Abstract
Current guidelines recommend patients with active and mild-to-moderate ulcerative colitis (UC), who have received initial therapy with 5-aminosalicylic acid (5-ASA). In this study, a novel drug delivery vehicle achieved by pH-sensitive hydrogels was applied to 5-ASA. In our previous work, a novel P(CE-MAA-MEG) pH-sensitive hydrogel was successfully synthesized by the heat-initiated free radical polymerization method. The aim of this study is to investigate its site-specific delivering of drugs to the colon and evaluate its colon-targeting characteristic in vivo. 5-ASA was chosen as a model drug and successfully loaded in the hydrogel. In vitro investigations were carried out to evaluate its release process. Above all, animal treatment results reveal an obvious effect on the UC healing. Therefore, all results suggested that the developed 5-ASA-P(CE-MAA-MEG) hydrogel (5-ASA-GEL) as a colon-targeting vector might have a great potential application in the UC therapy.
Introduction
Inflammatory bowel disease (IBD), which comprises Crohn’s disease (CD) and ulcerative colitis (UC), is characterized by relapsing and remitting episodes of active inflammation and chronic mucosal injury (Martin et al., Citationin press). Although many theories exist regarding the cause of UC, none has been proved (Sonu et al., Citation2013; Trivedi & Jena, Citation2012, Citation2013). Currently, some studies indicated that immunological abnormality as a part of interstinal mucosa may cause UC (Ardizzone et al., Citation2006; Römkens et al., Citation2012). Treatment with 5-aminosalicylic acid (5-ASA) may supposed to be effective on the grounds of good therapeutic clinical effect and is expected to reduce the inflammation in the large intestine by inhibiting the NF-kappa B and scavenging free radicals in order to relieve the symptoms of UC (Harris & Lichtenstein, Citation2011; Klotz, Citation2012; Thomas & Baumgart, Citation2012). For more than 30 years, mesalazine (5-ASA) has been used for the treatment of chronic IBD, especially in UC. During this time, various rectal and oral formulations have been developed. Such a drug targeting strategy is needed for its topical action and especially because local concentrations in the mucosa will determine the clinical outcome (Mura et al., Citation2011; Collnot et al., Citation2012; Huttunen et al., Citation2013; Shaikh et al., Citation2013; Wolk et al., Citation2013). Unfortunately, its clinical application was greatly limited by the great gastrointestinal stimulation. Simultaneously, 5-ASA within the gastrointestinal tract is not controllable (Ha et al., Citation1999; Li et al., Citationin press).
In order to solve the above-mentioned problems, some researchers carried out a large amount of investigations on the oral preparation of 5-ASA, including traditional oral dosage forms, such as tablets, capsules, etc. Currently, smart or intelligent hydrogels with a property of biocompatibility and resemblance to biological tissue were presented with a great potential in applying to the targeting drug delivery system (Cruise et al., Citation1998; Qiu & Park, Citation2001; Feng et al., Citation2009; Gann et al., Citation2009; He et al., Citation2009; Yin et al., Citation2009). Various pH-sensitive hydrogels have been widely investigated as site-specific drug delivery carriers to specific regions of the gastro-intestinal tract due to their adjustable swelling behavior in aqueous medium, which can control drug release rates in a mild manner. These pH-sensitive hydrogels bearing weakly acidic pendant groups would exhibit pH sensitivity due to the alternation of COOH/COO– upon pH changes (Scheme 1) (Chao et al., Citation2006; Horkay et al., Citation2006; Shim et al., Citation2006; Xu et al., Citation2006; Hamidi et al., Citation2008; Wei et al., Citation2008; Wang et al., Citation2009). In our previous work (Wang et al., Citation2010), a novel new kind of pH-sensitive hydrogels were successfully synthesized by the heat-initiated free radical polymerization method, which exhibits unique properties due to its ability to form reversible hydrogen-bonded complexes. The pH-sensitive hydrogel that we have prepared by biodegradable materials, without any organic solvent, might have a great potential application in oral drug delivery system, especially in colon-targeted therapy.
Scheme 1. Schematic illustration of drug release from pH-sensitive hydrogel and its application in UC therapy .
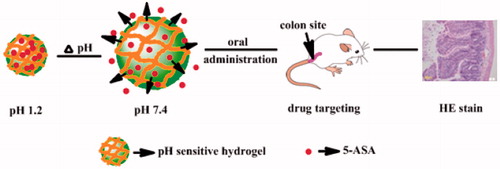
In this study, 5-ASA hydrogel was prepared without any organic solvent; and its good biocompatibility, low toxicity, pH sensitivity and good sustained release properties of the poly(methoxyl ethylene glycol-caprolactone-co-methacrylic acid-co-poly(ethylene glycol) methyl ether methacrylate) P(CE-MAA-MEG) hydrogel makes it a safe candidate for oral colon-specific drug delivery system. With desirable drug released behavior, all results indicated that the developed 5-ASA-GEL hydrogel, as an efficient colon-targeting vehicle, might have potential application in the therapy of UC healing.
Methods and materials
Material
Poly(methoxyl ethylene glycol-caprolactone)-acryloyl chloride (MPEG-PCL-AC) copolymers (synthesized by our lab), N,N′-Methylene-bis-acrylamide (BIS), methacrylic acid (MAA), poly(ethylene glycol) methyl ether methacrylate (MPEGMA, MEG), ammonium persulfate (98%) (APS) were all of analytical grade, and purchased from Aldrich Company, St. Louis, MO, USA. All the other reagents were also of analytical grade and used as-received.
BALB/c mice of both sexes weighing 25 ± 2 g were used in UC therapy test and histopathological observations. They were obtained from Laboratory Animal Center of Xi’an Jiaotong University; SCXK (Shaanxi) 2007-001, China. The mice were housed under a 12-h light–dark cycle at a constant ambient temperature (22–25 °C), with normal mice chow and water ad libitum. They were allowed to acclimatize for 1 week before the experiments were started. All mice were deprived of food for 48 h prior to the experimental procedure, but were allowed to have free access to tap water throughout.
Preparation of 5-ASA-P(CE-MAA-MEG) hydrogels
P(CE-MAA-MEG) hydrogel was synthesized by heat-initiated free radical polymerization with APS as heat-initiator and BIS as cross-linker. The drug was dissolved in the mixture of ethyl alcohol and disodium hydrogen phosphate, with a combination of the mixture of poly(methoxyl ethylene glycol-caprolactone)-acryloyl chloride macromonomer (40 wt%), MAA (30 wt%), MPEG-MA (20 wt%), BIS (10 wt%), and APS (6% w/w of the total monomers), which were dissolved in ethyl alcohol (6 ml). Then the mixture was poured into a weighing bottle and heated to 37 °C with nitrogen for about 1 h. In our previous work, we loaded drug into hydrogels by immersing the blank gel into the drug solution. However, low content of drug and non-uniform of drug distribution in the hydrogel were hindered off its further application. In this study, freshly 5-ASA-loaded P(CE-MAA-MEG) hydrogel complexes were prepared as following and they were used to assay in vitro release behavior of hydrophobic drugs. Briefly, the predetermined amounts of PCE-AC monomer, MAA, MPEG-MA, BIS and APS were added into an EP tube, then the prepared 5-ASA solution was added. After shaken to form a homogeneous mixed solution, these mixtures were incubated for 1 h at 40 °C. The gel was removed and dried at room temperature for 1 day, and then dried at 45 °C in vacuum for 3 days. The dried gel was cut into less than 4 mm square pieces and stored.
Scanning electron microscopy
Scanning electron microscopy (SEM) was employed to investigate morphology of P(CE-MAA-MEG) hydrogel and drug-loaded hydrogel. The hydrogels were frozen in liquid nitrogen and lyophilized for 72 h. Then, the hydrogels were sputtered with gold before observation. In this study, morphology of prepared particles was examined on JEOL SEM (JSM-5900LV, JEOL, Tokyo, Japan).
In vitro release of P(CE-MAA-MEG) hydrogel
The 0.1 g gels with different drug content were immersed in 20 ml of PBS (pH 7.4) or HCl/H2O solution (pH 1.2), respectively, and then were shaken at 100 rpm at 37 °C. At specific time intervals, all of release media were removed and replaced by fresh release media. After centrifuged at 13 000 rpm for 10 min, the supernatant of the removed release media were collected and stored at −20 °C before analysis. The collected supernatants were detected on HPLC to determine the concentration of 5-ASA. The cumulative release of 5-ASA was calculated according to Equation (3) (Jia et al., Citation2007):
(1)
where Q was cumulative release weight, and Cn was the 5-ASA concentration at time t. Vt was the volume of medium (Vt = 20 ml), and Vs was the volume of solution removed from supernatant (Vs = 3 ml).
Animal treatment
With the aim of evaluating the therapeutic value of 5-ASA-loaded hydrogels, the ulcerative colitis model was introduced into this experiment. The model colitis was induced in male Balb/c mice (average weight 25 ± 2 g, n = 10/group) by the use of dextran sulfate sodium (DSS) method: mice received 2.5% of DSS with their drinking water over a period of 7 days except for one group as a negative control. Starting on day 7, the different groups received 0.2 ml 5-ASA solution and 5-ASA-loaded hydrogels as oral administration once daily for 14 continuous days. Similarly, colitis group, which was adopted as positive control received normal saline instead of free drug or drug-loaded hydrogels. The treatment period was adjusted to 14 days on preliminary results as well as earlier studies with 5-ASA. The animals were sacrificed 24 h after the last drug loaded hydrogels administration. Their distal colons were resected and collected (Wang et al., Citation2012).
Specimens were taken from the collected colon, and then, the specimens were fixed in neutral formalin and embedded in paraffin. Tissues were sectioned, stained with hematoxylin and eosin (H&E) and then observed for histological assessment. After the treatment, the major immune organs, such as stomach, small intestine, heart, colon, liver, spleen, lung and kidney from the control and treated groups by a scissor and sliced them for H&E staining to histological evaluation.
Statistical analysis
All statistical analyses were performed with SPSS version 11.5 for Windows (Chicago, IL). Macroscopic score was compared by the Kruskal–Wallis non-parametric test and other parameters were compared by a one-way ANOVA, test. All results were expressed as mean ± SD. For all tests, p values less than 0.05 were considered to be statistically significant.
Results and discussion
Preparation and characterization of 5-ASA hydrogels
Preparation of P(CE-MAA-MEG) hydrogels
The P(CE-MAA-MEG) hydrogels were successfully synthesized by heat-initiated free radical polymerization method. Predetermined amount of PCE-AC macromonomer, MAA, MPEG-MA, BIS and APS was added in an EP tube, the mixtures were incubated for 1 h at 40 °C. In our previous work, its pH-responsibility and swelling capability were investigated in detail. With excellent pH-responsibility, this new kind of pH-sensitive hydrogels might play a good role in oral drug delivery system. Although the exact cause of UC remains uncertain, 5-ASA might be supposed to be an effective treatment on grounds of the good therapeutic effect in clinical. In this study, 5-ASA was chosen as model drug, and successfully loaded into the hydrogel through the heat-initiated polymerization process.
Morphological characterization
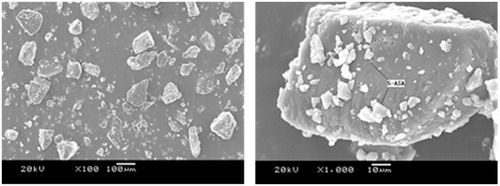
The typical scanning electron microphotographs of surfaces of drug-loaded hydrogels was presented in . According to this figure, drug-loaded hydrogel particles were apparently readable. Drugs were well attached to the section of hydrogel particles. Nevertheless, the great pH-responsibility of demonstrated hydrogels was still great to maintain after the drug was loaded into hydrogel. In all, drugs well-adhered into the hydrogel were benefited for the UC treatment.
In vitro release of P(CE-MAA-MEG) hydrogel
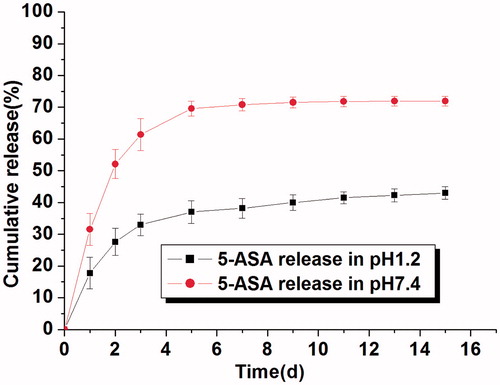
5-aminosalicylic acid, a multi-functional drug, has a great potential application in human disease therapy, especially in inflammatory therapy. In this study, 5-ASA was chosen as a model drug in this in vitro drug release study. The release of 5-ASA from P(CE-MAA-MEG) hydrogel with different pH (pH 1.2 and 7.4) was performed and its cumulative release profile is displayed in . From , we could find that pH value has a great effect on the release behavior of 5-ASA from this hydrogel. It is clear that the cumulative release rate of 5-ASA increased dramatically from 43.0% (pH 1.2) to 71.9% (pH 7.4) in a 15-day period. The burst release rates of 5-ASA were approximately 17.8 and 31.5% in the first 24 h, respectively. After that, 5-ASA could be released steadily from the hydrogel, which finally reached 43.0 and 71.9% of cumulative drug release rates in the next 14 days. The phenomenon of this drug release indicated that pH value has great effects on the 5-ASA release behavior. Generally, drug release behavior from hydrogel was driven by two forces: diffusion effect and degradation or erosion of the hydrogel. For hydrophobic drugs, low diffusion rate in water and strong intermolecular interaction with hydrogel dominated the drug release profile, which resulted in the high residual drug in hydrogel and low release rate. For pH-sensitive hydrogel, the water content in hydrogels and the type of releasing agent were greatly influenced the permeability and release rate of drugs. Hydrogels are capable of adsorbing a considerable amount of water, while maintaining their integrity in water, and they might also be used for the release of drugs that are poorly soluble in water. In our previous work, swelling behavior had been investigated, and we could find that larger water content absorption in high pH value than that in low pH value. Therefore, it seems that increasing the water content of this hydrogel at pH 7.4 increases the permeability of the solutes, which might accelerate the drug release rate.
Animal treatment
With the aim of evaluating the therapeutic value of 5-ASA-loaded hydrogels, the UC model was introduced into this experiment. Ulcerative colitis (is a subcategory of inflammatory bowel disease, which afflicts millions of people worldwide and even more, nowadays. Ulcerative colitis usually affects the colon and rectum and typically involves only the innermost lining or mucosa, manifesting as continuous areas of inflammation and ulceration, with no segments of normal tissue. Ulcerative colitis is always accompanied with a long course and recurrent attacks, prone to canceration and complicated in its clinical pathological changes. Now, there is still no clear and effective strategy to cure it. In this study, experimental colitis model was successfully introduced, and the treatment of UC therapy was evaluated by the following aspects.
Changes in weight of mice
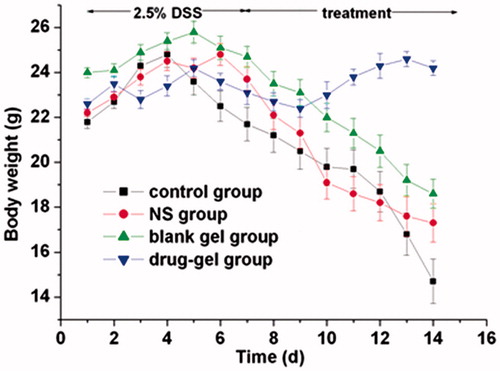
Body weight and hemafecia for all mice were recorded on every study day. Hemafecia in response to intestinal inflammation became visible on day 3 and weight loss could be seen after around 3 days and statistically significant () after day 5, after inducing experimental colitis successfully. During this period, they were listless, their hairs fell out and their weight lost gradually. After 3 days’ treatment, both in the administration of oral 5-ASA-loaded hydrogel or 5-ASA solution (control group) accelerated the weight loss and increase the food intake compared with the untreated colitis control group (DSS group). This observation became statistically significant on day 7. More effective treatment was observed with 5-ASA-loaded hydrogel group and the differences were statistically significant in comparison with the 5-ASA group (p < 0.05) and with the DSS group (p < 0.01).
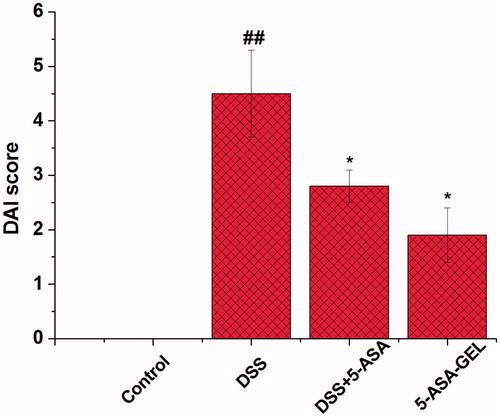
Effects of 5-ASA-loaded hydrogel on disease activity index score in the colon tissues
Disease activity index (DAI) score was the main parameter for evaluating the degree of colonic inflammation in IBD. On day 14 (24 h after the last drug administration), the mice were sacrificed to determine the DAI score in order to characterize the inflammation quantitatively. shows that the differences could be figured out significantly among these groups. From the 4th day, the feces were no longer coherent, and bloody diarrheas were appeared on the 7th day. Compared with the control group, the DSS group shows a significant increase in DAI activities in the colonic mucosa (p < 0.01). Treatment with 5-ASA solution (14.3 mg/kg) or 5-ASA-loaded hydrogel (14.3 mg/kg) significantly decreased the DAI scores (p < 0.05, p < 0.05) compared to the DSS group (). The therapeutic dose of 14.3 mg/kg, 5-ASA-loaded hydrogel shows a slightly more effective treatment compared to 5-ASA group in DAI activity (p < 0.05, p < 0.01). On the other side, we measured the change of colon length in order to evaluate the inflammation quantitatively.
Figure 4. Effects of DSS on colonic DAI score in mice with DSS-induced colitis. Data are means ± SD. n = 10 per group. ##p < 0.01 versus control group; *p < 0.05 versus DSS group; ##p < 0.01 versus DSS + 5-ASA-GEL group. DSS, dextran sodium sulfate; 5-ASA, 5-aminosalicylic acid; 5-ASA-GEL, 5-aminosalicylic acid-loaded hydrogel.
Change of colon length during the treatment process
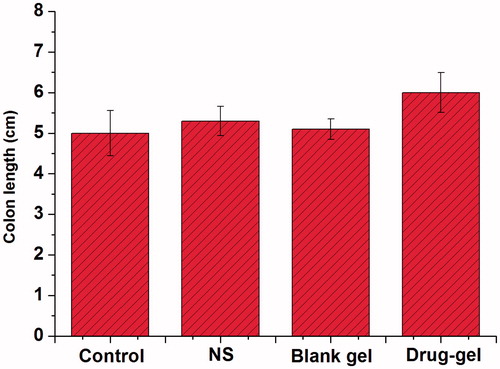
The colon length trends of each group mice were displayed in . On the day 14 (24 h after the last drug administration), the mice were sacrificed to determine the colon length in order to characterize the inflammation quantitatively. As a result, the length of normal colon was longer than that of the diseased colon, which is due to the DSS solution that is responsible for the formation of colonic ulcers. It is clear that the colon length was proportional according to the degree of inflammation. The colon length was increased slightly after treating with 5-ASA (control group), whereas it increased faster in the 5-ASA-GEL group. We could see that 5-ASA had a good efficacy for treating UC, and hydrogels entrapping 5-ASA could significantly enhance the colon targeting of drug and improve their efficacy.
Histopathological study
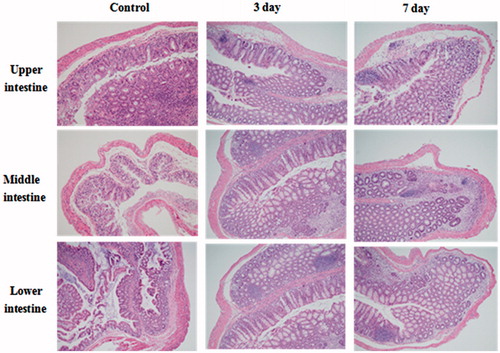
shows the interpretation of the pathological changes in those colon specimens. Under light microscope, the visible bleeding points, crypt destruction and secondary mucosal or submucosal inflammation in different colon section of control group, 5-ASA-GEL group were observed obviously in detail, and we could clearly find that these groups had serious inflammation, especially in lower intestine. There were crypts dropout, glands destruction and variable inflammations cells infiltration in colon tissues. After the 5-ASA-GEL treatment, inflammation cells had reduced obviously, and the crypts were repaired greatly. The inflammation was well treated and controlled. It could be illustrated that 5-ASA-GEL had positive effects on the treatment for UC healing. In this study, due to the stability in the acidic solution, hydrogel remained relatively integral formation in the stomach and the small intestine on the upper. And with the increase of the pH and the degree erosion of the hydrogel, the release rate increased significantly, especially in colon.
The safety of 5-ASA hydrogels
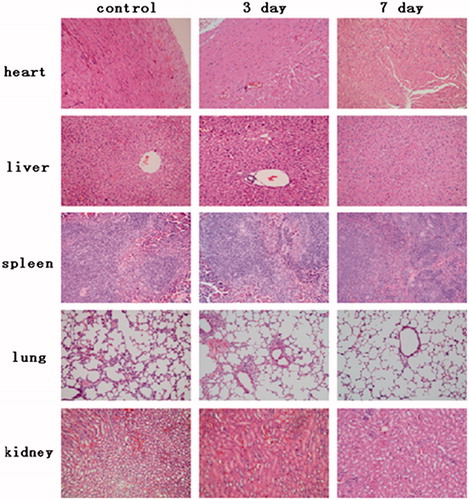
shows the pathological changes of major immune organs (cardiac muscle, liver, spleen, lung and kidney) treated by 5-ASA-GEL. Compared to the normal control, the cardiac muscle, liver, spleen, lung and kidney of 5-ASA-GEL group had no obvious pathological changes. Cardiac myocytes are clear and arranging in good order, and there was no hemorrhage or necrosis or inflammatory exudates. The classic structure of liver lobule with central vein is clearly observed. No lymphocyte, neutrophil and macrophage infiltration, also no hepatocellar degeneration or necrosis was found. Simultaneously, the normal tissue structure of spleen was observed in . It is clear that spleen sinus did not show pathologic changes. Lung tissue did not show significant difference compared with the control group. No bronchioles and alveoli ectasia or collapse, no alveolar epithelial denaturation, and no inflammatory cell infiltration surrounding bronchus were observed. Meanwhile, we can see that various kidney tubes and renal glomerulus show normal shape, no degeneration and bleeding and necrosis. In a word, all results indicated that the 5-ASA-GEL was in a safety dosage form for the ulcerative colitis treatment.
Conclusions
With the aim of evaluating the therapeutic value of 5-ASA-loaded hydrogels, the UC model was introduced into this experiment. P(CE-MAA-MEG) hydrogels that we prepared for UC could be a safe vehicle for drug delivery, and help 5-ASA to control the release ratio in vivo and in vitro, which administered orally could produce favorable results in treating UC. In this study, the model drug of 5-ASA were successfully loaded in the hydrogel according to the picture of SEM. The in vitro release behavior of drug gave an apparent evidence that this kind of hydrogel might be an effective vehicle for colon targeting drug delivery system. Animal treatment with 5-ASA-loaded hydrogel administered orally could produce favorable results in treating ulcerative colitis. In a word, this study provided the potential application for a novel colon targeting drug delivery vehicle.
Declaration of interest
This work was supported by the China Postdoctoral Science Foundation Funded Project (2014M562429) and Fundamental Research Funds for the Central Universities (xj08142016 and xjj2013054).
References
- Ardizzone S, Maconi G, Russo A, et al. (2006). Randomized controlled trial of azathioprine and 5-aminosalicylic acid for treatment of steroid dependent ulcerative colitis. Gut 55:47–53
- Chao GT, Deng HX, Huang Q, et al. (2006). Preparation and characterization of pH sensitive semi-interpenetrating network hydrogel based on methacrylic acid, bovine serum albumin (BSA), and PEG. J Polym Res 13:349–55
- Collnot EM, Ali H, Lehr CM. (2012). Nano-and microparticulate drug carriers for targeting of the inflamed intestinal mucosa. J Control Release 161:235–46
- Cruise GM, Scharp DS, Hubbell JA. (1998). Characterization of permeability and network structure of interfacially photopolymerized poly (ethylene glycol) diacrylate hydrogels. Biomaterials 19:1287–94
- Feng SS, Mei L, Anitha P, et al. (2009). Poly(lactide)-vitamin E derivative/montmorillonite nanoparticle formulations for the oral delivery of Docetaxel. Biomaterials 30:3297–306
- Gann MJM, Higginbotham CL, Geever LM, Nugent MJD. (2009). The synthesis of novel pH-sensitive poly(vinyl alcohol) composite hydrogels using a freeze/thaw process for biomedical applications. Int J Pharm 372:154–61
- Ha JC, Kim SY, Lee YM. (1999). Poly(ethylene oxide)-poly(propylene oxide) – poly(ethylene oxide) (pluronic)/poly(e-caprolactone) (PCL) amphiphilic block copolymeric nanospheres. I. Preparation and characterization. J Control Release 62:381–92
- Hamidi M, Azadi A, Rafiei P. (2008). Hydrogel nanoparticles in drug delivery. Adv Drug Deliver Rev 60:1638–49
- Harris MS, Lichtenstein GR. (2011). Review article: delivery and efficacy of topical 5-aminosalicylic acid (mesalazine) therapy in the treatment of ulcerative colitis. Aliment Pharm Therap 33:996–1009
- He CB, Cui FY, Yin LC, et al. (2009). A polymeric composite carrier for oral delivery of peptide drugs: bilaminated hydrogel film loaded with nanoparticles. Eur Polym J 45:368–76
- Horkay F, Han MH, Han IS, et al. (2006). Separation of the effects of pH and polymer concentration on the swelling pressure and elastic modulus of a pH-responsive hydrogel. Polymer 47:7335–8
- Huttunen KM, Tani N, Juvonen R, et al. (2013). Design, synthesis, and evaluation of novel cyclic phosphates of 5-aminosalicylic acid as cytochrome P450-activated prodrug. Mol Pharm 10:532–7
- Jia WJ, Liu JG, Zhang YD, Wang JW. (2007). Preparation, characterization, and optimization of pancreas-targeted 5-Fu loaded magnetic bovine serum albumin microspheres. J Drug Target 15:140–5
- Klotz U. (2012). The pharmacological profile and clinical use of mesalazine (5-aminosalicylic acid). Arzneimittelforschung 62:53–8
- Li YM, Wang YN, Liu Y, et al. (2014). The possible role of the novel cytokines IL-35 and IL-37 in inflammatory bowel disease. Mediators Inflamm DOI: org/10.1155/2014/136329
- Martin TD, Chan SSM, Hart AR. (2014). Environmental factors in the relapse and recurrence of inflammatory bowel disease: a review of the literature. Dig Dis Sci. DOI: 10.1007/S 10620-014-3437-3
- Mura C, Nàcher A, Merino V, et al. (2011). N-succinyl chitosan systems for 5-aminosalicylic acid colon delivery: in vivo study with TNBS-induced colitis model. Int J Pharm 416:145–54
- Qiu Y, Park K. (2001). Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev 53:321–39
- Römkens TEH, Kampschreur MT, Drenth JPH, et al. (2012). High mucosal healing rates in 5-ASA-treated ulcerative colitis patients: results of a meta-analysis of clinical trials. Inflamm Bowel Dis 18:2190–8
- Shaikh M, Kichenadasse G, Choudhury NR, et al. (2013). Non-vascular drug elutin stents as localized controlled drug delivery platform: preclinical and clinical experience. J Control Release 172:105–17
- Shim WS, Kim JH, Park H, et al. (2006). Biodegradability and biocompatibility of a pH- and thermo-sensitive hydrogel formed from a sulfonamide-modified poly(ε-caprolactone-co-lactide)-poly(ethylene glycol)-poly(ε-caprolactone-co-lactide) block copolymer. Biomaterials 27:5178–85
- Sonu I, Wong R, Rothenberg ME. (2013). 5-ASA induced recurrent myopericarditis and cardiac tamponade in a patient with ulcerative colitis. Dig Dis Sci 58:2148–50
- Thomas S, Baumgart DC. (2012). Targeting leukocyte migration and adhesion in Crohn’s disease and ulcerative colitis. Inflammopharmacology 20:1–18
- Trivedi PP, Jena GB. (2012). Dextran sulfate sodium-induced ulcerative colitis leads to increased hematopoiesis and induces both local as well as systemic genotoxicity in mice. Mutat Res-Gen Tox En 744:172–83
- Trivedi PP, Jena GB. (2013). Melatonin reduces ulcerative colitis-associated local and systemic damage in mice: investigation on possible mechanisms. Dig Dis Sci 58:3460–74
- Wang K, Fu SZ, Gu YC, et al. (2009). Synthesis and characterization of biodegradable pH-sensitive hydrogel based on poly(ε-caprolactone), methacrylic acid, and poly(ethylene glycol). Polym Degrad Stabil 94:730–7
- Wang K, Xu X, Wang YJ, et al. (2010). Synthesis and characterization of poly(methoxyl ethylene glycol-caprolactone-co-methacrylic acid-co-poly(ethylene glycol) methyl ether methacrylate) pH-sensitive hydrogel for delivery of dexamethasone. Int J Pharm 389:130–8
- Wang YJ, Wang C, Wang YJ, et al. (2012). Micelles of methoxy poly(ethylene glycol)-poly(ε-caprolactone) as a novel drug delivery vehicle for tacrolimus. J Biomed Nanotechnol 9:1–11
- Wei L, Wang JL, Zou LZ, Zhu SQ. (2008). Synthesis and characteristic of the thermo- and pH-sensitive hydrogel and microporous hydrogel induced by the NP-10 aqueous two-phase system. Eur Polym J 44:3688–99
- Wolk O, Epstein S, Dahan VL, et al. (2013). New targeting strategies in drug therapy of inflammatory bowel disease: mechanistic approaches and opportunities. Expert Opin Drug Del 10:1275–86
- Xu FJ, Kang ET, Neoh KG. (2006). pH- and temperature-responsive hydrogels from crosslinked triblock copolymers prepared via consecutive atom transfer radical polymerizations. Biomaterials 27:2787–97
- Yin LC, Ding JY, He CB, et al. (2009). Drug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanopariticles in oral insulin delivery. Biomaterials 30:5691–700