?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Delivery systems controlling drug release only in the colon holds great promises since they improve utilization of drug and decrease the dosing times comparison with conventional forms. The aim of the present study was to prepare polymeric microparticles on the basis of Ciprofloxacin via oral route for the treatment of inflammatory bowel disease. Ciprofloxacin was selected because of its extensive coverage for intestinal flora, relatively favorable side-effect profile and preliminary data suggesting its efficacy in the treatment of active Crohn's Disease. Microparticles were prepared using different acrylic compounds, namely Eudragit® RL (PO) and RS (PO) and a mixture of both. Spray-drying was used as a preparation method of Ciprofloxacin/Eudragit® microparticles using a Mini Spray Dryer B-290 (Büchi, Postfach, Switzerland). In vitro dissolution studies were performed to choose the best formulation and selected microparticles were characterized by size and morphology by environmental scanning electron microscopy. Yield and encapsulation efficiency were calculated and in vivo/ex vivo experiments were investigated both of which suggest that selected microparticles can be used for colon targeting of drugs increasing residence time of the drug in the affected area.
Introduction
The oral route is considered to be the most convenient for administration of drugs to patients. Normally it dissolves in intestinal fluid and absorb from these regions of gastrointestinal transit (GIT). At present, the specific drug delivery to the colon is considered as an important alternative for the treatment of serious local disease like inflammatory bowel disease (IBD) (Crohn's disease, ulcerative colitis), carcinoma and infection (Collnot et al., Citation2012; Coco et al., Citation2013). There has been considerable investigation for the design of colon drug delivery systems and targeting has been achieved by different methods, such as polymer coating systems (pH-dependent, time-dependent and bacteria-dependent polymers), osmotic-controlled systems, pressure-controlled systems and prodrugs (Singh et al., Citation2012; Verma et al., Citation2012; Reddy et al., Citation2013).
A reduction of drug carrier size has been recognized as an efficient way to prolong colonic passage time. Most IBD patients suffer from diarrhea due to pathophysiological changes in fluid and electrolyte absorption and secretion. This enhances the so called streaming phenomenon in the colon, i.e. the more rapid movement of solid phases and larger dosage forms compared to liquid phases. Reducing the diameter of a pharmaceutical formulation, the residence time in the large intestine can be increased and this also holds true for patients suffering from diarrhea (Collnot et al., Citation2012; Schmidt et al., Citation2013).
In recent years, polymeric nano- and microparticles have turned out to be promising for that (Lamprecht et al., Citation2001; Lautenschläger et al., Citation2014). Polymeric particles used for drug delivery are defined as colloidal systems made of solid polymers that may be classified according to their size and preparation processes. Different methods are described to obtain micro-nanoparticles: coacervation (simple and complex), solvent evaporation, fluidized-bed technology, ionic gelation, polymerization (in situ and interfacial) and spray drying process (Sae et al., Citation2007).
The principle of spray drying is based on the nebulization through a desiccating chamber of a polymer solution containing the active ingredient as solute or in suspension. The solvent is rapidly evaporated transforming the small droplets in solid microparticles, which are usually organic solvent free with respect to other preparation methods. The performance of a spray-drying process critically depends on the drop size produced by the atomizer and on the way the gaseous medium (used to dry the spray) mixes with the drops. The method is suitable to high throughput but not in treatments of temperature-sensitive streams (Richardson et al., Citation2002).
Methacrylate copolymers (Eudragit®) represent interesting candidates for the production of microparticles by spray drying since they are inert and freely soluble in organic solvent. Among the different types of Eudragit® commercialized, Eudragit® RS and RL are copolymers based on neutral methacrylic acid esters. In particular, Eudragit® RS is able to form permeable films since it contains only 5% w/w of hydrophilic units and it exhibits a very low permeability, enabling sustained release formulation manufacture (Otsuka et al., Citation1993; Kim et al., Citation1994).
The aim of the present study was to produce and characterize control release methacrylate microparticles using Eudragit® RL (PO), Eudragit RS (PO) and a mixture of both, prepared by spray drying method for the treatment of the Inflammatory Bowel Disease (IBD). In future studies, a dosage form was done to overcome the pH of the stomach. A lot of drugs have been reported for this disease: salicylates, corticoids, immunomodulators, biologic agents, probiotics and antibiotics. In recent years, several published reports have appeared in the medical literature suggesting that antibiotic treatment of CD might be beneficial (Prantera et al., Citation1996, Citation1998; Steinhart et al., Citation2002; Lal & Steinhart, Citation2006). In our study, Ciprofloxacin (Greenbloom et al., Citation1998; Arnold et al., Citation2002), a quinolone derivative, was selected because of its extensive coverage for intestinal flora, relatively favorable side-effect profile and preliminary data suggesting its efficacy in the treatment of active CD.
Materials and methods
Materials
Ciprofloxacin chlorhydrate (CF) was purchased from Guinama (La Pobla de Valbona, Spain). Eudragit® RL (PO), (ERL), Eudragit® RS (PO) (ERS) was supplied by Evonik Industries AG (Essen, Germany). Ethanol absolute was purchased from Scharlau (Sentmenat, Spain). Sodium chloride, disodium hydrogen phosphate and Potassium dihydrogen phosphate were obtained from Panreac (Castellar del Valles, Spain). Sodium hydroxide and potassium chloride were supplied by Guinama S.A. (La Pobla de Valbona, Spain) Hydrogen chloride was obtained from Prolabo (Sonora, Mexico). All chemicals used were of analytical reagent grade.
Preparation of micro/nanoparticles
Eudragit® RS, Eudragit® RL or a mixture of both was dissolved in ethanol absolute. Then CF was added and kept stirring for two hours. The resultant solution was spray dried in a Mini Spray Dryer B-290 (Büchi). The solvent in the droplets created by atomization was evaporated inducing the formation of solid microparticles from the drops under the conditions of: inlet air temperature 120 °C, outlet air temperature 80 °C and spray feed rate 5 ml/min. The dry product was then separated in a cyclone and settled down into a collector (Otsuka et al., Citation1993; Chong-Kook et al., Citation1994; Lorenzo-Lamosa et al., Citation1998; He et al., Citation1999; Esposito et al., Citation2000, Citation2002). The different formulations investigated are summarized in . The samples were kept in a desiccator until all the assays were performed.
Table 1. Batch specifications of micro/nanoparticles.
Physical tests
Particle size analysis
The particle size of microparticles was detected by Mastersizer 2000LF (Malvern Instruments Ltd, Worcestershire, United Kingdom) with a sample dispersion unit via minimum liquid volume Hydro 2000 μP (A).
Yield of microparticles
The yields of preparation were determined by weighing the product of spray-dried microparticles with respect to the weight of the initial polymer and the drug used.
Encapsulation efficiency
The encapsulation efficiency (%) was determined dissolving 10 mg of microparticles into 25 ml of pH = 1.2 HCl buffer and kept under stirring overnight. Then various dilutions were made and analyzed spectrophotometrically (Lambda 35, Perkin Elmer Inc., Waltham, MA) at 276 nm for ciprofloxacin content, using a calibration curve based on standard solutions.
Environmental scanning electron microscopy
The morphology of selected microparticles was examined with an environmental scanning electron microscopy (ESEM) (mod. Quanta 400, FEI, Hillsboro, OR), equipped with an EDT detector operating at 7000–12 000 × and at 10.0 kV.
In vitro drug release
The rate of release of the drug from the microspheres was studied in phosphate buffered saline (PBS) and was determined in a dissolution apparatus (AT7 Smart, Sotax, Matas, Spain) with the dissolution baskets assembly (USP Apparatus I) in order to simulate the colon where IBD is normally instituted. A total of 35 mg of microspheres were suspended in 1000 ml of PBS, pH = 7.4 (Lorenzo-Lamosa et al., Citation1998; Vinay et al., Citation2012), 37 °C, at 50 rpm agitation rate (Padhy et al., Citation2013; Rahman et al., n.d.). At predetermined time intervals, samples of 5 ml were withdrawn. The samples were suitably diluted if necessary and estimated spectrophotometrically at 276 nm by using Perkin Elmer UV/Visible double beam spectrophotometer (Lambda 35, Perkin Elmer Inc., Waltham, MA) and cumulative percentage drug release was calculated. As the sample volume was not replaced with fresh dissolution medium, a correction factor was used to account for the lost volume.
Bioavailability after oral administration
Twelve healthy white albino rabbits ranging in body weight from 1 to 1.2 kg were used (Harder et al., Citation1990; Fahmy & Abu-Gharbieh, Citation2014).
The animals were placed in poly-propylene cages, with free access to standard laboratory diet, and provided with tap water ad libitum. Lighting was on a standard 12 h on/12 h off cycle. Pharmacokinetics of CF from the prepared microparticles as well as free drug was studied after administration of an oral dose in normal rabbits.
The rabbits were randomly divided into two groups (n = 6). A single dose was given for each rabbit (approximately 212 mg drug per kg body weight). The first group received 500 mg of the microparticles containing 212 mg CF. The drug was prepared in a solution form and was administered similarly to the other group through the feeding tube orally.
Blood samples were collected through the central vein of the rabbits in heparinized glass centrifuge tubes using sterilized disposable plastic syringes at 0.5, 1, 2, 3, 4, 5, 6, 8, 12 and 24 h after the drug administration. The blood samples were centrifuged at 5000 rpm for 10 min to separate the plasma for analysis.
An equal amount of acetonitrile was added to the plasma samples to separate proteins. The mixture was vortexed for two minutes, and then centrifuged at 2000 rpm for 10 min. The supernatant was transferred to a vial and concentration of CF in plasma was determined by a high performance liquid chromatographic (HPLC) method (Khan & Khan, Citation2005) using a system equipped with a reversed – phase C18 analytical column (220 × 4.4 mm I.D., 5 µm particle size) proceeded by a guard column (15 × 3.2 mm, 7 µm particle size). The mobile phase consisted of a mixture of 2% acetic acid aqueous solution and Acetonitrile (ACN) (85:15, v/v). The flow rate was set at 1.0 ml/min. The injection volume was 20 μl and the effluent was monitored at 279 nm by a UV detector. The retention time was about 6.5 ± 0.26 min. Elution was carried out isocratically at ambient temperature. The method was selective with no interference from other components (Wu et al., Citation2008).
Ex-vivo intestinal permeation
Twelve male Wistar rats (weighing 200–250 g) were housed in a temperature and humidity controlled room (23 °C, 55% air humidity) with free access to water and standard diet. The rats were fasted overnight but supplied with water ad libitum before the experiment. Animals were sacrificed by spinal dislocation and the small intestine was immediately removed after sacrifice by cutting across the upper end of the duodenum and the lower end of the ileum and manually stripping the mesentery. The small intestine was washed out carefully with cold normal oxygenated saline solution (0.9%, weight/volume, NaCl) using a syringe equipped with a blunt end. The clean intestinal tract was cut into 8 ± 0.2 cm long sacs (Ruan et al., Citation2006; Shishu et al., Citation2012).
Six sacs were filled with water via a blunt needle with CF solution (equivalent to 20 mg of the drug) and the other six sacs were filled with 50 mg of the microparticles dispersed in water. The two sides of the intestine were tied tightly with a thread. Each sac was placed in a glass conical flask containing 10 ml of Ringer’s solution. The entire system was maintained at 37 °C in a shaking water bath operated at 100 rpm and aerated using a laboratory aerator. Samples were withdrawn from outside of the sac and the medium was totally replaced by fresh medium every 15 min for 2 h. After that, the sacs were taken homogenized with normal saline. The tissue specimens were homogenized with normal saline, and subjected to freezing and defreezing cycles with frequent vortexing. ACN was then added followed by centrifugation at 4000 rpm for 30 min. The supernatant was separated and analyzed for drug content. Samples were analyzed by HPLC as previously reported.
Results
Physical tests
Microparticles obtained with a small percentage of polymer (2:1) were relatively large (17.4–239.9 µm). On the other hand, those obtained with ratio 1:2 and 1:1 were similar, whatever the polymer used, being the particle size around 3.31–5 µm. Yield was similar and enough high at drug:polymer ratios 1:1 and 1:2.
In general, as polymer content increased, the encapsulation efficiency slightly decreased as seen in the comparing data of . This can be due to the fact that ERL being more permeable than ERS due to the presence of more quaternary ammonium groups leading to insufficient encapsulation of drug in polymeric matrix. Those results are in agreement with those obtained by Biswal et al. (Citation2011).
Table 2. Microparticles physical tests.
ESEM
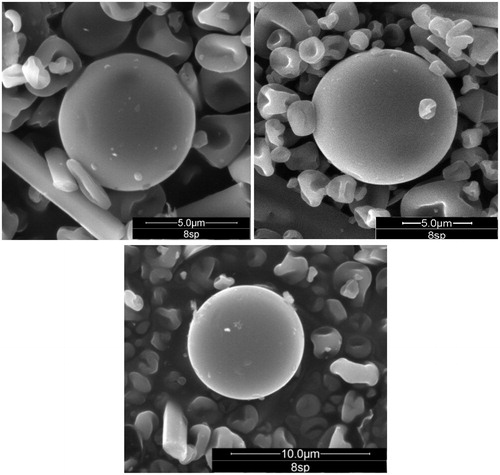
Environmental scanning electron microscopy of prepared microparticles with Eudragit® RL (formulation 8) revealed mostly spherical, smooth surface microparticles and in some cases distorted surface and without trend to aggregation ().
In vitro drug release
Preliminary studies were made to choose the best drug:polymer rate for the CF microparticles. The effect of varying the Eudragit type and the drug:polymer (w/w) ratio on CF dissolution rate from the microparticles are shown in and .
Figure 2. In vitro drug release of the microparticles with (a) ERS, (b) ERL and (c) mixture of ERS and ERL (1:1 w/w) expressed as a percentage of the amount of total drug content.
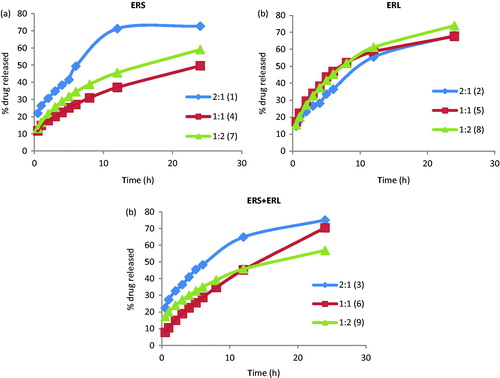
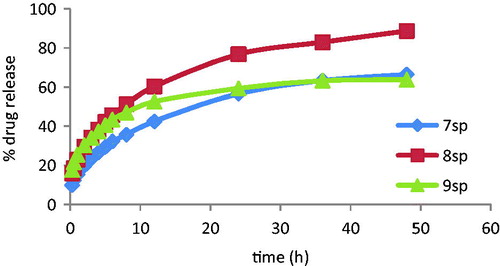
Figure 3. In vitro drug release of the microparticles with 1:2 drug:polymer ratio for 48 h expressed as a percentage of the amount of total drug content.
To make easy the comparison of different drug:polymer (w/w) ratios for each polymer type used, the dissolution curves obtained from microparticles prepared with ERS (), ERL () and ERS + ERL (1:1 w/w) () were shown separately. Those preliminary studies were made for 24 h and three replicates from each batch were tested.
A controlled release was reached in all studied microparticles after a burst effect attributed to the dissolution of free ciprofloxacin on the surface of the microparticles. In general, for the three polymers studied, the release of CF from de microparticles with 2:1 proportion was poorly controlled. When the polymer amount increased in the microparticles (1:1 and 1:2 ratio) the dissolution rates were slower, being the last in those of the best dissolution behavior. The microparticles showed similar dissolution behavior but a higher % amount of dissolved drug with ERL polymer that could be attributed to the number of quaternary ammonium groups of ERS and ERL (approximately 5% for ERS and 10% of ERL), in agreement with results obtained by Biswal et al. (Citation2011).
Consequently, a later in vitro dissolution study was made for microparticles with 1:2 drug:polymer ratio in order to choose the polymer with the highest percentage of CF released in 48 h (). The samples were six-fold analyzed.
Dissolution profiles data indicate that with the three assayed polymers, CF releasedfrom the microparticles was slow and gradual, highlighting microparticles with formulation 8 which released 90% of CF content after 48 h, while those based on the formulations 7 and 9 just got a CF release of 60% content. Consequently, the microparticles prepared using formulation 8 were selected for the further in vivo and ex vivo experiments.
In vivo and ex vivo experiments
The systemic bioavailability was assessed by measuring the plasma concentrations of the drug after oral administration of the free drug and the drug-loaded microparticles ().
Figure 4. Plasma concentrations of ciprofloxacin versus time after administration of a 212-mg oral dose of ciprofloxacin HCl and 500 mg loaded microparticles.
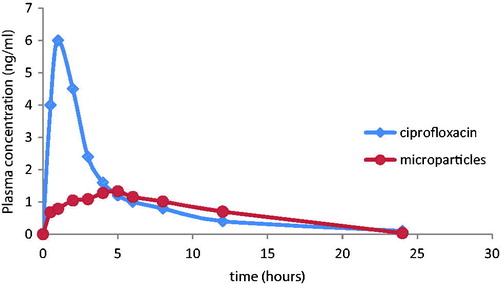
The oral administration of the free drug resulted in a relatively rapid increase in plasma concentrations with a peak value of 6.1 mg/l at about 1 h. On the other hand, after oral administration of the microparticles, the peak serum level was delayed to 5 h and was reduced to 1.38 mg/l. The area under the curve was reduced from 26.4 mg h/l in case of the free drug to 16.6 mg h/l in case of the microparticles. These results can be attributed to the fast absorption of the free drug resulting in rapidly high serum level. In case of microparticles, the sustained release is maintained for 12 h with constant serum levels of drug resulting in slower absorption and lower systemic bioavailability, allowing a prolonged action of the CF at the site of action (Makhlof et al., Citation2009).
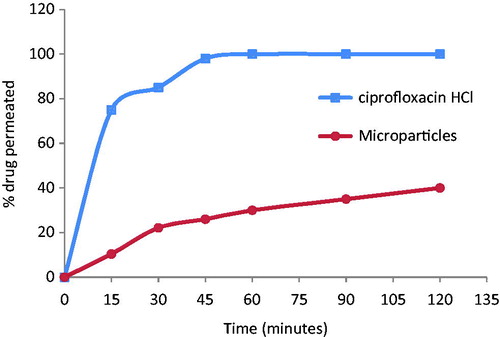
These results were further confirmed by an ex-vivo permeation study (). 100% of the free drug was detected in the receptor medium after 1 h. However, in case of the microparticles only 40% were detected in the receptor medium after 2 h. Analysis of the homogenate at the end of the experiment revealed that more than 50% of the drug was still present inside the intestinal sac suggesting its transfer to the colon after its transient time in the intestine where it is required to perform its effect. So microparticles prolong the release of the drug in the specific site of action because of the gradual permeation and the sustained release.
Conclusion
From the results of the present study, it can be concluded that ciprofloxacin microspheres prepared using Eudragit® RL (PO) with a drug:polymer ratio 1:2 could represent a candidate for its sustained release after in vivo administration for the treatment of IBD. This eventually leads to an increased residence time of the drug in the affected area. However, further in vivo studies are needed to ascertain the efficacy of the formulation in real life situation.
Currently studies are ongoing for incorporating the developed microparticles into suitable oral dosage form.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Arnold GL, Beaves MR, Pryjdun VO, Mook WJ. (2002). Preliminary study of ciprofloxacin in active Crohn’s disease. Inflamm Bowel Dis 8:10–15
- Biswal I, Dinda A, Das D, et al. (2011). Encapsulation protocol for highly hydrophilic drug using non-biodegradable polymer. Int J Pharm Sci 3:256–9
- Chong-Kook K, Mi-Jung K, Kyoung-Hee O. (1994). Preparation and evaluation of sustained release microspheres of terbutaline sulfate. Int J Pharm 106:213–19
- Coco R, Plapied L, Pourcelle V, et al. (2013). Drug delivery to inflamed colon by nanoparticles: comparison of different strategies. Int J Pharm 440:3–12
- Collnot E-M, Ali H, Lehr C-M. (2012). Nano- and microparticulate drug carriers for targeting of the inflamed intestinal mucosa. J Control Release 161:235–46
- Esposito E, Cervellati F, Menegatti E, et al. (2002). Spray dried Eudragit microparticles as encapsulation devices for vitamin C. Int J Pharm 242:329–34
- Esposito E, Roncarati R, Cortesi R, et al. (2000). Production of Eudragit microparticles by spray-drying technique: influence of experimental parameters on morphological and dimensional characteristics. Pharm Dev Technol 5:267–78
- Fahmy S, Abu-Gharbieh E. (2014). In vitro dissolution and in vivo bioavailability of six brands of ciprofloxacin tablets administered in rabbits and their pharmacokinetic modeling. BioMed Res Int 2014, 1–8. doi:10.1155/2014/590848
- Greenbloom SL, Steinhart AH, Greenberg GR. (1998). Combination ciprofloxacin and metronidazole for active Crohn’s disease. Can J Gastroenterol 12:53–6
- Harder S, Fuhr U, Beermann D, Staib A. (1990). Ciprofloxacin absorption in different regions of the human gastrointestinal tract. Investigations with the hf-capsule. Br J Clin Pharmacol 30:35–9
- He P, Davis SS, Illum L. (1999). Chitosan microspheres prepared by spray drying. Int J Pharma 187:53–65
- Khan MK, Khan MF. (2005). Extraction and separation of ciprofloxacin by HPLC from human plasma. Intl Chem Pharm Med 2:267–70
- Kim C-K, Mi-Jung K, Kyoung-Hee O. (1994). Preparation and evaluation of sustained release microspheres of terbutaline sulfate. Int J Pharm 106:213–19
- Lal S, Steinhart AH. (2006). Antibiotic therapy for Crohn’s disease: a review. Can J Gastroenterol 20:651–5
- Lamprecht A, Schäfer U, Lehr, C.-M. (2001). Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm Res 18:788–93
- Lautenschläger C, Schmidt C, Fischer D, Stallmach A. (2014). Drug delivery strategies in the therapy of inflammatory bowel disease. Adv Drug Deliv Rev 71:58–76
- Lorenzo-Lamosa ML, Remuñán-López C, Vila-Jato JL, Alonso MJ. (1998). Design of microencapsulated chitosan microspheres for colonic drug delivery. J Control Release 52:109–18
- Makhlof A, Tozuka Y, Takeuchi H. (2009). pH-Sensitive nanospheres for colon-specific drug delivery in experimentally induced colitis rat model. Eur J Pharm Biopharm Off J Arbeitsgemeinschaft Für Pharm Verfahrenstechnik EV 72:1–8
- Otsuka M, Onoe M, Matsuda Y. (1993). Hygroscopic stability and dissolution properties of spray-dried solid dispersions of furosemide with eudragit. J Pharm Sci 82:32–8
- Padhy SK, Sahoo D, Acharya D, Mallick J. (2013). Formulation and in-vitro evaluation of ciprofloxacin hydrochloride sustained release tablets using various viscosity grades of hydroxypropyl methylcellulose. Am J Pharm Tech Res 3:1433–43
- Prantera C, Berto E, Scribano ML, Falasco G. (1998). Use of antibiotics in the treatment of active Crohn’s disease: experience with metronidazole and ciprofloxacin. Ital J Gastroenterol Hepatol 30:602–6
- Prantera C, Zannoni F, Scribano ML, et al. (1996). An antibiotic regimen for the treatment of active Crohn’s disease: a randomized, controlled clinical trial of metronidazole plus ciprofloxacin. Am J Gastroenterol 91:328–32
- Rahman MM, Roy S, Das SC, et al. n.d. Formulation and evaluation of hydroxypropylmethylcellulose based matrix systems as oral sustained release drug delivery systems for ciprofloxacin hydrochoride. Int J Pharmaceut Sci Rev Res 6:34–41
- Reddy RB, Malleswari K, Prasad G, Pavani G. (2013). Colon targeted drug delivery system: a review. IJPSR 4:42–54
- Richardson JF, Backhurst J, Harker JH. (2002). Chemical engineering. Vol. 2. San Diego (CA): Butterworth-Heinemann [Imprint] Elsevier Science & Technology Books
- Ruan L-P, Chen S, Yu B-Y, et al. (2006). Prediction of human absorption of natural compounds by the non-everted rat intestinal sac model. Eur J Med Chem 41:605–10
- Schmidt C, Lautenschlaeger C, Collnot E-M, et al. (2013). Nano- and microscaled particles for drug targeting to inflamed intestinal mucosa - a first in vivo study in human patients. J Control Release 165:139–45
- Shishu Kamalpreet, Maheshwari M. (2012). Development and evaluation of novel microemulsion based oral formulations of 5-fluorouracil using non-everted rat intestine sac model. Drug Dev Ind Pharm 38:294–300
- Singh, Jagpreet Singh, Daisy Sharma, Anil Sharma. (2012). Colon specific drug delivery system: review on novel approaches. IJPSR 3:637–47
- Steinhart AH, Feagan BG, Wong CJ, et al. (2002). Combined budesonide and antibiotic therapy for active Crohn’s disease: a randomized controlled trial. Gastroenterology 123:33–40
- Verma S, Vipin Kumar V, Mishra DN, Singh SK. (2012). Colon targeted drug delivery: current and novel perspectives. IJPSR 3:1274–84
- Vinay KG, Gnanarajan G, Preeti Kothiyal. (2012). A review article on colonic targeted drug delivery system 1:14–24
- Vivian S, José Ramón H, Carlos P. (2007). Las microesferas como sistemas de liberación controlada de péptidos y proteínas
- Wu S-S, Chein C-Y, Wen Y-H. (2008). Analysis of ciprofloxacin by a simple high-performance liquid chromatography method. J Chromatogr Sci 46:490–5