Abstract
The aims of the present study were to develop a colon-specific gel formulation of melatonin with sodium alginate and to evaluate its in vitro characteristics and intracolonic performance on oxidative stress parameters, such as nitric oxide (NOx), malondialdehyde (MDA) and glutathione (GSH) levels in rats with acetic acid-induced colitis. The melatonin-alginate gel formulations were prepared and their physico-pharmaceutical properties were determined. Formulation M5, which contained 3% of sodium alginate and 20% polyethylene glycol, was used for in vivo studies. The in vivo studies were conducted in rats with acetic acid-induced colitis. NOx, MDA and GSH levels were determined and histological investigations were performed. It was found that formulation M5 was the most suitable formulation for the colon-specific melatonin gel, in terms of pH, viscosity, drug release and mucoadhesion properties. The MDA levels in the tissues of Group 2 (treated with an intracolonic gel formulation without melatonin) were found to be significantly higher than in Group 1 (the untreated group). NOx levels decreased with the intracolonic and systemic melatonin treatment in the colitis-induced rats. Neither intracolonic nor intra-peritoneal (IP) melatonin treatment affected GSH levels. The epitelization of the colon tissues in groups administered with intracolonic melatonin, IP melatonin, and the intracolonic gel formulation without melatonin was much better than that found in the untreated group. It was concluded that melatonin participated in various defense mechanisms against the colonic inflammatory process, and that the dose, route and formulation type were the most important parameters in the effectiveness of melatonin.
Introduction
Inflammatory bowel disease (IBD), which includes Crohn’s disease and ulcerative colitis, is a chronic inflammatory disorder of the gastrointestinal tract. The exact pathogenesis of the disease is not fully understood, although interaction between immune factors, genetic suspectibility and the environment seem to be the most important elements (Triantafillidis & Triantafillidis, Citation2009). Oxidative stress produced by free radicals is one of the risk factors of the disease. Oxidative stress arises when there is a marked imbalance between the production of reactive oxygen species (ROS) and antioxidant levels (Kruidenier & Verspaget, Citation2002). High free-radical levels and low antioxidant capability are well-known characteristics of IBD (Lih-Brody et al., Citation1996).
Melatonin (N-acetyl-5-methoxytryptamine), a multifunctional molecule, is synthesized from l-tryptophan in the pineal gland and involved in the control of circadian and photoperiodic systems in mammals. High melatonin concentrations are found in the gastrointestinal tract and enterochromaffin cells have been suggested as the source of its synthesis (Bubenik et al., Citation1998). It is known that melatonin and its metabolites are potent antioxidants, both as direct free radical scavengers and as indirect antioxidants (Tan et al., Citation1994; Reiter et al., Citation2007; Rybka et al., Citation2014).
As ROS play a significant role in the pathogenesis of IBD (Head & Jurenka, Citation2004), the antioxidant properties of melatonin may have a protective effect in colonic inflammation.
Various studies conducted with rodents have shown that melatonin administration reduces the severity of colitis. These effects were attributed to a variety of mechanisms, including inhibition of nitric oxide (NOx) production, inhibition of COX-2 expression, inhibition of NF-kappa activation, reduction of colon immunological injury through regulation of macrophage activity, reduction of proinflammatory cytokines, reduction of bacterial translocation, reduction of matrix metalloproteinase-2 and -9 activity, and modulation of signal transduction pathways and apoptosis (Terry et al., Citation2009).
Necefli et al. (Citation2006) reported that melatonin administration reduced mucosal damage in rats that had 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis. It significantly decreased the elevation of malondialdehyde (MDA) levels, while increasing the GSH in the colonic tissue of these rats. The mechanism of the protection associated with melatonin was attributed to its anti-oxidant, antiapoptotic and anti-inflammatory effects. Similarly, the intracolonic administration of melatonin attenuates the colon injury macroscopically and microscopically, reduced the myeloperoxidase activity (MPO), and ameliorated the degree of lipid peroxidation of the colon in the TNBS-induced colitis in rats (Mei et al., Citation2002).
It has been demonstrated that pre-treatment with melatonin reduces the signs of colonic inflammation induced by acetic acid in rats. This effect was independent of the route of melatonin administration, and similar results were obtained after IP and after intracolonic administration. It was explained that melatonin participates in various defense mechanisms against colonic inflammatory processes by preserving the important endogenous antioxidant reserve of glutathione (GSH), and by preventing lysosomal enzyme disruption through inhibiting enhanced MPO activity, thus reducing the extent of colonic damage, mainly in the early phase of colitis. It may be a promising therapeutic agent for ulcerative colitis (Nosál’ova et al., Citation2007).
Tahan et al. (Citation2011) reported that intraperitoneally administered melatonin inhibited not only oxidant damage but also inflammatory cytokines, and improved the colonic inflammation induced by acetic acid in rats. It has a dual action as an effective anti-inflammatory and an antioxidant.
Mucoadhesive drug delivery systems work by increasing the duration of drug residence at the site of activity. Alginates are biodegradable, biocompatible and mucoadhesive polymers (Gombotz & Wee, Citation1998). Their mucoadhesive features may increase their utility as potential delivery vehicles for drugs targeted to mucosal tissues (Rajinikanth et al., Citation2003). The adhesion of an alginate gel to mucosal tissues localizes the drug and prolongs the duration of drug residence, thereby potentially improving the overall effectiveness.
The aims of the present study were to develop a colon-specific gel formulation of melatonin with sodium alginate and to evaluate its in vitro characteristics and intracolonic performance on oxidative stress parameters, such as NOx, MDA and GSH levels in rats with acetic acid-induced colitis. In studies evaluating the antioxidant efficacy, melatonin with an isotonic saline solution has been administered either intracolonically or through the IP route (Cuzzocrea et al., Citation2001; Mei et al., Citation2005; Marquez et al., Citation2006; Necefli et al., Citation2006; Nosál’ova et al., Citation2007). To our knowledge, no in vitro or in vivo intracolonic formulation study has been performed with melatonin. The present study aimed to prolong the residence time of melatonin by using an intracolonic administration of a gel formulation.
Materials and methods
Materials
The active ingredient melatonin (assay ≥ 98%) was obtained from Sigma-Aldrich Corp., St. Louis, MO, USA. It was stored at 2–8 °C. Sodium alginate (Protonal LF 10/60) was obtained from FMC Biopolymer, Philadelphia, PA, USA. The viscosity of the sodium alginate (1% in acetic acid) was measured with a viscometer (DV-III + Rheometer TC502, Brookfield, Middleboro, MA, USA) and found to be 650 ± 8 cP at 37 °C.
Other materials used for the in vitro and in vivo studies, such as polyethylene glycol 400 (PEG 400), sodium dihydrogen phosphate monohydrate, disodium hydrogen phosphate anhydrous, disodium hydrogen phosphate dihydrate and sodium chloride, were obtained from Merck, Darmstadt, Germany.
Animals
Wistar rats, male (n = 28), weighing 200–300 g were housed in clear plastic cages and fed with pellets and tap water.
In vitro studies
Preparation of the gel formulations
The sodium alginate type Protonal LF10/60 is used as a wound care agent (Hnin-Ei et al., Citation2012). Poly(ethylene glycol) (PEG) is an uncharged hydrophilic polymer. The PEGylation enhances the stability of drug delivery systems in digestive fluids and mucus, so that degradation is delayed (Lautenschläger et al., Citation2014). Based on this knowledge, alginate and PEG were chosen for the preparation of the gel formulations in this study.
Melatonin was mixed with PEG 400 for 5 min. A sufficient quantity of water and sodium alginate (2 or 3%) was added and mixed for 15 min. The mixture was kept in an ultrasonic bath for 5 min to discard air bubbles and a clear gel formulation was obtained. The composition of the gel formulations are given in .
Table 1. Composition of the gel formulations.
The melatonin solution administered by the IP route was prepared in a sterile saline solution containing PEG 400 (4%), just before administration. After the melatonin was completely dissolved, the intraperitonal solution was filtered through a membrane filter (pore size 0.22 µm).
pH measurements
pH values of the gel formulations were measured with a pH meter (Schott CG 840, Mainz, Germany) at room temperature 24 h after preparation.
Measurement of viscosity
The viscosity of the gel formulations was determined with a viscometer at a temperature of 25.0 ± 0.5 °C (DV-III + Rheometer TC-502 Brookfield, Middleboro, MA, USA). The spindle speed was adjusted to provide the highest percent torque value in the 10–100% range, as recommended by the Brookfield instruction manual. Spindle Nos 52 and 20 rpm were used for measuring viscosity.
Mucoadhesion measurements
TA-XTPlus Texture Analyzer (Stable Micro Systems, London, UK) equipped with a 5 kg load cell was used for the mucoadhesion test. The in vitro mucoadhesion testing method was based on the measurement of the force (N), work (N·mm) and work of mucoadhesion (mJ/cm2) needed to detach a sample of rat colonic mucosa from a gel formulation.
Testing conditions and instrumental parameters had been determined in a previous study (Tugcu-Demiröz et al., Citation2013).
Drug release studies
In vitro release of melatonin from gel formulations was carried out by USP dissolution apparatus I at a rotating speed of 50 rpm in 500 mL of a pH 7.4 phosphate buffer solution at 37 ± 0.5 °C. A 2 mL sample of each formulation was placed in dialysis tubing (Dialysis bag: D-0405, Sigma-Aldrich Corp., St. Louis, MO, USA, pore size of 12 000 Da) after one end was tightly tied. The other end was then sealed to obtain a bag which was immediately put into a basket. At pre-determined intervals, samples were withdrawn and melatonin concentrations were assayed spectrophotometrically at 278 nm.
The release profile obtained was evaluated in terms of release kinetics by applying zero order, first order and Higuchi kinetics.
Stability studies of the M5 gel formulation
The M5 gel formulation was chosen for further in vivo studies, because of the results of its in vitro characterization. Therefore, stability studies were carried out on solely the M5 gel formulation under conditions of 25 °C/60% relative humidity and 40 °C/75% relative humidity for a period of 2 months. Samples were drawn at regular intervals for stability analysis.
In vivo studies
The experimental protocol was approved by the Local Ethical Committee of Gazi University (G.Ü.ET-09-009).
After an overnight fast, colitis was induced in the rats (under xylazine (10 mg/kg) + ketamine (100 mg/kg) anesthesia) by intrarectal administration of 1 mL of acetic acid solution (acetic acid 3% in saline solution) with a 8-cm long cannula. The acetic acid solution was retained in the colon for 30 s. Thereafter, the rats were kept for 10 min in a Trendelenburg position to avoid reflux. The gel formulations were administered 24 h after induction of colitis. The rats were kept for 15 min in a Trendelenburg position after the administration of the gel formulations.
After induction of colitis, the rats were randomly allocated into four groups with six animals per group as follows:
Group 1: Untreated for 7 days
Group 2: Treated with intracolonic gel formulation without melatonin for 7 days
Group 3: Treated with intracolonic gel formulation containing melatonin (10 mg/kg) for 7 days
Group 4: Treated with IP melatonin solution (10 mg/kg) for 7 days
The test groups were sacrificed 7 days after treatment. The distal 12 cm of the colon was excised. While some of the samples were kept at −80 °C, the others were fixed in formalin for histological examination. Another group (Group 0) which was sacrificed 1 day after the colitis had been induced by acetic acid was used for histological studies only.
The levels of MDA, NOx and GSH in the colonic tissues were measured.
Determination of NOx levels
The NOx levels, the stable end products of NOx of the tissue homogenates, were determined by the Griess reaction (Green et al., Citation1982; Miranda et al., Citation2001). The tissue samples (0.1 g) were homogenized in a phosphate buffer (0.1 M) and then centrifuged at 3500 rpm for 15 min. Equivalent amounts of VaCl3 were added to the 0.20 mL of supernatant for the reduction of nitrate to nitrite by VaCl3 and left to incubate for 30 min at 37 °C. Then the sodium phosphate buffer and a mixture of equal amounts of Griess I (0.2% naphthalene diethylamine dihydrochloride) reactant and Griess II (2% sulphanilamide in 10% phosphoric acid (H3PO4)) reactant were added. After incubation for 10 min at 37 °C, the absorbance values of the samples were measured at 540 nm by spectrophotometry.
Determination of MDA levels
The MDA level, as the last step of lipid peroxidation, was measured in the tissues spectrophotometrically (Casini et al., Citation1986). The tissue samples (0.2 g) were homogenized in 10% trichloroacetic acid (1:9) and centrifuged at 4000 rpm for 20 min. 0.75 mL of supernatant was added to the solution of 1% butyl hydroxyl toluene in ethanol and 0.67% thiobarbituric acid. The solution was then left to boil over a water bath. The absorbance was measured at 535 nm by spectrophotometry.
Determination of GSH levels
The reduced GSH levels were measured in the tissue samples. The tissue samples (0.2 g) were homogenized under ice-cold conditions with a 10% trichloroacetic acid (1:9) solution in a tissue homogenizer, and centrifuged at 4000 rpm for 20 min. 0.25 mL of supernatant was added to Na2HPO4·2H2O and the DTNB solutions (0.4 mg/mL in 1% sodium citrate). The mixture was assayed spectrophotometrically at 410 nm, immediately after mixing (Aykac et al., Citation1985).
Histological investigations
Colonic mucosal tissues to be used in light microscope studies were fixed in 10% formalin. They were dehydrated in alcohol, cleared in xylene and embedded in paraffin blocks. Each section of tissue in the blocks was deparaffinated with xylene and rehydrated, before finally being stained with hematoxylin/eosin and examined under a light microscope.
Statistical analysis
The data were presented as a mean ± standard deviation (SD). Statistical analyses were carried out using the GraphPad InStat (GraphPad Software, Inc., La Jolla, CA, USA) program’s one-way analysis of variance. Values of p < 0.05 were considered to be significant.
Results
pH and viscosity measurements
The pH and viscosity values of the prepared gel formulations are shown in . All of them were transparent and had a homogeneous structure. The pH values of the formulations ranged from 6.9 to 7.1 and were assumed to be suitable for colonic administration.
Table 2. Results of pH and viscosity values of the gel formulations.
The rheologic properties of the gel formulations were also studied. All formulations showed pseudoplastic flow, presenting an immediate flow after stress application. An increase in the amount of sodium alginate in the gel formulations led to an increase in viscosity. The M5 gel formulation was found to have the highest viscosity value whereas M1 had the lowest viscosity value.
Mucoadhesion studies
The mucoadhesion test was performed to measure the adhesive strength of the gel formulations to the rats’ colonic mucosa. The results obtained for maximum detachment force (Fmax), work of adhesion (Wad) and work of mucoadhesion (Wmucoad) are shown in . The highest values were determined in the M5 formulation that included sodium alginate (3%) and PEG 400 (20%).
Table 3. Mucoadhesion values of the gel formulations using texture analyzer with rat colonic mucosa (n = 6).
In vitro drug release studies
The drug release profiles from the gel formulations are depicted in and the main release kinetic characteristics of the gel formulations are gathered in . All the formulations showed similar release profiles except for the M1 formulation. About 96–99% of drug was released from the M2-M5 formulations at the end of 6 h.
Figure 1. Release profiles of melatonin from gel formulations. The data represent the mean ± confidence interval, n = 6.
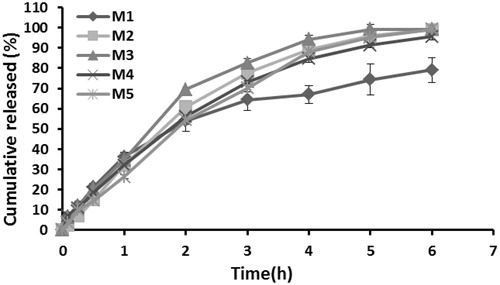
Table 4. Kinetic assessment of the release data of melatonin from gel formulations.
Stability studies
The results of the stability studies of the M5 gel formulation in two different test conditions are shown in . The prepared M5 gel formulation presented good stability in terms of viscosity and drug content. On the other hand, no macroscopical physical changes were observed during storage. It was observed that its viscosity value was varied, but its fluctuations remained within the limits of application. It was found that the M5 formulation was the most suitable formulation for a colon-specific MT gel in terms of pH, viscosity, release and mucoadhesion properties. Therefore, the in vivo efficacy of the M5 gel formulation was investigated in rats with the acetic acid induced colitis.
Table 5. The stability test results of the M5 gel formulation.
In vivo studies
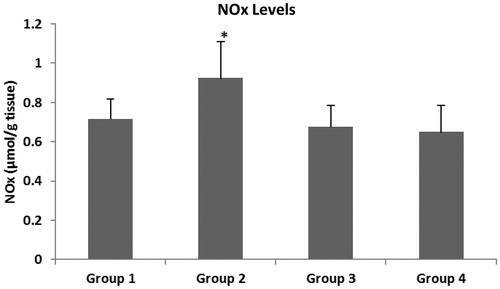
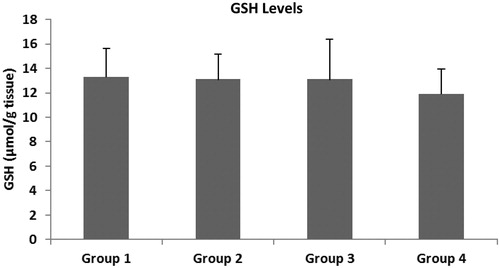
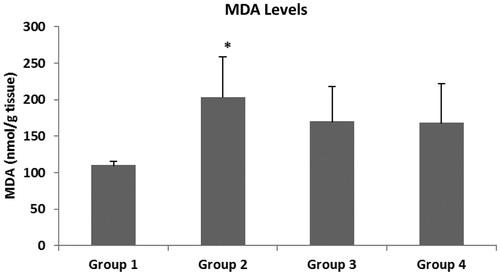
The main oxidative stress parameters were evaluated in the colonic tissue of the rats. show the MDA, NOx and GSH levels. It is known that a high level of MDA in tissues shows an increase of oxidative stress. Tissue MDA levels in Group 2 were found to be significantly higher than in Group 1 (p < 0.01). There was no statistically significant difference between Group 3 and Group 4 in terms of MDA levels of colon tissues (p > 0.05). It was observed that the NOx level, which is an indicator of oxidative stress, increased in colitis-induced rats, whereas it decreased after treatment with intracolonic and systemic melatonin. Neither intracolonic nor IP melatonin treatment affected the GSH level.
Figure 2. MDA levels in colonic mucosa. The data represent the mean ± SD, n = 6 (*p < 0.01, versus to Group 1).
Histological studies
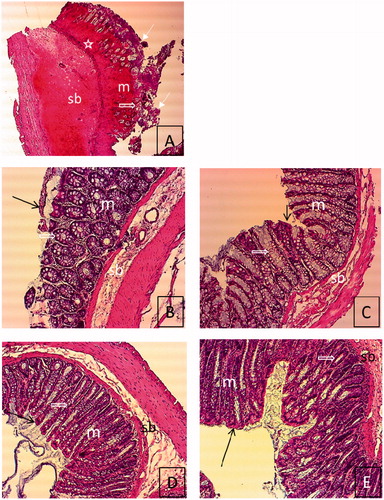
shows the histological appearance of the colonic tissues, under a light microscope. We observed a massive loss of epithelium, severe inflammatory cell infiltration, distortion of intestinal crypt and mucosal and submucosal edema in the rats where colitis had been induced by acetic acid (Group 0, ). In contrast, we found improvement in the structure of the intestinal crypt and inflammation, and disappearance of edema in other groups (Group 1–4, ). Epitelization of the tissue in Groups 2, 3 and 4 was observed to be better than in Group 1.
Figure 5. Histological features of rat colon sections in different groups. (A) Tissue image of group 0. (B) Tissue image of group 1, (C) Tissue image of group 2, (D) Tissue image of group 3, (E) Tissue image of group 4. Degeneration of epithelium and intestinal crypt, severe inflammatory cell infiltration, mucosal and submucosal edema in group 0 (A) (H&E. 4 × 10). Improvement in the structure of the intestinal crypt, incompleted epitelization, no edema in mucosa and submucosa in group 1 (B) (H&E. 10 × 10). Regular epithelium, improvement of the structure of the intestinal crypt, and no edema in mucosa and submucosa in Groups 2–4 (C–E) (H&E. 10 × 10). Epithelium (![]()
Discussion
In vitro studies
Colonic gels with various amounts of sodium alginate and PEG were formulated in order to modulate their rheological and mucoadhesive properties. The results of preliminary experiments allowed for the optimal amounts of both polymers to be used (data not shown). The physicopharmaceutical properties of the prepared gel formulations were suitable in terms of pH and viscosity as well as in macroscopic appearance.
Due to its bioadhesive property and high viscosity, the gel formulation was expected to remain in the colon and to release the drug over a relatively long period of time, thus enhancing the therapeutic effect. Mucoadhesion tests were carried out to measure the adhesive strength of the gel formulations. The experimental parameters were determined by preliminary tests on the basis of the literature (Tugcu-Demiroz et al., Citation2013) and then they were validated. The bioadhesion of the M1 gel formulation containing the least amount of alginate and PEG could not be measured: this system displayed no adhesion. In comparison with the remaining formulations, it was found that the M5 formulation, comprising the highest amount of polymers, had the greatest mucoadhesion value (). The combination of both polymers contributed to an increase in mucoadhesion work. The binary system exhibited good adhesive properties.
The experimental technique was used for the in vitro release study of melatonin from gel formulations, which had emanated from a previously published method (Koffi et al., Citation2008). The results of in vitro release studies showed that about 79% of the drug was released during 6 h for the formulation M1, while a 95.6–99.3% release was obtained at 6 h for the other four formulations. Sodium alginate slowed down the in vitro release of melatonin. The developed formulations exhibited a sustained release of drug over a period of 6 h, thus increasing the residence time of the drug. The increase in the PEG ratio increased the release of melatonin from the gel formulations.
The data of percent released versus time were assessed for evaluation of the release kinetics. Upon checking the results according to r2, it was found that Q√t was the most suitable kinetic equation. The comparison of the release constants showed that the M1 formulation had the lowest values, whereas the others had higher values that were similar to each other. The kinetic assessment of release data revealed that the release of the active ingredient can be assumed to be diffusion-controlled.
The results of in vitro studies results showed that the M5 formulation was suitable for colonic administration in rats, according to its in vitro release profile, physicochemical and mucoadhesive properties. Moreover, it was assumed that this formulation was also appropriate in terms of stability. Therefore, further in vivo studies were performed with the M5 formulation.
In vivo studies
Melatonin is effective in the prevention or treatment of gastric or colonic injury, and it seems to participate in various defense mechanisms against colonic inflammatory processes (Tan et al., Citation2002; Nosál’ova el al., Citation2007; Triantafillidis & Triantafillidis, Citation2009; Tahan et al., Citation2011). While it has been suggested that melatonin acts as an agent protecting the gastrointestinal mucosa, the mechanisms of the protective effects of melatonin have not been fully elucidated. In the present study, a mucoadhesive alginate-PEG gel formulation containing melatonin was prepared. The effects of its performance on oxidative stress parameters, such as NOx which are the final stable products of NOx, MDA which is the final product of lipid peroxidation and the antioxidant GSH levels. These parameters were measured and evaluated in order to understand the effects of melatonin on oxidative stress in rats with acetic acid induced colitis.
Acetic acid was used in varying ratios from 2 to 8% in the literature (Fabia et al., Citation1992; Nosál’ova et al., Citation2007; Oruç et al., Citation2008). We applied various ratios of acetic acid (2, 3, 4%) to the rats and investigated the colonic tissues macroscopically and histopathologically after 24 h. After the evaluation of the macroscopic and microscopic data (not shown), it was decided that the 3% acetic acid ratio was suitable for the induction of colitis. Intrarectal administration of 3% acetic acid caused macroscopic damage of the colon. The colonic mucosa appeared hemorrhagic and ulcerated. The literature shows that a similar acetic acid ratio has been used in other studies for the induction of colitis (Millar et al., Citation1996; Tahan et al., Citation2011).
The melatonin doses administered to rats in various studies vary over a wide range from 2.5 mg/kg to 100 mg/kg (Li et al., Citation2005). The duration of treatment also varies widely and ranges from 48 h to 27 days (Terry et al., Citation2009). After review of the literature, a moderate dose (10 mg/kg) was chosen and administered to the rats for seven days in the present study. At the end of the treatment, colonic tissue samples were evaluated macroscopically. At the end of the seventh day, the macroscopic properties of all groups were observed to be similar. This situation was similar to the findings of Fabia et al. (Citation1992).
An increase in lipid peroxidation level is expected following injury, due to an increased oxidative stress. Our results were evaluated in terms of MDA, and no significant difference was observed between Groups 3 and 4 ( and ). This finding suggested that either IP or intracolonic administration of melatonin was not effective in scavenging the ROS in colon tissues. However, the MDA level of Group 2 (203 nmol/g tissue) was not significantly different from that of Group 3 (170 nmol/g tissue) either, but there was a reduced MDA level in Group 3. The decreased MDA level was found in the intracolonic melatonin administered group (Group 3). This finding showed that intracolonic melatonin administration has a protective effect on colon cells against increased lipid peroxidation. This decrease in MDA may have arisen due to administration time and the dose of melatonin.
Table 6. NOx, MDA and GSH levels in colonic mucosa (mean ± confidence interval).
Cells have been known to be hypersensitive to hypertonic media and to show an increased oxidative damage (Koziol et al., Citation2005). In the present study, colon cells encounter multiple stress conditions, such as acetic acid-induced colitis and hyperosmotic stress with intracolonic gel containing Na-alginate. This specific condition stimulated the generation of ROS. Increased ROS triggered lipid peroxidation in the colon cells in Group 2. We also suggested that the damaged cells in the colitis model may cause water loss due to the water-retaining properties of Na-alginate. Therefore, hyperosmotic stress could be increased in the damaged colon cells. This study demonstrated that a hypertonic medium (containing Na-alginate gel) increased the generation of superoxide and other reactive species in colon cells in Group 2. The combined effect of a hypertonic medium and a breakdown of the oxidant-antioxidant balance in the damaged cells may have affected the generation of superoxide and nitrosative reactive species, and therefore increased the lipid peroxidation of colon tissue.
Glutathione peroxidase and MDA levels of damaged tissues were investigated in a study conducted on 47 patients with ulcerative colitis and Crohn’s disease (Tüzün et al., Citation2002). Similar to our study, no significant difference in MDA levels were observed. It was reported that free radicals were removed without causing lipid peroxidation.
It was observed that the NOx level, which is an indicator of oxidative stress, increased in our colitis-induced rats, whereas it decreased with the treatment of intracolonic and systemic melatonin, but this decrease was insignificant. However, a significant difference was determined between Groups 2 and 3 ( and ). The administration of the gel formulation with melatonin decreased the NOx level compared to that of the formulation without melatonin.
The NO level is at its highest in the inflammatory phase of the wound healing process. It is known that NO is produced by many different cell types in wounded tissue by three different isotypes of NOx synthase. Inducible NO (iNOS) is one of these isotypes. Fibroblasts, macrophages, keratinocytes and platelets produce NO by iNOS (Frank et al., Citation2002; Schwentker et al., Citation2002). When compared to the NOx levels of Group 1, the NOx levels of Group 2 were found to be significantly higher (p < 0.05). In other words, intracolonic Na-alginate administration without melatonin increased the NOx level in the colon cells. As mentioned before, in the presence of Na-alginate hyperosmotic stress could be increased in the damaged colon cells, which may affect the generation of superoxide and nitrosative reactive species. Intracolonic Na-alginate formulation may, therefore, have altered the colon tissue NOx levels in Group 2 (0.924 µmol/g tissue). There were no significant differences between the NOx levels of Group 3 (0.676 µmol/g tissue) and Group 4 (0.648 µmol/g tissue). This finding suggested that neither the IP administration of melatonin nor the intracolonic administration of melatonin changed the colon tissue’s NOx levels. However, the NOx level of Group 2 was significantly different from those of both Group 3 and 4. These findings bring to mind two possible cases: the first of these is that, both intracolonic melatonin formulation and IP administration of melatonin were successful in reducing the NOx levels when compared to Group 2. The second of these is that, intracolonic melatonin administration has revealed the protective effect of melatonin in reducing the tissue NOx levels which increased in the presence of Na-alginate. Both intracolonic melatonin gel formulation and IP administration of melatonin decreased the levels of NOx in colon cells, and it is known that melatonin administration inhibits NOx levels due to its antioxidant properties. Our results showed similarity with the results of previously conducted studies (Mei et al., Citation2005).
GSH is a primary component of physiological systems protecting against free-radical damage (Casini et al., Citation1986; Tahan et al., Citation2011). In our findings, when considered in terms of GSH levels, a significant difference was not observed among the groups ( and ). GSH is an antioxidant itself, and local and systemic administration of melatonin had no effect on the level of GSH. This finding may suggest that other antioxidants, such as SOD, vitamin C and vitamin E may scavenge the generation of ROS or nitrosative species, and melatonin may act directly as a powerful antioxidant when there is no opportunity to use other antioxidants.
Although a wound healing effect of sodium alginate has been reported (Paul & Sharma, Citation2004), its positive effect on colon damage was not observed in the present study. Alginate gel showing oxidative effects itself significantly increased the lipid peroxidation and the NOx levels, thus hiding the protective effect of melatonin was hidden.
One of the important findings of this study was that there was a parallelism between altering tissue MDA and NOx levels in all groups ( and ).
When evaluated in vivo experiments and observations it can be said that interference in the colon via a canula can create an additional stress effect. In this case, the dose and the duration of treatment of melatonin were insufficient for such topical application. Unlike the situation with a wound incision, the integrity of the tissues was excessively disrupted in the acetic acid-induced colitis rat, and consequently, the use of melatonin by these cells was significantly decreased and the protective effect of melatonin could not be observed. It was thought that in such studies, pre-treatment with melatonin for a certain time, to raise the antioxidant capacity of the tissues before the induction of colitis, may be suitable.
Histological investigations concluded that the epitelization of the colon tissues in intracolonic melatonin, IP melatonin and intracolonic gel formulation without melatonin administered groups was much better than in the untreated groups. Improvement in the structure of the intestinal crypt and inflammation, and disappearance of edema were observed to be the same in groups which were untreated (Group 1), treated with gel without melatonin (Group 2), given intracolonic treatment (Group 3), and given systemic melatonin (Group 4).
Conclusion
It was concluded that melatonin participated in various defense mechanisms against colonic inflammatory processes, but the dose, route and formulation type were the most important parameters in establishing the effectiveness of melatonin.
Acknowledgements
We would like to thank FMC Biopolymer (USA) for the gift samples of sodium alginate (Protonal LF 10/60).
Declaration of interest
This study was supported by a research grant from Gazi University Research Foundation (BAP 02/2009-10).
References
- Aykac G, Uysal M, Yalcin AS, et al. (1985). The effect of chronic ethanol ingestion on hepatic lipid peroxide, glutathione, glutathione peroxidase and glutathione transferase in rats. Toxicol 36:71–6
- Bubenik GA, Blask DE, Brown GM, et al. (1998). Prospects of the clinical utilization of melatonin. Biol Signals Recept 7:195–219
- Casini A, Ferrali M, Pompelam A, et al. (1986). Lipid peroxidation and cellular damage in extrahepatic tissues of bromobenzene intoxicated mice. Am J Pathol 123:520–31
- Cuzzocrea S, Mazzon E, Serraino I. (2001). Melatonin reduces dinitrobenzene sulfonic acid-induced colitis. J Pineal Res 30:1–12
- El-Kamel AH, Abdel-Aziz AA, Fatani AJ, et al. (2008). Oral colon targeted delivery systems for treatment of inflammatory bowel diseases: synthesis, in vitro and in vivo assessment. Int J Pharm 358:248–55
- Fabia R, Willen R, Ar’Rajab A, et al. (1992). Acetic acid-induced colitis in the rat: a reproducible experimental model for acute ulcerative colitis. Eur Surg Res 24:211–25
- Frank S, Kampfer H, Wetzler C, et al. (2002). Nitric oxide drives skin repair: novel functions of an established mediator. Kidney Int 61:882–8
- Gombotz WR, Wee SF. (1998). Protein release from alginate matrices. Adv Drug Del Rev 31:267–85
- Green LC, Wagner DA, Glogowski J, et al. (1982). Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 1126:131–8
- Head KA, Jurenka JS. (2004). Inflammatory bowel disease part II: Crohn’s disease-pathophysiology and conventional and alternative treatment options. Altern Med Rev 9:360–401
- Hnin-Ei Thu, Mohd HZ, Shiow-Fern N. (2012). Alginate based bilayer hydrocolloid films as potential slow-release modern wound dressing. Int J Pharm 434:375–83
- Koffi AA, Agnely F, Besnard M, et al. (2008). In vitro and in vivo characteristics of a thermogelling and bioadhesive delivery system intended for rectal administration of quinine in children. Eur J Pharm Biopharm 69:167–75
- Koziol S, Zagulski M, Bilinski T, et al. (2005). Antioxidants protect the yeast Saccharomyces cerevisiae against hypertonic stress. Free Radic Res 39:365–71
- Kruidenier L, Verspaget HW. (2002). Oxidative stress as a pathogenic factor in inflammatory bowel disease – radicals or ridiculous? Aliment Pharmacol Ther 16:1997–2015
- Lautenschläger C, Schmidt C, Fischer D, et al. (2014). Drug delivery strategies in the therapy of inflammatory bowel disease. Adv Drug Deliv Rev 71:58–76
- Li JH, Yu JP, Yu HG. (2005). Melatonin reduces inflammatory injury through inhibiting NF-κB activation in rats with colitis. Mediators Inflamm 4:185–93
- Lih-Brody L, Powell SR, Collier KP. (1996). Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci 41:2078–86
- Marquez E, Sanchez-Fidalgo S, Calvo JR, et al. (2006). Acutely administered melatonin is beneficial while chronic melatonin treatment aggravates the evolution of TNBS-induced colitis. J Pineal Res 40:48–55
- Mei Q, Xu JM, Xiang L, et al. (2005). Change of nitric oxide in experimental colitis and its inhibition by melatonin in vivo and in vitro. Postgrad Med J 81:667–72
- Mei Q, Yu JP, Xu JM, et al. (2002). Melatonin reduces colon immunological injury in rats by regulating activity of macrophages. Acta Pharmacol Sin 23:882–6
- Millar AD, Rampton DS, Chander CL, et al. (1996). Evaluating the antioxidant potential of new treatments for inflammatory bowel disease using a rat model of colitis. Gut 39:407–15
- Miranda KM, Espey MG, Wink DA, et al. (2001). Simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5:62–71
- Necefli A, Tulumoğlu B, Giriş M. (2006). The effect of melatonin on TNBS-induced colitis. Dig Dis Sci 51:1538–45
- Nosál'ová V, Zeman M, Cerná S, et al. (2007). Protective effect of melatonin in acetic acid induced colitis in rats. J Pineal Res 42:364–70
- Oruç N, Kutluana U, Sezak M, et al. (2008). Effects of leflunomide in acetic acid-induced acute colitis in rats. Akademik Gastroenteroloji Dergisi 7:67–72
- Paul W, Sharma CP. (2004). Chitosan and alginate wound dressings: a short review. Trends Biomater Artif Organs 18:18–23
- Rajinikanth PS, Sankar C, Mishra B. (2003). Sodium alginate microspheres of metoprolol tartrate for intranasal systemic delivery: development and evaluation. Drug Deliv 10:21–8
- Reiter RJ, Tan DX, Terron MP, et al. (2007). Melatonin and its metabolites: new findings regarding their production and their radical scavenging actions. Acta Biochim Pol 54:1–9
- Rybka J, Kedziora-Kornatowska K, Kupczyk D, et al. (2014). Antioxidant effect of immediate-versus sustained-release melatonin intype 2 diabetes mellitus and healthy controls. Drug Deliv, Early Online: 1–4. DOI: 10.3109/10717544.2014.917343
- Schwentker A, Vodovotz Y, Weller R, et al. (2002). Nitric oxide and wound repair: role of cytokines? Nitric Oxide 7:1–10
- Tahan G, Gramignoli R, Marongiu F, et al. (2011). Melatonin expresses powerful anti-inflammatory and antioxidant activities resulting in complete improvement of acetic-acid-induced colitis in rats. Dig Dis Sci 56:715–20
- Tan DX, Reiter RJ, Chen LD, et al. (1994). Both physiological and pharmacological levels of melatonin reduce DNA adduct formation induced by the carcinogen safrole. Carcinogenesis 15:215–18
- Tan DX, Reiter RJ, Manchester LC, et al. (2002). Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem 2:181–97
- Terry PD, Villinger F, Bubenik GA, et al. (2009). Melatonin and ulcerative colitis: evidence, biological mechanisms, and future research. Inflamm Bowel Dis 15:134–40
- Triantafillidis A, Triantafillidis JK. (2009). Melatonin: a potent antioxidant agent with anti-inflammatory and anti-apoptotic effects that might be useful in the treatment of IBD patients. Ann Gastroenterol 22:10–12
- Tugcu-Demiroz F, Acarturk F, Erdogan D. (2013). Development of long-acting bioadhesive vaginal gels of oxybutynin: formulation, in vitro and in vivo evaluations. Int J Pharm 457:25–39
- Tüzün A, Erdil A, Inal V, et al. (2002). Oxidative stress and antioxidant capacity in patients with inflammatory bowel disease. Clin Biochem 35:569–72