Abstract
In the present study, nanostructured lipid carriers (NLCs) along with various surfactants loaded with paclitaxel (PTX) were prepared by an emulsification technique using a Box-Behnken design. The Box-Behnken design indicated that the most effective factors on the size and PDI were at high surfactant concentration (1.5%), low lipids ratio (6:4) and medium homogenization speed (6000 rpm). Among all the formulations, Tween 20-loaded NLCs show least particle size compared to Tween 80 and Tween 60. Entrapment efficiency of Tween 20, Tween 80 and Tween 60-loaded formulations were 82.40, 85.60 and 79.78%, respectively. Drug release of Tween 80, Tween 20 and Tween 60-loaded NLCs is 64.9, 62.3 and 59.7%, respectively (within 72 h). Maximum cellular uptake was observed with Tween 20 formulation on Caco-2 cell lines. Furthermore, spray drying of resultant NLCs was showed good flow properties and was selected for drug delivery to deeper airways. In-vivo studies demonstrated the better localization of drug within the lungs using different surfactant-based pulmonary delivery systems. From this study, we have concluded that delivering drugs through pulmonary route is advantageous for local action in lungs as maximum amount of drug concentration was observed in lungs. The surfactants could prove to be beneficial in treating drug resistance lung cancer by inhibiting P-gp efflux in the form of nano lipidic carriers.
Introduction
Paclitaxel (PTX) is a mitotic inhibitor used in cancer chemotherapy. Paclitaxel is now widely used in the treatment of different types of cancer including lung, ovarian, breast, head and neck cancer, and advanced forms of Kaposi’s sarcoma (Chaudhary et al., Citation2014; Gagandeep et al., Citation2014). Cancer treatment found to be more challenging with the consequences of chemo-resistance (Garg, Citation2014). Anticancer drugs that cause MDR mostly are hydrophobic in nature with amphipathic character including taxanes (PTX and docetaxel), vinca alkaloids (vinorelbine, vincristine and vinblastine), anthracyclines (doxorubicin, daunorubicin and epirubicin), epipodophyllotoxins (etoposide and teniposide), etc.
Currently, PTX is commercially available as Taxol in a vehicle composed of 1:1 of Cremophor EL (CrEL) and ethanol to enhance water solubility of the drug. Some serious side effects associated with CrEL include hypersensitivity reactions, nephrotoxicity and neurotoxicity. In addition, Taxol has short-term physical stability upon dilution due to the precipitation of PTX in the aqueous media. Other problems with ethanol and CrEL include leaching of plasticizer from polyvinyl chloride infusion bags and sets during injection of the drug (Kim et al., Citation2006). Due to the above-aforementioned limitations, there is a need for new alternative formulations that can overcome poor aqueous solubility of PTX, deliver the drug more specifically to the target organs, and produce fewer side effects.
Several approaches, such as parenteral emulsions (Singh et al., Citation2014c), liposomes (Garg & Goyal, Citation2014b), nanoparticles complex with cyclodextrin (Singh et al., Citation2014b), water soluble prodrugs (Singh et al., Citation2014a), and polymeric micelles (Sharma et al., Citation2014c) have been employed to deliver PTX into the body or targeted tissue (Emami et al., Citation2012; Sharma et al., Citation2014b). Liposomal encapsulation enables increased antitumor efficacy (Sharma et al., Citation2014a) and decreased toxicity (Rohilla et al., Citation2014) due to higher accumulation of drug in tumors (Hao et al., Citation2005). In spite of these advantages, liposomes display some storage (Pabreja et al., Citation2014) and drug leakage problems (Morie et al., Citation2014) and a relatively high cost for large-scale production (Wong et al., Citation2007; Modgill et al., Citation2014). Stable formulations are required to take full advantage of the more specific drug distribution into solid tumors (EPR effect) (Marwah et al., Citation2014) and to maximize cell exposure (Drummond et al., Citation2010; Malik et al., Citation2014). The use of solid lipids in nanoparticle preparations is a very attractive alternative for overcoming some of the limitations associated with liposomes and has attracted increasing attention to the possible use of solid lipid nanoparticles (SLN) and related carriers since the 1990s (Muèller et al., Citation2000; Mehnert & Mäder, Citation2001; Kaur et al., Citation2014i). Solid lipid nanoparticles are sub-micron particles made from biocompatible lipids that are solid at room and body temperature (Muèller et al., Citation2000; Kaur et al., Citation2014h). When compared with liposomes, SLNs have a slower degradation rate in vivo (Kaur et al., Citation2014g), providing better control of drug release (Kaur et al., Citation2014f) and superior protection of the incorporated drug (Vivek et al., Citation2007; Kaur et al., Citation2014e). Furthermore, these systems are easily and cost-effectively produced on a large scale by high pressure homogenization techniques (HPH) already available in the pharmaceutical industry for the production of parenteral O/W emulsions (Muèller et al., Citation2000; Kaur et al., Citation2014d).
However, SLNs are associated with some potential limitations, namely, limited drug loading and drug expulsion during storage due to the crystallization of lipid matrix or lipid polymorphism (Kaur et al., Citation2014c). To overcome these limitations, nanostructured lipid carriers (NLCs) by mixing solid lipids with chemically very different liquid lipids (oils) have been developed to improve drug loading and release properties of conventional SLNs (Kaur et al., Citation2014b). By using special mixtures of solid lipids and liquid lipids the particles become solid after cooling but do not crystallize, which leads to more imperfections in the crystal and higher drug loading (Müller et al., Citation2002a; Kaur et al., Citation2014a). Nanostructured lipid carriers are an improved generation of SLNs produced by the controlled mixing of solid lipids with liquid lipids (Müller et al., Citation2002a; Kataria et al., Citation2014). These particles display improved drug incorporation and release (Joshi et al., Citation2014). The advantages associated with using SLN and NLC for cancer chemotherapy were recently reviewed by Wong et al. (Citation2007).
The objective of the present study was to develop surfactant-based NLCs for pulmonary delivery of PTX to the lung cancer cells. In this work for preparation of NLCs, oleic acid (OA) has been used as liquid lipid and glyceryl monostearate as solid lipid base. Cremophor EL was used as a component of marketable formulations of PTX (Taxol); however, this formulation was found to be toxic. Usually, non-ionic surfactants can improve dissolution in water insoluble moieties because of being highly hydrophobic and comparatively lesser toxicity to biological membranes (Rege et al., Citation2002; Johal et al., Citation2014). Further research reveals the capability of Tweens to inhibit efflux pumps. Lo (Citation2003) has established that Tween 20, Tween 80, Myrj 52 and Brij 30 enhance the epirubicin transport and decrease in efflux of diffusion chambers with excised rat intestinal mucosa. P-gp inhibition action is based on two essential parameters, i.e. surfactant concentration and hydrophilic–lipophilic balance (HLB) (Goyal et al., Citation2014b; Hussain et al., Citation2014). Surfactant concentrations which are non-hazardous to the intestinal mucosa may be usually preferred for the inhibition of P-gp (Goyal et al., Citation2014a). Greater than the crucial micelle concentration (CMC) surfactants become much beneficial in the solubilization of lipophilic substrates, while they would gave double achievement of solubilizing lipophilic drugs and inhibiting efflux (Goyal et al., Citation2013b). Though, the prototype of P-gp inhibition alters on the basis of the kind of excipients used (Garg et al., Citation2014c). On the basis of research, P-gp inhibitory activity raises when CMC is achieved and after CMC there is a failure of the inhibitory activity lead to drug (P-gp substrate) entrapment in the micelles (Garg et al., Citation2012b). In a further development, inhibitory action raises yet beyond CMC and this could be accredited to the information that substrate entrapped in micelles avoid P-gp-mediated efflux. The most advantageous HLB range of surfactant systems among appropriate hydrocarbon chains and polar part is a significant issue in designing potential drug formulations. The most favorable development on the intracellular aggregation of drug was attributing among intermediary HLB range of 10–17 (Lo, Citation2003; Goyal et al., Citation2013a).
Therefore, it is postulated that surfactant containing NLCs may concentrate in cancerous cells with high level of p-gp in drug resistance. After inhalation of the NLCs by pulmonary route, surfactant base of the nanoparticles inhibit p-gp for reversal of drug resistance (Garg et al., Citation2013). Nanoemulsions are in liquid form and undergo instabilities, such as flocculation, drug release, creaming, gelling and aggregation of particles; whereas, NLCs combine the advantages of polymeric nanoparticles, fat emulsions and liposome (Nishiyama & Kataoka, Citation2006; Garg et al., Citation2014b). But in our study, using surfactant simply inhibits p-gp confirmed by maximum cellular uptake on Caco-2 cell lines. Maximum cellular uptake of NLCs due to use on hydrophilic surfactants (Garg et al., Citation2014a), because hydrophilic surfactant solubilized the nanoparticles and gave smaller particle size (Garg et al., Citation2012a). Smaller particle size mediates internalization of the drug to the cancerous cells. In this circumstance, the drug is largely released inside the cancer cells resulting in enhanced efficiency (Garg & Goyal, Citation2014a).
The Box-Behnken design was used to optimize and evaluate the effect of different processing variables on characteristics of the NLCs. In aqueous dispersed form, NLCs show an increase in particle size after a short period of time. Hence, in a subsequent study, we aimed to increase the stability of NLCs using spray drying to convert into dry powder form, so as to easily deliver through pulmonary route. Moreover, we evaluated the organ distribution of drug incorporated in NLCs and compared with those of free drug solutions using the HPLC method.
Materials and methods
Materials
Glyceryl monostearate, oleic acid, Tween 80, Tween 20, Tween 60, heparin, l-leucine, mannitol sodium hydroxide were obtained from CDH Laboratory Reagents (New Delhi, India). Acetic acid glacial, ammonium acetate, fetal bovine serum, methanol, RPMI 1640 medium, trypsin-EDTA solution and Potassium dihydrogen phosphate were purchased from HIMEDIA (Mumbai, India). HPLC grade acetonitrile and methanol from Rankem Laboratory reagents (New Delhi, India). Human intestinal cancer cells (Caco-2) were obtained from NCC (Pune, India). Paclitaxel was purchased from Emcure Pharmaceuticals (Pune, India).
Methods
Preparation of NLC using an emulsification and ultrasonication method
Paclitaxel-loaded nanostructured lipid carriers (NLCs) were prepared by the emulsification and ultrasonication method (Vohra et al., Citation2013). The lipid and aqueous phases were prepared separately. The solid lipid/liquid lipid phase consisted of stearic acid (or glyceryl monostearate) and oleic acid at different ratios. Aqueous phase (20 ml) was prepared by taking desired amount of surfactants (Tween 80, Tween 20, Tween 40), and it was dissolved in water with continuous stirring under magnetic stirring (0683, SPINOT Ambala, Haryana, India) at low revolutions per minute. Lipid phase was prepared by taking desired amount of lipid (glyceryl monostearate and oleic acid) that was heated at 70 °C. Paclitaxel being insoluble in water was added to lipid phase and dissolved. Both aqueous phase and lipid phase were heated separately at 60–70 °C, and then aqueous phase was poured into lipid phase at the same temperature. This solution was allowed to homogenize for 3 min at 500 rpm, and this emulsion was immediately subjected to further size reduction using a probe sonicator (40050, Steryl Medi Equip System, Chennai, India). Emulsion was probe sonicated at 60% amplitude for time 4 min. The period of ultrasound burst was set 9 s with a pause of 3 s between two ultrasound bursts. Obtained solution was refrigerated at 2–3 °C for 15 min.
Experimental design and analysis
In this study, a 17-run, 3-factor, 3-level Box-Behnken design was employed to construct polynomial models for the optimization process, because it requires few runs with 3 or 4 variables. The Box-Behnken design was specifically selected since it requires fewer runs than a CCD in cases of three or four variables. This cubic design is characterized by set of points lying at the midpoint of each edge of a multidimensional cube and centre point replicates (n=3) whereas the “missing corners” help the experimenter to avoid the combined factor extremes. This property prevents a potential loss of data in those cases. A design matrix comprising of 17 experimental runs was constructed. The non-linear computer-generated quadratic equation is given as:
where, Y is the measured response associated with each factor level combination; b0 is an intercept; b1 to b3 are regression coefficients computed from the observed experimental values of Y from experimental run; and A, B and C are the coded level of independent variables representing the linear terms.
The dependent and independent variables selected are shown in along with their low, medium and high levels for the preparation of final optimized formulation. The solid lipid:liquid lipid, surfactant concentration and homogenization speed used for NLCs formation were taken as independent variables. These three independent variables were analyzed by the design for two responses which are particle size and PDI and these two responses were dependent variables.
Table 1. Variables in the Box-Behnken design.
Design matrix comprising of 17 experimental runs with a range of three variables, i.e. Solid lipid:Liquid lipid (X1), Surfactant concentration (X2) and Homogenization speed (X3) and the respective observed responses, i.e. Size and PDI. The responses obtained after the preparation of these 17 formulations were filled in the design. These data were analyzed by the design of the experiment (Design Expert® (Version 9.0.3.1, Stat-Ease Inc., Minneapolis, MN). The best fitting model was selected. The run which showed the minimal size range of the NLCs and having minimum range of PDI was found to be optimized. Finally, optimized was selected for further characterization.
Determination of particle size and zeta potential of NLCs
Size and zeta potential of NLCs were determined by laser diffractometry using the Delsa TM Nano C Particle analyzer (Beckman Coulter India Pvt. Ltd., Mumbai, India). All samples were diluted appropriately with the aqueous phase of the formulation for the measurements. Z-Average particle size, polydispersity index (PI) and zeta potential were measured in triplicate.
Determination of drug entrapment efficiency (EE) and drug loading (DL) for NLCs
Paclitaxel-loaded NLCs were separated from the NLCs suspension by centrifugation at 25–000 rpm for 1 h. Then 1 ml supernatant was taken and diluted up to 100 ml with methanolic PBS. Finally, drug content was analyzed at 217 nm using a UV-spectrophotometer (Perkin Elmer, Kyoto, Japan). EE was calculated using the following formula:
In-vitro drug release
In-vitro release was evaluated by using a dialysis bag diffusion technique under sink condition. The drug release from PTX-NLCs was performed in methanolic phosphate buffer saline (PBS) (pH 7.4) using dialysis diffusion bag. The suspension was placed in dialysis bags (MWCO 12 000 Da, Sigma Aldrich, USA) and the dialysis bag was subsequently placed in flask containing 200 ml dissolution medium Methanolic-PBS (pH 7.4) were stirred at 150 rpm at 37 °C in an incubator shaker. Aliquots of the dissolution medium were withdrawn at each time interval and the same volume of fresh dissolution medium was added to the flask to maintain a constant volume. Drug concentration in the dissolution medium was determined using a UV/Vis Spectrophotometry (Shimadzu 1700, Japan) at 217 nm. All experiments were carried out in triplicates. The release profile were fitted to kinetic equations (zero order, first order, Higuchi release and Korsmeyer-Peppas release, Hixson Crowell) to understand the release mechanisms.
In vitro culture studies
Cell cultures: Caco-2 cell culture
To track the entry of nanoparticles into the cells, SQV-NLCs were labeled with the fluorescent dye coumarin-6. Briefly, 5 mg of coumarin-6 was incorporated in the lipid phase of the formulation and the preparation continued as aforementioned. Caco-2 cells were grown in RPMI-1640 medium supplemented with 10% (v/v) inactivated fetal bovine serum, 1% (v/v) trypsin-EDTA solution, at 37 °C under a 10% CO2/90% air atmosphere. Caco-2 cells were poured into a 24-well plate and cultivated over 24 h. The permeability of PTX across intestinal in vitro models was evaluated by comparing free PTX with all the three surfactant-loaded PTX-NLC formulations, in Caco-2 cells. The experiments were conducted at 37 °C or 4 °C by adding a volume of 100 μl at 30 μg/ml PTX concentrations.
As mentioned previously, surfactants are well-known P-gp inhibitors. To evaluate the role of surfactant-based NLCs in the inhibition of P-gp, cells were pre-treated with a solution of 100 μM verapamil, a well-known P-gp inhibitor, for 1 h and nanoparticles were subsequently added on the apical side and incubated for 2 h in the presence of verapamil. The evaluation of surfactant-based NLCs suspension was also carried out under P-gp inhibition to confirm that surfactant was a P-gp substrate in our Caco-2 cell model. In all the assays carried out in the presence of inhibitors, several inserts were kept as controls and the transport studies were carried out in transport buffer instead of in inhibitor solutions.
Intracellular uptake of NLCs by Caco-2 cells
Entry of nanoparticles into Caco-2 cells was studied qualitatively by fluorescence microscope for which coumarin-6-loaded nanoparticles were employed. For this study, Caco-2 cells were seeded in 24-well cell culture plates at a density of 5 × 105 cells per well and allowed to adhere for 24 h until confluency. As for the transport studies, cells were co-incubated with 100 μL of a coumarin-6-loaded nanoparticles suspension in transport buffer. After 2 h of incubation with fluorescent nanoparticles, cells were washed three times with PBS and detached from the plates by trypsinization. Cells were then centrifuged at 1500 × g, the supernatant was discarded, the cells were resuspended in PBS and fluorescence was visualized under fluorescence microscope (Olympus-CKX41, NY, USA). Cell fluorescence was qualified by capturing the images of fluorescence of coumarin-6.
Spray-drying of NLCs
Spray dried powders were prepared by adding mannitol and leucine as excipients to improve yield and flow properties of dry powders. The inlet temperature was maintained at 80 °C, outlet temperature at 40–50 °C and vacuum at 100–120 °C of WC. 1% mannitol and 0.2% leucine was optimized on the basis of good flow properties. Incorporation of leucine into the formulation prior to spray-drying could improve process yield because of its antiadherent property.
Re-dispersibility of spray dried nanoparticles
A total of 10 mg drug loaded spray dried dry powder of NLCs samples dispersed in 10 ml of phosphate buffer (7.4) with continuous vortexing for 20 min, then centrifuged at 10 000 rpm for 30 min. The particles size and PDI of dispersion was measured with a Delsa-nano Zetasizer, New Delhi, India.
Solid state characterization of DPI
Solid state characterization of DPI formulations like Angle of repose, Both bulk density (BD) and tapped density (TD), Carr’s index (C), Hausner’s ratio determined. The Compressibility Index of the powder blend was determined by Carr’s compressibility index. The formula for Carr’s Index is as shown below:
Hausner’s ratio by using the formula:
H = 100/(100−C)
The angle of repose was calculated by:
tan θ = h/rwhere, θ is angle of repose, h is height of the particles pile and r is distance from the centre of the pile to the edge.
Particle morphology
Particle morphology of the DPI was performed by Scanning Electron Microscopy (SEM) and motic microscopy for determining the surface morphology, size and shape of formulation and to observe the aggregation property of NLC with carrier particles.
In-vivo studies
For animal experiments, the protocol complies with the particular recommendation and approval obtained for their protocols. Wistar rats of either sex (180–200 g) were used to study lung deposition of optimized formulations. Organ distribution studies were obtained after pulmonary administration of dry powder of NLCs in comparison to oral administration of PTX solution in methanolic PBS. Two groups of rats were taken for pharmacokinetic and lungs deposition study. Each group had nine rats.
Route of administration and withdrawal of blood sample
Concentration in organs was obtained from oral administration of PTX solution, inhalation of DPI of NLCs. Two groups of rats were taken with nine rats in each group. Rats were sacrificed and organs (lungs, liver, kidney and spleen) were removed. For organs distribution studies, organs were homogenized separately using phosphate buffer (pH 7.4). Then centrifugation was done at 6000 rpm for 30 min. Then supernatants were separated and collected in eppendorfs for further studies. 0.2 ml of the supernatants was taken and 1.8 ml of acetonitrile was added for the purpose of deproteinization. Vortexing was done for 5 min and then centrifugation was done for 15 min at 3000 rpm. After that, 1 ml of supernatant was removed from the centrifuged samples and is diluted with 1 ml of methanolic PBS and assayed spectrophotometrically.
Results and discussion
Optimization of NLCs by DOE
The results of the experimental design were analyzed using Design-Expert software, MN, USA, which provided considerable useful information and reaffirmed the utility of statistical design for conduct of experiments. The selected independent variables including the GMS/oleic acid ratio, surfactants (Tween 80, Tween 20 and Tween 60) concentration, and homogenization speed, significantly influenced the observed responses for particle size and PDI. Polynomial equations involving the main effect and interaction factors were determined based on estimation of statistical parameters, such as multiple correlation coefficient, adjusted multiple correlation coefficient and the predicted residual sum of squares generated by Design-Expert software.
Analysis of responses
All the responses observed for 17 formulations prepared were simultaneously fitted to first order, second order and quadratic models using Design Expert.
Response Y1 (particle size)
Analyzing this response required no transformation, the best-fitted model was observed to be the linear for Tween 80 loaded NLCs and quadratic for Tween 20 and Tween 60 loaded NLCs and the comparative values of R2, SD and % C.V. are given in along with the regression equation generated for this response. Only statistically significant (p < 0.05) coefficients are included in the equations.
Table 2. Summary of results of regression analysis for responses Y1, for fitting to linear and quadratic model.
Response Y2 (PDI)
Variation in PDI is a function related to the rate at which nanostructured lipid carriers are dispersed in the prepared suspension. Thus, a transformation is required while analyzing this response and applied, and the best-fitted model was observed to be quadratic in all the type of surfactant-loaded NLCs and the comparative values of R2, SD and % C.V. are given in along with the regression equation generated for this response. Only statistically significant (p < 0.05) coefficients are included in the equation.
Table 3. Summary of results of regression analysis for responses Y2, for fitting to quadratic model.
Response surface analysis for Tween 80 NLCs particle size and PDI
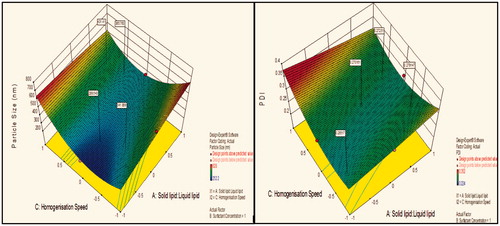
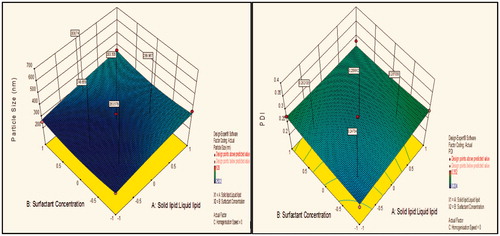
Contour plot analysis further explained that in all probable case of interactions, low level of lipids ratio (A), and high levels of surfactant concentration (B) and medium level of homogenization speed (C) was providing favorable range of minimum particle size as well as PDI. Surface response curve in further justified the above made conclusion.
Figure 1. Response surface plot showing effect of interaction between solid lipid:liquid lipid (A) and surfactant concentration (B) with medium level of homogenization speed (C) on response (size) and PDI.
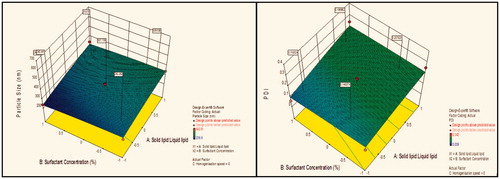
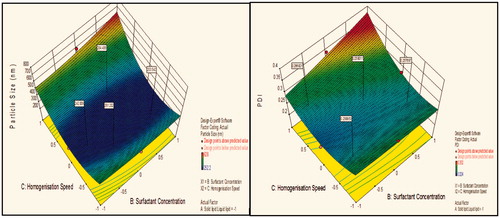
Figure 2. Response surface plot showing effect of interaction between solid lipid:liquid lipid (A) and homogenization speed (C) with high level of surfactant concentration (B) on response (size) and PDI.
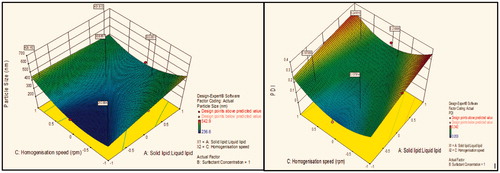
Figure 3. Response surface plot showing effect of interaction between surfactant concentration (B) and homogenization speed (C) with low level of lipids ratio (A) on response (size) and PDI.
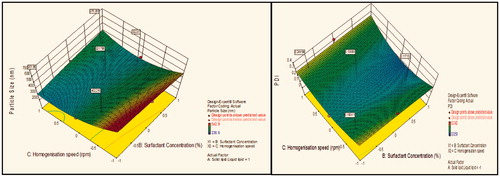
Figure 4. Response surface plot showing effect of interaction between solid lipid:liquid lipid (A) and surfactant concentration (B) with medium level of homogenization speed (C) on response (size) and PDI.
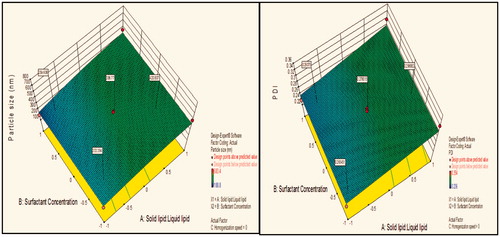
Figure 5. Response surface plot showing effect of interaction between solid lipid:liquid lipid (A) and homogenization speed (C) with high level of surfactant concentration (B) on response (size) and PDI.
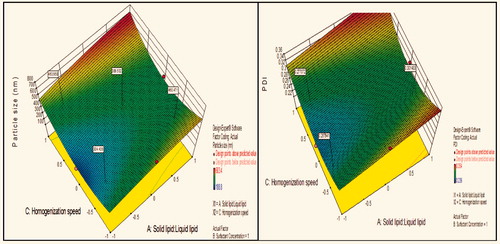
Figure 6. Response surface plot showing effect of interaction between surfactant concentration (B) and homogenization speed (C) with low level of lipids ratio (A) on response (size) and PDI.
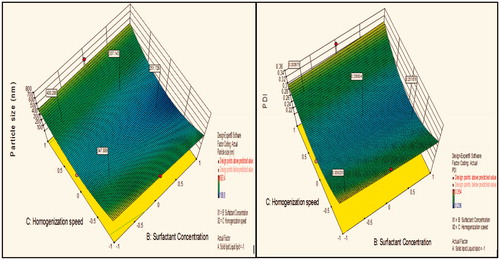
Figure 7. Response surface plot showing effect of interaction between solid lipid: liquid lipid (A) and surfactant concentration (B) with medium level of homogenization speed (C) on response (size) and PDI.
Effects of variables on particle size
Effect of homogenization speed on particle size
Contour plot analysis showed that in the case of interaction between the solid lipid:liquid lipid (A) and surfactant concentration (B), the effect of increased homogenization speed on the size response was observed. At low homogenization speed, the prevalence of red region was high. With the gradient inclination of speed, the acceptable blue region was increasing, which represents that the size was significantly decreases. Then, further increase in speed, the prevalence of green region shows again an increase in size. The phenomenon may be by keeping the ratio of lipids constant, the homogenization speed varied and concluded that the particle size was found to decrease at certain extent then increases with an increase in homogenization speed. This concluded that at low to medium speed, particle size decreases due to breakdown in particles, but drastic change was seen at high speed due to more breakdowns of vesicles which forms aggregates and ultimately size increases.
Effect of surfactant concentration on particle size
Contour plot analysis showed that in the case of interaction between the solid lipid:liquid lipid (A) and homogenization speed (C), the effect of increased Tween 80 concentrations on the size response was observed. At lower levels of Tween 80, the prevalence of acceptable blue region was quite low. With the gradient inclination of Tween 80, the acceptable blue region was increasing, which represents that the size was significantly decreases. The phenomenon may be by keeping the amount of lipid constant, the concentration of surfactant varied and concluded that the particle size was found to decrease with an increase in surfactant concentration. In emulsification, due to high shearing droplet size usually gets reduced and also droplet have tendency to form aggregate in order to reduce their surface energy. But presence of surfactant molecule stabilizes the emulsion by providing a thick protective layer around the droplet to solve aggregation problem. Similarly, at low concentration of surfactant, amount of lipid increased, size generally increases due to a lack of ability of surfactant solution to stabilize emulsion at low concentration.
Effect of solid lipid:liquid lipid on particle size
Contour plot analysis showed that in the case of interaction between the surfactant concentration (B) and homogenization speed (C), the effect of increased ratio of lipids on the size response was observed. At a low lipid ratio, the prevalence of blue region was high. With the gradient inclination of lipids ratio, the acceptable blue region was decreasing, which represents that the size was significantly increases. The phenomenon may be by keeping the surfactant ratio constant, the lipids ratio varied and concluded that the particle size was found to increase with increase in lipids ratio. This concluded that at low to high lipid ratio, particle size increases due to an accumulation of vesicles which forms aggregates and ultimately size increases.
Effects of variables on PDI
Effect on PDI
Contour plot analysis for response of PDI showed same results as in particle size, because if large is the particle size, value of the PDI is too greater. As such, small particle size shows less PDI. This is because the PI is directly related to the particle size. Contour plot analysis further explained that in all probable case of interactions, low level of lipids ratio (A), and high levels of surfactant concentration (B) and medium level of homogenization speed (C) was providing favorable range of minimum PDI.
Although, same trend was followed in design for all the three cases, i.e. Tween 80, Tween 20 and Tween 60 but still Tween 20 gave more compact size with narrow range of PDI could be better lipid in order to develop the final drug-loaded optimized formulation. The particle size and particle size distribution are critical factors in the performance of nanoparticles, as batches with wide particle size distribution may show significant variations in drug loading, drug release, bioavailability and efficacy. Formulation of nanoparticles with a narrow size distribution will be a challenge if emulsion cannot be produced with a narrow droplet size distribution. As nanoparticles are internalized into cells by endocytosis, an increase in particle size will decrease uptake and potentially affect bioavailability of the drug.
Particle size and zeta potential of NLCs
The properties of PTX-loaded NLCs, such as particle size, PI, zeta potential, entrapment efficiency, drug loading and release percent are listed in .
Table 4. Various parameters of different surfactant-loaded NLCs.
It was found that the size of drug-loaded particles was higher than that of drug-free ones due to the incorporation of drug in NLC matrix. On the other hand, the most effective factor on particle size relates to the drug content. Increasing the concentration of surfactant decreased the particle sizes of nanoparticles. Müller et al. (Citation2002b) have shown that increasing the poloxamer concentration to 1% was effective in producing smaller size SLN composed of tripalmitin, cetylpalmitate and glycerylmonostearate. In the present study, it seems that 1.5% surfactant is sufficient to cover the surface of nanoparticles effectively and prevents agglomeration during the process. Zeta potential is often a key factor to understand the stability of colloidal dispersion. As shown in , the absolute value of zeta potential in most formulation shows the stability of formulations.
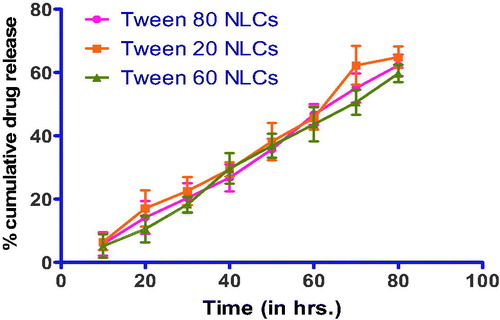
In vitro release of PTX from NLC
shows the drug release from NLCs. Surfactant type, sonication time and homogenization rate did not significantly affect the release rate. For all formulations, a biphasic drug release pattern was observed, which is due to the burst release at the initial stage and is followed by a sustained release in the later times.
Uptake study of different surfactant-loaded formulation on Caco-2 cell lines
Different surfactant-loaded NLCs labeled with the fluorescent dye coumarin-6 were prepared to track the entry of nanoparticles into the cells. Prepared formulations was poured into 24-well plates with grown cultured cells and then visualized by fluorescence B-excitation filter on 20 × magnification objective. Tween 20-loaded NLCs show maximum cellular uptake, which was selected for further studies, i.e. formulation of dry powder inhaler by spray drying using its characterization and in-vivo studies. The fluorescent images of plain drug and all the surfactant-loaded NLCs confirming cellular uptake are shown in .
Figure 11. Cellular uptake of (A) Plain drug solution; (B) Tween 80 loaded NLCs; (C) Tween 20 loaded NLCs and (D) Tween 60 loaded NLCs.
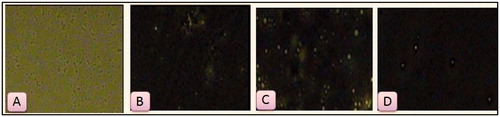
From the above results we can conclude that cellular uptake was more in the form of NLCs as compared with plain drug solution. Moreover, Tween 20 loaded formulation shows maximum uptake which successfully confirms the surfactant-based formulation. More is cellular uptake; greater is p-gp efflux inhibition. P-gp efflux inhibition is due to the presence of p-glycoprotein in Caco-2 cell lines. Evidently, inhibition of p-gp may treat drug resistance.
However, the efflux rate of this treatment was significantly greater than that of free PTX. The cellular uptake of NLCs was size-dependent, i.e. formulation C>B>D. This finding is consistent with Rejman et al. (Citation2004), who also reported a tendency to decreased internalization with an increased particle size. These authors studied the pathway of entry and subsequent fate of commercial latex nanoparticles inside the cell and concluded that particles with a diameter of less than 200 nm enter the cell via clathrin-mediated endocytosis whereas larger particles (200 nm–1 μm) enter preferentially via caveolae-mediated endocytosis. Moreover, the surface hydrophobicity of the nanoparticles may also determine nanoparticle entrance into Caco-2 cell because the larger uptake into the cells is correlated with the higher nanoparticle surface hydrophobicity (formulation C>B>D). In our study, nanoparticle size and surface hydrophobicity were major factors influencing NLC entrance into the cell.
Particle morphology, size, PDI and zeta potential of spray dried formulation (DPI)
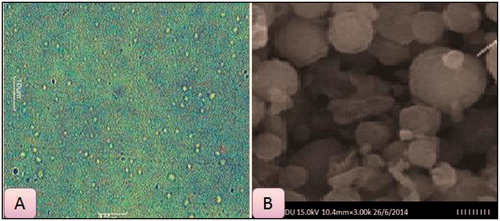
The size of the PTX-loaded NLCs was found to be 283.4 ± 4.5 nm and PDI was 0.226 ± 0.94. In order to confirm the size of NLC and to observe surface morphological characteristics, microscopic images and SEM were carried out, respectively. Microscopic and SEM images () showed uniform size; mono-dispersed round shaped NLCs. Slight rough surface could be observed in majority of NLCs in SEM photographs. Zeta potential of NLVs estimated by electrophoretic mobility technique using zetameter was found to be −25.12 mV indicating the stability of nanoparticle during storage.
Solid state characteristics
Solid state characteristics like angle of repose, bulk and tapped density, Carr’s index, Hausner’s ratio, percentage yield and aerodynamic diameter are essential parameters that determine the delivery efficiency of DPI formulations. These characteristics determined for DPI formulations are given in .
Table 5. Flow properties of Tween 20 loaded NLCs after spray drying.
Improved aerodynamic properties of nanoparticulate formulations have confirmed the finding to previous reports that mannitol have been used to improve the yield and fine particle fraction of the aerosol powder. Similarly, leucine was used because of its dispersibility enhancing properties, which improved the aerosolization characteristic of powders. The effect of leucine on particle surface morphology of spray dried powders containing mannitol was obvious. SEM photographs of these powders presented spherical particles with rough surface. This surface roughness could improve the aerosolization capability of produced spray dried powders. By adding leucine to the excipients, particle size of spray dried powders decreased significantly. It has been reported that incorporation of leucine into the formulation prior to spray-drying could improve process yield because of its antiadherent property. The data tabulted in indicates good flow property of the powders.
In-vitro release studies of PTX NLCs and DPI
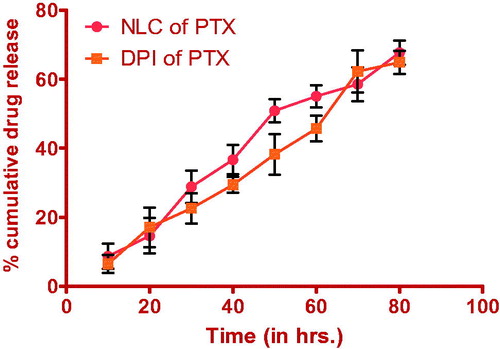
In-vitro drug release profile of optimized formulation after and before spray drying using dialysis bag in methanolic PBS (pH 7.4) was shown in .
Drug release study on optimized formulation after redispersion showed 67.7% drug release from NLCs and 64.9% drug release from DPI. As shown in , there is no significant change in the burst effect and drug release content from NLCs before and after lyophilization. Drug release profile shows the Higuchi model having R2 = 0.871, which shows release of drug from formulations by diffusion. The initial drug release (up to 8–10 h) is because of the burst effect that does not correspond to the real mechanism of the drug release from NLCs. It is due to immediate dissolution of drug particles adsorbed on the surface of NLCs. About 50% of drug was released from NLCs within the first 48 h and the 70% drug was released up to 72 h. Drug release followed the Higuchi kinetics indicating the release predominantly by diffusion. Increased diffusional distance due to the formation of drug depletion zone at particle surface and presence on the surrounding lipid shell barrier might have slowed down the release rate resulting in a sustained drug release profile.
In-vivo studies
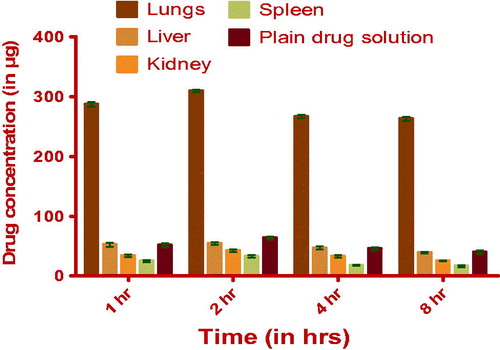
Organ distribution studies of optimized formulations at different time intervals were done on male Wistar rats (180–220 g). Doses were delivered through pulmonary route by using insufflators (Penny Century, PA, USA). The bio-analytical method was developed by HPLC spectroscopy. The chromatographic conditions were optimized as WATERS, C-18 column (250 mm*4.6 mm*5 µ) as stationary phase, Buffer (Na2HPO4): ACN (43:57) as mobile phase and flow rate was 1 ml/min. The amount of drug distributed in different organs (lungs, liver, spleen and kidney) is shown in and .
Table 6. Amount of drug present in organs (lungs, liver, kidney, spleen), homogenate of tested and control animals.
Lungs uptake of the drug from the DPI was higher as compared to plain drug suspension. Because in case of lyophilized NLC, clearance of the drug from the organ was less due to slow release of the drug from the NLC. PTX-DPI longevity in lung tissue was a direct consequence of the retention of the lipidic nanocarriers at the site and the retention of the drug in lipid nanoparticles. The potential for pulmonary delivery of may have clinical significance in the light of recent data demonstrating that PTX-NLC-DPI was highly effective, following inhalation in rat lungs. Drug concentration in lungs was higher because of the direct exposure of the PTX-DPI to lungs.
Conclusion
In this study, drug-loaded NLCs were successfully prepared using different surfactants. NLCs were prepared by the emulsification technique using a Box-Behnken design and displayed small, homogeneous size, high entrapment efficiency and satisfactory drug loading. The Box-Behnken design indicated that the most effective factors on the size and PDI were at high (1.5%) surfactant concentration, low lipids ratio (6:4) and medium homogenization speed (6000 rpm). Maximum cellular uptake was observed with Tween 20 formulation was shown on Caco-2 cell lines. Furthermore, spray drying of resultant NLCs showed good aerodynamic behavior and were found suitable for drug delivery to deeper airways. In-vivo studies confirmed the therapeutic efficacy of developed formulation and demonstrated the better localization of drug within the lungs using different surfactant-based pulmonary delivery systems. We can conclude that delivering drugs through pulmonary route for local action is advantageous. The surfactants were helpful in treating drug resistance lung cancer by inhibiting P-gp efflux in the form of nano lipidic carriers. This work can thus give us the better idea to work in the direction of surfactant-based delivery system by inhibiting P-gp efflux inhibition for treating lung cancer via pulmonary route.
Declaration of interest
The authors confirm that this article content has no conflicts of interest.
Author Dr Amit K. Goyal is thankful to the Department of Biotechnology (DBT), New Delhi (under IYBA scheme; BT/01/IYBA/2009 dated 24 May 2010), for providing financial assistance to carry out research.
References
- Chaudhary S, Garg T, Murthy RS, et al. (2014). Recent approaches of lipid-based delivery system for lymphatic targeting via oral route. J Drug Target 1–12
- Drummond DC, Noble CO, Guo Z, et al. (2010). Development of a highly stable and targetable nanoliposomal formulation of topotecan. J Control Release 141:13–21
- Emami J, Rezazadeh M, Varshosaz J. (2012). Formulation of LDL targeted nanostructured lipid carriers loaded with paclitaxel: a detailed study of preparation, freeze drying condition, and in vitro cytotoxicity. J Nanomater 2012:3
- Gagandeep G, Garg T, Malik B, et al. (2014). Development and characterization of nano-fiber patch for the treatment of glaucoma. Eur J Pharm Sci 53:10–16
- Garg T, Goyal AK, Arora S, Murthy R. (2012a). Development, optimization & evaluation of porous chitosan scaffold formulation of gliclazide for the treatment of type-2 diabetes mellitus. Drug Deliv Lett 2:251–61
- Garg T, Goyal AK. (2014a). Biomaterial-based scaffolds - current status and future directions. Expert Opin Drug Deliv 11:767–89
- Garg T, Goyal AK. (2014b). Liposomes: targeted and controlled delivery system. Drug Deliv Lett 4:62–71
- Garg T, Kumar A, Rath G, Goyal AK. (2014a). Gastroretentive drug delivery systems for therapeutic management of peptic ulcer. Crit Rev Ther Drug Carrier Syst 31:531–57
- Garg T, Rath G, Goyal AK. (2014b). Ancient and advanced approaches for the treatment of an inflammatory autoimmune disease-psoriasis. Crit Rev Ther Drug Carrier Syst 31:331–64
- Garg T, Rath G, Goyal AK. (2014c). Comprehensive review on additives of topical dosage forms for drug delivery. Drug Deliv
- Garg T, Singh O, Arora S, Murthy R. (2012b). Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst 29:1–63
- Garg T, Singh S, Goyal AK. (2013). Stimuli-sensitive hydrogels: an excellent carrier for drug and cell delivery. Crit Rev Ther Drug Carrier Syst 30:369–409
- Garg T. (2014). Current nanotechnological approaches for an effective delivery of bio-active drug molecules in the treatment of acne. Artif Cells Nanomed Biotechnol 1–8
- Goyal AK, Rath G, Garg T. (2013a). Nanotechnological approaches for genetic immunization. DNA and RNA Nanobiotechnologies in Medicine: Diagnosis and Treatment of Diseases 67–120
- Goyal G, Garg T, Malik B, et al. (2013b). Development and characterization of niosomal gel for topical delivery of benzoyl peroxide. Drug Deliv
- Goyal G, Garg T, Rath G, Goyal AK. (2014a). Current nanotechnological strategies for an effective delivery of drugs in treatment of periodontal disease. Crit Rev Ther Drug Carrier Syst 31:89–119
- Goyal G, Garg T, Rath G, Goyal AK. (2014b). Current nanotechnological strategies for treating glaucoma. Crit Rev Ther Drug Carrier Syst 31:365–405
- Hao YL, Deng YJ, Chen Y, et al. (2005). In-vitro cytotoxicity, in-vivo biodistribution and anti-tumour effect of PEGylated liposomal topotecan. J Pharm Pharmacol 57:1279–87
- Hussain T, Garg T, Goyal AK, Rath G. (2014). Biomedical applications of nanofiber scaffolds in tissue engineering. J Biomim Biomater Tissue Eng 4:600–23
- Johal HS, Garg T, Rath G, Goyal AK. (2014). Advanced topical drug delivery system for the management of Vaginal candidiasis. Drug Deliv 1–14
- Joshi D, Garg T, Goyal AK, Rath G. (2014). Advanced drug delivery approaches against periodontitis. Drug Deliv 1–15
- Kataria K, Sharma A, Garg T, et al. (2014). Novel technology to improve drug loading in polymeric nanofibers. Drug Deliv Lett 4:79–86
- Kaur M, Garg T, Narang RK. (2014a). A review of emerging trends in the treatment of tuberculosis. Artif Cells Nanomed Biotechnol 1–7
- Kaur M, Garg T, Rath G, Goyal AK. (2014b). Current nanotechnological strategies for effective delivery of bioactive drug molecules in the treatment of tuberculosis. Crit Rev Ther Drug Carrier Syst 31:49–88
- Kaur M, Malik B, Garg T, et al. (2014c). Development and characterization of guar gum nanoparticles for oral immunization against tuberculosis. Drug Deliv
- Kaur N, Garg T, Goyal AK, Rath G. (2014d). Formulation, optimization and evaluation of curcumin-beta-cyclodextrin-loaded sponge for effective drug delivery in thermal burns chemotherapy. Drug Deliv 1–10
- Kaur P, Garg T, Rath G, et al. (2014e). Surfactant-based drug delivery systems for treating drug-resistant lung cancer. Drug Deliv 1–12
- Kaur R, Garg T, Das Gupta U, et al. (2014f). Preparation and characterization of spray-dried inhalable powders containing nanoaggregates for pulmonary delivery of anti-tubercular drugs. Artif Cells Nanomed Biotechnol 1–6
- Kaur R, Garg T, Malik B, et al. (2014g). Development and characterization of spray-dried porous nanoaggregates for pulmonary delivery of anti-tubercular drugs. Drug Deliv 1–6
- Kaur R, Garg T, Rath G, Goyal AK. (2014h). Advanced aerosol delivery devices for potential cure of acute and chronic diseases. Crit Rev Ther Drug Carrier Syst 31:495–530
- Kaur V, Garg T, Rath G, Goyal AK. (2014i). Therapeutic potential of nanocarrier for overcoming to P-glycoprotein. J Drug Target 1–12
- Kim J-H, Kim Y-S, Kim S, et al. (2006). Hydrophobically modified glycol chitosan nanoparticles as carriers for paclitaxel. J Control Release 111:228–34
- Lo Y-L. (2003). Relationships between the hydrophilic–lipophilic balance values of pharmaceutical excipients and their multidrug resistance modulating effect in Caco-2 cells and rat intestines. J Control Release 90:37–48
- Malik R, Garg T, Goyal AK, Rath G. (2014). Polymeric nanofibers: targeted gastro-retentive drug delivery systems. J Drug Target 1–16
- Marwah H, Garg T, Goyal AK, Rath G. (2014). Permeation enhancer strategies in transdermal drug delivery. Drug Deliv 1–15
- Mehnert W, Mäder K. (2001). Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev 47:165–96
- Modgill V, Garg T, Goyal AK, Rath G. (2014). Permeability study of ciprofloxacin from ultra-thin nanofibrous film through various mucosal membranes. Artif Cells Nanomed Biotechnol 1–6
- Morie A, Garg T, Goyal AK, Rath G. (2014). Nanofibers as novel drug carrier - an overview. Artif Cells Nanomed Biotechnol 1–9
- Muèller RH, Maèder K, Gohla S. (2000). Solid lipid nanoparticles (SLN) for controlled drug delivery – a review of the state of the art. Eur J Pharm Biopharm 50:161–77
- Müller R, Radtke M, Wissing S. (2002a). Nanostructured lipid matrices for improved microencapsulation of drugs. Int J Pharmaceut 242:121–8
- Müller R, Radtke M, Wissing S. (2002b). Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev 54:S131–55
- Nishiyama N, Kataoka K. (2006). Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol Therapeut 112:630–48
- Pabreja S, Garg T, Rath G, Goyal AK. (2014). Mucosal vaccination against tuberculosis using Ag85A-loaded immunostimulating complexes. Artif Cells Nanomed Biotechnol 1–8
- Rege BD, Kao JP, Polli JE. (2002). Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur J Pharmaceut Sci 16:237–46
- Rejman J, Oberle V, Zuhorn I, Hoekstra D. (2004). Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem J 377:159–69
- Rohilla R, Garg T, Goyal AK, Rath G. (2014). Herbal and polymeric approaches for liver-targeting drug delivery: novel strategies and their significance. Drug Deliv 1–17
- Sharma A, Garg T, Aman A, et al. (2014a). Nanogel-an advanced drug delivery tool: current and future. Artif Cells Nanomed Biotechnol 1–13
- Sharma R, Garg T, Goyal AK, Rath G. (2014b). Development, optimization and evaluation of polymeric electrospun nanofiber: a tool for local delivery of fluconazole for management of vaginal candidiasis. Artif Cells Nanomed Biotechnol 1–8
- Sharma R, Singh H, Joshi M, et al. (2014c). Recent advances in polymeric electrospun nanofibers for drug delivery. Crit Rev Ther Drug Carrier Syst 31:187–217
- Singh B, Garg T, Goyal AK, Rath G. (2014a). Recent advancements in the cardiovascular drug carriers. Artif Cells Nanomed Biotechnol 1–10
- Singh H, Sharma R, Joshi M, et al. (2014b). Transmucosal delivery of Docetaxel by mucoadhesive polymeric nanofibers. Artif Cells Nanomed Biotechnol
- Singh O, Garg T, Rath G, Goyal AK. (2014c). Microbicides for the treatment of sexually transmitted hiv infections. J Pharmaceut 1–18
- Vivek K, Reddy H, Murthy RS. (2007). Investigations of the effect of the lipid matrix on drug entrapment, in vitro release, and physical stability of olanzapine-loaded solid lipid nanoparticles. AAPS Pharmscitech 8:16–24
- Vohra T, Kaur I, Heer H, Murthy RR. (2013). Nanolipid carrier-based thermoreversible gel for localized delivery of docetaxel to breast cancer. Cancer Nanotechnol 4:1–12
- Wong HL, Bendayan R, Rauth AM, et al. (2007). Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv Drug Deliv Rev 59:491–504