?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The present study was designed to determine the role of curcumin-β-cyclodextrin-loaded sponge on burn wound healing in rats. Curcumin-β-cyclodextrin complex was prepared by the solvent evaporation encapsulation method. Molecular inclusion complex of curcumin-β-cyclodextrin was incorporated into gelatin sponge. The developed sponge was characterized for drug entrapment, drug release and morphology. The biological activity of optimized formulation was determined on burn wounds which were made on rats. The burn wound healing efficacy was analyzed through physical and histological changes observed at the wound sites. There was a significant decrease in rate of wound contraction in experimental groups then the control group. Curcumin-β-cyclodextrin-loaded sponge treated wound was found to heal in rate comparable to marketed formulation with no sign of adverse consequence. The result clearly substantiates the beneficial effects of curcumin-β-cyclodextrin-loaded sponge in the acceleration of wound healing.
Introduction
A burn is an injury to skin caused by heat (thermal), electricity, chemicals, friction or radiation (Chaudhary et al., Citation2014). Among them, the most serious burns usually result from heat, known as thermal burns. Thermal burn injuries are associated with a series of biological events, including inflammation, proliferation and remodeling (Garg et al., Citation2012b). The ideal wound dressing protects wound tissue from microbial infection (Gagandeep et al., Citation2014) and accelerates the tissue regeneration by spatial control of inflammatory (Garg, Citation2014) and pro-inflammatory mediators involved in the process of inflammation (Garg et al., Citation2011). Curcumin has been reported to accelerate wound healing efficacy through their antimicrobial and free radical scavenging property. However, their biological activity is limited due to its poor aqueous solubility (Garg & Goyal, Citation2014a). Far year’s scientists put extensive efforts to improve the solubility, permeability and stability of curcumin in order to increase bioavailability (Garg et al., Citation2014). Uses of β-cyclodextrin, lipid complex and nano solid dispersion were among the promising approach that have been used successfully to improve the aqueous solubility of curcumin. Furthermore, it is widely accepted that a moist environment facilitate tissue regeneration (Goyal et al., Citation2013). Therefore, an ideal burn dressing material should combine the feature of moist wound healing with appropriate fluid absorbance (Kaur et al., Citation2014e), antimicrobial (Marwah et al., Citation2014) and anti-inflammatory property (Rohilla et al., Citation2014) to promote tissue regeneration in burn tissue (Johal et al., Citation2014). Gelatin due to its nontoxic, biodegradable and hydrocolloids properties have been used in number of biomedical applications such as wound dressing, tissue engineering and drug delivery (Joshi et al., Citation2014). Therefore, our aim is to prepare curcumin-β-cyclodextrin-loaded gelatin sponge as a topical formulation for burn wound healing. In the present study, curcumin is used because it exhibits anti-inflammatory and anti-oxidant activity (free radical scavenging activity). Topical applications of curcumin have shown to improve significantly burn wound healing and protect tissues from oxidative damage (Durgaprasad et al.). Curcumin is a natural compound as mentioned above but it has poor solubility in water as well as less stable at normal conditions, that is, it degrades easily at room temperature. Thus to enhance the solubility profile of curcumin, complex of curcumin with β-cyclodextrin has been prepared in this project work (Yadav et al., Citation2010).
Further gelatin sponge is used as a base material. High porosity and hydrocolloids properties of sponge allow the permeation of oxygen and help to maintain a moist environment at the wound bed, likely to promote the wound healing efficacy of developed formulation (Kaur et al., Citation2014b). Further reports suggesting that gelatin dressing stimulate the production of α-interleukin, pro-inflammatory cytokines, such as α-interleukin-6, consider to be advantageous to wound healing. Wound healing efficacy of the developed formulation was determined by measuring the physical and histological changes observed at the wound bed. Further a comparable efficacy of the developed product was determined by comparing the wound healing efficacy of developed formulation with marketed formulation (Kaur et al., Citation2014c).
Material and method
Materials
Acetone, β-cyclodextrin, curcumin, disodium hydrogen phosphate, formaldehyde, gelatin and resorcinol were purchased from CDH Laboratory Reagents, New Delhi. Dimethyl sulfoxide (DMSO) was purchased from Loba Cheme Pvt. Ltd., New Delhi, and Thiopental sodium from Neon Laboratories Ltd., Mumbai. The obtained drug sample (curcumin) was identified for purity according to standard procedures. All other chemicals and reagents used in this study were of analytical grade.
Preparation, optimization and characterization of curcumin-β-cyclodextrin inclusion complex
Inclusion complex of curcumin in β-cyclodextrin was prepared by the solvent evaporation encapsulation method as reported by Yadav et al. (Citation2010). In the present study, quantity of β-CD (80 mg) was kept constant; while concentration of curcumin was varied in an increasing order. β-CD was dissolved in 16 ml of deionized water. Different concentrations of curcumin solution were prepared separately in acetone. The solution in an open system was stirred overnight under constant temperature; allow the solvent to evaporate at 1500 rpm for 5 min. Supernatant containing highly water soluble curcumin-β-cyclodextrin inclusion complexes (Cur-CD) were recovered by freeze drying (Alpha 12Lo Plus, Christ, Shropshire, UK). The inclusion complex curcumin-β-cyclodextrin was stored at 4 °C until further use.
Determination of curcumin loading
Curcumin-β-cyclodextrin (1 mg) inclusion complex was dissolved in 50 ml DMSO to extract curcumin in DMSO for the loading estimations. The curcumin-β-cyclodextrin sample in DMSO was gently shaken on a shaker incubator (LSB-1005RE, Daihan Labtech Co. Ltd., Korea) for 24 h at room temperature in the dark. The curcumin extracted DMSO solution was centrifuged (3 K-30, Sigma, Shropshire, UK) at 14 000rpm to remove clumps of β-cyclodextrin and a clear supernatant DMSO solution containing curcumin was collected and used for the estimations. The curcumin concentration was determined by using standard curve of curcumin in DMSO. Characterization of optimized complex was done by the FT-IR spectroscopy (Thermo Nicolet 380, Thermo Scientific, Madison, WI) (Singh et al., Citation2014b).
Preparation and optimization of sponge
Gelatin was soaked in 100 ml of water for 30 min to get solution at the varying concentrations (1–5%) of gelatin. The suspension was stirred and heated till gelatin was solubilized. The gelatin solution was then allowed to cool up to 40 °C. Formaldehyde in varying concentrations (0.1–0.5%) was added to gelatin solution to induce the cross-linking of gelatin, solution was then stirred vigorously for 25 min using homogenizer (T25 D S 22, Cole-Paramer India Pvt. Ltd., Maharashtra, India) to form stable foam. Foam was washed several times to remove residue of formaldehyde and then subjected to lyophillization to convert it into sponge. Optimum conditions for foam preparation was determined by measuring pore size, foam stability, swelling behavior, apparent foam density and foam volume as an in-process indicator (Wang et al., Citation2006).
Pore size
Pore size of developed foam was determined microscopically. Briefly a thin section of the foam was mounted on the slide. The slide was observed using optical microscope (CK 147, Olympus, Tokyo) using 100× magnifications. The pore size of 50 pores was determined. The mean pore size was determined and reported as average pore size of developed formulation (Singh et al., Citation2014a; Sharma et al., Citation2014b).
Measurement of density of lyophilized sponge
The bulk density of sponge was determined from the ratio of weight of lyophilized sponge to its volume. The density of sponge was calculated using sample with a volume of 1 × 1 × 1 cm3 and the final density was obtained from the average of three specimens (Parnami et al., Citation2013; Sharma et al., Citation2014a).
Determination of swelling behavior of sponge
The fluid absorbing capacity of the developed formulation is one of the important properties for establishing a moist environment in the wound site. One centimeter cube of the lyophilized sponge was placed in 50 ml water and the beaker was incubated at 37 °C. At predetermined intervals, the weight of sponge was measured after removing the extra water with the help of a filter paper. Weight of sponge was noted until equilibrium swelling was reached (Denkbaş & Odabaşi, Citation2000; Modgill et al., Citation2014; Morie et al., Citation2014). Swelling behavior of sponge was calculated according to the following equation:
where Ws is the weight of wet sponge and Wd is the weight of dry sponge.
In vitro study
Drug release study from sponges with or without formaldehyde content
This study was performed using type-I USP dissolution rate tester apparatus. Sponge (2 cm2) was placed in basket which was then introduced in a 100 mL of phosphate buffer pH 7.4 contained in 900 ml vessel of the dissolution apparatus. The drug release study was performed at 37 ± 0.5 °C. Samples (5 ml) were withdrawn after 0.15, 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9……21 h. The volume was replaced with fresh medium to maintain the sink condition. Collected samples were filtered through Whatman filter paper and the drug in the solution was determined by the UV Spectrophotometer (Ribatti et al., Citation2006; Kaur et al., Citation2014a, Citationd).
Digestibility
A piece of prepared gelatin sponge weighing about 50 mg was placed in a beaker containing water. The piece was kneaded gently between the fingers until thoroughly wet and all the entrapped air has been removed, taking care not to break the tissue. Sponge piece was lifted from the water and excess water was removed with absorbent paper. The wetted sample was placed in a 150 ml flask, contained 100 ml of 1% solution of pepsin in 0.1 N HCl and mixed continuously until digestion was complete. The average digestion time of three determinations was taken as the reading (Cenni et al., Citation2000; Garg et al., Citation2013; Goyal et al., Citation2014).
Residual formaldehyde content
Residual formaldehyde content in developed sponge was determined quantitatively. In brief, a piece of sponge weighing about 20 mg was taken, and 2 mg of resorcinol was added to it, then 2 ml of concentrated sulfuric acid was added carefully down the side of the tube. The presence of violet ring at the junction of two liquid confirmed the presence of formaldehyde in developed lyophilized sponge (Garg et al., Citation2012a).
Drug entrapment
The developed sponges of 2 × 2 cm2 sizes were soaked overnight at 25 °C in 100 ml of phosphate buffer; pH 7.4. The preparation was sonicated for 10 min; sample leach ate was then filtered onto 0.2 μm membrane filter. Each filter was then rinsed with 3 ml of phosphate buffer saline, the concentration of drug in the solution was determined by the UV spectrophotometric (UV 1700 Pharm Spec, Shimadzu, Japan) method and percentage drug entrapment was calculated (Foda et al., Citation2007; Garg & Goyal, Citation2014b).
In vivo studies
Burn wounds were inflicted on overnight-starved animals under thiopental sodium anesthesia, by placing hot cylindrical metal rod (10 mm diameter) on the shaven back of the animals (Sanwal & Chaudhary, Citation2011). Immediately after the injury, 30 wistar rats (180–220 gm) were randomized into five groups consisting of six animals in each group. Group-I received plain β-cyclodextrin treatment, Group II received gelatin gel treatment, Group III received plain curcumin treatment, Group IV received silver sulfadiazine treatment and Group V received curcumin-β-cyclodextrin-loaded sponge treatment. The in vivo studies were carried out according to the guidelines of the council for the purpose of control and supervision of experiments on animals (CPCSEA), Ministry of Social Justice and Empowerment, Government of India (ISF/CPCSEA/IAEC/2014/84).
Histopathology study
On 21st day of the experiment, animals from each group were sacrificed and the wounds were excised together with the surrounding skin. They were fixed in 10% neutral-buffered formalin. Histological evaluation was performed on hematoxylin and eosin stained 5–6 µm thin paraffin sections of wound bed material. The stained sections were examined for collagen and inflammatory cells (Wu et al., Citation2012).
Skin irritation test
Dermal irritation is defined as the production of “reversible damage of the skin following the application of a test substance”. It is generally assessed by the potential of a certain substance to cause erythema/eschar and/or edema after a single topical application on rat skin. For this study, the burn wounds of rats covered with experimental formulations were observed for any kind of edema or erythema on the skin during the period of healing (Goll et al., Citation1963).
Assessment of burn healing
Animals were inspected daily and the healing was assessed on the basis of rate of wound contraction.
Rate of wound contraction
The wound contraction rate was measured as percentage reduction in wound size after 5, 10, 15 and 20 days interval. Progressive decrease in the wound size was monitored periodically by tracing the boundary.
Statistics
Statistical analysis was performed using Graph Pad Prism version 5.01 for Windows (Graph Pad Software, San Diego, CA). Two-ways analysis of variance was used to compare the treatment groups with control.
Results and discussion
Preparation and optimization of inclusion complex
Curcumin-β-cyclodextrin (Cur-CD) inclusion was prepared according to the procedure mentioned and further it was optimized on the basis of curcumin loading in the hydrophobic core of β-cyclodextrin. Curcumin loading was estimated for each batch of inclusion complex. shows the loading efficacy of different formulations.
Table 1. Loading efficacy of different formulations.
Results indicated that batch 5 exhibit highest curcumin entrapment among other. However, batch 4 results better loading capacity than batch 5. A gradual increase in curcumin loading from batches 1–3 indicated the availability of more hydrophobic sites for the formation of inclusion complex. Negligible increase in curcumin loading from batch 4 to batch 5 determined the establishment of the equilibrium between hydrophobic site of β-cyclodextrin and curcumin, therefore increasing the concentration beyond 40 mg of curcumin per 80 mg of cyclodextrin, there is a negligible increase in curcumin loading (Loftsson & Brewster, Citation1996).
Characterization of optimized inclusion complex
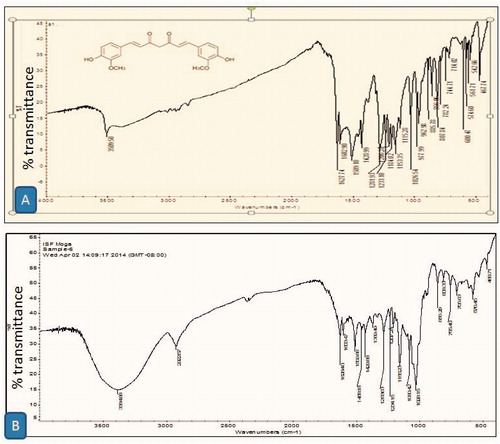
Infra-red spectrum of curcumin and curcumin-β-cyclodextrin inclusion complex is shown in and , respectively. Result suggested that all the sharp peaks belonging to β-cyclodextrin and only few characteristic peaks of curcumin were observed in inclusion complex. Curcumin-related peaks at 1628.40, 1603.4 and 1028.15 cm−1 was the characteristic peaks observed in the inclusion complex confirm the formation of curcumin-β-cyclodextrin complex.
Optimization of gelatin
Once the concentration of curcumin was established, the preparation method for the gelatin foam was optimized. The concentration of gelatin and whipping technique found to be rate limiting parameter in determining foam quality. The result () suggested that 5% w/v of gelatin at 5000 homogenization speed found to be optimized. Concentration above and below 5% w/v gelatin produce unstable foam. Foam produced below 5% w/v was unstable with pore diameter >400 µm. Large pore size is attributed to large bubble, poor mechanical strength of bubble and low bubble density. Although a bubble, result from gelatin concentration above 5% w/v was stable with pore size of 80–120 µm. However, inadequate water present at a high gelatin concentration, unable to emulsify the total gelatin, results in incomplete bubbling of gelatin. Further, the high concentration of gelatin increased the hydrophobic interaction between the non-emulsified gelatin induces Ostwald flocculation leading to destabilization of foam.
Table 2. Optimization of gelatin, formaldehyde and curcumin-β-cyclodextrin complex concentration.
Optimization of formaldehyde
The stability of gelatin foam was increased by using chemical cross-linking technique. The concentration of formaldehyde (cross-linking agent) was optimized. From the study, it has been found that 0.3% v/v formaldehyde produced stable foam (). Formaldehyde at concentration below 0.3% v/v was not sufficient to fix the primary amine results from the 5% w/v gelatin. 0.3% v/v formaldehyde found to be engaged at primary amine in the cross-linking process results in the formation of stable foam (Berlowitz-Tarrant et al., Citation1998). Although formaldehyde concentration more than 0.3% v/v was sufficient to engage all the primary amine, However high concentration of formaldehyde was discarded for medical application due to toxicity (Geiger et al., Citation2003).
Optimization of curcumin-β-cyclodextrin complex
Optimization of curcumin-β-cyclodextrin was accessed by measuring the pore size and foam volume of prepared formulation. The result () indicated that the 2% w/v concentration of curcumin-β-cyclodextrin-yielded optimum foam property. Concentration of curcumin-β-cyclodextrin above 2% w/v results unstable foam with high pore size and lower foam volume. This could be attributed to high foam density. High foam density overcomes the mechanical strength of foam film results in the disruption of foam (Garg & Goyal, Citation2012). Furthermore, the results were also supported with the density studies, where density studies indicate that there is a gradual increase in density with increasing amount of curcumin-β-cyclodextrin complex. While concentration of curcumin-β-cyclodextrin complex below 2% w/v results stable foam, since the objective of the present study is to produce batch with better loading efficacy therefore 2% w/v concentration of curcumin-β-cyclodextrin complex was optimized and selected for further study.
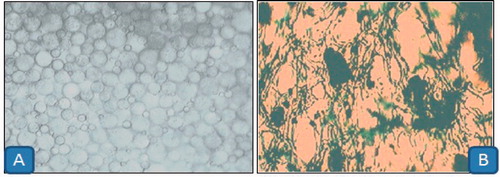
Foam morphology
The optimal microscopy study indicated that the foam was discrete dose and uniform in nature. The pore size of optimized formulation was found to 80–120 µm. Large pore size in batch with 0.1–0.2% w/v formaldehyde yield low foam volume and large foam size is attributed to incomplete cross-linking of primary amine resulted from 5% w/v gelatin. The result further indicated that there is no significant different in pore size and foam volume after the addition of curcumin-β-cyclodextrin complex. However, curcumin-β-cyclodextrin complex beyond 2% w/v concentration start to destabilize the foam integrity. This could be due to increase hydrophilicity of the preparation and increase in foam density. High foam density overcomes the mechanical strength of the bubble film leads to the disruption of bubble ().
Foam density
Foam density of 2% w/v of curcumin cyclodextrin complex is 0.063 gm/cm3. The result of density study indicated that there is gradual increase in density with the addition of curcumin-β-cyclodextrin complex. Two percent curcumin-β-cyclodextrin complex exhibited a density of 0.063 gm/cm3. The ultra-low density of the prepared formulation indicated a high degree of porosity. Further high porosity ensures the excellent adsorption capacity of the developed preparation for the absorption of wound secretion.
Swelling index
Swelling index of developed preparation was determined to measure absorption behavior of the developed formulation. The result demonstrated that the optimized formulation absorb approximately 85% water of its own weight. The absorption efficacy is measurement of pore size and moisture content of the final product preparation. Sponge with large pore size shows poor swelling index is associated with poor foam density and low porosity (Parks & Beckman, Citation1996).
In vitro studies
Drug entrapment
The drug entrapment in the cross-linked sponge is 95%. The drug entrapment is more in the cross-linked sponge than the plain sponge. This could be due to the reduced permeability of cross-linked sponge which restricts the diffusion of entrapped inclusion complex from the foam whereas the high permeability of plain sponge results as a low entrapment of the drug. Further, the reduced pore size could be a measure of higher percentage entrapment in cross-linked sponge.
In vitro drug release
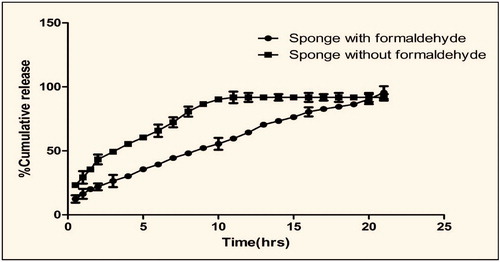
In vitro release suggested that there was a significance difference in the release profile between plain foam and cross-linked foam (). This could be attributed to rigid foam substance in cross-linked formulation. Cross-linking further delay the erosion of gelatin in dissolution medium and further a stable micro porous structure reduces the permeability of dissolution medium, thereby delay the release of entrapped curcumin (Prasad & Kalyanasundaram, Citation1993) whereas the foam without cross-linking agent was more soluble and permeable to dissolution medium allow faster release of entrapped drug. Release study further confirmed that formaldehyde causes extensive cross-linking of gelatin foam and thereby prove the mechanical stability of foam. Further the cross-linking of gelatin with formaldehyde reduces the permeability and hydrophilicity of the developed formulation.
Digestibility
Digestibility is an important criterion to check the digestion of sponge in the presence of proteolytic enzymes secreted at the wound site. The digestibility of sponge is an indication of biocompatibility of sponge inside the body (Cenni et al., Citation2000). The result suggested that there is a significant difference in the digestive time between plain and cross-linked gelatin sponge. It was observed that plan sponge digest completely in 45 min whereas cross-linked sponge take 2 h for complete digestion. This could clearly indicate that the primary amine engaged in the cross-linking process significantly reduces the hydrophilicity of developed sponges.
Residual formaldehyde content
The curcumin-β-cyclodextrin-loaded sponge shows orange color when piece of sponge weighing about 20 mg was treated with resorcinol and 2 ml of concentrated sulfuric acid. When same sponge treated with 0.1 ml of formaldehyde and then with resorcinol and sulfuric acid shows violet color. From this study, we observed that our formulation has no residual formaldehyde content and eliminate the probability of adverse consequences associated with formaldehyde.
In vivo studies
Histopathology study
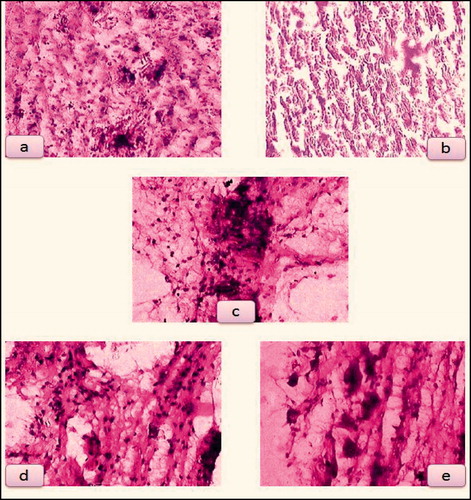
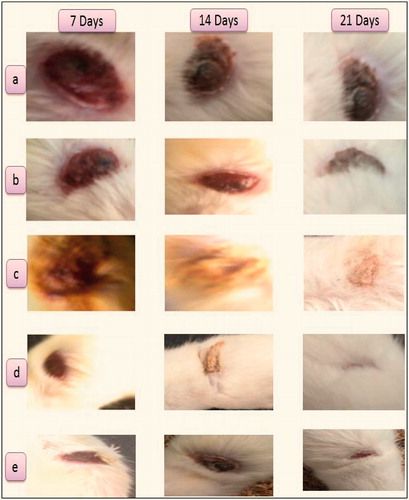
Histopathological result () demonstrates that even after 21 days, plain cyclodextrin-treated group shows more inflammatory cells, gelatin gel-treated group shows few inflammatory cells, plain curcumin-treated group shows fibroblast formation, silver sulfadiazine cream and curcumin-β-cyclodextrin-treated group shows granulation tissue with collagen. Thus, histopathological study showed that silver sulfadiazine and curcumin-β-cyclodextrin-loaded sponge is almost equally effective in the tissue regeneration and wound healing efficacy. In the present study, topical application of curcumin-β-cyclodextrin-loaded sponge significantly enhanced the rate of wound healing associated with more collagen, and less inflammatory cells. Enhanced healing activity has been attributed to increased collagen formation and angiogenesis, and combination of anti-oxidant activity of curcumin (Durgaprasad et al.). The moist environment produced by gelatin sponge promotes the synthesis of collagen (Deng et al., Citation2007) which is a principal component of connective tissue, plays a key role in the healing of wounds and provides a structural framework for the regenerating tissue.
Skin irritation test
The applied curcumin-β-cyclodextrin-loaded sponge did not show any kind of the physical changes of the skin. There was no sign of any erythema/eschar and/or edema on rat skin which indicates the compatibility of the curcumin-β-cyclodextrin-loaded sponge but marketed formulation, that is, silver sulfadiazine shows the sign of irritation (redness). Furthermore, the studies demonstrate that the excipients used in curcumin-β-cyclodextrin-loaded sponge were comparatively safe and effective for burn wounds.
Physical estimation of wound healing
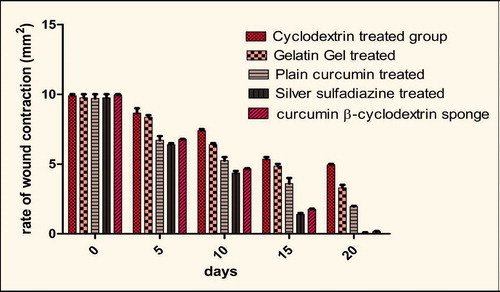
The physical estimation of wound healing can be clearly observed in . It showed a clear comparison of the rate at which the wounds of different animal models healed. Wounds that were dressed with curcumin-β-cyclodextrin-loaded sponge rapidly decreased in size (6.8 mm2) during the first five days following treatment, and then seemed to continue at the same pace even after five days which ultimately led to highest rate of healing in the animal model as compared with other formulations. The wounds covered with plain cyclodextrin, gelatin gel and plain curcumin healed slowly in comparison to curcumin-β-cyclodextrin-loaded sponge. Finally, wounds healed under the curcumin-β-cyclodextrin-loaded sponge demonstrated better tissue quality with less scarring. The wounds that were treated with marketed formulation (SSD) showed considerable healing at a very good rate which was almost similar to curcumin-β-cyclodextrin-loaded sponge. Wounds that received plain cyclodextrin treatment had the slowest rates of healing. The area of wounds for all the five groups during the entire period of healing at regular intervals is reported in .
Figure 5. (a) Plain cyclodextrin-treated group, (b) gelatin gel-treated group, (c) plain curcumin-treated group, (d) silver sulfadiazine-treated group and (e) curcumin-β-cyclodextrin-loaded sponge treated group.
Further plain curcumin shows better wound healing efficacy than the plain cyclodextrin and gelatin gel, could be attributed to broad antimicrobial action and free radical scavenging property of curcumin (Durgaprasad et al.). Moreover, the combination of curcumin and gelatin sponge establishes a moist environment which promotes the tissue regeneration at the wound site. Further the control release of entrapped pharmaceuticals provides a better pharmacological management of curcumin with high wound healing efficacy (Patel et al., Citation2009).
Conclusion
This study concludes that curcumin-β-cyclodextrin-loaded gelatin sponge serves as an ideal dressing material for burn wound. Furthermore, the inclusion complex of curcumin is successfully accomplished with β-cyclodextrin. Moreover, the proposed strategy successfully explores the therapeutic potential of gelatin and curcumin in the management of wound healing in thermal burn.
Declaration of interest
Authors Dr. Amit K. Goyal and Goutam Rath thankful to Department of Biotechnology (DBT), New Delhi, INDIA for providing financial assistance to carry out research. The authors report no conflict of interest.
References
- Berlowitz-Tarrant L, Shivkumar S, Tukumo T. (1998). Algal plastics. Google Patents US5779960 A
- Chaudhary S, Garg T, Murthy RS, et al. (2014). Recent approaches of lipid-based delivery system for lymphatic targeting via oral route. J Drug Target 1–12
- Deng C-M, He L-Z, Zhao M, et al. (2007). Biological properties of the chitosan-gelatin sponge wound dressing. Carbohydr Polym 69:583–9
- Denkbaş EB, Odabaşi M. (2000). Chitosan microspheres and sponges: preparation and characterization. J Appl Polym Sci 76:1637–43
- Durgaprasad S, Reetesh R, Hareesh K, et al. (2011). Effect of a topical curcumin preparation (BIOCURCUMAX) on burn wound healing in rats. J Pharm Biomed Sci (JPBMS) 8:1–3
- Cenni E, Ciapetti G, Stea S, et al. (2000). Biocompatibility and performance in vitro of a hemostatic gelatin sponge. J Biomater Sci Polym Ed 11:685–99
- Foda NH, El-Laithy HM, Tadros MI. (2007). Implantable biodegradable sponges: effect of interpolymer complex formation of chitosan with gelatin on the release behavior of tramadol hydrochloride. Drug Dev Ind Pharm 33:7–17
- Gagandeep GT, Malik B, Rath G, et al. (2014). Development and characterization of nano-fiber patch for the treatment of glaucoma. Eur J Pharm Sci 53:10–6
- Garg T. (2014). Current nanotechnological approaches for an effective delivery of bio-active drug molecules in the treatment of acne. Artif Cells Nanomed Biotechnol 1–8
- Garg T, Bilandi A, Kapoor B. (2011). Scaffold: tissue engineering and regenerative medicine. Int Res J Pharm 2:37–42
- Garg T, Goyal AK. (2012). Iontophoresis: drug delivery system by applying an electrical potential across the skin. Drug Deliv Lett 2:270–80
- Garg T, Goyal AK. (2014a). Biomaterial-based scaffolds – current status and future directions. Expert Opin Drug Deliv 11:767–89
- Garg T, Goyal AK. (2014b). Liposomes: targeted and controlled delivery system. Drug Deliv Lett 4:62–71
- Garg T, Goyal AK, Arora S, et al. (2012a). Development, optimization & evaluation of porous chitosan scaffold formulation of gliclazide for the treatment of type-2 diabetes mellitus. Drug Deliv Lett 2:251–61
- Garg T, Rath G, Goyal AK. (2014). Comprehensive review on additives of topical dosage forms for drug delivery. Drug Deliv
- Garg T, Singh O, Arora S, et al. (2012b). Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst 29:1–63
- Garg T, Singh S, Goyal AK. (2013). Stimuli-sensitive hydrogels: an excellent carrier for drug and cell delivery. Crit Rev Ther Drug Carrier Syst 30:369–409
- Geiger M, Li R, Friess W. (2003). Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev 55:1613–29
- Goll DE, Bray R, Hoekstra W. (1963). Age-associated changes in muscle composition. The isolation and properties of a collagenous residue from bovine musclea. J Food Sci 28:503–09
- Goyal G, Garg T, Malik B, et al. (2013). Development and characterization of niosomal gel for topical delivery of benzoyl peroxide. Drug Deliv
- Goyal G, Garg T, Rath G, et al. (2014). Current nanotechnological strategies for an effective delivery of drugs in treatment of periodontal disease. Crit Rev Ther Drug Carrier Syst 31:89–119
- Johal HS, Garg T, Rath G, et al. (2014). Advanced topical drug delivery system for the management of vaginal candidiasis. Drug Deliv 1–14
- Joshi D, Garg T, Goyal AK, et al. (2014). Advanced drug delivery approaches against periodontitis. Drug Deliv 1–15
- Kaur M, Garg T, Rath G, et al. (2014a). Current nanotechnological strategies for effective delivery of bioactive drug molecules in the treatment of tuberculosis. Crit Rev Ther Drug Carrier Syst 31:49–88
- Kaur M, Malik B, Garg T, et al. (2014b). Development and characterization of guar gum nanoparticles for oral immunization against tuberculosis. Drug Deliv
- Kaur P, Garg T, Rath G, et al. (2014c). Surfactant-based drug delivery systems for treating drug-resistant lung cancer. Drug Deliv 1–12
- Kaur R, Garg T, Das Gupta U, et al. (2014d). Preparation and characterization of spray-dried inhalable powders containing nanoaggregates for pulmonary delivery of anti-tubercular drugs. Artif Cells Nanomed Biotechnol 1–6
- Kaur R, Garg T, Malik B, et al. (2014e). Development and characterization of spray-dried porous nanoaggregates for pulmonary delivery of anti-tubercular drugs. Drug Deliv 1–6
- Loftsson T, Brewster ME. (1996). Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J Pharm Sci 85:1017–25
- Marwah H, Garg T, Goyal AK, et al. (2014). Permeation enhancer strategies in transdermal drug delivery. Drug Deliv 1–15
- Modgill V, Garg T, Goyal AK, et al. (2014). Permeability study of ciprofloxacin from ultra-thin nanofibrous film through various mucosal membranes. Artif Cells Nanomed Biotechnol 1–6
- Morie A, Garg T, Goyal AK, et al. (2014). Nanofibers as novel drug carrier – an overview. Artif Cells Nanomed Biotechnol 1–9
- Parks KL, Beckman EJ. (1996). Generation of microcellular polyurethane foams via polymerization in carbon dioxide. II: foam formation and characterization. Polym Eng Sci 36:2417–31
- Parnami N, Garg T, Rath G, et al. (2013). Development and characterization of nanocarriers for topical treatment of psoriasis by using combination therapy. Artif Cells Nanomed Biotechnol
- Patel R, Singh S, Singh S, et al. (2009). Development and characterization of curcumin loaded transfersome for transdermal delivery. J Pharm Sci Res 1:71–80
- Prasad M, Kalyanasundaram M. (1993). Ionotropic crosslinking of sodium carboxymethylcellulose and sodium carboxymethylcellulose-gelatin matrices and their erosion properties. J Appl Polym Sci 49:2075–9
- Ribatti D, Nico B, Vacca A, et al. (2006). The gelatin sponge-chorioallantoic membrane assay. Nat Protoc 1:85–91
- Rohilla R, Garg T, Goyal AK, et al. (2014). Herbal and polymeric approaches for liver-targeting drug delivery: novel strategies and their significance. Drug Deliv 1–17
- Sanwal R, Chaudhary AK. (2011). Wound healing and antimicrobial potential of Carissa spinarum Linn. in albino mice. J Ethnopharmacol 135:792–6
- Sharma A, Garg T, Aman A, et al. (2014a). Nanogel-an advanced drug delivery tool: current and future. Artif Cells Nanomed Biotechnol 1–13
- Sharma R, Singh H, Joshi M, et al. (2014b). Recent advances in polymeric electrospun nanofibers for drug delivery. Crit Rev Ther Drug Carrier Syst 31:187–217
- Singh B, Garg T, Goyal AK, et al. (2014a). Recent advancements in the cardiovascular drug carriers. Artif Cells Nanomed Biotechnol 1–10
- Singh H, Sharma R, Joshi M, et al. (2014b). Transmucosal delivery of docetaxel by mucoadhesive polymeric nanofibers. Artif Cells Nanomed Biotechnol
- Wang X, Yan Y, Zhang R. (2006). A comparison of chitosan and collagen sponges as hemostatic dressings. J Bioactive Compatible Polym 21:39–54
- Wu X-B, Luo X-Q, Gu S-Y, et al. (2012). The effects of Polygonum cuspidatum extract on wound healing in rats. J Ethnopharmacol 141:934–7
- Yadav VR, Prasad S, Kannappan R, et al. (2010). Cyclodextrin-complexed curcumin exhibits anti-inflammatory and antiproliferative activities superior to those of curcumin through higher cellular uptake. Biochem Pharmacol 80:1021–32