?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective: To explore the antitumor effects of low-intensity focused ultrasound (LIFU) mediated localized drug delivery of adriamycin-microbubble-PLGA nanoparticle complexes on rabbits VX2 liver tumor.
Methods: ADM-NMCs were prepared by covalent linking of ADM-PLGA nanoparticles (ADM-NPs) to the shell of the microbubbles. A fixed water bag filled with microbubbles was subjected to LIFU and non-focused ultrasound respectively, and the ultrasound images of which were recorded before and after ultrasonication. A total of 54 VX2 liver tumor-burdened rabbits were divided into six groups randomly, including control, ADM-NPs combined with LIFU, microbubbles combined with LIFU, ADM-NPs and microbubbles combined with LIFU, ADM-NMCs combined with LIFU and ADM-NMCs combined with Non-FUS. The tumor volume and volume inhibition rate (VIR) of tumor progression were calculated and compared. Apoptotic cells were labeled by terminal deoxyuridine nick end. Proliferating cell nuclear antigen was detected by immunohistochemistry. The median survival time of the animals were recorded and compared.
Results: ADM-NMCs were successfully prepared with an average diameter of 1721 nm. The highest VIR and apoptotic index (AI) were found in the group of ADM-NMCs combined with LIFU while the lowest proliferating index (PI) was simultaneously observed in this group. The median survival time of the rabbits in the ADM-NMCs combined with LIFU group was the longest (71days) among all groups.
Conclusions: ADM-NMCs combined with LIFU could inhibit the rabbits VX2 liver tumor progress by delaying the tumor proliferation and accelerating apoptosis, which presents a novel process for liver tumor targeting chemotherapy.
Introduction
Diagnostic and therapeutic agents such as computed tomography (CT), magnetic resonance imaging (MRI), and ultrasound agents have been of interest to many researchers. For many decades, gas-filled microbubbles have been used as contrast agents in ultrasound imaging (Berstad et al., Citation2009; Blomley et al., Citation2001; Frausher et al., Citation2001). During the last decade, ultrasound and microbubbles have enjoyed increasing attention as devices for drug and gene delivery (Ferrara et al., Citation2007; Lindner, Citation2004). Microbubbles can be used for non-invasive ultrasound imaging as well as ultrasound-induced drug delivery. Various kinds of antitumor drug-loaded microbubbles have been successfully developed for tumor chemotherapy (Ibsen et al., Citation2011; Tartis et al., Citation2006). However, microbubbles have limited drug loading capacity owing to their thin shell and gas core resulting in an unsatisfactory therapeutic effect (Hernot & Klibanov, Citation2008). In addition, the lipid shell of microbubbles restricts the type of drug that can be incorporated (hydrophobic drugs) in the shell (Unger et al., Citation1998). This allows for a scope to increase the drug loading capacity of microbubbles to be used as a potential for drug delivery.
Adriamycin, also known as doxorubicin (DOX), is extensively used as a chemotherapy drug for numerous cancers. However, clinical application of DOX is restricted as it results in severe side effects such as cardiac toxicity and nephrotoxicity (Patil et al., Citation2008). It has been reported that DOX encapsulated in poly lactic-co-glycolic acid (PLGA) polymeric carrier or liposomes could overcome these limitations, and shows a better efficacy than free DOX (Eisenbrey et al., Citation2010; Lin et al., Citation2005). Previous studies have demonstrated that acoustically-active DOX-liposome-loaded microbubbles produced a better antitumor effect than DOX-liposome alone in vitro (Lentacker et al., Citation2009). Other groups have also demonstrated that fluorescently labeled PLGA nanoparticles covalently linked to albumin-shelled microbubbles can improve the fluorescence signals in the skeletal muscle when subjected to ultrasound (Burke et al., Citation2011a). These studies suggest that linking drug-carriers (liposomes or PLGA nanoparticles) to microbubbles can improve the drug loading capacity of microbubbles and help in delivering a higher load to the tissues in response to ultrasound. PLGA has been a preferential polymeric candidate used to fabricate drug-loaded nanoparticles for tumor therapy (Makadia & Siegel, Citation2011). PLGA nanoparticles could successfully transport drug or gene into cells and release them over time (Chen et al., Citation2011). PLGA can be conjugated to multiple drugs for application in drug delivery due to desirable properties that can be tuned according to the drug conjugated (Grayson et al., Citation2005; Yang et al., Citation2005). PLGA is stable, biocompatible and biodegradable, and ensures a wide range of circulation time and safety in biomedical applications. Most importantly, drug delivery products based on PLGA polymers have been approved by the US FDA. Therefore, we can expect that PLGA nanoparticles (NPs) linked to the shell of lipid microbubbles may also increase the drug loading capacity of each bubble and widen the type of materials encapsulated in microbubbles. This study aims to combine the advantages of microbubbles and PLGA nanoparticles to prepare adriamycin-PLGA nanoparticle-microbubble complexes (ADM-NMCs) by linking ADM-loaded PLGA nanoparticles to the shell of lipid microbubbles. Indeed, previous “DOX-liposome loaded-microbubbles” described in literature were synthesized by coupling DOX-containing-liposomes to the lipid shell of microbubbles through avidin-biotin bridges (Lentacker et al., Citation2009). However, avidin, as a foreign protein, will cause adverse immune response in vivo (mammals) (Hernot & Klibanov, Citation2008). Therefore, it is desirable to seek a safe linking scheme. It has been reported that linking ligand to bubbles through covalent chemical bridge is a preferential linking scheme (Bloemen et al., Citation1995). In this study, we designed Adriamycin-PLGA nanoparticles-microbubble complexes via carbodiimide chemistry of Adriamycin-containing-PLGA nanoparticles to the lipid shell of microbubbles. These ADM-NMCs are especially attractive, because the NPs linked to microbubbles would be able to cross the endothelial gap of the tumor (300-800 nm) for cellular interaction after being subjected to activation by ultrasound. Accordingly, the NPs may accumulate in the tumor due to enhanced permeability and retention (EPR) effect of the tumor (Iyer et al., Citation2006; Torchilin, Citation2011). In addition, the large particle size of ADM-NMCs would restrict the drug extravasation to undesired tissues and therefore decrease the unwanted side effects. These features would ensure maximal delivery of the drug to the tumor tissue and thereby improve the therapeutic effect.
Ultrasound-targeted microbubble destruction (UTMD) is a potential delivery tool for targeted drug and gene therapy (Mayer et al., Citation2008; Tsutsui et al., Citation2004). The extracorporeal sonication is non-invasive and, can effectively induce drug release from the carrier. This process can also dramatically perturb cell membranes thereby improving drug absorption by tumor cells (Gao et al., Citation2008; Rapoport, Citation2004; Rapoport et al., Citation2006). However, the ultrasonic device usually applied is non-focused ultrasound (non-FUS). This continuous plane wave of non-focused ultrasound can destroy microbubbles in non-targeted regions leading to unwanted side effects and thereby negatively influence the reperfusion of microbubble into the targeted region, requiring further improvement of the targeting method. It has been reported that focused ultrasound in combination with microbubbles could selectively open the blood brain-barrier (Ting et al., Citation2012). A low-intensity focused ultrasound (LIFU)-triggered drug delivery system could be an alternative method for a localized drug delivery in ultrasound-treated regions.
Taking the advantages of microbubble-nanocarrier complexes and LIFU-triggered drug delivery system, we investigated the localized drug delivery of LIFU in combination with ADM-NMCs on VX2 rabbits liver tumor.
Materials and methods
Preparation of ADM-NMCs and characterization
Amino lipid microbubble fabrication and characterization
To prepare amino lipid microbubble (NH2-MB), 5 mg dipalmitoyl phosphatidylcholine (DPPC) (Avanti Polar lipids, AL, USA), 2 mg amino-polyethylene glycol (peg) - two stearoyl phosphatidyl ethanolamine (NH2-PEG-DSPE) (Avanti Polar lipids, Alabaster, AL), 450 μl of 10% polylysine (PLL) and 50 μl glycerin were dissolved in a 1.5 ml vial and incubated at 45 °C for 30 min. Subsequently, the solution was overlaid with perfluoropropane above the aqueous dispersion and mechanically shaken for 50 s using an agitator (YJT-1; Anhui, China). The resulting microbubbles were sealed under perfluoropropane atmosphere and stored at 4 °C in a refrigerator. Mean microbubble diameter was analyzed using a Laser Particle Size Analyzer System (Zeta SIZER 3000HS; Malvern, Chester, PA).
Preparation of ADM-NPs and characterization
ADM-PLGA (poly lactide-co-glycolide) nanoparticles were prepared using a double emulsion–solvent evaporation technique. 100 mg PLGA (lactide: glycolide=50:50, MW=12 000 Da, Daigang BIO Engineer Limited Co, Zhanjiang, China) was dissolved in 2 ml methylene chloride. Once PLGA was completely dissolved, 200 μl of adriamycin solution (50 mg/ml) was added and the solution was sonicated for 50 s at 104 W using an acoustic vibration meter Heat System (XL2020; Heat System, Connecticut newtown, USA). Thermo Syncronis C18 (Thermo Scientific, Shanghai, China). to achieve an emulsion. Subsequently, 10 ml poly (vinyl alcohol) (PVA,MW=25 000 Sigma-Aldrich, St. Louis, MO) solution (4% w/v) was added and dispersed using a homogenizer (FJ-200; Shanghai, China) for 5 min at 5000 rpm to form the second emulsion. Thereafter, 20 ml propyl alcohol solution (2% v/v) was added and the solution was vortexed using a rotatory evaporator (RE-52A; Shanghai, China) for 2 h at 25 °C to evaporate methylene chloride. After that, the solution was centrifuged at 5000 rpm for 5 min and this process was repeated three times. The supernatant was discarded and the precipitate was collected and washed using deionized water. The final precipitate was stored at 4 °C for further use. The size distribution of the ADM-NPs was analyzed using a Laser Particle Size Analyzer System (Zeta SIZER 3000HS; Malvern, USA). To determine Adriamycin encapsulation efficiency and loading capacity, the ADM-NPs were analyzed using high-performance liquid chromatography system (HPLC, Shimadzu, Japan). The mobile phase contained 50:50 mixture of methanol and sodium acetate buffer (pH=7.3). A Thermo Syncronis C18 (4.6 × 150 mm, 5um) chromatographic column was used with a flow rate of 1.0 ml/min, and column temperature of 25 °C. Detection was carried out using a UV detector at 254 nm. The adriamycin content was determined by calculating the area under the characteristic peak at the retention time of 8.75 min. Briefly, ADM-NPs were centrifuged for 10 min at 12 000 rpm and the adriamycin content of the supernatant after filtrate (Wres) was measured using HPLC. Drug loading capacity (WPLGA) was defined as the difference between the total Adriamycin (Wtot) and Wres. Drug loading and encapsulation efficiency were calculated according to the following formula:
where WT was the total weight of ADM-NPs.
Synthesis of ADM-NMCs and characterization
ADM-NMCs were synthesized by covalently linking the microbubbles and nanoparticles. Briefly, ADM-NPs were activated by mixing ADM-NPs with EDC (Sigma, USA)/sulfo-NHS (Sigma, USA) at a molar ratio of 1:5:15 in 2 ml MES buffer, pH 5.4. The solution was allowed to react for 10 min before NHS was added at room temperature. To purify ADM-NPs, the solution was centrifuged three times for 5 min at 5000 rpm and then the reaction was allowed for 1 h at room temperature after the addition of NHS. Meanwhile, NH2-MBs were prepared and washed three times with MES buffer, pH 8.0 to remove excess lipids. Then, excess EDC and NHS were removed from the solution by three consecutive centrifugations at 5000 rpm for 5 min each. The last, two centrifugations were carried out in MES buffer, pH 8.0. Subsequently, activated ADM-NPs were added drop-wise into washed NH2-MBs and the mixture was incubated for 2 h at room temperature with continuous rocking. The reaction was carried out for 24 h at 4 °C. To purify the ADM-NMCs, the supernatant was washed off using saline followed three consecutive centrifugations at 200 g. The morphology of ADM-NMC was examined using optical microscopy. The mean diameter of ADM-NMCs was measured using a laser particle size analyzer system. The ADM amount per MB was determined as follows: ADM-NMCs were redissolved in methylene dichloride, and ADM amount (WADM) in the filtrate was determined by HPLC. Microbubble concentration was determined by a blood cell count plate. The ADM amount per MB was calculated according to the following equation:
In order to assess the release behavior of ADM-NMCs exposed to LIFU, the release of ADM in vitro was determined. Acoustic frequency of 1MHz, acoustical intensity at 1.2 W/cm2, a 50% duty cycle and a duration time of 60 s were used as LIFU parameters for sonication. 1 ml ADM-NMCs were diluted into 4 ml saline and transferred into dialysis bags (MWCO: 10 000 Da), which were then placed in a container of 50 mL of saline with stirring at 100 rpm at 37 °C. The ADM-NMCs were then treated with LIFU radiation (LM.SC051 ACA, Institute of Ultrasound Imaging of Chongqing Medical Sciences, Chongqing, China). 1 mL of medium was removed from the sample at 2, 4, 8, 12, 24, 36, 48, 72 h, respectively and then 1 mL of saline was added into the container to keep a constant volume. The concentration of ADM of each sample was analyzed by HPLC. The accumulative ratios of the released ADM were calculated as a function of time. The controls were performed according to the above steps, but without LIFU radiation.
Animal studies
All animal studies were performed in accordance with the guidelines approved by the animal care committee of Chongqing medical University. Rabbits weighing 2.0–2.5 kg were obtained from the Animal Center of Chongqing Medical University. Rabbits carrying VX2 liver tumors were donated by the Ultrasound Engineering Institute of Chongqing Medical University.
Preparation of VX2 tumor tissue mass and establishment of VX2 liver tumor model
Tumor-burdened rabbits were anesthetized by injecting 3% (w/v) sodium pentobarbital (1 ml/kg) intraperitoneally. Hair on the belly was removed using 3% (w/v) sodium sulfide. Subsequently, rabbits were placed on the operating table a supine position, VX2 tumor tissue was harvested from the liver and washed with normal saline. 54 recipient rabbits were prepared in the same way and then a cube of active VX2 tumor tissue (approximately 1 × 1 × 1 mm) was implanted about 1–2 mm depth in the left lobe of liver of the recipients. The incisions were sutured layer by layer without any significant bleeding and penicillin was injected for 3 days in order to prevent infection. All procedures were carried out under aseptic conditions.
Ultrasound imaging of ADM-NMCs in vivo
Two weeks after implantation, the tumor was examined using an ultrasonic diagnostic system (9 L, logIQ9, GE, USA, frequency = 10MHz) under fundamental wave conditions with B-mode to evaluate the morphology and echo intensity characteristics. The longest and shortest axes of each tumor were determined and stored for later use. 1 ml ADM-NMCs solution was injected via ear vein to evaluate the enhanced ultrasound imaging contrast. Pure lipid microbubbles were used as control. The enhanced ultrasound signal was obtained using a second harmonics mode and monitored for 30 min.
Local targeting activity of LIFU in vitro and in vivo
Latex water bags (home-made, 100 × 100 × 60 mm) were filled with lipid microbubbles (prepared by mechanical vibration, concentration: 1.5 × 108/ml) were used to evaluate the targeting ability of LIFU. LIFU transducer (LM.SC051 ACA, frequency=1MHz, acoustic intensity=1.2 W/cm2; duty cycle=50%, duration time=10 s) was placed perpendicular to the surface of the water bag. A diagnostic ultrasonic probe (5C; IU22; Philips Medical Systems, Bothell, WA, frequency = 3.5 MHz) was placed parallel and perpendicular to LIFU beams, respectively, to determine the lateral and longitudinal destruction range of LIFU targeted destruction microbubbles. A similar procedure was used for non-focused ultrasound (UTG 1025, Institute of Ultrasound Imaging of Chongqing Medical Sciences, Chongqing, China) using the same parameters (frequency=1 MHz, acoustic intensity=1.2 W/cm2; duty cycle=50%, duration time=10 s) as the LIFU.
A total of 20 healthy rabbits were used to confirm the targeting activity of LIFU. The gall bladder was primarily observed using B-mode; LIFU beams (acoustic intensity: 0.3 w/cm2, 0.6 w/cm2, 0.9 w/cm2, 1.2 w/cm2) were focused on the liver adjacent to (10 mm) the fundus of the gallbladder 60 s after the injection of ADM-NMCs into rabbit ear vein. A water sac filled with degassed water was used to ensure that the focal point of LIFU (the focal distance was 15 mm) was at the same depth. Liver tissues at different distances (every 10 mm) from the focal point were harvested 1 h later. The tissues were weighted and homogenized with saline and adjusted to a pH 7.3 with acetic acid. The homogenized tissues were extracted with a mixture of chloroform and methanol (4:1 v/v). The tissue suspensions were centrifuged at 12 000 rpm for 5 min, then 40 μl supernatant was injected into the HPLC system to measure the ADM level.
Therapeutic schedule with LIFU and treatment evaluation
A total of fifty-four rabbits receiving tumors were randomly divided into six groups (n = 9): (1) control group; (2) ADM-nanoparticles combined with LIFU (ADM-NPs + LIFU) group; (3)microbubble combined with LIFU(MB + LIFU) group; (4) the mixture of ADM-nanoparticles and microbubble combined with LIFU(ADM-NPs+MB + LIFU) group; (5) ADM-NMCs combined with LIFU (ADM-NMCs + LIFU) group; (6) ADM-NMCs combined with Non-FUS (ADM-NMCs + Non-FUS) group. In the control group, 4 ml saline was injected through marginal ear veins; in the ADM-NPs + LIFU group,0.8 ml of ADM-NPs was diluted into 4 ml and injected, followed by exposure to LIFU; in the MB+LIFU group,1.2 ml of pure microbubbles were diluted to a final volume of 4 ml and injected, followed by exposure to LIFU; in the ADM-NPs + MB + LIFU group, 1.2 ml of pure microbubbles were mixed with 0.8 ml of ADM-NPs and the final volume was diluted into 4 ml and injected, followed by LIFU irradiation; in the ADM-NMCs + LIFU group and the ADM-NMCs + Non-FUS group, 1.2 ml ADM-NMCs diluted into 4 ml and injected, and then was exposed to LIFU and Non-FUS respectively. LIFU parameters were same in the groups (2), (3), (4), (5). The parameters of Non-FUS were same to LIFU. A LIFU treatment system (LM.SC051 ACA, focal distance=15 mm, Institute of Ultrasound Imaging of Chongqing Medical Sciences, Chongqing, China) and non-FUS system were used to aid the microbubble destruction and release the ADM in tissue. Based on the previous determined maximum ADM release in vivo and in vitro, acoustic frequency of 1MHz, acoustical intensity at 1.2 w/cm2, a 50% duty cycle and a duration time of 60 s were used as parameters for LIFU treatment.
It has been shown that the administration of several small doses of a drug can generate an equal or even enhanced effect when compared to a single bolus administration at the maximum tolerated dose (Bouffet et al., Citation2010). Therefore, to evaluate the anti-tumor effect in each group, treatment was carried out on days 15, 17, and 19 after tumor implantation. Tumor volume was monitored by diagnostic ultrasound system (9L, GE, logIQ9, USA, frequency=10 MHz) on days 15 and 26. Tumor volume was calculated in accordance to the following formula: V = (a × b2)/2.where: ‘a’ is the longest axis, and ‘b’ is the shortest axis of the tumor. The volume inhibition rate (VIR) was calculated in accordance to the following formula:
VIR = [(average tumor volume of control group − average tumor volume of experimental group)/average tumor volume of control group] ×100%.
Three rabbits of each group were sacrificed and tumor masses were harvested on day 21. The tumor tissues were washed in saline and placed in polyoxymethylene (4% w/v) for proliferating cell nuclear antigen (PCNA) detection by immunohistochemical examination and apoptosis by terminal deoxyuridine nick end labeling. The remaining tumor-bearing rabbits from each group were monitored until their cachexia death and the median survival time was recorded to compare the antitumor effects. The survival rate of each group was analyzed with the Kaplan–Meier curve.
Cytotoxicity test of ADM-NMCS in vivo
To determine the cytotoxicity of ADM-NMCs to heart, 15 healthy rabbits were randomly divided into three groups with a group size of n = 5: (1) saline- control group; (2) ADM group (ADM concentration 0.5 mg/ml) and (3) ADM-NMCs group (ADM concentration 0.5 mg/ml). Serum was collected from all animals at 12 and 24 h after the corresponding treatment. CK levels were measured to evaluate acute injury to heart.
Statistics
All data is presented as the mean ± standard error and was analyzed by SPSS 18.0 analysis software (Chicago, IL). One-way ANOVA was used to evaluate differences between multiple groups. p < 0.05 was considered significant.
Results
Characterization of ADM-NMCs
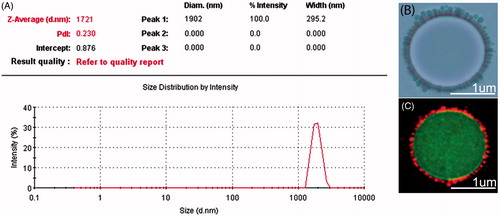
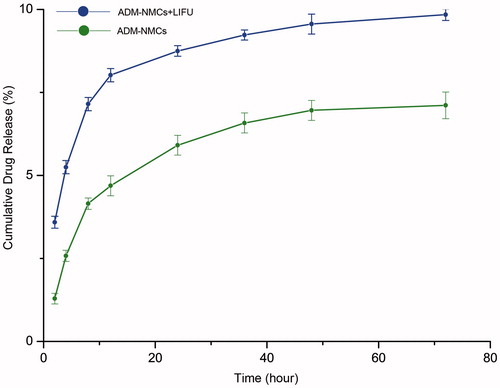
ADM-NMCs were synthesized using a double emulsion method including O/W encapsulation of ADM and subsequent use of carbodiimide chemistry linker to covalently bind ADM-NPs to the shell of the microbubbles. There are abundant amino and carboxyl-groups on the surface of NH2-MB and ADM-NPs, respectively. Carboxyl-groups on the surface of the ADM-NPs are subjected to a series of activation by EDC/NHS to enable the reaction with the amino groups of the NH2-MB. Optical microscopy () was performed to visualize the morphology and size of ADM-NMCs. Fluorescence microscopy () were used to observe the DiI-labeled-NPs loaded on FITC-labeled-microbubble, NPs coated the surface of the microbubbles in a structure reminiscent of a garland. The mean diameter of the ADM-NPs was 249.8 ± 52.94 nm. ADM encapsulation efficiency was 86.1% and loading efficiency was 7.8%. The diameter of NH2-MB was determined as 1032 ± 12.61 nm. Covalently linking ADM-NPs to their surface to form ADM-NMCs increased their size to 1721 ± 295.2 nm (), which was consistent with the size of pure (i.e. ADM-free) NMCs. ADM-NMCs contained an average of 0.28 ng ADM of per MB as determined by HPLC. The release profiles of ADM from ADM-NMCs with/without LIFU were measured to evaluate the effects of ADM-NMCs release properties exposed to LIFU. The release profile of ADM-NMCs with/without LIFU was represented by the percentage of released ADM as a function of time. As shown in , the release rate of ADM with LIFU radiation was faster than that without LIFU with approximately 90% of the ADM was released after 2 days. However, for the ADM-NMCs without LIFU radiation less than 60% of the ADM was released. The average T1/2 (50% of the ADM to be released) values for ADM with/without LIFU were about 4 and 15 h, respectively.
Ultrasound imaging of ADM-NMCs in vivo
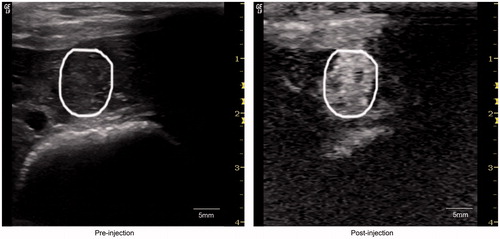
To study whether ADM-NMCs could enhance ultrasound contrast intensity, ultrasonography in vivo was performed. Both ADM-NMCs and pure lipid microbubbles produced enhanced ultrasound imaging on VX2 liver tumor (). The ultrasound signal intensity in the tumors reached a peak 20 s after ADM-NCMs administration. It is noteworthy that the peak intensity time, peak height and clearance time of ADM-NCMs was similar to that of pure lipid microbubbles (data not shown). This demonstrates that ADM-NMCs retained the favorable acoustic characteristics of microbubbles.
Local targeting activity of LIFU and optimization of therapeutic parameters in vitro and in vivo
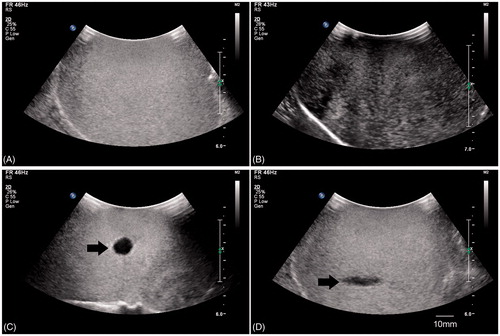
To test whether LIFU is superior in its ability to locally disintegrate the microbubbles when compared to non-focused ultrasound under the same ultrasonic conditions in vitro, a water bag filled with microbubbles was used. shows two-dimensional ultrasound imaging results prior to the destruction of microbubbles. These microbubbles presented with high ultrasound signal intensity prior to destruction. Decreased echo intensity signifies microbubbles destruction in response to the ultrasonic pulse. shows a decreased ultrasound signal upon exposure to non-focused ultrasound triggering. We can observe a wide range of microbubble destruction in response to non-focused ultrasound. However, LIFU resulted in a circular region of decreased signal intensity perpendicular to the direction of propagation of the ultrasound waves (). It also resulted in a fusiform region of decreased signal intensity along direction of propagation of ultrasound waves (). These findings support the hypothesis that LIFU created a highly focused destruction area in contrast to non-focused ultrasound.
Figure 4. (A) Ultrasound image (5C; IU22; Philips Medical Systems, Bothell, WA) of microbubbles before rupture; (B) ultrasound image of microbubbles after rupture with non-focused ultrasound; (C) ultrasound image of broken microbubbles perpendicular to ultrasound beams with LIFU; (D) ultrasound image of broken microbubbles along the ultrasound beams with LIFU.
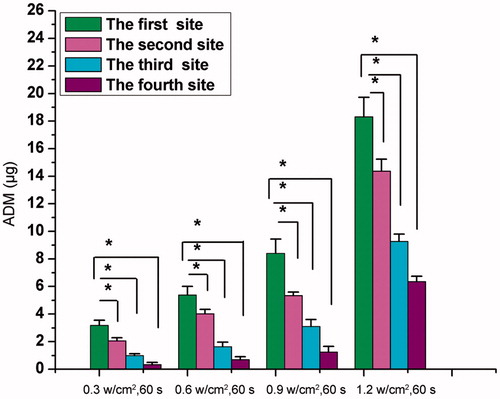
We then tested the targeted release of ADM when LIFU was combined with ADM-NCMs. indicates that the ADM levels in the target area were significantly higher than the surrounding areas and the ADM levels increased with the increased acoustic power (p < 0.05).
Antitumor activity in vivo
In order to assess the antitumor effect of LIFU in combination with ADM-NMCs for the treatment of liver cancer, we primarily optimized the ultrasound parameters according to the maximal drug release and 1.2 w/cm2 acoustic power, 50% duty cycle and 60 s duration time were set as the final therapy parameters.
To demonstrate that LIFU combined with ADM-NMCs could inhibit tumor growth, the tumor growth was evaluated by the expression of proliferating cell nuclear antigen (PCNA) and apoptotic cells, the VIR. The PCNA expression appeared as brown granules in nucleus, and the positive apoptosis was indicated by brown staining in nucleus. The results showed that the brown granules and apoptotic cells were detectable in all groups ( and ). shows that the PCNA expression and proliferation index (PI, denoted by PCNA) in LIFU + ADM-NMCs group was obviously lower than that in other groups (p < 0.05). The apoptotic cells and apoptosis index (AI, denoted by percentage of apoptotic cells) in LIFU + ADM-NMCS group was the highest amongst the groups (p < 0.05) (). The results also indicate that the volume inhibition rate (VIR) of the LIFU + ADM-NMCs group was 56.73% (), which is significantly higher than all other groups (p < 0.01). LIFU combined with ADM-NMCs treatment increased the median survival from 31 days in the control group to 71 days (). As shown in , survival rate decreased in all groups over time, however, the survival rate of LIFU + ADM-NMCs group was obviously higher than that of other groups (p < 0.01).
Figure 6. The left panel shows the expression of PCNA in tumor using immunohistochemical staining after each treatment. Less PCNA-positive cells were seen in group E when compared to the other groups (*p < 0.05). (A) Control group; (B) ADM-NPs + LIFU; (C) MBs + LIFU; (D) ADM-NPs + MBs + LIFU; (E) ADM-NMCs + LIFU; (F) ADM-NMCs + non-FUS. The right panel shows the proliferation index (PI) of tumor tissue in each group (*p < 0.05).
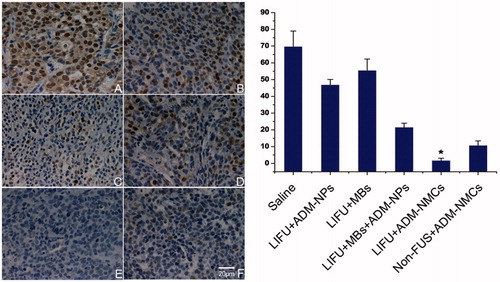
Figure 7. The left panel indicates the expression of apoptotic cells in tumor using immunohistochemical staining after each treatment. More apoptotic cells were seen in group E in comparison to the other groups (*p < 0.05). (A) Control group; (B) ADM-NPs + LIFU; (C) MBs + LIFU; (D) ADM-NPs + MBs + LIFU; (E) ADM-NMCs + LIFU; (f) ADM-NMCs + non-FUS. The right is the apoptotic index (AI) of tumor tissue in each group (*p < 0.05).
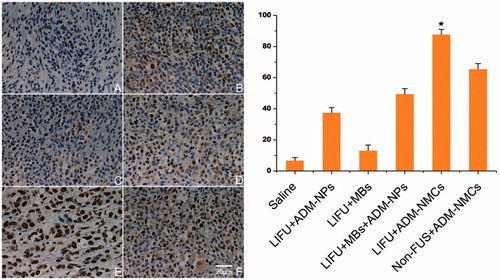
Figure 8. Kaplan–Meier curve of each group. The survival rate of LIFU + ADM-NMCs group was obviously higher than that of other groups (*p < 0.01).
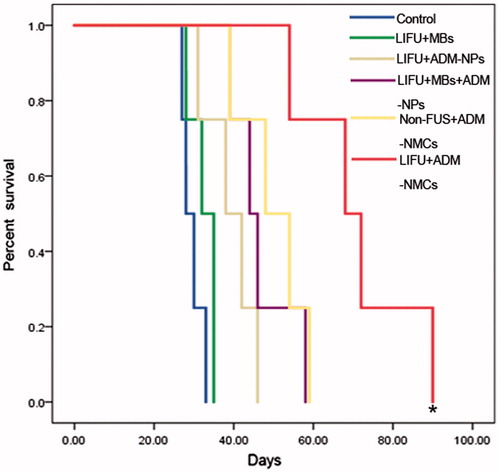
Table 1. The tumor volume and volume inhibition rate (VIR) of each group on days 26 (the second day after last treatment).
Table 2. The median survival of each group.
Heart function evaluation
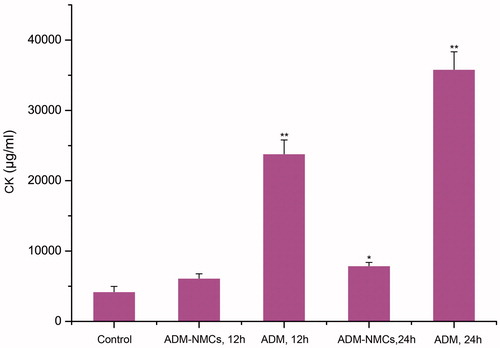
As the most harmful effect of ADM was cardiotoxity, we determined CK levels to evaluate impact of the treatments on heart function. showed that injection of ADM significantly enhanced the CK level at 12 h (p < 0.01). Both ADM (p < 0.05)and ADM-NMCs (p < 0.01) increased CK level at 24 h compared to control group; however, we noted that CK level in the ADM-NMCs group was significantly lower than that of the ADM group (P < 0.01). It implied that ADM encapsulated in NPs could protect the heart from the damage to some degree.
Figure 9. CK level of each group. Injection of ADM significantly enhanced the CK level at 12 h (**p < 0.01). Both ADM (*p < 0.05)and ADM-NMCs (**p < 0.01) increased CK level at 24 h compared to control group, however, CK level in the ADM-NMCs group was significantly lower than that of the ADM group (**p < 0.01).
Discussion
Adriamycin is an effective broad-spectrum chemotherapy drug and plays an important role in tumor chemotherapy. The mechanisms of Adriamycin action against tumors include DNA intercalation, lipid peroxidation and inhibition of topoisomerase-II. These mechanisms of action of Adriamycin are non-specific in nature and can also kill normal cells. Therefore, Adriamycin was encapsulated into PLGA nanoparticles owing to its side effects (Lin et al., Citation2005). As demonstrated in a previous study, DOX-loaded microbubbles were at least two times more effective than DOX liposomes when exposed to ultrasound (Lentacker et al., Citation2009). In order to further study the antitumor effects of these complexes in vivo, we prepared a novel drug carrier of ADM-NMCs, which combines the advantages of microbubbles and PLGA. In this study, ADM-NMCs were prepared and characterized successfully. The properties of derived complexes such as morphology, size distribution, ADM amount carried by per MB, the ultrasound imaging ability were determined. ADM-NMCs could enhance the ultrasound contrast of VX2 liver tumor, which indicated that ADM-NMCs could be used for visual monitoring of the tumor sites. The synthesized ADM-NMCs were stable when stored at 4 °C for two weeks. The results showed that the creatine kinase (CK) levels after ADM-NMCs injection were significantly lower than that of the ADM (p < 0.01), implying that ADM-NMCs were safer than free ADM on heart function in vivo. Ultrasound targeted microbubble destruction (UTMD) is an attractive technology employed for drug and gene delivery. Drug loaded microbubbles could be destroyed after intravenous injection upon exposure to a predefined ultrasound intensity which released the drug into a desired region. However, an ideal effect of chemotherapy is to maximize the cytotoxicity to tumor tissue while minimizing the undesired side effects to the surrounding normal tissue. Previous studies have shown that focused ultrasound combined with drug-loaded microbubbles could selectively open the blood–brain barrier (BBB) and deliver the antitumor drug to targeted site (Ting et al., Citation2012). In this study, we choose the LIFU for the treatment, in order to minimize the unwanted side effects to the normal tissue and enhance therapeutic effect. To further evaluate the hypothesis, we compared the targeting specificity of microbubbles upon exposure to LIFU and non-focused ultrasound in vitro. The results showed that non-focused ultrasound induced extensive microbubble destruction, while LIFU induced a small-scale microbubble destruction. Extensive microbubble destruction not only causes tissue damage but also deposits the antitumor drug in the normal tissue. Therefore, the LIFU technique is potentially ideal in mediating chemotherapy effect in a specified tissue area.
In the second section of this study, we evaluate the antitumor effect in vivo, and optimized the parameters of LIFU in vitro and in vivo. Based on the maximum ADM release from ADM-NMCs, 1 MHz frequency, 1.2 w/cm2 acoustic power, 50% duty cycle and 60 s duration time were set as the final therapy parameters. 50% duty cycle implies that the irradiation time was equal to the time interval, which ensured reperfusion of ADM-NMCs to achieve sufficient concentration at the targeted site. The synthesized ADM-NMCs were stable for up to two weeks in the refrigerator. Methods to improve the stability would further contribute to the use of this technique for chemotherapeutic purposes. We then established a VX2 liver tumor model to evaluate the chemotherapeutic effect of LIFU combined with ADM-NMCs. Just as we expected, the combination of LIFU led to a stronger tumor volume inhibition. We also found the antitumor activity for the other groups to a certain extent. In our study, the combination of LIFU and pure lipid microbubbles induced an obvious tumor inhibition effect, we attribute this effect to cellular and capillary mechanisms resulting from cavitation of ultrasound in combination with MBs, which resulted in the tumor inhibition. This process of inhibition tumor cells has been previously described (Burke et al., Citation2011b). LIFU combined with ADM-NPs produced an inferior antitumor effect probably because of the extravasation of ADM-NPs from the vasculature before arriving at the tumor site. LIFU combined with the mixture of microbubbles and ADM-NPs produced a lower antitumor activity than the combination of LIFU and ADM-NMCs. This decreased activity of LIFU + MB + ADM - NPs might be attributed to the dilution of ADM-NPs while in the blood stream, while the stable covalent bond of ADM-NMCs could tolerate the impact of bloodstream and arrived at the targeted site. In non-FUS + ADM-NMCs group, ADM-NMCs would remain intact when arrived at desired region as in LIFU + ADM-NMCs group. However, only after Non-FUS-mediated release of ADM-NPs, microbubbles destruction could occur especially in non-targeted region, which resulted in ADM release and physical injury to non-targeted region. Therefore, in contrast to LIFU + ADM-NMCs group, Non-FUS + ADM-NMCs group showed lower antitumor effect. We can infer that the tumor inhibition effect of LIFU/ADM-NMCs is a result of interaction of many factors, including the cavitation effect, sonoporation, membrane permeability alteration (Domenici et al., Citation2013), local microbubbles destruction effect of LIFU, antitumor effect of ADM, EPR effect, and the covalent bond against blood stream impact. The mechanism of cavitation effect resulting from ultrasound combined with microbubbles is different from the mechanism of thermal treatment resulting from a combination of ultrasound with temperature-sensitive liposomes (Kheirolomoom et al., Citation2013). Compared to thermal treatment-mediated drug release, which requires a longer heating time (20 min), the cavitation effect mediated drug release may be a safer method of drug-delivery as longer heating times can result in thermal damage to the normal tissues.
Conclusions
In this study, we successfully produced novel drug-loaded microbubbles by covalently linking NPs to the microbubble shell. As an ultrasound contrast agent, ADM-NMCs significantly enhanced the ultrasound signal of VX2 liver tumor, and could be employed as a drug delivery carrier to delivery antitumor drug to desired tumor tissue when irradiated by LIFU. It was demonstrated that LIFU combined with ADM-NMCs could inhibit the tumor growth more markedly in comparison to other groups. LIFU was employed as an ultrasound irradiation system to mediate microbubble destruction. It was found that the ability of LIFU to drive the targeted destruction of microbubbles was superior in comparison to the non-focused ultrasound. The results in our present study indicate that ADM-NMCs combined with LIFU could be used for future clinical study on tumor chemotherapy.
Declaration of interest
The authors report no conflicts of interest. This work was supported by the National Nature Science of China (Grant No.30770565, 81227801, 81161120548, 81130025, 81371579) the Chongqing Science Research Project (Grant No.CSTC, 2009AB5216), and the found of Chongqing university scientific innovation team 2013(KJTD201303).
Acknowledgements
The authors are grateful to Prof. Yuanyi Zheng for assistance with English correction.
References
- Berstad AE, Brabrand K, Foss A. (2009). Clinical utility of microbubble contrast-enhanced ultrasound in the diagnosis of hepatic artery occlusion after liver transplantation. Transplant International 22:954–60
- Bloemen PGM, Henricks PAJ, Van Bloois L, et al. (1995). Adhesion molecules: a new target for immunoliposome-mediated drug delivery. FEBS Lett 357:140–4
- Blomley MJ, Cooke JC, Unger EC, et al. (2001). Science, medicine, and the future: Microbubble contrast agents: a new era in ultrasound. BMJ 322:1222
- Bouffet E, Tabori U, Huang A, Bartels U. (2010). Possibilities of new therapeutic strategies in brain tumors. Cancer Treat Rev 36:335–41
- Burke CW, Hsiang YHJ, Alexander E, et al. (2011a). Covalently linking poly (lactic-co-glycolic acid) nanoparticles to microbubbles before intravenous injection improves their ultrasound-targeted delivery to skeletal muscle. Small 7:1227–35
- Burke CW, Klibanov AL, Sheehan JP, et al. (2011b). Inhibition of glioma growth by microbubble activation in a subcutaneous model using low duty cycle ultrasound without significant heating: laboratory investigation. J Neurosurg 114:1654–61
- Chen YS, Alany RG, Young SA, et al. (2011). In vitro release characteristics and cellular uptake of poly (D,L-lactic-co-glycolic acid) nanoparticles for topical delivery of antisense oligodeoxynucleotides. Drug Deliv 18:493–501
- Domenici F, Giliberti C, Bedini A, et al. (2013). Ultrasound well below the intensity threshold of cavitation can promote efficient uptake of small drug model molecules in fibroblast cells. Drug Deliv 20:285–95
- Eisenbrey JR, Burstein OM, Kambhampati R, et al. (2010). Development and optimization of a doxorubicin loaded poly (lactic acid) contrast agent for ultrasound directed drug delivery. J Control Release 143:38–44
- Ferrara K, Pollard R, Borden M. (2007). Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Annu Rev Biomed Eng 9:415–47
- Frausher F, Halpern EJ, Horninger W, Bartsch G. (2001). Detection of prostate cancer with a microbubble ultrasound contrast agent. Lancet 357:1849–50
- Gao Z, Kennedy AM, Christensen DA, Rapoport NY. (2008). Drug-loaded nano/microbubbles for combining ultrasonography and targeted chemotherapy. Ultrasonics 48:260–70
- Grayson ACR, Cima MJ, Langer R. (2005). Size and temperature effects on poly (lactic-co-glycolic acid) degradation and microreservoir device performance. Biomaterials 26:2137–45
- Hernot S, Klibanov AL. (2008). Microbubbles in ultrasound-triggered drug and gene delivery. Adv Drug Deliv Rev 60:1153–66
- Ibsen S, Benchimol M, Simberg D, et al. (2011). A novel nested liposome drug delivery vehicle capable of ultrasound triggered release of its payload. J Control Release 155:358–66
- Iyer AK, Khaled G, Fang J, Maeda H. (2006). Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today 11:812–18
- Kheirolomoom A, Lai C Y, Tam S M, et al. (2013). Complete regression of local cancer using temperature-sensitive liposomes combined with ultrasound-mediated hyperthermia. J Control Release 172:266–73
- Lentacker I, Geers B, Demeester J, et al. (2009). Design and evaluation of doxorubicin-containing microbubbles for ultrasound-triggered doxorubicin delivery: cytotoxicity and mechanisms involved. Mol Ther 18:101–8
- Lin R, Ng SL, Wang CH. (2005). In vitro study of anticancer drug doxorubicin in PLGA-based microparticles. Biomaterials 26:4476–85
- Lindner JR. Microbubbles in medical imaging: current applications and future directions. (2004). Nat Rev Drug Discov 3:527–33
- Makadia HK, Siegel SJ. (2011). Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 3:1377–97
- Mayer CR, Geis NA, Katus HA, Bekeredjian R. (2008). Ultrasound targeted microbubble destruction for drug and gene delivery. Expert Opin Drug Deliv 5:1121–38
- Patil RR, Guhagarkar SA, Devarajan PV. (2008). Engineered nanocarriers of doxorubicin: a current update. Crit Rev Ther Drug Carrier Syst 25:1–61
- Rapoport N. (2004). Combined cancer therapy by micellar-encapsulated drug and ultrasound. Int J Pharm 277:155–62
- Rapoport NY, Gao Z, Kamaev P, Christensen DA. (2006). Ultrasound-enhanced localized chemotherapy of drug-sensitive and multidrug resistant tumors [C]//Therapeutic Ultrasound: 5th International Symposium on Therapeutic Ultrasound. 829:481–5
- Tartis M S, McCallan J, Lum A F H, et al. (2006). Therapeutic effects of paclitaxel-containing ultrasound contrast agents. Ultrasound Med Biol 32:1771–80
- Ting CY, Fan CH, Liu HL, et al. (2012). Concurrent blood–brain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment. Biomaterials 33:704–12
- Torchilin V. (2011). Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Deliv Rev 63:131–5
- Tsutsui JM, Xie F, Porter RT. (2004). The use of microbubbles to target drug delivery. Cardiovasc Ultrasound 2:23–7
- Unger EC, McCreery TP, Sweitzer RH, et al. (1998). Acoustically active lipospheres containing paclitaxel: a new therapeutic ultrasound contrast agent. Invest Radiol 33:886–92
- Yang R, Chen T, Chen H, et al. (2005). Microfabrication of biodegradable (PLGA) honeycomb-structures and potential applications in implantable drug delivery. Sensors Actuators B 106:506–11