Abstract
Most of the new drugs, biological therapeutics (proteins/peptides) and vaccines have poor performance after oral administration due to poor solubility or degradation in the gastrointestinal tract (GIT). Though, vesicular carriers exemplified by liposomes or niosomes can protect the entrapped agent to a certain extent from degradation. Nevertheless, the harsh GIT environment exemplified by low pH, presence of bile salts and enzymes limits their capabilities by destabilizing them. In response to that, more resistant bile salts-containing vesicles (BS-vesicles) were developed by inclusion of bile salts into lipid bilayers constructs. The effectiveness of orally administrated BS-vesicles in improving the performance of vesicles has been demonstrated in researches. Yet, these attempts did not gain considerable attention. This is the first review that provides a comprehensive overview of utilizing BS-vesicles as a promising pharmaceutical carrier with a special focus on their successful applications in oral delivery of therapeutic macromolecules and vaccines. Insights on the possible mechanisms by which BS-vesicles improve the oral bioavailability of the encapsulated drug or immunological response of entrapped vaccine are explained. In addition, methods adopted to prepare and characterize BS-vesicles are described. Finally, the gap in the scientific researches tackling BS-vesicles that needs to be addressed is highlighted.
Introduction
To improve drug–receptor interactions, many new designed potent drug molecules based on contemporary drug discovery programs possess high degree of lipophilicity that unfortunately causes poor solubility in GI fluids accompanied by low and variable bioavailability (Boyd, Citation2008). Furthermore, the progress in biotechnological engineering has led to the evolvement of new biological-based therapeutics and vaccines that are usually characterized by limited ability to cross cell membranes accompanied by poor in vivo performance due to degradation in the gastrointestinal tract (GIT) (Boyd, Citation2008; Li et al., Citation2012). Different strategies that include encapsulating therapeutic agents or vaccines into nano-colloidal delivery systems (polymeric or lipid-based nanoparticles, nanoemulsions, nanostructured lipid carriers, mixed micelles and vesicular structures) have been adopted by researchers to cope with impediments associated with delivering these challenging agents to specific target tissues (Malik et al., Citation2007; Tiwari et al., Citation2010; Li et al., Citation2012; Gupta et al., Citation2013; Demetzos & Pippa, Citation2014). Among different classes of colloidal systems, vesicular carriers have gained particular attention in delivering poorly soluble drugs and proteins/peptides (Torchilin, Citation2005). Vesicular carriers comprise of unilamellar or multilamellar spherical structures formed of lipid molecules gathered into bilayers orientation and capable of encapsulating drug molecules (Sinico & Fadda, 2009). Conventional vesicular systems exemplified by liposomes and niosomes have demonstrated an appealing potential in augmenting the oral bioavailability of therapeutic agents and immunogenic response of vaccines (Torchilin, Citation2005; Bramwell & Perrie, Citation2006). Nevertheless, the efficacy of conventional vesicles has been compromised by their instability in the GIT, which necessitated modification in their bilayers constructs to improve their in vivo resistance (Andrieux et al., Citation2009). Different research efforts conducted over the past decade has demonstrated the effectiveness of including bile salts into vesicular systems bilayers in improving their in vivo resistance and performance after oral administration (Chen et al., Citation2009; Niu et al., Citation2014). However, these attempts have not gained considerable attention in relation to their demonstrated positive outcomes. Accordingly, the aim of this review is to provide a comprehensive overview of the fundamentals of bile salts-containing vesicles (BS-vesicles) with a focus on their successful applications in oral delivery of poorly water soluble drugs, therapeutic proteins/peptides and vaccines. Some insights on the possible mechanisms by which BS-vesicles improve the oral bioavailability of the entrapped drug or in case of vaccine delivery, protect the antigen from the hostile GIT environment are vividly explained and diagrammatically illustrated. In addition, different methods adopted to prepare and characterize BS-vesicles are described. Finally, this article concludes by highlighting the gap in scientific researches tackling BS-vesicles that needs to be addressed in the near future.
Bile salts in drug delivery systems
Bile salts, ionized form of bile acids, are distinctive amphipathic molecules that consist of a steroid nucleus with a hydrophilic side encompassing hydroxyl groups (concave α-side) and a hydrophobic side containing methyl groups (convex β-side) (Holm et al., Citation2013). This makes bile salts dissimilar from traditional pharmaceutical surfactants that are formed of a hydrophilic polar head group and a long hydrophobic tail group (Holm et al., Citation2013). The chemical structure of bile salts commonly used in BS-vesicles and a representation of the hydrophilic and hydrophobic sides of bile salts are depicted in .
Figure 1. (A) Chemical structure of bile salts commonly used in bile salts-containing vesicles and (B) an illustration depicting the hydrophilic side and hydrophobic side of a bile salt molecule.
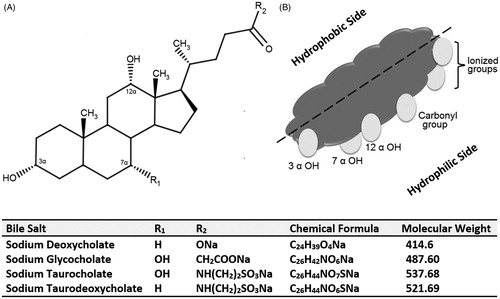
Bile salts and acids play a major role in emulsifying and solubilizing dietary fats through the formation of mixed micelles (Holm et al., Citation2013). In the latter, bile salts are compressed between the phospholipids (PL) polar heads with their hydrophilic sides facing the aqueous medium (Hofmann & Hagey, Citation2008). The ability of bile salts to improve the passage of lipophilic drug molecules across biological membranes and thus augment their oral bioavailability has been demonstrated for many drug candidates (Mikov et al., Citation2006). It was reported that the absorption of sulfadiazine and indomethacin increased when bile secretion was simulated (Nightingale et al., Citation1971; Miyazaki et al., Citation1981). Taking advantage of the aforementioned property, various mixed micelles systems based on bile acids and salts were successfully employed for improving the solubility of extremely lipophilic drugs. Mixed micelles composed of sodium cholate with PL, linoleic acid and lecithin were found to improve the solubility and intestinal absorption of silybin (Yu et al., Citation2010), clofazimine (O'Reilly et al., Citation1994) and Cyclosporine A (Guo et al., Citation2005), respectively. Furthermore, the applicability of bile salts in the preparation of nanoparticulate systems was demonstrated in different researches. Zhang et al. (Citation2013) reported that the incorporation of sodium cholate improved the intestinal absorption of candesartan from cilexetil-loaded lipid nanocarriers. Liu et al. (Citation2007) employed sodium cholate in solid lipid nanoparticles to improve the lipo-solubility of insulin. Zeng et al. (Citation2013) attempted to functionalize nanoparticles with cholic acid to improve docetaxel delivery to cervical cancer. Furthermore, deoxycholic acid was used to augment the oral bioavailability of biodegradable nanoparticles (Samstein et al., 2008) and to impart negative charge to nanoemulsions designed for enhancing the oral bioavailability of paclitaxel (Tiwari & Amiji, Citation2006). Significant improvement in the bioavailability of itraconazole from pegylated bile acids self-emulsifying system was reported by Le Dévédec et al. (Citation2013). On the other hand, transdermally, bile salts were employed as potent penetration enhancers for topically administrated drugs due to the membrane destabilizing activity of these agents. Sodium cholate and combinations of taurocholate and glycholate were found to enhance the percutaneous penetration of indomethacin (Chiang et al., Citation1991) and elcatonin (Ogiso et al., Citation1991), respectively. In addition to that, the applicability of utilizing bile salts as nasal absorption promoters was investigated. Chavanpatil & Vavia (Citation2005) confirmed the effectivity of sodium deoxycholate (SDC) in enhancing the nasal absorption of sumatriptan succinate. Different research groups have utilized bile salts/fatty acids mixed micelles to promoting nasal absorption insulin (Tengamnuay & Mitra, Citation1990), acyclovir (Shao & Mitra, Citation1994) and gentamicin (Duchateau et al., Citation1986). Likewise, the influence of bile salts on conjunctival penetration of drug molecules have been highlighted. Morimoto et al. (Citation1987) demonstrated the effectiveness of sodium taurodeoxycholate (STDC) in increasing the ocular adsorption of hydrophilic compounds. Regarding the buccal route of administration, sodium glycocholate (SGC) was capable of enhancing the buccal absorption of the ionized form of flecainide (Deneer et al., Citation2002). Furthermore, Mahalingam el al. (Citation2007) reported that sodium deoxyglycocholate and SGC increased the buccal permeation of the decitabine about 40-fold. Regarding the inhaled delivery systems, sodium taurocholate (STC) was added to inhaled itraconazole dry nanoparticles powders to minimize the irreversible aggregation of the nanoparticles inducing by spray-drying. Recently in 2014, Gangadhar et al. (Citation2014) synthesized SDC sulfate from deoxycholic acid to formulate lipid-based Amphotericin B nanoparticles with reduced toxicity for the treatment of lung fungal infections via inhalation.
In summary, the aforementioned studies presented various benefits associated with including bile salts in drug delivery systems administrated through oral, topical, transmucosal and inhalation routes.
Vesicular carriers
Pharmaceutical vesicular carriers are composed of water-filled colloidal unilamellar or multilamellar vesicles comprising lipid amphiphilic molecules arranged in a bilayers conformation (Honeywell-Nguyen & Bouwstra, Citation2005; Torchilin, Citation2005; Sinico & Fadda, Citation2009). Hydrophilic drugs are encapsulated within the internal aqueous compartment of the vesicles, whereas hydrophobic drugs are associated with the hydrophobic lipid bilayers (Honeywell-Nguyen & Bouwstra, Citation2005; Torchilin, Citation2005). Broadly, vesicular carriers are divided into two main classes: liposomes, which represent the ground-breaking discovery of Alec Bangham in the 1960s and are composed mainly of phosphatidylcholine (PC) and cholesterol (CH) bilayers, and niosomes that were revealed later in 1970, by L’Oreal, and denote non-ionic surfactant vesicles (Torchilin, Citation2005). Following that, newer forms of lipid vesicles were presented by various researchers and includes tranferosomes (Bragagni et al., Citation2012), emulsomes (Vyas et al., Citation2006), enzymosomes (Gaspar et al., Citation2003), ethosomes (Pandey et al., Citation2014) and pharmacosomes (Semalty et al., Citation2009).
In this review, the term “conventional liposomes” is used to describe traditional PL- and CH-based liposomes, whereas the term “conventional vesicles” is employed to collectively denote traditional liposomes and niosomes. In general, any changes undertaken in the composition of the vesicles’ bilayers influence their physicochemical characteristics that include size, charge, bilayer elasticity and stability, which simultaneously impact their in vivo behavior and effectiveness as pharmaceutical carriers (Sinico & Fadda, Citation2009; Torchilin, Citation2005).
Though, oral vesicular carriers have been widely used to improve drugs bioavailability, yet, the pH gradient, enzymes and physiological bile salts can easily cause vesicles’ destabilization (Martins et al., Citation2007). Nevertheless, the extent to which the vesicular carriers preserve their structural integrity after oral administration during transfer through the GIT is extremely crucial to ensuring effective protection of the entrapped therapeutic agent or antigen in case of vaccine immunization (Martins et al., Citation2007; Neutra & Kozlowski, Citation2006).
Vesicles destruction in GIT
The ability of bile salts to interrupt lipid bilayers by intercalating amongst the PL and forming mixed micelles is well demonstrated (Andrieux et al., Citation2009). It is hypothesized that upon reaching the small intestine and exposing to bile salts, conventional vesicles are effectively destroyed due to inclusion of bile salts monomers into the lipid bilayers of the vesicles (Schubert et al., Citation1983; Wilkhu et al., 2013). Further increase in bile salt concentration induces vesicle–micelle transition (Andrieux et al., Citation2009) followed by formation of new aggregates. The latters have served as vehicles for enhancing the absorption of poorly water-soluble drug molecules in the GIT (Nightingale et al., Citation1971; Miyazaki et al., Citation1981).
Based on the research published by Subuddhi & Mishra (Citation2007), vesicles solubilization by bile salts occurs in three stages depending on bile salt concentrations. At low bile salts concentration, the surfactants partition into the lipid bilayer. When the concentration of bile salts increase, vesicles and micelles co-exist and the bilayer permeability for the bile salts intensifies allowing more bile salts to associate with the bilayer converting them into mixed micelles. In the last stage, surfactants are at their CMC, disrupting the vesicles’ bilayers completely and solubilizing them into mixed micelles.
BS-vesicles
Conventional vesicular carriers can protect peptides and proteins to a certain extent from degradation in the GIT (Bramwell & Perrie, Citation2006). Yet, the existence of intestinal bile salts limit the capabilities of these carriers by cause membrane deformation and vesicles lysis resulting in the release of entrapped macromolecules from the vesicle preceding reaching its planned site of action (Wilkhu et al., 2013). In response to this, modified stabilized vesicles were developed by inclusion of bile salts into the lipid bilayers making them more resistant against bile salts disruption in the GIT. The stability imposed by BS-vesicles was attributed to the repulsion between the bile salts already present in the vesicle bilayer and the intestinal bile salts in the GIT (Conacher et al., Citation2001). Hence, providing protection for the entrapped agents from the hostile environment in the GIT (Beg et al., Citation2013; Sahdev et al., Citation2014) (). Different ingredients utilized in the preparation of BS-vesicles are collectively listed in .
Figure 2. A schematic representation of the behavior of conventional liposome and niosome in comparison to bilosomes in presence of intestinal bile salts. (A) Disruption and lysis of conventional vesicle by intestinal bile salts and (B) The bilosome remains stable in presence of intestinal bile salts (redrawn from Henriksen-Lacey & Perrie, Citation2013).
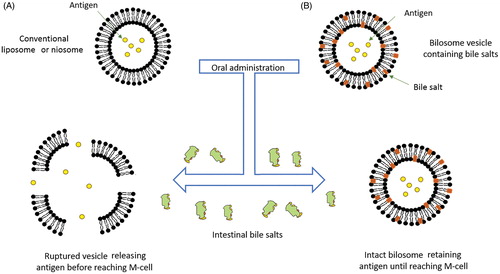
Table 1. Commonly used lipids, surfactants and bile salts for preparing BS-vesicles.
Bile salts-containing liposomes (BS-liposomes)
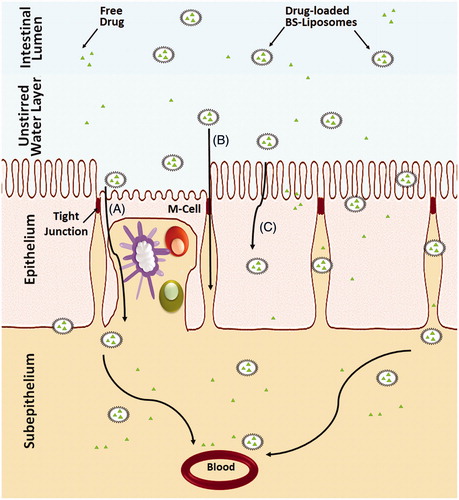
Many efforts have been undertaken to increase the stability and integrity of liposomes in the GIT either by coating the vesicles with polymers, such as chitosan, polyethylene glycol and pectin (Immordino et al., Citation2006; Nguyen et al., Citation2011; Parmentier et al., 2011; Kowapradit et al., Citation2012), or by inclusion of tetraether lipids or gangliosides within their structures (Torchilin, Citation2005). Recently, BS-liposomes have been investigated as stable carriers for delivering sensitive therapeutic agents and proteins by replacing a fraction of the CH in the lipid bilayers with bile salts (Guan et al., Citation2011; Niu et al., Citation2011, Citation2012, Citation2014). Schematic illustrations of different types of liposomes comprising bile salts in comparison to conventional liposome are presented in . Several studies have demonstrated the superiority of BS-liposomes to conventional liposomes in improving the oral absorption of different therapeutic agents and proteins (Chen et al., Citation2009; Niu et al., Citation2011). Although the exact underlying mechanisms of oral absorption enhancement of drugs or proteins loaded in BS-liposomes have not been comprehensively elucidated, several authors acknowledged the capability of intact BS-liposomes to permeate across the unstirred water layer followed by uptake of the vesicles by membranous epithelial cells (M-cells) in the Payer’s patch (Aramaki et al., Citation1993) and transcellular through enterocytes (Niu et al., Citation2014) in addition to the facilitated transport through the paracellular pathway (Chen et al., Citation2009; Niu et al., Citation2012). Furthermore, it is expected that upon contact with the intestinal epithelial, the included bile salts may increase the membrane fluidity followed by membrane-destabilizing effect, which facilitate internalization of the vesicles (Chen et al., Citation2009). Chen et al. (Citation2009) related the ultra-deformability of liposomes due to the inclusion of bile salts to the enhanced carrier-mediated transmembrane absorption. Furthermore, BS-liposomes can freely convert into mixed micelles in the GI environment and hence enhance transmembrane drug absorption (Chen et al., Citation2009). Using imaging method, Niu et al. (Citation2014) demonstrated that the in vivo residence time of oral administrated BS-liposomes in the GIT was longer than its conventional counterpart. The transenterocytic internalization of BS-liposomes in Caco-2 cells was confirmed using fluorescence imaging (Niu et al., Citation2014). The contribution of paracellular permeability to oral absorption from BS-liposomes was predicted by measurement of transepithelial electrical resistance (TEER) (Niu et al., Citation2014). The TEER of Caco-2 cell monolayers is significantly reduced when tight junctions are opened. BS-liposomes significantly reduction the TEER compared to conventional liposomes, indicating better paracellular permeability by reversibly opening the epithelial tight junctions (Ward et al., Citation2000). Schematic illustration of the mechanisms of drug absorption via BS-liposomes is presented in .
Figure 3. Structural composition of different types of liposomes. (A) Conventional liposome, (B) Bile salts-containing liposome and (C) Bile salts coated liposome.
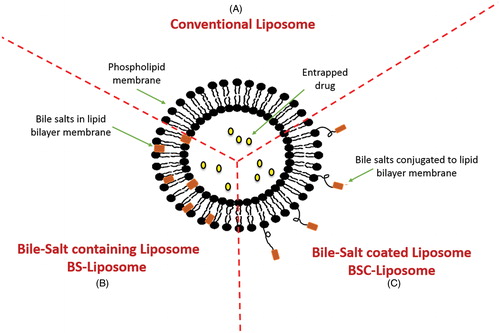
Figure 4. Schematic representation of the uptake of bile salts liposomes by intestinal epithelium after oral administration. (A) Uptake of the vesicles by M-cells in the Payer’s patch, (B) Transport of the vesicles through the paracellular pathway and (C) Transcellular uptake of vesicles through the enterocytes.
Bile salts-containing niosomes (bilosomes)
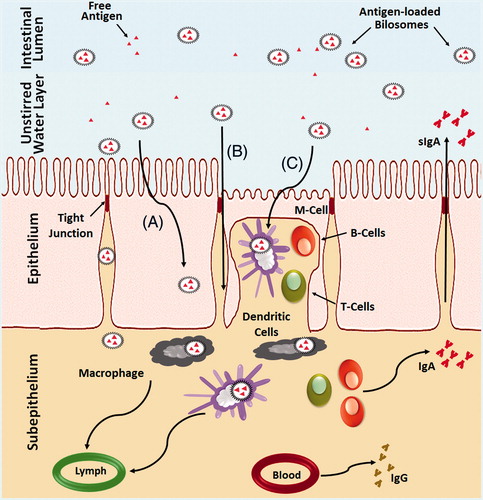
Bilosomes, first described by Conacher et al. (Citation2001), are closed bilayer structures of non-ionic amphiphiles closely correlated to non-ionic surfactant vesicles (niosomes) but incorporating bile salts. Several research groups have demonstrated the potential of utilizing bilosomes in facilitating successful oral vaccine delivery (Mann et al., Citation2006, Citation2009; Shukla et al., Citation2008, Citation2010a,Citationb, Citation2011). Schematic illustrations of different types of bilosomes are depicted in . The mechanisms of oral immunization that take place in the intestinal mucosa differ from that of the oral bioavailability enhancement of protein drugs loaded in BS-liposomes. In the GIT, a single layer of epithelial cells, linked by tight junctions and containing gut-associated lymphoid tissues (GALT), face the complex intestinal luminal environment (McGhee et al., Citation1992; Neutra & Kozlowski, Citation2006). Orally administrated vaccines face defense mechanisms in GIT similar to that imposed on microbial pathogens. The vaccines are diluted and arrested in the mucus gels, denaturated by enzymes and excluded by epithelial barriers (Neutra & Kozlowski, Citation2006; Jain et al., Citation2011). Feeble immune responses associated with oral vaccines arise due to inadequate absorption of antigens by the Peyer’s patches (Neutra & Kozlowski, Citation2006; Jain et al., Citation2011), which nessicates the administration of relatively large doses of vaccine. Bilosomes address these challenges by offering several advantages for oral vaccine delivery. It is postulated that bilosomes protect the vaccine antigen enclosed within the lipid bilayer from premature release and/or degradation in the hostile environment of GI tract (Mann et al., Citation2006, Citation2009; Shukla et al., Citation2008, Citation2010a,Citationb, Citation2011). Using radiolabeled I-125 insulin entrapped in bilosomes, Mann et al. (Citation2006) confirmed the aforementioned and proved that bilosomes are stable in the stomach and intestine. Induction of mucosal immune responses require efficient uptake of antigen by GALT that consist primarily of Peyer’s patches overlying M-cells present between the mucosal epithelial and intraepithelial dendritic cells (DCs). The M-cell take up the bilosomes by endocytosis and transport them to the antigen-presenting cells (APCs), such as macrophages and DCs, that present the antigen to B or T cell initiating antigen-specific mucosal and systemic immune responses (McGhee et al., Citation1992; Neutra & Kozlowski, Citation2006; Jain et al., Citation2011). In this context, fluorescence microscopy studies conducted by Shukla et al. (Citation2011, Citation2008) established effective uptake of bilosomes by the M-Cells, thereby confirming the capability of bilosomes to transport the vaccine antigens to Peyer’s patches.
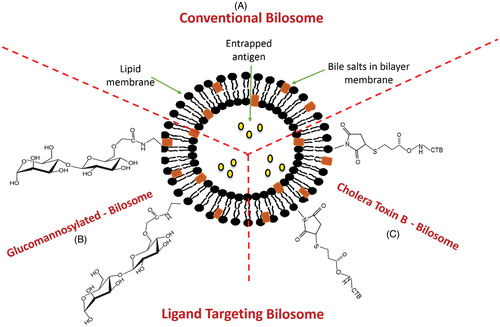
Figure 5. Structural composition of different types of bilosomes. (A) Conventional bilosome, (B) Glucomannan bilosome and (C) Cholera toxin B-bilosome.
Additional mechanisms, such as direct intracellular delivery of antigens through phagocytosis of particles have also been proposed to explain the potent immunological adjuvant activity of bilosomes (Mann et al., Citation2009). Schematic illustration of the mechanisms of immune response associated with bilosomes is presented in .
Figure 6. Schematic representation of the uptake of bilosomes containing antigen through the intestinal epithelium after oral administration. (A) Transcellular uptake of bilosomes through the enterocytes, (B) Transport of the bilosomes through paracellular pathway and (C) Uptake of bilosomes by M-cells and dendritic cells.
Modified forms of bile salts vesicles (BS-vesicles)
Modified versions of BS-vesicles via anchoring specific ligands to the vesicles surface were endeavored by several research groups to enhance their uptake by certain target cells (, ) (Singh et al., Citation2004; Shukla et al., Citation2010a; Jain et al., Citation2014a,Citationb).
Cholera toxin B-modified bilosomes
The adjuvanticity of cholera toxin B subunit (CTB) is related to their affinity to Glucomannan-modified (GM)1 ganglioside receptors on M-cells (Dertzbaugh & Elson, Citation1993). To accomplish efficient systemic and mucosal antibody responses following oral administration, CTB was conjugated to the surface of bilosomes to permit them to specifically bind to GM1 ganglioside receptors on M-cells membrane of Peyer’s patches (Singh et al., Citation2004; Shukla et al., Citation2010a). The superiority of CTB-conjugated bilosomes in terms of effective localization in the M-cells of the GALT compared to non-conjugated bilosomes was verified using fluorescence microscopy (Shukla et al., Citation2010a).
GM bilosomes
To increase the stability of bilosomes and enhance their uptake through the mannosyl receptors of APCs, Jain et al. (Citation2014a,Citationb) decorated the bilosomes surface with glucomannan (GM). Results confirmed that GM-bilosomes were stable against digestive enzymes due the polymeric nature of the ligand. Furthermore, better immune response was elicited after oral administration of GM-bilosomes compared to conventional bilosomes. This was related to the existence of high density of mannose molecules over the bilosomes surface that resulted in more precise recognition and binding of bilosomes to mannose receptors, thereby, enhance uptake through APCs.
Bile salt-coated liposomes (BSC-liposomes)
Bile salts are taken from the blood stream into the hepatocytes with the aid of a well-defined transport system. Pütz et al. (Citation2005) took advantage of this property and examined the capability of bile salts in serving as ligands to target liposomes to the hepatocytes. For that purpose, the bile salt lithocholyltaurine was covalently linked with PL moiety. The formed bile salt-coated liposomes (BSC-liposomes) having the covalently linked bile salt–lipid conjugate showed hepatocyte-specific targeting, thereby opened promising possibilities for hepatocyte-specific drug delivery.
Preparation of BS-vesicles
Based on the reviewed literature, BS-vesicles were prepared using different methods collectively presented in . Brief overview of these methods are presented hereafter and schematically illustrated in .
Figure 7. Representation of different methods adopted for preparation of bile salts-containing vesicles. (A) Reverse phase evaporation method, (B) Thin film hydration method and (C) Hot homogenization method.
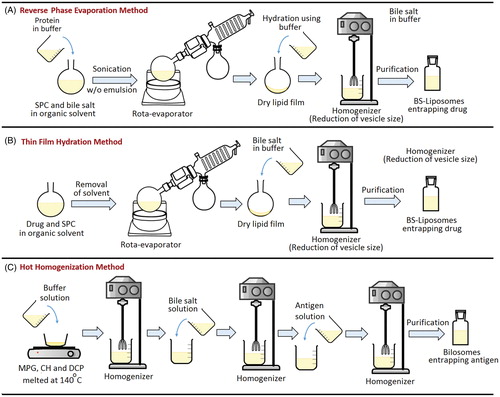
Table 2. Formulation details, preparation methods, and vesicles characteristics of bile salt-containing liposomes (BS-liposomes).
Table 3. Formulation details, preparation methods and vesicles characteristics of bilosomes.
Table 4. Formulation details, preparation methods and vesicles characteristics of modified bilosomes.
Reverse-phase evaporation method
Reverse-phase evaporation method was used to formulate BS-liposomes loaded with insulin (Niu et al., Citation2011, Citation2012, Citation2014) or hexamethylmelamine (Sun et al., Citation2010). In this approach, soybean phosphatidylcholine (SPC) and bile salts were dissolved in ethyl ether to which buffered solution containing insulin was added followed by ultra-sonication to form reverse w/o emulsion (Niu et al., Citation2011, Citation2012, Citation2014). The solvent was then removed from the emulsion using a rota-evaporator under reduced pressure. The dried lipids formed were eventually hydrated by buffer to form homogenous aqueous vesicles dispersion that was extruded through a high pressure homogenizer to obtain BS-liposomes entrapping protein. For loading the poorly water-soluble drug, hexamethylmelamine, it was dissolved with the lipid components in the organic solvent in first step.
Thin film hydration method
This method was adopted to prepare both BS-liposomes and bilosomes. For preparing drug-loaded BS-liposomes, the drug and SPC were dissolved in organic solvent, which was then evaporated in a rota-evaporator. The formed thin film of SPC on the wall of the flask was hydrated with buffered bile salt solution and then rotated to obtain a crude dispersion of multilamellar liposomes. The latter was then sonicated followed by homogenization to obtain small unilamellar structure of BS-liposomes (Chen et al., Citation2009). Slight differences in the preparation steps were found between different research groups where, in some cases, all the components of liposomes namely, SPC, bile salts and drug, were initially dissolved in the organic solvent, then followed the same aforementioned procedures (Guan et al., Citation2011; Dai et al., Citation2013).
For preparing antigen-loaded bilosomes using thin-film hydration method, the lipid components, surfactant, CH and dicetylphosphate (DCP) were dissolved in an organic solvent in a round-bottomed flask followed by solvent evaporation under reduced pressure using rota-evaporator. The thin film formed on the glass wall of the round-bottomed flask was then hydrated with buffer containing the bile salt and the antigen to form large multilamellar vesicles that were transformed to small unilamellar vesicles by ultrasonication (Dai et al., Citation2013) or extrusion (Shukla et al., Citation2008, Citation2011). Thin film hydration method was used to prepare bilosomes loaded with diphtheria toxoid (DTx) (Shukla et al., Citation2011), hepatitis B surface antigen (HBsAg) (Shukla et al., Citation2011) and bovine serum albumin (BSA) (Jain et al., Citation2014b).
Hot homogenization method
For preparing bilosomes using this approach, the lipid components, namely, monopalmitoyl glycerol, CH and DCP were melted at 140 °C for 5 min and subsequently hydrated with buffered solution. The lipid mixture was then homogenized followed by the addition of bile salt solution to form dispersion containing empty vesicles that was homogenized. Thereafter, the antigen buffered solution was added to the homogenate, and protein entrapment was achieved by further homogenization (Gebril et al., Citation2014) or several freeze–thaw cycles (Mann et al., Citation2009). This method was used to entrap influenza A antigen (Gebril et al., Citation2014) and gonadotropin-releasing hormone (GnRH) immunogens (Gebril et al., Citation2014) to bilosomes. It is worth mentioning that the antigen was added at the final stage to minimize long exposure to homogenization (Wilkhu et al., 2013).
Proniosomal method
Preparation of bile salts-containing proniosomes was initiated one decade ago by Song et al. (Citation2005) to facilitate handling of liposomes and improve their long-term stability by converting them to powder form. In this method, the carrier, usually sorbitol particles, was located in a round-bottomed flask and vacuum dried on the rota-evaporator. Then, solution of PC, bile salt and drug dissolved in organic solvent were added drop wise into the flask to load them onto sorbitol particles. Following that, the loaded sorbitol particles were freeze-dried to confirm the complete evaporation of any organic solvents residues. The resultant proliposomal powder was converted to liposomes following manual agitation in water.
In vitro characterizations and variables influencing BS-vesicles
The in vitro characterizations of BS-vesicles included determination of the vesicle size, polydispersity index (PI), zeta potential, vesicles morphology, entrapment efficiency, in vitro release and cellular uptake studies. The characterization techniques identified in the published literature are listed in . A brief description of the characterization techniques and variables influencing some properties of BS-vesicles are provided hereafter.
Table 5. Major methods for BS-vesicles characterizations.
Particle size, PI, morphology and zeta potential
Particle size of BS-vesicles exerts substantial impact on their in vitro and in vivo performances (Sun et al., Citation2008; Mann et al., Citation2009). Analytical instruments constructed on dynamic light scattering principle basis, i.e. photon correlation spectroscopy, were used to estimate the particle size and PI of the BS-vesicles dispersions (Chen et al., Citation2009; Dai et al., Citation2013). The vesicle size of BS-vehicles ranged from 97.3 ± 1.6 nm (Dai et al., Citation2013) to around 3 μm (Conacher et al., Citation2001).
Based on the published literature, several variables affect the particle size and PI of BS-vesicles. These variables include bile salts content, pH of the hydration buffer, homogenization (Chen et al., Citation2009) or ultrasonication conditions adopted during vesicles preparation (Dai et al., Citation2013).
Regarding the influence of bile salts content, substantial decrease in particle size and PI of BS-vesicles was associated with the increase in bile salt contents due to the enhanced flexibility and decrease in surface tension of the vesicles (Chen et al., Citation2009; Guan et al., Citation2011; Wilkhu et al., 2013; Dai et al., 2013). However, further increase in bile salts content caused vesicles enlargement bound with increase in PI due to the increase in medium viscosity (Guan et al., Citation2011). The pH of the hydration medium also influences the particle size of the vesicles due to the ionization of bile salts at higher pH which in turn increase its surface activity (Chen et al., Citation2009). Likewise, different processing variables influenced the vesicles particle size. The multilamellar micron-size BS-vesicles produced by thin film dispersion method were converted into small unilamellar nano-sized vesicles using extrusion through membrane (Shukla et al., Citation2008, Citation2011), ultrasonication (Dai et al., Citation2013) or homogenization (Guan et al., Citation2011). It was reported that increasing either homogenization pressure or cycles reduced the size of BS-liposomes (Chen et al., Citation2009; Niu et al., Citation2011). Oppositely, extremely high homogenization pressure increased particle size and PI of BS-vesicles due to rupturing and combination of the vesicles under high homogenization force (Guan et al., Citation2011).
The preparation method also influences the vesicle particle size. Mann et al. (Citation2009) reported that homogenization method produced single population of small vesicles; however, thin-film method produced a mixture of large vesicles combined with smaller ones.
It is worth mentioning that the uptake of bilosomes by Peyer’s patches and mesenteric lymph tissue is somehow dependent on the vesicles size (Mann et al., Citation2009). Bilosomes with size between 5 and 10 μm are retained within the Peyer’s patches offering mucosal immunoglobulin A (IgA) response, whereas particles less than 5 μm are conveyed into the efferent lymphatics inside macrophages inducing predominantly circulating antibody response (Eldridge et al., Citation1990).
Likewise, particle size of BS-liposomes influences their in vivo cellular uptake via endocytosis. Niu et al. (Citation2014) reported that SGC-liposomes with particle size of 80–400 nm showed more cellular uptake in comparison to their counterpart with size of 2000 nm. Endocytosis is an energy-dependent process, and the greater extent of uptake shown for smaller particle is related to the less energy required during the endocytosis process (Gaumet et al., Citation2009; Niu et al., Citation2014).
Transmission electron microscopy (TEM) was used to ascertain the formation of BS-vesicles and visualize their morphology (Chen et al., Citation2009). The TEM micrographs presented in different studies indicates that BS-vesicles were spherical or nearly spherical in nature. Discernable lamella was observed for SGC-liposomes loaded with insulin (Niu et al., Citation2011). Likewise, the bilosomes appeared to be unilamellar vesicles utilizing TEM. Other microscopic techniques that include cryogenic TEM (cryo-TEM) (Chen et al., Citation2009) and freeze fracture electron microscopy (Mann et al., Citation2006) were employed for visualization of BS-vesicles.
Zeta potential is the measure of overall charges acquired by particles in a particular medium. In general, vesicles with surface charge are more stable against accumulation than uncharged ones. Opposing conventional liposomes that showed a tendency to agglomerate, BS-liposomes were negatively charged due the presence of bile salts that stimulated the zeta potential and hindered the aggregation of the vesicles (Dai et al., Citation2013). Regarding in vivo performance, it is reported that negatively charged vesicles are favorably taken up by the Peyer’s patches (Wilkhu et al., 2013).
Entrapment efficiency percent
For the use of vesicles in pharmaceutical applications, one of the most important parameter to assess is the entrapment efficiency percent (EE%). After preparation of BS-vesicles suspension, the unencapsulated drug/protein was separated from the total amount of drug by techniques such as ultra-centrifugation (Song et al., Citation2005; Mann et al., Citation2009; Gebril et al., Citation2014) or size exclusion chromatography using Sephadex column (Chen et al., Citation2009; Dai et al., Citation2013; Niu et al., Citation2014). The amount of encapsulated agents was quantified by HPLC (Niu et al., Citation2011, Citation2012, Citation2014), UV spectrophotometer (Guan et al., Citation2011; Dai et al., Citation2013) or using different protein analysis methods like bicinchoninic acid (Shukla et al., Citation2011; Jain et al., Citation2014a,Citationb) and modified ninhydrin assay (Mann et al., Citation2006; Mann et al., Citation2009; Conacher et al., Citation2001; Premanand et al., Citation2013).
Similar to the particle size, a variety of variables affected drug or protein entrapment in BS liposomes (Niu et al., Citation2011). It was observed that the entrapment of different drugs (hexamethylmelamine, Cyclosporine A and fenofibrate) increased with the increase in bile salt contents in the vesicles. Bile salts, with surface-active properties, can integrate perpendicularly into the exterior of the bilayer membrane, perturbating the acyl chains of the lipid matrix, and thereby increasing the elasticity and solubility of drug in the membrane (Chen et al., Citation2009; Sun et al., Citation2010). However, further increase in the content of bile salts simultaneously increases the drug solubility in the dispersion medium, thereby compromising the entrapment efficiency (Niu et al., Citation2014). Furthermore, the fluidizing effect of bile salts on the lipid bilayers causes leakage of the entrapped drug (Niu et al., Citation2011). The PC content in the vesicles as well contributes to the EE. Significant increase in the amount of cyclosporine A and hexamethylmelamine entrapped was bound with the increase of SPC content, which was attributable to the fact that higher lipid content propose more space for drug molecules (Sun et al., Citation2010; Guan et al., Citation2011). The hydration pH affects the EE% as its affects the bile salt dissociation, which in turn affects the particle size and ability of the bile salts to hold the drug molecules (Chen et al., Citation2009; Niu et al., Citation2014).
In vitro release and cellular uptake studies
Regarding BS-vesicles, the in vitro release study was evaluated by dynamic dialysis method to circumvent the leakage of vesicles into the external release medium (Chen et al., Citation2009; Guan et al., Citation2011; Dai et al., Citation2013). In vitro release studies are essential indicators for in vivo performance of a drug delivery system. It is worth stating that their main drawbacks are the absence of complete mimicking of in vivo digestion and failing to take into account the probability of vesicular disruption in physiological conditions (Andrieux et al., Citation2009). In dynamic dialysis study, compared to conventional liposomes, BS-liposomes released more drug due to the enhance lipid bilayer fluidity that allowed drug leakage from the vesicles (Chen et al., Citation2009). Besides, the uptake of BS-vesicles was assessed in different biological cells like human colon adenocarcinoma cell line (Caco-2 cells) (Song et al., Citation2005; Niu et al., Citation2014), spontaneously derived human corneal epithelial cells (Dai et al., Citation2013), and murine macrophage cell line (RAW 264.7) (Jain et al., Citation2014a,Citationb).
In vivo performance of bile salt vesicles (BS-vesicles)
Improvement in oral drug bioavailability
Based on the published literature, incorporation of drugs or proteins into BS-liposomes substantialy enhanced their bioavailability and in vivo efficacy as depicted in and diagrammatically illustrated in . Chen et al. (Citation2009) reported that orally administrated SDC-liposomes loaded with fenofibrate exhibited 1.57-fold increase in bioavailability compared to conventional liposomes in beagle dogs (Chen et al., Citation2009). The observed enhancement was related to the uptake of the vesicles through M-cells in the Peyer’s patch and facilitated transmembrane absorption of fenofibrate due to the ultra-deformability of BS-liposomes. Likewise, the superiority of BS-liposomes in enhancing the oral bioavailability of cyclosporine A relative to the marketed microemulsion formulation (Sandimmune Neoral) and conventional liposomes was confirmed by Guan et al. (Citation2011). Facilitated absorption of intact BS-liposomes vesicles rather than enhancing drug solubility and release rate was the main reason for the increase in cyclosporine A bioavailability. Besides, Sun et al. (2010) achieved about 9.76- and 1.21-fold higher bioavailability form hexamethylmelamine-loaded SDC liposomes in animal model relative to drug solution and drug loaded in conventional liposomes, respectively. The oral delivery of insulin loaded in BS-liposomes experimented in animal models generated encouraging results indicating that this carrier system may be an interesting oral alternative for parenteral insulin in the near future. Using luminescence imaging, Niu et al. (Citation2014) confirmed that orally administrated SGC-liposomes loaded with recombinant human insulin (rhINS) were more stable in vivo than conventional liposomes due to the dual protective effects imposed by SGC vesicles against damaging effects of bile salts and enzymes in the GIT. Besides, the enhanced absorption of rhINS-loaded SGC-liposomes was related to the improved transenterocytic internalization and permeation of rhINS across the biomembranes. As for selecting the bile salt with more protective action, Niu et al. (Citation2012) observed that the hypoglycemic efficiency and oral bioavailability of insulin from different BS-liposomes were in the sequence of SGC > STC > CH > SDC indicating that SGC in liposomes offer better protection against enzymatic degradation.
Figure 8. In vivo performance of orally administrated bile salts-containing liposomes in comparison to conventional liposomes. (A) Fenofibrate-loaded bile salts-containing liposomes (Chen et al., Citation2009) and (B) Recombinant insulin-loaded bile salts-containing liposomes (Niu et al., Citation2014).
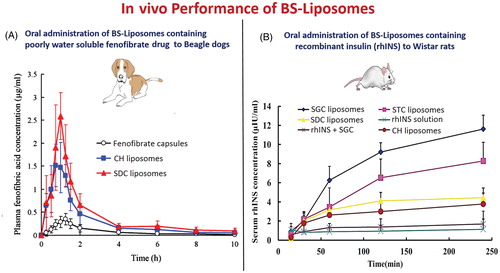
Table 6. In vivo performances of BS-liposomes in different animal models.
The suitability of utilizing different bile salts as absorption enhancers in free-flowing proliposomal powder loaded with salmon calcitonin was examined by Song et al. (Citation2005). STDC-proliposomes exhibited balanced permeation enhancement across Caco-2 cell monolayers and minimized intestinal epithelial cells damage. Besides, STDC-proniosomes resulted in 7.1-fold increase in salmon calcitonin bioavailability when administered duodenally to rats compared to proliposomes without bile salts. In addition to the intrinsic ability of bile salts to fluidize biological membranes, STDC was capable of forming a lipophilic ion-pair complex with salmon calcitonin characterized by increase permeability across biological membranes.
Enhancement of vaccine immunogenicity
After introducing bilosomes by Conacher et al. in Citation2001, considerable evidence in literature, collectively listed in , affirmed the suitability of employing bilosomes as carrier for vaccines to provide transmucosal immunization ().
Figure 9. In vivo performance of orally administrated bilosomes. (A) Confocal laser scanning microscopy (CLSM) of the intestine (Shukla et al., Citation2011) and (B) Immunological study and measurement of specific IgA and IgG.
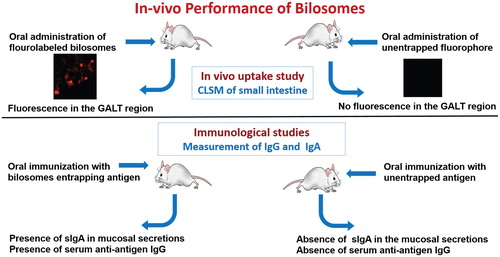
Table 7. In vivo performances of bilosomes in animal models.
Conacher et al. (Citation2001) reported that orally administrated bilosomes induced cell-mediated responses against synthetic peptides and high antibody titers against protein antigens comparable to those engendered succeeding systemic immunization.
The potential of utilizing SDC-bilosomes loaded with either HBsAg or DTx in providing transmucosal immunization was investigated by Shukla et al. (Citation2008, Citation2011). In both studies, orally administrated bilosomes loaded with high dose of antigen produced systemic immunoglobulin G (IgG) response in mice comparable to those induced by intramuscular (IM) administrated antigens. In addition, bilosomes elicited measurable secretory IgA in mucosal secretions that were not induced by IM administrated antigens. Similarly, the combinatorial bilosomes formulation containing high dose of HBsAg and tetanus toxoid (TTx), prepared by Shukla et al. (Citation2010b), produced anti-HBsAg-IgG and anti-TTx-IgG levels mice serum similar to IM administered HBsAg and TTx. Likewise, Mann et al. (Citation2006) reported positive results regarding the effectiveness of the bilosomes containing TTx in inducing significant systemic and mucosal immune response after oral administration. The obtained end-point antibody titers from TTx-loaded bilosomes were superior to the oral administration of the un-entrapped antigen, but analogous to parenterally administrated ones. Premanand et al. (Citation2013) confirmed that baculovirus displaying VP1 (Bac-VP1) linked with bilosomes provoked significantly higher immune responses relative to bilosomes non-linked with Bac-VP1 suggesting that bilosomes have intrinsic adjuvant characters when linked with antigen. Wilkhu et al. (2013) confirmed the protective property of bilosomes to antigens as low levels of antigen (39%) were measured across the GIT after oral administration of free antigen to mice in compared to 55% for antigen-loaded bilosomes. The influence of bilosomes particle size on the associated immune response after oral administration was assessed by Mann et al. (Citation2009). Results indicated that mixed population of small and large vesicles entrapped influenza A antigen caused higher protection measured as symptom score and higher responder number than bilosomes with smaller vesicle size.
To increase their concentration in the intestinal immune cells, targeted bilosomes with surface attached ligands capable of recognizing and linking to the cells of interest have been examined. In this context, Singh et al. (Citation2004) depicted that the oral dose of CTB-conjugated bilosomes produced nearly comparable immune reaction to that of parenteral administrated antigen containing Freund’s complete adjuvant. Using the same approach, Shukla et al. (Citation2010b) showed that HBsAg-loaded CTB-conjugated bilosomes (20 µg) produced anti-HBsAg IgG antibody titre response comparable to either IM alum-adsorbed HBsAg (10 µg) or HBsAg-loaded bilosomes (50 µg).
As far as the current literature is concerned, Jain et al. (Citation2014a,Citationb) reported the foremost studies that examined the efficiency of anchoring GM polymer to bilosomes encapsulating TTx or BSA. In both cases, orally administrated GM-bilosomes superseded niosomes and conventional bilosomes in eliciting better immune response due to their selective and enhanced uptake through APCs in mucosal linings.
Opposing the aforementioned positive outcomes associating with loading the antigen into bilosomes, recently, negative result was reported by Gebril et al. (Citation2014) where orally administrated bilosomes entrapping GnRH immunogen failed in inducing any antibody response. This indicated that non-pathogenic origin peptides such as GnRH are not suitably delivered by bilosomes.
Stability considerations of BS-vesicles
Stability studies (whether in-process, in simulated fluids or during storage) are of vital prominence for successful development of pharmaceutical carrier systems. Decomposition of entrapped therapeutic agents may lead to decrease in their potency, whereas in the case of immunological preparations, denaturation of antigens may cause inadequate immune response making the subject prone to diseases.
In processing stability
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
Different research groups utilized sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) to examine the chemical stability of entrapped peptides (rhINS) or antigens (DTx, TTx and BSA) in BS-vesicles after being exposed to the preparation stress conditions (Niu et al., Citation2011; Shukla et al., Citation2011; Jain et al., Citation2014a,Citationb). SDS-PAGE is a widely used procedure to isolated proteins according to their electrophoretic mobility. Sodium dodecyl sulfate (SDS), as an anionic surfactant, was added to protein specimens to linearize proteins and impart negative charge thus fractionate them according to estimated size through electrophoresis (Rath et al., Citation2009). In all the published studies, symmetrical position of bands between the pure as well as extracted antigens/proteins along with the absence of additional bands were evident confirming that the preparation method, and the quantity of bile salts included did induce any irreversible accumulation or decomposition of the entrapped agents.
Circular dichroism spectroscopy analysis
Extracted antigens and proteins from BS-vesicles samples were subjected to conformational stability studies using circular dichroism (CD) spectroscopy (Niu et al., Citation2011, Citation2012; Jain et al., Citation2014a,Citationb). CD is displayed by molecules because of their levorotary and dextrorotary constituents and CD spectroscopy analysis is used to study the conformations adopted by proteins as they have CD spectral signatures representative of their structures (Martin & Schilstra, Citation2008). The entrapped agents were found conformationally stable throughout the preparation process as evident by the superimposed CD spectra of test samples with that of standard, thus, further supporting the appropriateness of BS-vesicles in delivering antigens and therapeutic proteins.
Storage stability
Stability studies were carried out to explore the leaching of the entrapped agent from the vesicles during storage (). Shukla et al. (Citation2011) examined the amount of DTx retained in the bilosomes after storage at refrigerated (5 ± 3 °C) and room temperature (25 ± 2 °C) at 70% relative humidity. After one-month storage, around 94% of the antigen remained in bilosomes stored at room temperature, whereas more than 98% of the antigen was found in samples stored at refrigerated conditions. The stability of the formulations was credited to the negative charge induced by DCP on the bilosomes surface that caused electrostatic repulsion preventing fusion and aggregation of vesicles upon storage.
Table 8. Percent antigen retained in different bilosomes formulations challenged by simulated biological media.
Stability in simulated biological milieu
It is crucial to interpret the stability of vesicular carriers and encapsulated peptides and proteins when confronted by gastric pH, bile salts and GI enzymes as the intact fraction of the vesicles predominantly influences the associated in vivo response. However, it is somehow challenging, to perceive the fate of the vesicles in the GI tract by sampling or imaging tools. As a result, different research groups took an alternative easier approach by incubating the BS-vesicles in in vitro-simulated GI media and bile salts solutions of concentration between 5 and 20 mM, similar to the bile salts concentration range in healthy humans (Westergaard, Citation1977). The superiority of bilosomes to niosomes, affirmed in terms of their ability to protect the loaded antigen in simulated biological fluids, was attributed to repulsion between the bile salts in the vesicle bilayer and the external bile salts in the solution (Conacher et al., Citation2001). In this domain, Conacher et al. (Citation2001) reported that niosomes lost all of their initially entrapped BSA content in bile salt concentration of 20 mM in contrast to bilosomes that retained 85% of its initial BSA, thereby, confirming the stabilizing role of bile salts in vesicle constructs. In accordance with the aforementioned result, Shukla et al. (Citation2008, Citation2011) reported that bilosomes loaded with either HBsAg or DTx retained considerable amount of entrapped antigen during stability studies conducted in simulated GIF and bile salts solutions (5 mM and 20 mM concentration). Following a comparable approach, Wilkhu et al. (2013) examined the changes in antigen load of bilosomes-incubated GIF. The initial antigen loaded (32%) was retained in gastric media; however, it decreased to around 8.5–9.5% when the bilosomes were simultaneously transferred to intestinal medium due to the degradation of antigen located on the surface of bilosomes.
Different authors have compared the stability of BS-liposomes in simulated GI media in comparison to conventional liposomes. Hu et al. (2013) reported that SGC-liposomes retained most of the insulin load as compared with conventional liposomes in either simulated GI fluids (including pancreatin or pepsin) or in ex vivo GI fluids obtained from rats. The protective effect was credited to the enzyme-inhibiting ability of SGC and its associated membrane stabilization aptitude. The influence of incorporating different bile salts (SGC, STC, SDC) on the integrity of rhINS-loaded BS-liposomes against different protease enzymes (pepsin, trypsin and α-chymotrypsin) was tested by Niu et al. (Citation2011) who further affirmed the superiority of SGC in formulating BS-liposomes due to its enzyme-inhibiting ability compared to the other investigated bile salts.
Regarding the modified bilosomes, Singh et al. (Citation2004) reported that CTB-bilosomes exhibited better stability than niosomes incubated at 20 mM bile salt solution as they retained about 84% of its initial BSA-load compared to niosomes that retained around 52% of its initial load.
Similarly, GM-bilosomes exhibited enhanced stability, in terms of significant higher percentage of TTx or BSA retained within the system, compared to bilosomes and niosomes due to the additional polymeric coating barrier (Jain et al., Citation2014a,Citationb).
Cytotoxicity of BS-vesicles
Bile salts are reported to cause irritation and toxicity when used as penetration enhancers because their enhancement effect is somehow bound to damaging the intestinal epithelial barrier (Saettone et al., Citation1996). However, some researches have shown that PL can reduce the noxiousness of bile salts (Dial et al., Citation2008). In this domain, Niu et al. (Citation2014) evaluated the cytotoxicity of BS-liposomes in concentration range of 0.25–6.25 mmol/L using cell growth inhibition assay employing Caco-2 cell monolayers. Although, there was a slight reduction in cell viability as the liposomes concentration increased, non-significant difference was observed among various bile salts (SGC, STC and SDC) used or between different concentration after incubation for 4 h. The obtained findings implied that BS-liposomes exhibited non-significant toxicity toward Caco-2 cells at the investigated concentrations. Cell apoptosis detection was carried out by the same research group to further evaluate cytotoxicity of BS-liposomes to Caco-2 cells as their hydrophobicity is correlated with apoptosis induction. Complementing their previous result, non-significant difference was found in apoptosis between BS-liposomes and negative control confirming the low cytotoxicity of BS-liposomes. Similarly, Dai et al. (Citation2013) evaluated the toxicity of BS-liposomes prepared using SGC, STC and SDC in comparison to conventional liposomes on the viability of corneal epithelial cells. After incubation for 12 h, at low lipid concentration (<4 mg/mL), the viability of the cells were more than 85% for all liposomal formulations; however, at high lipid concentration (>6 mg/mL), both SGC and STC showed low toxicity affirming their suitability for ocular drug delivery opposite to SDC-liposomes that had greater toxicity to corneal cells.
Critical opinion
Based on the reviewed literature, BS-liposomes, not only were they capable of enhancing the bioavailability of poorly water soluble drugs in animal models but also they demonstrated the ability to protect the entrapped peptides and proteins after oral administration. Moreover, after the first breakthrough of utilizing bilosomes for oral vaccines delivering by Conacher et al. in Citation2001, investigations by different research groups in BALB/c mice confirmed the usefulness of oral bilosomes entrapping antigens in alerting both mucosal and systemic immune responses. In addition, surface engineered bilosomes (CTB-bilosomes and GM-liposomes) prepared via anchoring ligands to the bilosomal surface demonstrated the ability of targeting the antigens into specific immune cells. Likewise, covalently linked bile salt-lipid conjugate to liposomes (BSC-liposomes) showed promising specific hepatocytes targeting. Though much have been learned from studies conducted in animals about the in vivo effectiveness of BS-vesicles, endeavoring to scale up the obtained positive results from animals to humans is usually problematic. The current challenge that faces the researchers is applying the gained knowledge to carry out safe trials in human subjects to systematically monitor all parameters of the elicited immune response and elucidate the exact immune mechanism after oral administration of bilosomes. Besides, the precise mechanisms of facilitated absorption and the interaction between the BS-liposomes and biological environment have not yet been explained due to the complexity of the GI physiology. Nevertheless, it is of remarkable significance to explicate the exact underlying mechanisms of improved absorption of macromolecules by BS-liposomes to serve as basis for their optimization. Further studies should also include the assessment of the influence of the ingested food on the in vivo performance of BS-vesicles.
In addition to the aforementioned, certain criteria must be fulfilled in BS-vesicles to ensure their acceptance as pharmaceutical carriers. An efficient and adequate process for large industrial scale production that ensures high and reproducible levels of protein/peptide entrapment should be addressed. Furthermore, the long-term storage stability of BS-vesicles should be scrutinized in details, which is still yet far from being accomplished due to the limited researches that address long-term stability.
Conclusion
Although, conventional lipid-based vesicular carriers, such as liposomes or niosomes, showed the potential for delivering different macromolecules, yet, after oral administration, the intestinal bile salts and enzymes disrupt the bilayers constructs of the vesicles, causing the release of entrapped macromolecules prior to reaching the intended site of action. In response to that, different forms of bile salts-containing vesicular carriers, namely BS-liposomes, bilosomes and surface-engineered BS-vesicles, have been developed as stable replacements for conventional vesicles that decomposition within the harsh environment in the GIT. The presented studies in this review confirmed that BS-vesicles have superior characteristics to conventional liposomes or niosomes for enhancing the oral bioavailability of therapeutically challenging agents and immunological response of vaccines. In addition, research on BS-vesicles are currently accelerating, stimulated by the present information regarding their positive in vivo performance combined with the simplicity in preparation.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Andrieux K, Forte L, Lesieur S, et al. (2009). Solubilisation of dipalmitoylphosphatidylcholine bilayers by sodium taurocholate: a model to study the stability of liposomes in the gastrointestinal tract and their mechanism of interaction with a model bile salt. Eur J Pharm Biopharm 71:346–55
- Aramaki Y, Tomizawa H, Hara T, et al. (1993). Stability of liposomes in vitro and their uptake by rat Peyer’s patches following oral administration. Pharm Res 10:1228–31
- Beg S, Samad A, Nazish I, et al. (2013). Colloidal drug delivery systems in vaccine delivery. Curr Drug Targets 14:123–37
- Boyd BJ. (2008). Past and future evolution in colloidal drug delivery systems. Expert Opin Drug Deliv 5:69–85
- Bragagni M, Mennini N, Maestrelli F, et al. (2012). Comparative study of liposomes, transfersomes and ethosomes as carriers for improving topical delivery of celecoxib. Drug Deliv 19:354–61
- Bramwell VW, Perrie Y. (2006). Particulate delivery systems for vaccines: what can we expect? J Pharm Pharmacol 58:717–28
- Chavanpatil MD, Vavia PR. (2005). Nasal drug delivery of sumatriptan succinate. Pharmazie 60:347–9
- Chen Y, Lu Y, Chen J, et al. (2009). Enhanced bioavailability of the poorly water-soluble drug fenofibrate by using liposomes containing a bile salt. Int J Pharm 376:153–60
- Chiang CH, Lai JS, Yang KH. (1991). The effect of pH and chemical enhancers on the percutaneous absorption of indomethacin. Drug Dev Ind Pharm 17:91–111
- Conacher M, Alexander J, Brewer JM. (2001). Oral immunisation with peptide and protein antigens by formulation in lipid vesicles incorporating bile salts (bilosomes). Vaccine 19:2965–74
- Dai Y, Zhou R, Liu L, et al. (2013). Liposomes containing bile salts as novel ocular delivery systems for tacrolimus (FK506): in vitro characterization and improved corneal permeation. Int J Nanomedicine 8:1921–33
- Demetzos C, Pippa N. (2014). Advanced drug delivery nanosystems (aDDnSs): a mini-review. Drug Deliv 21:250–7
- Deneer VH, Drese GB, Roemelé PE, et al. (2002). Buccal transport of flecainide and sotalol: effect of a bile salt and ionization state. Int J Pharm 241:127–34
- Dertzbaugh MT, Elson CO. (1993). Comparative effectiveness of the cholera toxin B subunit and alkaline phosphatase as carriers for oral vaccines. Infect Immun 61:48–55
- Dial EJ, Rooijakkers SH, Darling RL, et al. (2008). Role of phosphatidylcholine saturation in preventing bile salt toxicity to gastrointestinal epithelia and membranes. J Gastroenterol Hepatol 23:430–6
- Duchateau GSMJE, Zuidema J, Merkus FWHM. (1986). Bile salts and intranasal drug absorption. Int J Pharm 31:193–9
- Eldridge JH, Hammond CJ, Meulbroek JA, et al. (1990). Controlled vaccine release in the gut-associated lymphoid tissues. 1 Orally administered biodegradable microspheres target the Peyer’s patches. J Control Release 11:205–14
- Gangadhar KN, Adhikari K, Srichana T. (2014). Synthesis and evaluation of sodium deoxycholate sulfate as a lipid drug carrier to enhance the solubility, stability and safety of an amphotericin B inhalation formulation. Int J Pharm 471:430–8
- Gaspar MM, Martins MB, Corvo ML, Cruz ME. (2003). Design and characterization of enzymosomes with surface-exposed superoxide dismutase. Biochim Biophys Acta 1609:211–17
- Gaumet M, Gurny R, Delie F. (2009). Localization and quantification of biodegrad-able particles in an intestinal cell model: the influence of particle size. Eur J Pharm Sci 36:465–73
- Gebril AM, Lamprou DA, Alsaadi MM, et al. (2014). Assessment of the antigen-specific antibody response induced by mucosal administration of a GnRH conjugate entrapped in lipid nanoparticles. Nanomedicine 10:971–9
- Guan P, Lu Y, Qi J, et al. (2011). Enhanced oral bioavailability of cyclosporine A by liposomes containing a bile salt. Int J Nanomedicine 6:965–74
- Guo J, Wu T, Ping Q, et al. (2005). Solubilization and pharmacokinetic behaviors of sodium cholate/lecithin-mixed micelles containing cyclosporine A. Drug Deliv 12:35–9
- Gupta S, Jain A, Chakraborty M, et al. (2013). Oral delivery of therapeutic proteins and peptides: a review on recent developments. Drug Deliv 20:237–46
- Henriksen-Lacey M, Perrie Y. (2013). Designing liposomes as vaccine adjuvants. In: Flower DR, Perrie Y, eds. Immunomic discovery of adjuvants and candidate subunit vaccines. New York: Springer, Chapter 10, 181–203
- Hofmann AF, Hagey LR. (2008). Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol Life Sci 65:2461–83
- Holm R, Müllertz A, Mu H. (2013). Bile salts and their importance for drug absorption. Int J Pharm 453:44–55
- Honeywell-Nguyen PL, Bouwstra JA. (2005). Vesicles as a tool for transdermal and dermal delivery. Drug Discov Today Technol 2:67–74
- Hu S, Niu M, Hu F, et al. (2013). Integrity and stability of oral liposomes containing bile salts studied in simulated and ex vivo gastrointestinal media. Int J Pharm 441:693–700
- Immordino ML, Dosio F, Cattel L. (2006). Stealth liposomes: review of the basic science, rationale, and clinicl applications, existing and potential. Int J Nanomedicine 1:297–315
- Jain S, Harde H, Indulkar A, Agrawal AK. (2014a). Improved stability and immunological potential of tetanus toxoid containing surface engineered bilosomes following oral administration. Nanomedicine 10:431–40
- Jain S, Indulkar A, Harde H, Agrawal AK. (2014b). Oral mucosal immunization using glucomannosylated bilosomes. J Biomed Nanotechnol 10:932–47
- Jain S, Khomane K, K Jain A, Dani P. (2011). Nanocarriers for transmucosal vaccine delivery. Curr Nanoscience 7:160–77
- Kowapradit J, Apirakaramwong A, Ngawhirunpat T, et al. (2012). Methylated N-(4-N,N-dimethylaminobenzyl) chitosan coated liposomes for oral protein drug delivery. Eur J Pharm Sci 47:359–66
- Le Dévédec F, Strandman S, Hildgen P, et al. (2013). PEGylated bile acids for use in drug delivery systems: enhanced solubility and bioavailability of itraconazole. Mol Pharm 10:3057–66
- Li P, Nielsen HM, Müllertz A. (2012). Oral delivery of peptides and proteins using lipid-based drug delivery systems. Expert Opin Drug Deliv 9:1289–304
- Liu J, Gong T, Wang C, et al. (2007). Solid lipid nanoparticles loaded with insulin by sodium cholate-phosphatidylcholine-based mixed micelles: preparation and characterization. Int J Pharm 340:153–62
- Mahalingam R, Ravivarapu H, Redkar S, et al. (2007). Transbuccal delivery of 5-aza-2 -deoxycytidine: effects of drug concentration, buffer solution, and bile salts on permeation. AAPS PharmSciTech 13;8:E55
- Malik DK, Baboota S, Ahuja A, et al. (2007). Recent advances in protein and peptide drug delivery systems. Curr Drug Deliv 4:141–51
- Mann JF, Scales HE, Shakir E, et al. (2006). Oral delivery of tetanus toxoid using vesicles containing bile salts (bilosomes) induces significant systemic and mucosal immunity. Methods 38:90–5
- Mann JF, Shakir E, Carter KC, et al. (2009). Lipid vesicle size of an oral influenza vaccine delivery vehicle influences the Th1/Th2 bias in the immune response and protection against infection. Vaccine 27:3643–9
- Martin SR, Schilstra MJ. (2008). Circular dichroism and its application to the study of biomolecules. Methods Cell Biol 84:263–93
- Martins S, Sarmento B, Ferreira DC, Souto EB. (2007). Lipid-based colloidal carriers for peptide and protein delivery-liposomes versus lipid nanoparticles. Int J Nanomedicine 2:595–607
- McGhee JR, Mestecky J, Dertzbaugh MT, et al. (1992). The mucosal immune system: from fundamental concepts to vaccine development. Vaccine 10:75–88
- Mikov M, Fawcett JP, Kuhajda K, Kevresan S. (2006). Pharmacology of bile acids and their derivatives: absorption promoters and therapeutic agents. Eur J Drug Metab Pharmacokinet 31:237–51
- Miyazaki S, Yamahira T, Nadai T. (1981). Effect of bile flow on indomethacin absorption in rats. Acta Pharm Suec 18:135–8
- Morimoto K, Nakai T, Morisaka K. (1987). Evaluation of permeability enhancement of hydrophilic compounds and macromolecular compounds by bile salts through rabbit corneas in vitro. J Pharm Pharmacol 39:124–6
- Neutra MR, Kozlowski PA. (2006). Mucosal vaccines: the promise and the challenge. Nat Rev Immunol 6:148–58
- Nguyen S, Alund SJ, Hiorth M, et al. (2011). Studies on pectin coating of liposomes for drug delivery. Colloids Surf B Biointerfaces 88:664–73
- Nightingale CH, Axelson JE, Gibaldi M. (1971). Physiologic surface-active agents and drug absorption. 8. Effect of bile flow on sulfadiazine absorption in the rat. J Pharm Sci 60:145–7
- Niu M, Lu Y, Hovgaard L, et al. (2012). Hypoglycemic activity and oral bioavailability of insulin-loaded liposomes containing bile salts in rats: the effect of cholate type, particle size and administered dose. Eur J Pharm Biopharm 81:265–72
- Niu M, Lu Y, Hovgaard L, Wu W. (2011). Liposomes containing glycocholate as potential oral insulin delivery systems: preparation, in vitro characterization, and improved protection against enzymatic degradation. Int J Nanomedicine 6:1155–66
- Niu M, Tan Y, Guan P, et al. (2014). Enhanced oral absorption of insulin-loaded liposomes containing bile salts: a mechanistic study. Int J Pharm 460:119–30
- Ogiso T, Iwaki M, Yoneda I, et al. (1991). Percutaneous absorption of elcatonin and hypocalcemic effect in rat. Chem Pharm Bull 39:449–53
- O'Reilly JR, Corrigan OI, O'Driscoll CM. (1994). The effect of mixed micellar systems, bile salt/fatty acids, on the solubility and intestinal absorption of clofazimine (B663) in the anaesthetised rat. Int J Pharm 109:147–54
- Parmentier J, Thewes B, Gropp F, Fricker G. (2011). Oral peptide delivery by tetraether lipid liposomes. Int J Pharm 415:150–7
- Pandey V, Golhani D, Shukla R. (2014). Ethosomes: versatile vesicular carriers for efficient transdermal delivery of therapeutic agents. Drug Deliv. [Epub ahead of print]
- Premanand B, Prabakaran M, Kiener TK, Kwang J. (2013). Recombinant baculovirus associated with bilosomes as an oral vaccine candidate against HEV71 infection in mice. PLoS One 8:e55536
- Pütz G, Schmider W, Nitschke R, et al. (2005). Synthesis of phospholipid-conjugated bile salts and interaction of bile salt-coated liposomes with cultured hepatocytes. J Lipid Res 46:2325–38
- Rath A, Glibowicka M, Nadeau VG, et al. (2009). Detergent binding explains anomalous SDS-PAGE migration of membrane proteins. Proc Natl Acad Sci USA 106:1760–5
- Saettone MF, Chetoni P, Cerbai R, et al. (1996). Evaluation of ocular permeation enhancers: in vitro effects on corneal transport of four β-blockers, and in vitro/in vivo toxic activity. Int J Pharm 142:103–13
- Sahdev P, Ochyl LJ, Moon JJ. (2014). Biomaterials for nanoparticle vaccine delivery systems. Pharm Res 31:2563–82
- Samstein RM, Perica K, Balderrama F, et al. (2008). The use of deoxycholic acid to enhance the oral bioavailability of biodegradable nanoparticles. Biomaterials 29:703–8
- Schubert R, Jaroni H, Schoelmerich J, Schmidt KH. (1983). Studies on the mechanism of bile salt-induced liposomal membrane damage. Digestion 28:181–90
- Semalty A, Semalty M, Rawat BS, et al. (2009). Pharmacosomes: the lipid-based new drug delivery system. Expert Opin Drug Deliv 6:599–612
- Shao Z, Mitra AK. (1994). Bile salt-fatty acid mixed micelles as nasal absorption promoters. III. Effects on nasal transport and enzymatic degradation of acyclovir prodrugs. Pharm Res 11:243–50
- Shukla A, Katare OP, Singh B, Vyas SP. (2010a). M-cell targeted delivery of recombinant hepatitis B surface antigen using cholera toxin B subunit conjugated bilosomes. Int J Pharm 385:47–52
- Shukla A, Bhatia A, Amarji B, et al. (2010b). Nano-bilosomes as potential vaccine delivery system for effective combined oral immunization against tetanus and hepatitis B. J Biotech 150:98–9
- Shukla A, Khatri K, Gupta PN, et al. (2008). Oral immunization against hepatitis B using bile salt stabilized vesicles (bilosomes). J Pharm Pharm Sci 11:59–66
- Shukla A, Singh B, Katare OP. (2011). Significant systemic and mucosal immune response induced on oral delivery of diphtheria toxoid using nano-bilosomes. Br J Pharmacol 164:820–7
- Singh P, Prabakaran D, Jain S, et al. (2004). Cholera toxin B subunit conjugated bile salt stabilized vesicles (bilosomes) for oral immunization. Int J Pharm 278:379–90
- Sinico C, Fadda AM. (2009). Vesicular carriers for dermal drug delivery. Expert Opin Drug Deliv 6:813–25
- Song KH, Chung SJ, Shim CK. (2005). Enhanced intestinal absorption of salmon calcitonin (sCT) from proliposomes containing bile salts. J Control Release 106:298–308
- Subuddhi U, Mishra AK. (2007). Effect of sodium deoxycholate and sodium cholate on DPPC vesicles: a fluorescence anisotropy study with diphenylhexatriene. J Chem Sci 119:169–74
- Sun J, Deng Y, Wang S, et al. (2010). Liposomes incorporating sodium deoxycholate for hexamethylmelamine (HMM) oral delivery: development, characterization, and in vivo evaluation. Drug Deliv 17:164–70
- Sun W, Zou W, Huang G, et al. (2008). Pharmacokinetics and targeting property of TFu-loaded liposomes with different sizes after intravenous and oral administration. J Drug Target 16:357–65
- Tengamnuay P, Mitra AK. (1990). Bile salt-fatty acid mixed micelles as nasal absorption promoters of peptides. II. In vivo nasal absorption of insulin in rats and effects of mixed micelles on the morphological integrity of the nasal mucosa. Pharm Res 7:370–5
- Tiwari SB, Amiji MM. (2006). Improved oral delivery of paclitaxel following administration in nanoemulsion formulations. J Nanosci Nanotechnol 6:3215–21
- Tiwari AK, Gajbhiye V, Sharma R, Jain NK. (2010). Carrier mediated protein and peptide stabilization. Drug Deliv 17:605–16
- Torchilin VP. (2005). Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov 4:145–60
- Vyas SP, Subhedar R, Jain S. (2006). Development and characterization of emulsomes for sustained and targeted delivery of an antiviral agent to liver. J Pharm Pharmacol 58:321–6
- Ward PD, Tippin TK, Thakker DR. (2000). Enhancing paracellular permeability by modulating epithelial tight junctions. Pharm Sci Technol Today 3:346–58
- Westergaard H. (1977). Duodenal bile acid concentrations in fat malabsorption syndromes. Scand J Gastroent 12:115–22
- Wilkhu JS, McNeil SE, Anderson DE, Perrie Y. (2013). Characterization and optimization of bilosomes for oral vaccine delivery. J Drug Target 21:291–9
- Yu JN, Zhu Y, Wang L, et al. (2010). Enhancement of oral bioavailability of the poorly water-soluble drug silybin by sodium cholate/phospholipid-mixed micelles. Acta Pharmacol Sin 31:759–64
- Zeng X, Tao W, Mei L, et al. (2013). Cholic acid-functionalized nanoparticles of star-shaped PLGA-vitamin E TPGS copolymer for docetaxel delivery to cervical cancer. Biomaterials 34:6058–67
- Zhang Z, Gao F, Jiang S, et al. (2013). Bile salts enhance the intestinal absorption of lipophilic drug loaded lipid nanocarriers: mechanism and effect in rats. Int J Pharm 452:374–81
