DA. (1999). A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol 57:727–41
- Graf A, Mcdowell A, Rades T. (2009). Poly(alkycyanoacrylate) nanoparticles for enhanced delivery of therapeutics-is there real potential? Expert Opin Drug Deliv 6:371–87
- Grankvist K, Henriksson R. (1987). Doxorubicin and epirubicin iron-induced generation of free radicals in vitro. A comparative study. Biosci Rep 7:653–8
- Greish K, Nagamitsu A, Fang J, Maeda H. (2005). Copoly(styrene-maleic acid)-pirarubicin micelles: high tumor-targeting efficiency with little toxicity. Bioconjug Chem 16:230–6
- Greish K, Sawa T, Fang J, et al. (2004). SMA-doxorubicin, a new polymeric micellar drug for effective targeting to solid tumours. J Control Release 97:219–30
- Juan BSD. (2005). The analysis of doxorubicin-loaded poly(butyl cyanoacrylate) nanoparticles in in vitro glioma models. Chemistry, Biochemistry and Pharmacy. Frankfurt am Main: Johann-Wolfgang-Goethe University
- Kamura T, Ushijima K. (2013). Chemotherapy for advanced or recurrent cervical cancer. Taiwan J Obstet Gynecol 52:161–4
- Kisich K, Gelperina S, Higgins M, et al. (2007). Encapsulation of moxifloxacin within poly (butyl cyanoacrylate) nanoparticles enhances efficacy against intracellular Mycobacterium tuberculosis. Int J Pharm 345:154–62
- Kroemer G, Galluzzi L, Vandenabeele P, et al. (2009). Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 16:3–11
- Larson N, Greish K, Bauer H, et al. (2011). Synthesis and evaluation of poly(styrene-co-maleic acid) micellar nanocarriers for the delivery of tanespimycin. Int J Pharm 420:111–7
- Lince F, Bolognesi S, Marchisio DL, et al. (2011). Preparation of poly (MePEGCA-co-HDCA) nanoparticles with confined impinging jets reactor: experimental and modeling study. J Pharm Sci 100:2391–405
- Mahajan N, Sakarkar D, Manmode A, et al. (2011). Biodegradable nanoparticles for targeted delivery in treatment of ulcerative colitis. Adv Sci Lett 4:349–56
- Mao S, Guo C, Shi Y, Li LC. (2012). Recent advances in polymeric microspheres for parenteral drug delivery–part 2. Expert Opin Drug Deliv 9:1209–23
- Matteoni R, Kreis TE. (1987). Translocation and clustering of endosomes and lysosomes depends on microtubules. J Cell Biol 105:1253–65
- Minotti G, Menna P, Salvatorelli E, et al. (2004). Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev 56:185–229
- Miyazaki S, Takahashi A, Kubo W, et al. (2003). Poly n-butylcyanoacrylate (PNBCA) nanocapsules as a carrier for NSAIDs: in vitro release and in vivo skin penetration. J Pharm Pharm Sci 6:238–45
- Nicolas J, Couvreur P. (2009). Synthesis of poly (alkyl cyanoacrylate)-based colloidal nanomedicines. Wiley Interdiscip Rev Nanomed Nanobiotechnol 1:111–27
- Oowaki H, Matsuda S, Sakai N, et al. (2000). Non-adhesive cyanoacrylate as an embolic material for endovascular neurosurgery. Biomaterials 21:1039–46
- Ouyang L, Shi Z, Zhao S, et al. (2012). Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif 45:487–98
- Paudel KR, Rauniar GP, Bhattacharya SK, Das BP. (2008). Recent advancement in drug delivery system. Kathmandu Univ Med J 6:262–7
- Pinto-Alphandary H, Andremont A, Couvreur P. (2000). Targeted delivery of antibiotics using liposomes and nanoparticles: research and applications. Int J Antimicrob Agents 13:155–68
- Plosker GL, Faulds D. (1993). Epirubicin: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in cancer chemotherapy. Drugs 45:788–856
- Ramge P, Unger RE, Oltrogge JB, et al. (2000). Polysorbate-80 coating enhances uptake of polybutylcyanoacrylate (PBCA)-nanoparticles by human and bovine primary brain capillary endothelial cells. Eur J Neurosci 12:1931–40
- Riedl SJ, Shi Y. (2004). Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol 5:897–907
- Schwartz D, Rotter V. (1998). p53-dependent cell cycle control: response to genotoxic stress. Semin Cancer Biol 8:325–36
- Simeonova M, Ivanova G, Enchev V, et al. (2009). Physicochemical characterization and in vitro behavior of daunorubicin-loaded poly(butylcyanoacrylate) nanoparticles. Acta Biomater 5:2109–21
- Torchilin V. (2010). Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev 63:131–5
- Tzeng SY, Green JJ. (2013). Therapeutic nanomedicine for brain cancer. Ther Deliv 4:687–704
- Uskokovic V. (2013). Entering the era of nanoscience: time to be so small. J Biomed Nanotechnol 9:1441–70
- Vasir JK, Labhasetwar V. (2007). Biodegradable nanoparticles for cytosolic delivery of therapeutics. Adv Drug Deliv Rev 59:718–28
- Vats A, Pathak K. (2013). Exploiting microspheres as a therapeutic proficient doer for colon delivery: a review. Expert Opin Drug Deliv 10:545–57
- Vauthier C, Labarre D, Ponchel G. (2007). Design aspects of poly (alkylcyanoacrylate) nanoparticles for drug delivery. J Drug Target 15:641–63
- Von Briesen H, Ramge P, Kreuter JR. (2000). Controlled release of antiretroviral drugs. AIDS Rev 2:31–8
- Weiss CK, Kohnle MV, Landfester K, et al. (2008). The first step into the brain: uptake of NIO-PBCA nanoparticles by endothelial cells in vitro and in vivo, and direct evidence for their blood-brain barrier permeation. ChemMedChem 3:1395–403
- World Health Organization. (2013). Human papillomavirus (HPV) and cervical cancer, Fact sheet No. 380, Media Centre, World Health Organization, http://www.who.int/mediacentre/factsheets/fs380/en/
- Wohlfart S, Khalansky AS, Bernreuther C, et al. (2011). Treatment of glioblastoma with poly(isohexyl cyanoacrylate) nanoparticles. Int J Pharm 415:244–51
- Yordanov G. (2012). Poly(alkyl cyanoacrylate) nanoparticles as drug carriers: 33 years later. Bulgarian J Chem 1:61–73
- Yordanov G. (2013). Advanced strategies for drug delivery in nanomedicine. In: Kralchevsky P, Miller R, Ravera F, eds. Colloid and interface chemistry for nanotechnology. Progress in Colloid and Interface Science. Boca Raton, USA: CRC Press, 3–36
- Yordanov G, Evangelatov A, Skrobanska R. (2013). Epirubicin loaded to pre-polymerized poly(butyl cyanoacrylate) nanoparticles: preparation and in vitro evaluation in human lung adenocarcinoma cells. Colloids Surf B Biointerfaces 107:115–23
- Yordanov G, Skrobanska R, Evangelatov A. (2011). Entrapment of epirubicin in poly(butyl cyanoacrylate) colloidal nanospheres by nanoprecipitation: formulation development and in vitro studies on cancer cell lines. Colloids Surf B Biointerfaces 92:98–105
- Zhou Q, Sun X, Zeng L, et al. (2009). A randomized multicenter phase II clinical trial of mitoxantrone-loaded nanoparticles in the treatment of 108 patients with unresected hepatocellular carcinoma. Nanomedicine 5:419–23
- Zimmer A, Mutschler E, Lambrecht G, et al. (1994). Pharmacokinetic and pharmacodynamic aspects of an ophthalmic pilocarpine nanoparticle-delivery-system. Pharm Res 11:1435–42