Abstract
Archaeosomes as liposomes made with one or more ether lipids that are unique to the domain of Archaeobacteria, found in Archaea constitute a novel family of liposome. Achaean-type lipids consist of archaeol (diether) and/or caldarchaeol (tetraether) core structures. Archaeosomes can be produced using standard procedures (hydrated film submitted to sonication, extrusion and detergent dialysis) at any temperature in the physiological range or lower, therefore making it possible to encapsulate thermally stable compounds. Various physiological as well as environmental factors affect its stability. Archaeosomes are widely used as drug delivery systems for cancer vaccines, Chagas disease, proteins and peptides, gene delivery, antigen delivery and delivery of natural antioxidant compounds. In this review article, our major aim was to explore the applications of this new carrier system in pharmaceutical field.
Introduction
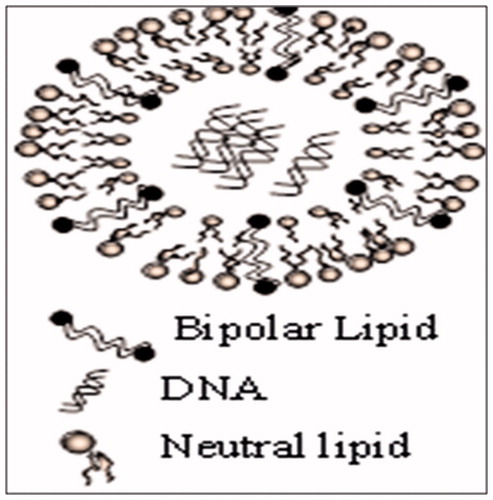
Archaeosomes (derived from a combination of the words archaea and liposomes) as liposomes made with one or more ether lipids that are unique to the domain of Archaeobacteria, including vesicles made from several combination of lipids that include archaea bacterial ether lipids in their composition (Chaudhary et al., Citation2014). The lipid membrane of archaeosomes can be found in the form of a bilayer (if made exclusively from monopole archaeol lipids or with lipid mixtures containing archaeols and nonarchaeobacterial monopolar lipids), or a monolayer (if made exclusively from bipolar caldarchaeol lipids), or a combination of mono and bilayers (if made from caldarchaeol lipids and archaeols or other monopolar lipids) (Benvegnu et al., Citation2009).
Archaeosomes prepared with one or more ether lipids found in Archaea constitute a novel family of liposome (Gagandeep et al., Citation2014). Achaean-type lipids consist of archaeol (diether) and/or caldarchaeol (tetraether) core structures (Garg, Citation2014). Archaeosomes' structures induce the presence of both hydrophobic and hydrophilic domains; they are able to entrap both hydrophilic and hydrophobic molecules, which are mainly attractive for encapsulation and drug delivery applications (Garg & Goyal, Citation2012). The definition of archaeosomes includes the use of synthetically derived lipids that have the unique structural characteristics of archaeobacterial ether lipids, that is frequently branched phytanyl chains attached via ether bonds at sn-2, 3 glycerol carbons (Goyal et al., Citation2013a). The lipid membrane of archaeosomes can be found in the bilayer form if made completely from monopolar archaeol (diether) lipids, or a monolayer if made completely from bipolar caldarchaeol (tetraether) lipids, or a grouping of monolayers and bilayers if made from caldarchaeol lipids in addition to archaeol lipids or standard bilayer-forming phospholipids (Yamauchi et al., Citation1992). The large range of lipid structures reflects the need for Archaea to adjust their core lipid structures in order to ensure membrane functions despite harsh destabilizing environmental conditions (Garg & Goyal, Citation2014b). Differences between archaeosomes and liposomes are shown in .
Table 1. Differences between archaeosomes and liposomes.
A typical characteristic should be particularly useful for the preparation of highly stable archaeosomes (Benvegnu et al., Citation2009).
(1) The ether linkages are additionally stable than esters over a wide range of pH (Garg & Goyal, Citation2014c).
(2) The branching methyl groups help both to reduce crystallization (membrane lipids in the liquid crystalline state at ambient temperature) and membrane permeability (steric hindrance of the methyl side groups) (Garg & Goyal, Citation2014a).
(3) The saturated alkyl chains would impart stability toward oxidative degradation (Garg et al., Citation2012a).
(4) The unusual stereochemistry of the glycerol backbone (opposite to mesophilic organisms) would ensure resistance from the attack by phospholipases released by other organisms (Garg et al., Citation2014a).
(5) The bipolar lipids span the membranes and enhance their stability properties (Garg et al., Citation2014d).
(6) The addition of cyclic structures (in particular five membered rings) in the transmembrane portion of the lipids appears to be thermo-adaptive response, resulting in enhanced membrane packing and reduced membrane fluidity (Garg et al., Citation2014b).
Archaeosomes can be produced using standard procedures (hydrated film submitted to sonication, extrusion and detergent dialysis) at any temperature in the physiological range or lower, therefore making it possible to encapsulate thermally stable compounds (Garg et al., Citation2014c). They can be prepared and stored in the presence of air/oxygen without any degradation (Garg et al., Citation2011a). The in vitro and in vivo studies point out that archaeosomes are safe and do not elicit toxicity in mice (Garg et al., Citation2011b). represents the basic structure of archaeosomes.
Source of archaea
Three major sources of archaea were found which will be very helpful for the availability for the same.
Methanogens: Methanococcus voltae, Methanosaeta concilii, Methanosphaera stadtmanae, Methanobacterium espanolae, Methanospirillum hungatei, Methanosarcina mazei, Methanococcus jannaschii, Methanobrevibacter smithii.
Halophiles: Halobacterium cutirubrum, Natronobacterium magadii.
Thermoacidophiles: Thermoplasma acidophilum (Benvegnu et al., Citation2009).
Advantages of archaeal lipids
Archaeal lipids play an important role from the production of pharmaceutical product to their stability maintainer. Some important advantages of archaeal lipids were described below:
The archaeal lipids are more stable than other phospholipids (Patel & Sprott, Citation1999).
The chemical modified archaeal lipid derivative provides more stability to the hydrolysis with other enzymes (Réthoré et al., Citation2007).
The addition of cyclic structure in the lipids appears to be thermo-adaptive response, by which enhanced membrane packing and reduced membrane fluidity (Réthoré et al., Citation2007).
Stability toward oxidative degradation would be improved by saturated alkyl chains (Benvegnu et al., Citation2013).
It does not require cholesterol in the formulation (Szoka & Papahadjopoulos, Citation1980).
The unusual stereochemistry of the glycerol backbone would ensure resistance to attack by phospholipases released by other organisms (Patel & Sprott, Citation1999).
It can be used for specific organ targeting (Patel & Chen, Citation2006).
The bipolar lipids enhance their stability properties hence they can be prepared and stored in the presence of air/oxygen without any degradation (Brown et al., Citation2009).
Due to high thermo-stability archaeosomes formulations can be sterilized by autoclaving (Brown et al., Citation2009).
Archaeal lipids have more thermo-labile stability in environment (Brown et al., Citation2009).
The phagocytic cells uptake is greater which ensures good adjuvant activity (Krishnan & Dennis Sprott, Citation2003).
Archaeal lipids act as self adjuvant drug delivery systems (Krishnan et al., Citation2000).
Types of archaea lipids
Archaea lipids can be classified into three major categories as follows.
Natural lipids
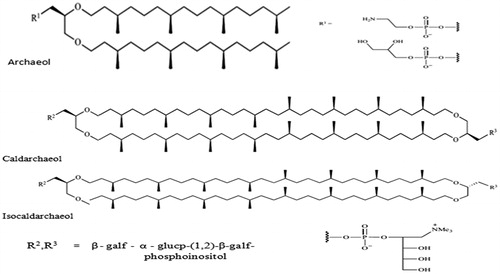
The structures of archaeal lipids are closely linked to the organisms from which they are extracted (Garg et al., Citation2012b). Archaea can be categorized into several species based on their living environment and each type of species has developed characteristic lipids. It is usually admitted that halophilic polar lipids are derived from monopolar dieter compounds (archaeal), whereas acidophilic polar lipids derived from bipolar tetraethers (caldarchaeol) (Garg et al., Citation2013). However, most of archaeal lipid membranes result from a mixture of lipids bearing various types of polar heads (phosphate, glycerophosphate, sugar, etc.); moreover, several archaea organisms such as methanogens have developed mixtures of both diether and tetraether type polar lipids (Jain et al., Citation2014). Natural archaeal lipids extracted from representative archaea organisms that cover the scope of the archaeal lipid structures. Several research groups cultivated extracted and purified total polar lipids (TPL) from methanogens, halophiles and thermoacidophiles (Goyal et al., Citation2013b). Archaea were grown under opmental living environmental conditions before undergoing extensive organic solvent extraction. TPL were finally obtained by the precipitation with acetone from a chloroform/methanol (2:1 v/v) solution (Goyal et al., Citation2014a). The resulting extracts were analyzed by thin layer chromatography (TLC) and mass spectrometry (MS) and were used as such or were further purified by preparative TLC to isolate pure polar lipid components (Benvegnu et al., Citation2009). Diether structures are based on a glycerol moiety bearing two phytanyl chains on the sn-2,3 positions. The main polar head groups of such archaeal derivatives are negatively charged phosphoethanolamine and phosphoglycerol (Goyal et al., Citation2014b). Tetraether components are characterized by the presence of two diphytanyl chains linked at both ends to two glycerol residues in an antiparallel manner (caldarchaeol) or in a parallel manner (isocaldarchaeol), while the configuration of the glycerol remaining the same. Sugar neutral head groups can complete the polar lipid structure as well as negative phosphoinositol or zwitterionic groups () (Ren et al., Citation2014).
Chemically modified natural lipids
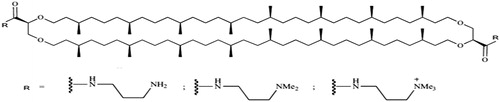
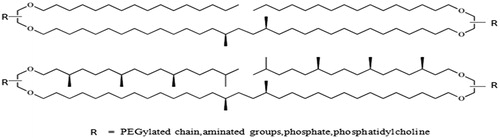
The polar head groups found in natural polar lipids from archaea lipid extracts are usually sensitive to acidic hydrolysis (1 M HCl–methanol). This chemical step therefore, results in the isolation of corresponding dihydroxyl archaeal lipid cores (Hussain et al., Citation2014). The Syrinx Diagnostika Company patented several new structures of archaeal lipids derived from T. acidophilum lipid extracts (Johal et al., Citation2014). Indeed after hydrolysis of the original polar head groups, a few organic synthetic steps such as oxidation of primary alcohols to carboxylic acids followed by activation and coupling with suitable amines afforded efficient access to the desired archaeal lipids bearing aminated polar head groups () (Benvegnu et al., Citation2009).
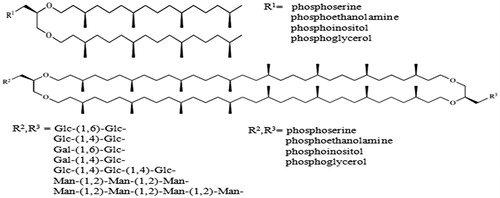
Both modified diether and tetraether lipid cores originally from, Halobacterium salinarum and T. acidophilum, respectively. Archaeols were then functionalized with sugar or phosphorylated head groups and caldarchaeols were symmetrically or unsymmetrically functionalized with the same polar head groups. Sugars were chosen from di-, tri- and tetrasaccharides of glucose, galactose or mannose. Phosphorylated groups were derived from phosphoserine, phosphoethanolamine, phosphoinositol and phosphoglycerol ().
Totally synthetic lipids
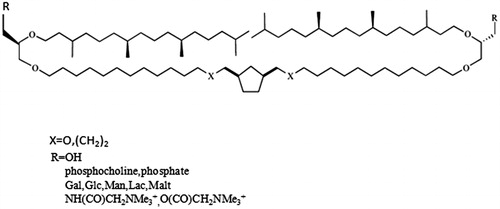
Although extraction of natural archaeal lipids led to significant amounts of pure compounds with some difficulties, total synthesis of archaeal lipid analogs was investigated by several academic or company research teams (Joshi et al., Citation2014a). A series of archaeal lipid analogs based on a symmetrical 1,3-cyclopentane ring bearing two alkyl (X = (CH2)2) or alkoxy (X = O) chains linked to two glycerol units ().
Finally, a phytanyl arm on each glycerol residue results in a quasimacrocyclic tetraether structure. The stereochemistry of the glycerol moieties was conserved with respect to natural archaeal lipids derived from isocaldarchaeol (parallel arrangement of the glycerol groups). A range of polar head groups (OH, sugars, glycine betaine, phosphorylated groups, PEG chains, ligands) was introduced symmetrically or unsymmetrically at both terminal ends of the tetraether core (Joshi et al., Citation2014b). These tetraethers are based on a C32 chain core bearing two branched methyl groups within the middle of the bridging chain. Here also two phytanyl or linear C16 arms led to quasimacrocyclic backbones. The stereochemistry (not shown) is the same as in natural archaeal lipids. Both caldarchaeol and isocaldarchaeol analogs were designed and functionalized by several polar head groups such as a PEG chain, aminated groups or phosphorylated groups () (Benvegnu et al., Citation2009).
Preparation techniques
The lipid extraction is commenced from suitable archaeobacterial species. The total natural archaeal lipid extract (TLE) composed of TPL and neutral lipids like Squalene and other hydrocarbons. Chloroform/methanol/water extraction from freeze-thawed biomass extracted from the selected archaea species provides such TLE. From TLE, TPL and neutral lipids are separated by precipitation of TPL using acetone (Kalia et al., Citation2014). These TPL made from isoprenoid ether lipids of opposite sn-2,3 stereochemistry can be stored in chloroform or chloroform/methanol (2:1) solutions without special conditions. It is worth to mention that only glycol lipid sulfate and phospatidylglycerophosphate fraction form the vesicle. Pure archaeal lipids can be further obtained by chromatography, either column chromatography or preparative thin layer chromatography (Patel & Sprott, Citation1999). The resulting pure archaeal lipids can be chemically modified in order to introduce specific head groups. One can also synthesize the archaeal lipid phosphatidylmyoinositol is one polar group and either glucopyranose or galactopyranosyl glucopyranose as the other polar head group is essential for structural stability (Perrie et al., Citation2007). Further, it is very difficult to hydrate such lipids. Starting from natural, chemically modified or synthetic archaeal lipids, it was possible to prepare formulations of archaeosomes and encapsulate/associate hydrophilic or hydrophobic compounds using methods developed for the preparation of conventional liposomes (Szoka & Papahadjopoulos, Citation1980).
Mechanical dispersion method
All methods covered under this category begin with a lipid solution in organic solvent and end up with lipid dispersion in water. The various components are typically combined by co-dissolving the lipid in an organic solvent and the organic solvent is removed, the solid–lipid mixture is hydrated using aqueous buffer (Kataria et al., Citation2014). The lipids spontaneously swell and hydrate to form archaeosomes. At this point, methods incorporate some diverge processing parameters in various ways to modify their ultimate properties. These post-hydration treatments include vortexing, sonication, freeze thawing and high pressure extrusion (Szoka & Papahadjopoulos, Citation1980).
Lipid hydration method
In this method, archaea lipid is dissolved in solvent mixture of chloroform–methanol (2:1) in rotary evaporator flask and dried thin film of lipid is made using rotary evaporator. This process was performed at room temperature (30 °C), speed (60 rpm) and time for 15 min. In this process, lipid hydration is performed by adding 5 ml of saline phosphate buffer containing drug/antigen to be encapsulated. Rotary evaporator was used for making uniform suspension. The archaeobacterial polar ether lipids are in a liquid crystalline-like or fluid state at room temperature, and hence they can be hydrated at room temperature so it was kept 2 h at room temperature to complete swelling process. By this process Multilamellar vesicles (MLVs) can be obtained. Archaeosomes will be annealed even at refrigeration temperatures (Kaur et al., Citation2014a). For example, betamethasone dipropionate (BMD)-loaded archaeosomes and conventional liposomes were prepared by the thin-lipid film procedures, using, correspondingly, archaeal lipids extracted from archaea H. salinarum and enriched soy phosphatidylcholine (González-Paredes et al., Citation2010). Ultra deformable archaeosomes (UDA) are vesicles made of soybean phosphatidylcholine (SPC), sodium cholate (NaChol) and polar lipids from Halorubrum tebenquichense (3:1:3 wt/wt). UDA were prepared by lipid hydration method for topical applications (Higa et al., Citation2012).
Membrane extrusion
Size of prepared archaeosomes is reduced by gently passing them through membrane filter of defined pore size and this can be achieved at much lower pressure. The vesicles content are extrude with the dispersion medium during breaking and resealing of polar phospholipids as they pass through the polycarbonate membrane in order to achieve high entrapment (Kaur et al., Citation2014b). The archaeosomes produced by this method have been termed as Large unilamellar vesicles by extrusion (LUVETs) and 30% encapsulation can be obtained using high lipid concentration. The polar lipid methanol fractionwas used for preparing archaeosomes. The dry lipid film was hydrated with KPB buffer consisting of 250 nM sucrose, 10 mM phosphate (K2HPO4/KH2PO4) and 1 mM MgCl2, pH 7.4, and then vortexes to obtain multilamellar (ML) vesicles. These were then transformed to unilamellar (UL) vesicles by extrusion through a 400-nm membrane (Nucleopore Track-Etch membrane, Whatman, UK) (Zavec et al., Citation2014).
Freeze–thaw method
In this method, freezing of UL dispersion and thawing (melting) to room temperature for 15 min and subjected to a sonication cycle. Archaeosomes themselves fuse and markedly increase in size (Kaur et al., Citation2014c). Then, the sonication considerably reduces the permeability of the archaeosomes membrane, by accelerating the rate at which the packing defects can be eliminated. For producing giant vesicles >1 μm of diameter, the sonication step can be replaced with the dialysis against hypo-osmolar buffer (Kaur et al., Citation2014d). Small unilamellar vesicles (SUVs) are mixed with salt solution followed by freeze thawing. During the dialysis, the large vesicles formed by freeze thawing swell and rupture as a result of the osmotic lysis, where they prepared as giant vesicles. Archaeosomes composed of polar lipid fraction E (PLFE) and conventional liposomes composed of EPC/cholesterol (3:2 molar ratio) were prepared by freeze thawing method for oral vaccine delivery (Li et al., Citation2011).
Sonication
Archaeosomes could also be formed from the polar lipid fraction “PLF” of Sulfolobus solfataricus, without the need for exogenous lipid supplementation, by sonication at 60 °C (Relini et al., Citation1996). At 0 °C sonication was also successful to produce archaeosomes from Sulfolobus acidocaldarius polar lipids (Relini et al., Citation1996). For example, BMD-loaded archaeosomes and conventional liposomes were prepared by the sonication procedures, archaeal lipids extracted from archaea H. salinarum and enriched soy phosphatidylcholine (González-Paredes et al., Citation2010). For example, sonicated vesicles were prepared by sonicating MLVs dispersions at 80% of amplitude during 4 min with a Hielscher UP50H ultrasonic disintegrator (Teltow, Germany) for topical delivery (González-Paredes et al., Citation2011).
French pressure cell extrusion
Liquid sample of preformed MLVs is introduced into the sample cavity, after that position of piston and pressure is set up, fill the sample in the outlet hole, and power is switched on. At room temperature 40 °C and high pressure (2000 psi) MLVs are extruded through small orifice, which are then collected in suitable container. Uni-or-oligo lamellar archaeosome can obtain using this method (Kaur et al., Citation2014f). For example, UL vesicles were generated from ML vesicles by extrusion 10 times using a lipid extruder (Lipex, Vancouver, Canada) at the desired temperature (65 °C for archaeosomes) through two stacked polycarbonate membranes (pore size = 200 nm) under nitrogen gas pressure (Brown et al., Citation2009).
Solvent dispersion method
In this method, lipids are first dissolved in an organic solution, which is then brought into contact with aqueous phase containing materials to be entrapped within the archaeosomes. The lipids align themselves at the interface of organic and aqueous phase forming monolayer of lipids, which form half of the bilayer of archaeosomes (Kaur et al., Citation2014e). Methods used in solvent dispersions can be categorized on the basis of miscibility of the organic solvent and aqueous solution. These include the conditions where the organic solvent is miscible with the aqueous phase; the organic solvent is immiscible with the aqueous phase, the latter being in excess and the case where the organic solvent is in excess, and immiscible with the aqueous phase (Kaur et al., Citation2014g).
Reverse phase evaporation
In this method, the removal of solvent from emulsion was performed by evaporation method. Generally, polar lipids are dissolved in organic solvents that are sonicated by bath sonication which form emulsion (w/o) and then emulsion is dried down to a semisolid gel using rotary evaporator under reduced pressure. Finally, LUVs can be obtained by bringing about a certain proportion of water droplets by vigorous mechanical shaking with a vortex mixer. (Kaur et al., Citation2014h).
Detergent dialysis method
In this method, the ethereal phospholipids are brought into close contact with the aqueous phase via the mediator of detergents, which link with phospholipid molecules and serve to screen the hydrophobic portions of the molecule from water (Kaur et al., Citation2014i). Detergent reduction is achieved by four following approaches:
Dialysis: The dialysis can be performed in dialysis bags wrapped up in large detergent free buffers (equilibrium dialysis) or using continuous flow cells (Kaur et al., Citation2014j).
Gel filtration: In this method, the detergent is washed-out by size exclusive chromatography. Any size of filters (such as SephadexG-50 to SephacrylS200–S1000) can be used for gel filtration. The archaeosomes will not penetrate into the pores of the beads packed in a column (Kaur et al., Citation2014k).
Adsorption using bio-beads: Detergent adsorption is achieved by shaking of mixed micelle solution with beaded organic polystyrene absorbers, such as XAD-2 beads and Bio-beads SM2. The advantage of the using detergent absorbers is that they can remove detergents with a very low critical micelle concentration, which are not completely depleted by dialysis or gel filtration methods (Malik et al., Citation2014).
Dilution: On dilution of aqueous mixed micellar solution of detergent and phospholipids with buffer, the micellar size and the polydispersity increases significantly, and as the system is diluted away from the mixed micellar phase boundary, an amorphous change from poly disperse micelles to mono disperse vesicles occurs. The detergent/dialysis method can result in poor entrapment due to the leakage of loaded molecules during the dialysis process (Marwah et al., Citation2014).
Hallmarks of an ideal adjuvant
Safety, stability, bioavailability, cost effectiveness.
Prevention of autoimmune responses.
Induction of nonpathological inflammatory responses.
Promotion of cross talk between innate and acquired immune systems.
Augmentation of specific (humoral) antibody response.
Induction of cytotoxic T-cell (cell mediated) response.
T-helper cell activation (Modgill et al., Citation2014a).
In vitro archaeosome stability
Stability expectations and current problems
It is generally required that archaeosomes formulations should have a minimum shelf-life of 1–2 years. This implies physical stability (freedom from fusion, which can cause loss of encapsulated drug, and aggregation which can alter pharmacokinetics) and chemical stability (oxidation, hydrolysis) during storage. Archaeobacterial lipids with saturated phytanyl chains are not oxidized in air, and these lipids can be stored in chloroform/methanol for years at room temperature without any alteration in the molecular ions (m/z) of the lipid structures (Choquet et al., Citation1993). We have stored UL archaeosomes for up to 16 months (at about 5 °C) in the presence of air without evidence of vesicle aggregation or significant loss of encapsulated aqueous markers. Cholesterol is often incorporated into conventional liposome formulations (usually at 33 mol% ratio of cholesterol to the phospholipids) to help decrease membrane permeability by making the membrane more rigid and hence improve stability. However, cholesterol itself can be oxidized, the resultant oxidation products causing loss of the encapsulated compound (Edgerton & Levine, Citation1993). Cholesterol is also reported to affect the extent of phagocytosis of liposome, depending on the bulk lipid used and whether serum is present. Because the caldarchaeol lipids of Archaeobacteria form a monolayer structure that spans the vesicle membrane, these structures are more rigid and stable than those obtained with mono polar lipids (Alving et al., Citation1995). Indeed, the Archaeobacteria that grow optimally at extremely high temperatures, with few exceptions, tend to be rich in membrane-spanning caldarchaeol lipids. Although the use of synthetic saturated lipids such as DMPC, DPPC and DSPC may alleviate the auto oxidation-related problems, the presence of ester bonds in these, and other conventional phospholipids, makes them susceptible to chemical and enzymatic hydrolysis. Such hydrolysis of ester lipids results in the formation of lysolecithins and fatty acids that cause the liposome to become more permeable (faster leakage of the encapsulated compound), leading to suggestions for the use of synthetic ether lipids to minimize such problems. However, archaeobacterial polar lipids with the ether bonds at the sn-2,3 glycerol carbons leads to the expectation that archaeosomes made; therefore, they would be resistant to such hydrolysis and its related complications (Gregoriadis, Citation1995). The in vitro stability of liposome is usually evaluated by following the leakage of encapsulated aqueous markers such as the dyes 5(6)-carboxyfluorescein (CF) and calcein, or radio labeled compounds such as 14C-sucrose or glucose. The advantage of using a self-quenching fluorescent dye, such as CF (fully quenched at about 100 mM and unquenched at 3–30 mM) is that the fluorescence increases as the dye leaks from the vesicles and is diluted into the external (to the vesicle) environment, and there is no need to separate the liposome from the suspension to assess the amount of dye leaked out, whereas CF is pH sensitive, calcein is less so (Patel & Sprott, Citation1999).
Thermal stability
The in vitro stability of vesicles prepared from purified polar lipid sub fractions, and mixtures of these with ester lipids, have focused primarily on thermal stability. Work with the Monophosphoryl lipid A (MPL) fraction (caldarchaeol lipid) of Total polar lipids (TPL) from T. acidophilus indicated that compared with vesicles made from conventional lipids, such as egg lecithin, DPPC or DSPC, the MPL vesicles were considerably less permeable to glycerol at 62 °C, were more resistant to leakage of encapsulated CF caused by alcohols (ethanol, methanol, propanol) and phenol, and were more stable to the destabilizing effect of detergents such as sodium dodecyl sulfate and Triton X-100 (Vossenberg et al., Citation1995). The maximum thermal stability (60 °C) of vesicles made from S. solfataricus polar lipid fraction Pressurized liquid extraction (PLE) when mixed with egg Phosphatidylcholine (PC) was reported when the lipids were used at a molar ratio of 1:2, and these vesicles were more thermo-stable than those made from the PLE lipid alone (Gregoriadis, Citation1995). The stability of vesicles made from mixtures of TPL (consisting of natural mixtures of archaeol and caldarchaeol lipids) with egg PC demonstrated that the stability at 37 °C was related to the greater content of the polar ether lipids in the mixture (Kikuchi et al., Citation1991). Comparative thermal stability of vesicles made from a highly purified caldarchaeol subfraction from TPL of S. acidocaldarius with those made from unsaturated palmitoyl-oleoyl phosphatidylcholine, or saturated diphytanoyl phosphatidylcholine, showed that unlike the latter two that become very permeable at 35–40 °C, the caldarchaeol lipid vesicles were not sensitive to leakage up to 60 °C, and at 80 °C released < 5% of encapsulated CF marker dye. It was observed that the archaeosomes from TPL containing only archaeol lipids were also thermally more stable (4–65 °C) than conventional liposomes, and, in vesicles made from TPL consisting of archaeol/caldarchaeol lipids, increased thermal stability correlated to the presence of greater amounts of caldarchaeol lipids (Zuidam et al., Citation1993). Synthetic ether lipid analogs of ester lipids suggest that the presence of branched phytanyl chains and bipolar caldarchaeol lipids may be major contributors to the thermal stability and proton permeability characteristics of archaeosomes. However, compositional differences other than the archaeol/caldarchaeol content and phytanyl chains also impact on stability, as reflected by the differences in the stability of vesicles prepared from 100% archaeol lipids. Overall, correctly selected archaeosomes would have an advantage in biotechnology applications where thermal stability is desired (Patel & Sprott, Citation1999).
Heat sterilization
Sterility of liposomal formulations is a requirement for in vivo applications, especially for systemic administration. Heat sterilization of liposomes made from ester phospholipids can cause aggregation of vesicles and release of water soluble compounds from negatively charged vesicles and hydrolysis of the ester lipids (Woodle & Lasic, Citation1992). Filter sterilization may not remove virus contaminants and is a slow process for large-scale preparation. The leakage during heat sterilization can be affected by the charge of the lipids used and the interaction with the compound encapsulated in the liposomes (Choquet et al., Citation1996). In contrast to conventional liposomes, UL archaeosomes (15–20 mg lipid/ml) prepared from the TPL of various archaeobacteria can be sterilized by autoclaving (15 min at 121 °C, 103.4 kPa), without significant alteration in the respective average vesicle diameter. Leakage of encapsulated CF indicated that vesicles prepared from TPL containing higher amounts of caldarchaeol lipids generally had lower amounts of CF leakage. The potential benefit of caldarchaeol lipids in preventing leakage was confirmed by the observation that the leakage from M. mazei TPL vesicles (100%archaeols) was reduced after incorporation of progressively higher proportions of TPL from T. acidophilum, which contains about 90% caldarchaeols (Kikuchi et al., Citation1991). The stability of M. mazei archaeosomes could alternatively be improved by incorporation of cholesterol similar to that seen with ester liposomes. The analyses of archaeosomes lipids by fast atom bombardment mass spectrometry, before and after autoclaving indicated very similar spectral profiles (relative signal heights) of molecular ions (m/z), indicating that there were no observed chemical changes in the archaeobacterial polar lipids. Archaeosomes may be sterilized empty and subsequently loaded with filter-sterilized compounds that may be heat labile (Patel & Sprott, Citation1999).
Stability in serum
High-density lipoproteins (HDL) in blood, plasma and serum can remove the phospholipids from the outer leaflet of the ester lipid–liposome bilayer, resulting in vesicle destabilization and significant serum induced leakage of encapsulated compounds. Although HDL is largely responsible for serum-induced liposome destabilization, generalizations cannot be made regarding its interaction with vesicles made from various different lipid compositions; vesicles composed of Sphingomyelin, DSPC or DPPC are generally less reactive (their higher phase transition temperatures make the bilayers more rigid at physiological temperature) with plasma lipoproteins than liposomes made from more fluid compositions containing egg PC or DMPC. Supplementation of up to 30 mol% cholesterol greatly improves stability of liposomes prepared from these lipids, and cholesterol has been shown to reduce the interaction of plasma proteins with liposomes. In addition to the effect on liposome stability, incorporation of cholesterol contributes to longer blood circulation half-life of the vesicles, and it appears that the fluidity and hydrophobicity of the liposomal membrane contributes greatly to interactions with serum opsonins, which determine the phagocytic fate of the vesicles (Conti et al., Citation2000). It has been reported that cholesterol can be readily incorporated into the lipid monolayer formed from the MPL fraction from T. acidophilum, but its effect on stability was not reported. UL archaeosomes made from TPL containing archaeol lipids only indicated that the in vitro stability in fetal bovine serum (20–100%) was similar to that of egg PC liposomes. However, the stability of caldarchaeol-containing archaeosomes in 100% serum (37 °C) was much better (<25% leakage of calcein after 5 h) than the archaeol containing archaeosomes or egg PC vesicles (about 50% leakage of marker). The stability of egg PC liposomes in serum could be improved by supplementing with archaeobacterial lipids containing caldarchaeol lipids. Archaeobacterial polar lipids can form stable common phases with mono polar ester phospholipids. One reason for an improved stability of archaeosomes containing caldarchaeol lipids could be that the membrane-spanning caldarchaeol lipids stabilize the outer layer of the vesicle membrane against exchange with HDL (Choquet et al., Citation1992). Research with UL vesicles made with phosphatidylcholine and its various synthetic ether analogs suggested that the presence of an ester bond at the sn-2 glycerol carbon enhances the interaction with HDL or lipid exchange proteins and contributes to liposome instability in human and porcine serum. The relatively poor serum stability of archaeosomes made from TPL containing archaeol lipids only it would appear that the ether bond at the sn-2 position per se may not be important for stability of archaeosomes in serum, and that additional factors, such as the content of caldarchaeol lipids play a dominant role (Patel & Sprott, Citation1999).
pH stability
One of the major interests in drug/vaccine delivery research has been the desire to develop an efficient oral delivery system, especially for proteins and peptides. Oral vaccine would be to obtain a protective mucosal immune response to an associated antigen. A major limitation of liposomes and of other delivery systems has been the poor stability of these vesicles in the relatively harsh environment of the gastrointestinal (GI) tract; for example, pH as low as 1 in the stomach, and susceptibility to attack by proteases, amylases, lipases and bile salts. Improvements in the stability of liposomes in the GI tract have been sought by the incorporation of large amounts of cholesterol by coating the liposome surface with protective polymers, or by polymerizing the liposomes. However, this has had limited success in the delivery of the vesicles and their cargo to the serosal side (Gregoriadis, Citation1995). Therefore, archaeosomes with their unique lipid characteristics provide an alternative to explore. During transit in the GI tract, the pH in the stomach can be as low as 1.4, and the average pH in the intestines is between 5.5 and 7.0. Compared with UL liposomes made from egg PC and DPPC (40% and 58% loss of encapsulated 14C-sucrose, respectively), many archaeosomes were more stable (25 to 30% loss of sucrose) during storage at pH 3.0 for 21 days, but one formulation (N. magadii TPL) was very unstable, loosing up to 90% of the sucrose. In general, most other archaeosomes were more stable than the two ester lipid formulations at pH 5.3–6.0 and 10.0. It has been reported that ML liposomes made from DSPC–cholesterol mixtures (2:1 or 7:2 molar ratio) are quite stable at pH 2.0, leaking less than 10% of the glucose or polyvinylpyrrolidone marker in 60–120 min incubations). Therefore, stability at low pH per se may not present a problem in the use of these liposomes. Also, instability at such low pH can be alleviated by administering the liposomes together with a bicarbonate solution to decrease the stomach acidity. ML archaeosomes indicated that those made from the TPL of T. acidophilum retained up to 80% and 20% of the sucrose marker, respectively, after 90 min at pH 2.0 and 1.5. Moreover, after 1.5 and 24 h at pH 1.5, about 48% and 29% of the initial vesicles, respectively, appeared to be intact, indicating that leakage was not due to total disruption of the vesicles. For each type of archaeosomes prepared from the TPL of T. acidophilum, M. espanolae or M. mazei, ML vesicles were more stable at low pH than were the corresponding UL vesicles (Patel & Sprott, Citation1999).
Stability against action of lipases
Phospholipase A2 activity is detected in human and several animal sera, as well as between 1.5 and 16 U/ml in pancreatic juices. Unlike conventional liposomes made from egg PC, Phosphatidylglycerol or DPPC, which lost 80–100% of encapsulated CF after 1–4 h exposure to phospholipase A2 at 37 °C, archaeosomes made from archaeol lipids lost less than 20% CF and those from caldarchaeol lipids less than 5% CF, after 4 h exposure. The stability of egg PC liposomes could be improved dramatically by incorporation of archaeobacterial TPL (1:1 ratio by weight) (Kirilenko & Gregoriadis, Citation1993). Therefore, for those routes of drug delivery where such phospholipase activity could be encountered in vivo, archaeosomes are expected to provide better stability and protection to the cargo drug. Pancreatic lipase in the intestines can cause severe hydrolysis of the liposomal lipids, causing a rapid release of encapsulated compounds. Of all conventional liposomes tested, ML vesicles made from mixtures of DSPC/cholesterol were reported to be the most stable to attack by pancreatic lipase (10 000 U/mol lipid), leaking < 20% of the encapsulated marker in 60–120 min incubation at 37 ° (Napotnik et al., Citation2013). Comparative studies with UL vesicles from ester lipids and from archaeobacterial TPL indicated that where DPPC liposomes leaked >90% of the CF in 3 h, the archaeosomes lost only 7–18% of the marker after 5 h incubation with porcine pancreatic lipase. Phospholipase B caused 40–100% CF release from conventional liposomes and less than 2% from the archaeosomes, except from those of H. cutirubrum TPL, which were less stable. The variable effects of phospholipase C, which caused a relatively dramatic release of CF from archaeosomes, composed of TPL from M. mazei or H. cutirubrum, compared with the other archaeosomes. This showed that phosphatidylinositol specific phospholipase C hydrolyzed phosphoinositol archaeol and caldarchaeol lipids, but at a much slower rate than the control phosphoinositol diester lipid. So, archaeobacterial ether lipid structures that the vesicles made from these lipids would be relatively more stable to attack by lipases, compared with those made from ester lipids (Patel & Sprott, Citation1999).
Stability in bile salts
The total bile salts concentration in the GI tract of humans can range between 3 and 45 mM, with the bulk of it being cholic acid and the average amount in the human duodenum being 10.8 mM. Human bile consists of a mixture of sodium glycocholate (30%), sodium glycochenodeoxycholate (30%), sodium glycodeoxycholate (15%), sodium taurocholate (10%), sodium taurochenodeoxycholate (10%) and sodium taurodeoxycholate (5%) and this mixture of salts (10 mM each, at the indicated %, v/v) in phosphate-buffered saline (10 mM phosphate, pH of 6.2, 160 mM NaCl) is referred to as simulated human bile (SHB). Bile salts can act as biological detergents and form mixed micelles with phosphatidylcholine to help solubilize it, as well as fats and cholesterol. Specific bile salts have varying degrees of destabilizing effects on liposomes, and permeation of bile salts into the bilayers tends to make the membranes more fluid and hence leakier. In general, bile salts (10 mM or lower concentration) cause a rapid and drastic disruption of UL and ML liposomes made from conventional ester lipids, resulting in loss of most of the encapsulated marker such as sucrose, glucose, polyvinylpyrrolidone, chromate or CF within 5–30 min of incubation at 37 °C. It was also observed that liposomes made with lipids with a high phase transition temperature (e.g. DSPC, 58 °C) had somewhat better resistance to disruption by bile salts than those made with lipids (DMPC, 23 °C; DLPC, 0 °C) that had lower phase transition temperatures. Incorporation of cholesterol or fat-soluble vitamins such as A, D3 and E in some conventional liposomes can help improve the stability somewhat, against certain bile salts, based on the lipid used (Kirilenko & Gregoriadis, Citation1993). However, MLV from egg lecithin/cholesterol were still highly unstable, releasing greater than 95% of entrapped CF within 5 min incubation (pH 7.0, 37 °C) in 10 mM taurocholic acid. MLV made with DSPC/cholesterol (7:2 or 2:1 molar ratio) were the most stable in 10 mM bile salts mixture (37 °C), but these still released 24% of encapsulated glucose (2 h) or 100% of CF (1 h) at pH 7.0 or 16% of 125I-labeled polyvinylpyrrolidone at pH 6.2. Exposure of UL archaeosomes (80–130 nm in diameter), prepared from TPL of T. acidophilum, M. jannaschii, M. hungatei or M. mazei, to SHB showed that the leakage of calcein after 20 min was 100% for all vesicles, except those from T. acidophilum, which lost about 15% of the marker. The vesicles from egg PC and M. mazei TPL lost 100% of the marker within a 2-min exposure, but those from the other TPL retained greater than 50%. None of the UL vesicles retained marker by the end of a 60-min incubation period. However, work with ML vesicles made from TPL of T. acidophilum or M. espanolae indicated that after a 90-min incubation in SHB about 15% of the initially encapsulated CF was retained in these vesicles (Patel & Sprott, Citation1999).
Stability against the combined effect of lipase and bile salts
Most of the in vitro stability studies earlier, for the GI tract environment, evaluated the individual effects of stressors such as low pH, phospholipase and bile salts on vesicle stability. However, a combination of both phospholipase and bile salts together are encountered in the GI tract, and this assay condition would be most relevant in predicting in vivo stability. ML liposomes composed of DSPC/cholesterol, one of the most stable conventional liposomes, lost <20% of the encapsulated CF when exposed (5 min at 37 °C) to either pancreatic lipase (10 000 U/mol lipid) or 10 mM sodium taurocholate, but the leakage (80%) was significantly greater in the combined presence of both stressors, with almost 100% leakage occurring in 30 min. In vivo work in a rat model, using orally administered MLV and ULV (DSPC/cholesterol) containing polethyleneglycol (PEG-4000), salicylic acid or hydrocortisone as the cargo, indicated that there were no differences in the excretion profiles of the free and the corresponding liposomal administered compound indicating that these liposomes were not stable. The results with MLV made from egg lecithin/cholesterol were similar. Hence, these liposomes did not influence oral absorption of the entrapped compounds. Of the various cholesterol and ester lipid (egg PC, egg PC/PS, DPPC/PS, DSPC/PS) combinations used for making liposomes for oral delivery in rats, the uptake of DSPC/PS/cholesterol vesicles by Payer's patches (based on rhodamine B-PE as the marker for the lipids) was twice that of egg PC/PS/cholesterol or free rhodamine-PE. The polymerization of the liposomes was done at 60 °C (5 h), and this can be an impediment for heat labile drugs/antigens (Bangham, Citation1993). However, polymerization of UL DODPC liposomes with Na2S2O5 and K2S2O8 at room temperature (overnight) yielded vesicles that were stable enough for uptake from the GI tract of mice into various tissues (3.2% of the administered dose), especially when lectins were immobilized on the vesicle surface (10.5% of administered dose of vesicles) for better targeting and uptake by the Payer's patches. However, the toxicity, extent of biodegradability and release of entrapped drug in vivo of polymerized liposomes need to be examined. The interactions between certain methacryloyl-based polymerizable lipids and blood components indicate potential concerns regarding biocompatibility of these lipids and their polymerized counterparts (Whitcomb & Lowe, Citation2007).
There is not much comparable published work on the in vitro evaluation of archaeosomes to susceptibility of combined effect of lipases and bile salts. However, recent results indicated that unlike ester phospholipid liposomes, pancreatic lipase (3333 U/ml) and SHB (in PBS at pH 6.2, 1 mM calcium, 37 °C) had no synergistic effect on the release of entrapped CF from UL or ML archaeosomes prepared from the TPL of T. acidophilum, M. espanolae or M. mazei, the effect being the same as that of SHB alone. ML archaeosomes from T. acidophilum TPL were the most stable, retaining about 15% CF after 90 min incubation. Also, when ML T. acidophilum TPL archaeosomes above were assayed for stability under conditions (10 mg/ml sodium taurocholate, 5 U/ml phospholipase A2, 3 mM calcium, pH 7.4 PBS, 37 °C) used to evaluate DODPC polymerized liposomes, only about 50% of the CF leaked out after 90 min, and it took between 5 and 7 h for 100% leakage. There was no difference in these observations when the taurocholate was replaced by SHB at pH 7.4 (Alving et al., Citation1995). The in vitro stability of archaeosomes suggests that they would be more stable in the GI tract environment than liposomes made from conventional ester lipids and cholesterol. Although not as stable as some polymerized liposomes, archaeosomes appear to provide sufficient stability to merit in vivo evaluation for oral delivery applications, and for possible surface modification of vesicles (as attempted with other liposomes) for improving the stability and targeting for uptake from the GI tract (Patel & Sprott, Citation1999).
Miscellaneous stability issues
The effect of cations in the buffer, especially divalent such as calcium and magnesium, on the properties, such as membrane curvature, dehydration, lateral phase separation and polymorphic phase transition in anionic lipid membranes has been well known. Calcium can enhance or decrease the permeability of acidic phospholipid membranes, based on the threshold level of the cation and the type of the lipid. UL vesicles made from the caldarchaeol containing polar lipid fraction (PLE) from S. solfataricus were more leaky (at 60 °C) in the presence of 15 mM calcium in the buffer, and this was attributed not only to a fusion process, but also to a change in the membrane stability and permeability. The increase in leakiness of vesicles (at 60 °C) made from PLE, by 15 mM calcium added in the buffer of preformed vesicles, who demonstrated that addition of polyethylene glycol (20%, w/v) to the buffer also destabilized the vesicles. Recently, it was reported that vesicles made from the bipolar, membrane spanning caldarchaeol lipids from another species of Sulfolobus, S. solfataricus, can be made to undergo nonleaky, calcium dependent fusion (4.5 mM calcium chloride, 15 mM potassium phosphate, pH 7.4, 25 °C), albeit as a relatively slow process. In contrast to these studies that evaluated the effect of cations present in the external environment of preformed membranes, recently we showed that the specific salt form of the TPL from M. mazei, before hydration and vesicle formation, affects the permeability of the archaeosomes. When the TPL was predominantly in the monovalent cationic form (sodium and potassium), the resultant vesicles in PBS were very leaky to encapsulated CF, but conversion of the TPL to the predominantly calcium or magnesium form yielded vesicles that were significantly less leaky. The positive effect on vesicle permeability of the divalent salt form of TPL was almost completely diminished when 10 mM Ethylene glycol tetra acetic acid (EGTA) (a strong chelator for divalent cations) was added to the hydration buffer, indicating that these cations had a role in lipid packing to form a less permeable membrane. This effect of the specific salt form of TPL, prior to lipid hydration, on vesicle permeability was not observed with the TPL of H. cutirubrum or M. espanolae, indicating the importance of lipid composition in this effect. Phosphatidylserine–Ca2+ interaction can lead to tighter molecular packing and the TPL of M. mazei can contain from 3% to 16% relative abundance of archaeol lipids with a phosphoserine polar head group. Hence, some archaeosomes can be made fusogenic, and the permeability of others can be modified by the specific salt form of the lipid (Patel & Sprott, Citation1999).
Applications of archaeosome formulations
Self-adjuvanting drug delivery systems for cancer vaccines
Archaeosomes adjuvants formulated as archaeal ether glycolipid vesicles induce strong CD4+ as well as CD8+ CTL responses to entrapped soluble antigens. Host CD8+ CTL responses are critical for long-term protection against tumors. In mice, spontaneous tumors extend in the absence of CD8+T-cell cytotoxicity (Modgill et al., Citation2014b). In humans, host CD8+ CTL responses to tumor-associated antigens have been shown to be highly useful to patients, particularly those with metastatic disease. Thus, there is considerable interest in the generation of cancer immune therapies. The success of cancer vaccines depends on two key aspects: identification of specific antigenic targets and the ability to evoke a strong and appropriate immune response (Krishnan et al., Citation2003). Archaeal ether glycerolipid vesicles (archaeosomes) efficiently deliver exogenous antigen for initiation of humoral and cell-mediated immunity. Because initiation of CD8+ cytotoxic T cells is critical for protective vaccination against tumors, when compared the ability of various archaeosomes lipid compositions to evoke a strong CD8+ CTL response to entrapped antigen (Krishnan & Sprott, Citation2007). Immunization of mice with ovalbumin (OVA) entrapped in all archaeosomes lipid compositions evoked a primary (day 10) splenic CTL response indicating processing for MHC class I presentation. Polar lipid compositions from halophilic archaea were very potent to adjuvant this early CTL response (Gonzalez et al., Citation2009). The lytic units reduced significantly by weeks 6–7. At 50 weeks, only M. smithii and T. acidophilum both rich in bipolar membrane-spanning caldarchaeols, recognized evoke memory CTLs. Immunization of mice with OVA entrapped in M. smithii, H. salinarum and T. acidophilum vesicles provide prophylactic protection against challenge with OVA-expressing solid tumors at 6 weeks. Even a dose of 3 mg OVA in archaeosomes significantly delayed tumor growth. Tumor protection was noted in a therapeutic design wherein OVA-archaeosomes were injected concurrent with the tumor challenge (Morie et al., Citation2014). Antigen-free T. acidophilum but entrapped antigen H. salinarum archaeosomes provided innate therapeutic protection. Vaccination with a CTL peptide epitope from the melanoma separation antigen, tyrosinase-related protein 2, in archaeosomes induced a protective CD8+ response against B16OVA metastasis, representing potential for targeting self, tumor antigens (Patel et al., Citation2002). Lipid structural properties of archaea may differentially change primary, long-term and/or innate immunity, impacting adjuvant choice for vaccine design (Krishnan & Dennis Sprott, Citation2003).
Immunoadjuvant for a vaccine against Chagas disease
American trypanosomiasis (Chagas disease) is a neglected tropical disease caused by the protozoan parasite Trypanosoma cruzi and has a widespread distribution in Latin America (Pabreja et al., Citation2014). WHO estimated that over 15 million are infected worldwide. Fifty-thousand children and adults are affected annually by clinical complications of T. cruzi induced heart disease and leading to death due to the lack of effective treatment. The risk of transmission of the disease is high because the infection has been detected in nonendemic areas of the Americas and Europe due to large-scale migrations. It is essential to develop new strategies for the prevention and control of Chagas disease (Rohilla et al., Citation2014a). At present, vaccines and immune therapies targeted at T. cruzi infection are practically nonexistent. In parallel with the efforts toward the identification of vaccine candidates, several adjuvants have been assayed to generate protective immunity to T. cruzi, but with limited success (Rohilla et al., Citation2014b). An increasing body of evidence has revealed the strong adjuvant properties of Archaeosomes (ARC). These vesicles enclosed by one or more bilayers prepared with TPL extracted from microorganisms belonging to the domain Archaea are more avidly internalized, both in vitro and in vivo, by macrophages and antigen presenting cells than conventional liposomes (Sharma et al., Citation2014a). They also differ from liposomes in the inclusion of immune modulators is not necessary to improve the adjuvancy beyond that of a simple depot effect, favoring scale up production. T. cruzi antigens can be incorporated successfully into ARC and, upon sc inoculation in mice, the resulting immunogen is capable of priming a protective response against an intracellular parasite infection. ARCs show promise as safe and helpful carrier-adjuvant for the design of future vaccines against this human pathogen (Higa et al., Citation2013).
As novel gene delivery systems
Novel cationic liposomes based on mixtures of neutral/cationic bilayer-forming lipids and archaeobacterial synthetic tetraether-type bipolar lipids show efficient in vitro gene transfection properties and represent a new approach for modulating the lipidic membrane fluidity of the complexes they form with DNA. Archaeobacterial lipids can be used as cationic lipids or co-lipids for in vitro gene transfection (Sharma et al., Citation2014b). The potential of combining conventional bilayer-forming lipids with monolayer-forming lipids modulate the membrane properties of CL–DNA complexes. The potential of novel archaeoplexes for in vivo gene transfection into the airway epithelium by nasal instillation or aerosolization with a view to lung-directed gene therapy for cystic fibrosis (Réthoré et al., Citation2007).
As carriers for oral delivery of proteins and peptides
Oral administration of peptide and protein drugs faces a big challenge partly due to the hostile GI environment. Lipid-based delivery systems are smart because they offer some protection for peptides and proteins. A special lipid-based oral delivery system archaeosomes, made of the PLFE extracted from S. acidocaldarius, and explore its potential as an oral drug delivery vehicle. Archaeosomes have superior stability in simulated GI fluids, and enable fluorescent labeled peptides to exist in for longer periods in the GI tract after oral administration (Sharma et al., Citation2014c). Although archaeosomes have slight effect on the transport of insulin across the Caco-2 cell monolayers, the in vivo experiments showed that archaeosomes containing insulin induced lower levels of blood glucose than a conventional liposome formulation. Archaeosomes obtained from PLFE were relatively stable in simulated GI tract conditions in vitro and facilitated to the slow transit of fluorescently labeled peptide in the GI tract in vivo. As a carrier of oral insulin, archaeosomes were superior in reducing the blood glucose levels in diabetic mice, compared with conventional liposomes. The hypoglycemic effect seemed to be modest, this may result from their poor permeability of intestinal epithelium after oral administration of the formulation (Li et al., Citation2010).
As novel antigen delivery systems
The humoral immune response mounted in BALB/c mice against bovine serum albumin or cholera toxin B subunit was compared when the antigens were associated with liposomes composed of either archaeal ether lipids or conventional lipids (Krishnan et al., Citation2000). Antibody titers in sera from mice immunized intraperitoneal were elevated to an extent comparable to those achieved with Freund's adjuvant by encapsulating bovine serum albumin in archaeal lipid vesicles (archaeosomes) of about 200 nm diameter (Singh et al., Citation2014a). Similarity among six archaeosomes and three conventional liposome compositions established that archaeosomes were generally much superior in potentiating an immune response (Patel & Chen, Citation2005). Further, only two immunizations, at the most, were needed to achieve close to the maximum antibody titer, as shown with archaeosomes composed of the polar lipids from M. smithii, an occupier of the human colon. A positive response to present the more immunogenic cholera B subunit protein to the immune system of mice was exposed for M. smithii archaeosomes. Encapsulation of the antigen in the archaeosomes was required to achieve the full humoral response (Sprott et al., Citation1997).
Drug delivery systems for natural antioxidant compounds
A novel kind of liposomes made up of archaeal polar lipids, multilamellar (MLVs) and unilamellar (SUVs), as a topical delivery system for natural antioxidant compounds recovered from olive mill waste. SUVs were smaller than MLVs, showing size values smaller than 200 nm, which was maintained throughout the stability study (Singh et al., Citation2014b). Transmission electron microscopy showed spherical morphology for conventional liposomes as archaeosomes had more irregular membranes. Vesicle encapsulation efficiency was relatively similar in both formulations and was enough to ensure a very good antioxidant activity. Stability studies were performed 1 month after the preparation of formulations, which showed a high stability with no change in the early characteristics of the suspensions. Moreover, the possibility of incorporating the liposomal suspensions in different excipients (Carbopol-940® and Pluronic-127®) for topical administration was studied. To determine the release behavior of the different systems prepared, in vitro diffusion studies were performed using vertical diffusion Franz cells. Incorporation of the vesicles into the gels lead in a sustained release for 24 h. Archaeosomes gels released the similar amount of phenolic compounds regardless the excipient used. Although in liposomal gels large release differences were found between carbopol and pluronic gel. Archaeosomes appeared as a suitable carrier for topical delivery of antioxidant phenolic compounds due to their characteristics of stability, entrapment efficiency and antioxidant activity, which were comparable with those obtained with classical phosphatidylcholine liposomes. Moreover, archaeosomes seemed to be more versatile than conventional liposomes for incorporation into gels (Singh et al., Citation2012a). Thus, the release using carbopol® or pluronic® is quite similar when using archaeosomes, so both excipients could be used indiscriminately, while it does not occur when conventional liposomes were used and so the selection of one excipient may be based on the desired effect (González-Paredes et al., Citation2011).
New drug carrier for delivery of Paclitaxel to breast cancer
Paclitaxel loaded archaeosomes reduce side effects and improve its therapeutic index. Carriers have made a big development in the treatment of many diseases. Lipid carriers are of individual significance among carriers. Archaeosomes is one of the most important lipid carriers (Patel & Chen, Citation2006). Paclitaxel is a drug, which is used in the treat breast cancer has some unwanted side effects, despite its therapeutic effects. Archaeosomes were obtained from methanogenic archi bacteria and synthesized with a certain ratio of Paclitaxel in PBS. Drug releasing of archaeosomes Paclitaxel was examined within 26 h which showed that the most drug release occur during first 3 h. The cytotoxicity effect of archaeosomes Paclitaxel on breast cancer's cell line was evaluated by MTT assay which results showed that the cytotoxicity result of archaeosomes Paclitaxel on breast cancer's cell line is more than that of the standard Paclitaxel formulation. New drug delivery of Paclitaxel using archaeosomes, increases the therapeutic index of the drug (Alavi et al., Citation2014).
A carrier for topical delivery of BMD
Archaeosomes, lipid vesicles made from archaea polar lipids, and conventional phospholipids liposomes was carried out, to evaluating the properties and the potential of archaeosomes as novel colloidal carriers for successful drug delivery to the skin. BMD-loaded archaeosomes and conventional liposomes were prepared by the thin-lipid film and sonication procedures, using, correspondingly, archaeal lipids extracted from archaea H. salinarum and enriched soy phosphatidylcholine (Singh et al., Citation2012b). Vesicular formulations were characterized by assessing vesicle size, zeta potential, entrapment efficiency and morphology. To investigate the effect of the incorporation in the two different colloidal carrier systems on the (trans) dermal delivery of drug, in vitro drug permeation studies through full-thickness pig skin were carried out by using Franz diffusion vertical cells by testing both archaeal and liposomal dispersions. Archaeosomes appeared to be the most effective carriers for the model drug, achieving a major drug penetration and accumulation in the skin strata and in the epidermis. Archaeosomes may hold great promise as a delivery vehicle for topical applications. Archaeosomes incorporation may be a novel, promising strategy to enhance the efficacy of an anti-inflammatory drug in the treatment of skin disorders where a local effect is required (González-Paredes et al., Citation2010).
Archaeosomes delivery to different types of cargo into epithelial cells grown in vitro
Archaeosomes are a type of liposomes prepared from the polar lipids of various archaeobacteria. These have exclusive structural features that increase the lipid bilayer's stability even under high temperatures, low or high pH, phospholipases and bile salts. This makes them perfect as basis for the growth of new drug, gene and vaccine delivery systems. Zavec et al. (Citation2014) prepared large UL archaeosomes (400 nm size) from Aeropyrum pernix K1 and demonstrated their potential as base for the development of an efficient and universal system for drug or therapy delivery to epithelial cells. These archaeosomes may be used to deliver small fluorescent molecules (calcein), smaller proteins (60 kD like alisteriolysin), large protein aggregates (e.g. keratin 14) and plasmid DNA, into epithelial cells grown in culture. The delivery effectiveness for small molecules is fairly high at this initial stage of development, around 40%. Prepared UL archaeosomes are also not toxic to keratinocytes even at high dose (500 μg/ml) (Zavec et al., Citation2014).
UDA as topical adjuvant
The skin is rich in potent antigen-presenting cells (APC) that are not readily accessible to parenteral vaccination except by intradermal route, which is difficult to carry out. The topical route is an attractive alternative enabling a much closer contact to the skin APC. Topical vaccination has more advantages than injectable, such as increased patient compliance, the reduced potential reinfection by contaminated material, and as well as the need for particular trained personnel and sterilized materials, and maintenance of cold chain. These advantages are more or less outweighed by dose variability and the need for strong immunomodulators, that is bacterial ADP-ribosylating exotoxins (cholera toxin, Escherichia coli and their mutants) (Higa et al., Citation2012). UDA are vesicles prepared from SPC, NaChol and polar lipids from H. tebenquichense (3:1:3wt/wt). Ultra deformable liposomes (UDL made of SPC and NaChol at 6:1 wt/wt) and UDA were neither captured nor caused cytotoxicity on keratinocytes; UDA was mostly captured by macrophages, their possibility being reduced by 0.4–1.6 mg/ml phospholipids by 25–60%. UDL were poorly capture and cause no toxicity. Balb/C mice immunized by the topical route with four doses of Ovalbumin (OVA)-loaded UDA, at 75 μg OVA/600 μg phospholipids (125 nm mean size and −42 mV zeta potential), induce IgG titers tenfold to 100-fold higher than those immunized with OVA-loaded UDL at the same dosage. Both matrices penetrate to the similar skin depth (nearly 10 μm after 1 h on human skin), being the higher topical adjutancy and higher phagocytic uptake of UDA related to its glycolipid content. Conventional liposomes fuse and do not penetrate the intact skin mouse beyond 1-μm depth (Singh et al., Citation2014c). However, the lipid matrix of UDL penetrates at least the entire depth of SC, delivering the aqueous content to the viable epidermis. UDL overcomes conventional liposomes as topical adjuvants because of their special mechanical behavior or ultra-deformability, gained by the presence of edge activators such as NaChol, polysorbate or ethanol in the phospholipid matrix. After SC penetration the UDL were lipids taken up by viable skin cells. After sc administration in mice, archaeosomes compose potent adjuvants for the induction of Th1, Th2 and CD8 + T cell responses to the entrapped soluble Ag (Carrer et al., Citation2014). shows some important applications of archaeosomes-based formulations.
Table 2. Applications of archaeosomes based formulations.
Conclusions
Finally, we can conclude that the above nano-carrier system has a huge prospect for the designing of a novel, low-dose and effective treatment systems to control various diseases. An inspiring emblem is the cumulative amount of clinical trials involving archaeosomes and lipid-based products. Several companies are aggressively affianced in the development and evaluation of archaeosomes products for use against several diseases. Archaeosomes have been recognized as tremendously useful carrier systems and helpful in the delivery of drugs, genes and cell to the target sites are promising and may serve as a handle for focus of future research.
Acknowledgements
Author Dr Amit K. Goyal is thankful to Department of Biotechnology (DBT), New Delhi (under IYBA scheme; BT/01/IYBA/2009 dated May 24, 2010).
Declaration of interest
The authors confirm that this article content has no conflicts of interest.
References
- Alavi SE, Mansouri H, Esfahani MKM, et al. (2014). Archaeosome: as new drug carrier for delivery of paclitaxel to breast cancer. Indian J Clin Biochem 29:150–3
- Alving C, Koulchin V, Glenn GM, Rao M. (1995). Liposomes as carriers of peptide antigens: induction of antibodies and cytotoxic T lymphocytes to conjugated and unconjugated peptides. Immunol Rev 145:5–31
- Bangham A. (1993). Liposomes: the Babraham connection. Chem Phys Lipids, 64:275–85
- Benvegnu T, Lemiègre L, Cammas-Marion S. (2009). New generation of liposomes called archaeosomes based on natural or synthetic archaeal lipids as innovative formulations for drug delivery. Recent pat Drug Deliv Formul 3:206–20
- Benvegnu T, Lemiègre L, Dalençon S, Jeftic J. (2013). Applications of extremophilic archaeal lipids in the field of nanocarriers for oral/topical drug delivery. Curr Biotechnol 2:294–303
- Brown DA, Venegas B, Cooke PH, et al. (2009). Bipolar tetraether archaeosomes exhibit unusual stability against autoclaving as studied by dynamic light scattering and electron microscopy. Chem Phys Lipids 159:95–103
- Carrer DC, Higa LH, Tesoriero MVD, et al. (2014). Structural features of ultradeformable archaeosomes for topical delivery of ovalbumin. Colloids Surf B Biointerfaces 121:281–9
- Chaudhary S, Garg T, Murthy RS, et al. (2014). Recent approaches of lipid-based delivery system for lymphatic targeting via oral route. J Drug Target 10:871–82
- Choquet CG, Patel GB, Beveridge T, Sprott G. (1992). Formation of unilamellar liposomes from total polar lipid extracts of methanogens. Appl Environ Microbiol 58:2894–900
- Choquet CG, Patel GB, Sprott GD. (1996). Heat sterilization of archaeal liposomes. Can J Microbiol 42:183–6
- Choquet CG, Patel GB, Sprott GD, Beveridge TJ. (1993). Stable liposomes formed from archaeal ether lipids. Advances in bacterial paracrystalline surface layers. Springer US, 252:257–68
- Conti S, Polonelli L, Frazzi R, et al. (2000). Controlled delivery of biotechnological products. Curr Pharm Biotechnol 1:313–23
- Edgerton M, Levine MJ. (1993). Biocompatibility: its future in prosthodontic research. J Prosth Dent 69:406–15
- Gagandeep, Garg T, Malik B, et al. (2014). Development and characterization of nano-fiber patch for the treatment of glaucoma. Eur J Pharm Sci 53:10–16
- Garg T. (2014). Current nanotechnological approaches for an effective delivery of bio-active drug molecules in the treatment of acne. Artif Cells Nanomed Biotechnol. Early Online, 1–8. DOI: 10.3109/21691401.2014.916715
- Garg T, Goyal AK. (2012). Iontophoresis: drug delivery system by applying an electrical potential across the skin. Drug Deliv Lett 2:270–80
- Garg T, Goyal AK. (2014a). Biomaterial-based scaffolds – current status and future directions. Exp Opin Drug Deliv 11:767–89
- Garg T, Goyal AK. (2014b). Liposomes: targeted and controlled delivery system. Drug Deliv Lett 4:62–71
- Garg T, Goyal AK. (2014c). Medicated chewing gum: patient compliance oral drug delivery system. Drug Deliv Lett 4:72–8
- Garg T, Goyal AK, Arora S, Murthy R. (2012a). Development, optimization & evaluation of porous chitosan Scaffold formulation of gliclazide for the treatment of type-2 diabetes mellitus. Drug Deliv Lett 2:251–61
- Garg T, Kumar A, Rath G, Goyal AK. (2014a). Gastroretentive drug delivery systems for therapeutic management of peptic ulcer. Crit Rev Ther Drug Carrier Syst 31:531–57
- Garg T, Rath G, Goyal AK. (2014b). Ancient and advanced approaches for the treatment of an inflammatory autoimmune disease-psoriasis. Crit Rev Ther Drug Carrier Syst 31:331–64
- Garg T, Rath G, Goyal AK. (2014c). Biomaterials-based nanofiber scaffold: targeted and controlled carrier for cell and drug delivery. J Drug Target. Early Online, 1–20. DOI: 10.3109/1061186X.2014.992899
- Garg T, Rath G, Goyal AK. (2014d). Comprehensive review on additives of topical dosage forms for drug delivery. Drug Deliv. Early Online, 1–19. DOI: 10.3109/10717544.2013.879355
- Garg T, Singh O, Arora S, Murthy R. (2011a). Dendrimer—a novel scaffold for drug delivery. Int J Pharm Sci Rev Res 7:211–20
- Garg T, Singh O, Arora S, Murthy R. (2011b). Patented microencapsulation techniques and its application. J Pharmacy Res 4:2097–102
- Garg T, Singh O, Arora S, Murthy R. (2012b). Scaffold: a novel carrier for cell and drug delivery. Crit Rev Ther Drug Carrier Syst 29:1–63
- Garg T, Singh S, Goyal AK. (2013). Stimuli-sensitive hydrogels: an excellent carrier for drug and cell delivery. Crit Rev Ther Drug Carrier Syst 30:369–409
- Gonzalez RO, Higa LH, Cutrullis RA, et al. (2009). Archaeosomes made of Halorubrum tebenquichense total polar lipids: a new source of adjuvancy. BMC Biotechnol 9:71
- González-Paredes A, Clarés-naveros B, Ruiz-martínez M, et al. (2011). Delivery systems for natural antioxidant compounds: archaeosomes and archaeosomal hydrogels characterization and release study. Int J Pharm 421:321–31
- González-Paredes A, Manconi M, Caddeo C, et al. (2010). Archaeosomes as carriers for topical delivery of betamethasone dipropionate: in vitro skin permeation study. J Liposome Res 20:269–76
- Goyal AK, Rath G, Garg T. (2013a). Nanotechnological approaches for genetic immunization. DNA and RNA nanobiotechnologies in medicine: diagnosis and treatment of diseases. Springer Berlin Heidelberg 67–120
- Goyal G, Garg T, Malik B, et al. (2013b). Development and characterization of niosomal gel for topical delivery of benzoyl peroxide. Drug Deliv. Early Online, 1–16. doi: 10.3109/10717544.2013.855277
- Goyal G, Garg T, Rath G, Goyal AK. (2014a). Current nanotechnological strategies for an effective delivery of drugs in treatment of periodontal disease. Crit Rev Ther Drug Carrier Syst 31:89–119
- Goyal G, Garg T, Rath G, Goyal AK. (2014b). Current nanotechnological strategies for treating glaucoma. Crit Rev Ther Drug Carrier Syst 31:365–405
- Gregoriadis G. (1995). Engineering liposomes for drug delivery: progress and problems. Trends Biotechnol 13:527–37
- Higa LH, Corral RS, Morilla MJ, et al. (2013). Archaeosomes display immunoadjuvant potential for a vaccine against Chagas disease. Human Vac Immunother 9:409–12
- Higa LH, Schilrreff P, Perez AP, et al. (2012). Ultradeformable archaeosomes as new topical adjuvants. Nanomed Nanotechnol Biol Med 8:1319–28
- Hussain T, Garg T, Goyal AK, Rath G. (2014). Biomedical applications of nanofiber scaffolds in tissue engineering. J Biomater Tissue Eng 4:600–23
- Jain S, Caforio A, Driessen AJ. (2014). Biosynthesis of archaeal membrane ether lipids. Microbial Physiol Metab 5:641–59
- Johal HS, Garg T, Rath G, Goyal AK. (2014). Advanced topical drug delivery system for the management of vaginal candidiasis. Drug Deliv. Early Online, 1–14. DOI: 10.3109/10717544.2014.928760
- Joshi D, Garg T, Goyal AK, Rath G. (2014a). Advanced drug delivery approaches against periodontitis. Drug Deliv. Early Online, 1–15. DOI: 10.3109/10717544.2014.935531
- Joshi D, Garg T, Goyal AK, Rath G. (2014b). Development and characterization of novel medicated nanofibers against periodontitis. Curr Drug Deliv. Early Online, 1–12. DOI: 10.2174/1567201811666141117144332
- Kalia V, Garg T, Rath G, Goyal AK. (2014). Development and evaluation of a sublingual film of the antiemetic granisetron hydrochloride. Artif Cells Nanomed Biotechnol. Early Online, 1–5. DOI: 10.3109/21691401.2014.984303
- Kataria K, Sharma A, Garg T, et al. (2014). Novel technology to improve drug loading in polymeric nanofibers. Drug Deliv Lett 4:79–86
- Kaur M, Garg T, Narang RK. (2014a). A review of emerging trends in the treatment of tuberculosis. Artif Cells Nanomed Biotechnol. Early Online, 1–7. DOI: 10.3109/21691401.2014.962745
- Kaur M, Garg T, Rath G, Goyal AK. (2014b). Current nanotechnological strategies for effective delivery of bioactive drug molecules in the treatment of tuberculosis. Crit Rev Ther Drug Carrier Syst 31:49–88
- Kaur M, Malik B, Garg T, et al. (2014c). Development and characterization of guar gum nanoparticles for oral immunization against tuberculosis. Drug Deliv. Early Online, 1–7. DOI: 10.3109/10717544.2014.894594
- Kaur N, Garg T, Goyal AK, Rath G. (2014d). Formulation, optimization and evaluation of curcumin-beta-cyclodextrin-loaded sponge for effective drug delivery in thermal burns chemotherapy. Drug Deliv. Early Online, 1–10. DOI: 10.3109/10717544.2014.963900
- Kaur P, Garg T, Rath G, et al. (2014e). Development, optimization and evaluation of surfactant-based pulmonary nanolipid carrier system of paclitaxel for the management of drug resistance lung cancer using Box–Behnken design. Drug Deliv. Early Online, 1–14. DOI: 10.3109/10717544.2014.993486
- Kaur P, Garg T, Rath G, et al. (2014f). Surfactant-based drug delivery systems for treating drug-resistant lung cancer. Drug Deliv. Early Online, 1–12. DOI: 10.3109/10717544.2014.935530
- Kaur P, Garg T, Vaidya B, et al. (2014g). Brain delivery of intranasal in situ gel of nanoparticulated polymeric carriers containing antidepressant drug: behavioral and biochemical assessment. J Drug Target 1–12. DOI: 10.3109/1061186X.2014.994097
- Kaur R, Garg T, Das Gupta U, et al. (2014h). Preparation and characterization of spray-dried inhalable powders containing nanoaggregates for pulmonary delivery of anti-tubercular drugs. Artif Cells Nanomed Biotechnol. Early Online, 1–6. DOI: 10.3109/21691401.2014.930747
- Kaur R, Garg T, Malik B, et al. (2014i). Development and characterization of spray-dried porous nanoaggregates for pulmonary delivery of anti-tubercular drugs. Drug Deliv. Early Online, 1–6. DOI: 10.3109/10717544.2014.920428
- Kaur R, Garg T, Rath G, Goyal AK. (2014j). Advanced aerosol delivery devices for potential cure of acute and chronic diseases. Crit Rev Ther Drug Carrier Syst 31:495–530
- Kaur V, Garg T, Rath G, Goyal AK. (2014k). Therapeutic potential of nanocarrier for overcoming to P-glycoprotein. J Drug Target 1–12. DOI: 10.3109/1061186X.2014.947295
- Kikuchi H, Carlsson A, Yachi K, Hirota S. (1991). Possibility of heat sterilization of liposomes. Chem Pharm Bull 39:1018–22
- Kirilenko V, Gregoriadis G. (1993). Fat soluble vitamins in liposomes: studies on incorporation efficiency and bile salt induced vesicle disintegration. J Drug Target 1:361–8
- Krishnan L, Dennis Sprott G. (2003). Archaeosomes as self-adjuvanting delivery systems for cancer vaccines. J Drug Target 11:515–24
- Krishnan L, Dicaire CJ, Patel GB, Sprott GD. (2000). Archaeosome vaccine adjuvants induce strong humoral, cell-mediated, and memory responses: comparison to conventional liposomes and alum. Infect Immun 68:54–63
- Krishnan L, Sad S, Patel GB, Sprott GD. (2003). Archaeosomes induce enhanced cytotoxic T lymphocyte responses to entrapped soluble protein in the absence of interleukin 12 and protect against tumor challenge. Cancer Res 63:2526–34
- Krishnan L, Sprott GD. (2007). Archaeosome vaccine adjuvants for cross-priming CD8 + T cell immunity. Vaccine adjuvants and delivery systems. Wiley Online Library 263–94
- Li Z, Chen J, Sun W, Xu Y. (2010). Investigation of archaeosomes as carriers for oral delivery of peptides. Biochem Biophys Res Commun 394:412–17
- Li Z, Zhang L, Sun W, et al. (2011). Archaeosomes with encapsulated antigens for oral vaccine delivery. Vaccine 29:5260–6
- Malik R, Garg T, Goyal AK, Rath G. (2014). Polymeric nanofibers: targeted gastro-retentive drug delivery systems. J Drug Target 1–16. DOI: 10.3109/1061186X.2014.965715
- Marwah H, Garg T, Goyal AK, Rath G. (2014). Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. Early Online, 1–15. DOI: 10.3109/10717544.2014.935532
- Modgill V, Garg T, Goyal AK, Rath G. (2014a). Permeability study of ciprofloxacin from ultra-thin nanofibrous film through various mucosal membranes. Artif Cells Nanomed Biotechnol. Early Online, 1–6. DOI: 10.3109/21691401.2014.924007
- Modgill V, Garg T, Goyal AK, Rath G. (2014b). Transmucosal delivery of linagliptin for the treatment of type-2 diabetes mellitus by ultra-thin nanofibers. Curr Drug Deliv. Early Online, 1–10. doi: 10.2174/1567201811666141117144332
- Morie A, Garg T, Goyal AK, Rath G. (2014). Nanofibers as novel drug carrier – an overview. Artif Cells Nanomed Biotechnol 1–9
- Napotnik T, Valant J, Gmajner D, et al. (2013). Cytotoxicity and uptake of archaeosomes prepared from Aeropyrum pernix lipids. Human Exp Toxicol 32:950–9
- Pabreja S, Garg T, Rath G, Goyal AK. (2014). Mucosal vaccination against tuberculosis using Ag85A-loaded immunostimulating complexes. Artif Cells Nanomed Biotechnol. Early Online, 1–8. DOI: 10.3109/21691401.2014.966195
- Patel GB, Chen W. (2005). Archaeosome immunostimulatory vaccine delivery system. Curr Drug Deliv 2:407–21
- Patel GB, Chen W. (2006). Archaeosomes as drug and vaccine nanodelivery systems. Nanocarrier technologies. Springer Netherlands, 17–40
- Patel GB, Omri A, Deschatelets L, Dennis Sprott G. (2002). Safety of archaeosome adjuvants evaluated in a mouse model 1. J Liposome Res 12:353–72
- Patel GB, Sprott GD. (1999). Archaeobacterial ether lipid liposomes (archaeosomes) as novel vaccine and drug delivery systems. Crit Rev Biotechnol 19:317–57
- Perrie Y, Kirby D, Bramwell VW, Mohammed AR. (2007). Recent developments in particulate-based vaccines. Recent Pat Drug Deliv Formul 1:117–29
- Relini A, Cassinadri D, Fan Q, et al. (1996). Effect of physical constraints on the mechanisms of membrane fusion: bolaform lipid vesicles as model systems. Biophys J 71:1789–95
- Ren X, Liu K, Zhang Q, et al. (2014). Design, fabrication, and characterization of archaeal tetraether free-standing planar membranes in a PDMS- and PCB-based fluidic platform. ACS Appl Mater Interfaces 6:12618–28
- Réthoré G, Montier T, Le Gall T, et al. (2007). Archaeosomes based on synthetic tetraether-like lipids as novel versatile gene delivery systems. Chem Commun 20:2054–6
- Rohilla R, Garg T, Bariwal J, et al. (2014a). Development, optimization and characterization of glycyrrhetinic acid–chitosan nanoparticles of atorvastatin for liver targeting. Drug Deliv. Early Online, 1–8. DOI: 10.3109/10717544.2014.977460
- Rohilla R, Garg T, Goyal AK, Rath G. (2014b). Herbal and polymeric approaches for liver-targeting drug delivery: novel strategies and their significance. Drug Deliv. Early Online, 1–17. DOI: 10.3109/10717544.2014.945018
- Sharma A, Garg T, Aman A, et al. (2014a). Nanogel – an advanced drug delivery tool: current and future. Artif Cells Nanomed Biotechnol. Early Online, 1–13. DOI: 10.3109/21691401.2014.930745
- Sharma R, Garg T, Goyal AK, Rath G. (2014b). Development, optimization and evaluation of polymeric electrospun nanofiber: a tool for local delivery of fluconazole for management of vaginal candidiasis. Artif Cells Nanomed Biotechnol. Early Online, 1–8. DOI: 10.3109/21691401.2014.966194
- Sharma R, Singh H, Joshi M, et al. (2014c). Recent advances in polymeric electrospun nanofibers for drug delivery. Crit Rev Ther Drug Carrier Syst 31:187–217
- Singh B, Garg T, Goyal AK, Rath G. (2014a). Recent advancements in the cardiovascular drug carriers. Artif Cells Nanomed Biotechnol 1–10
- Singh H, Sharma R, Joshi M, et al. (2014b). Transmucosal delivery of Docetaxel by mucoadhesive polymeric nanofibers. Artif Cells Nanomed Biotechnol. Early Online, 1–10. doi: 10.3109/21691401.2014.885442
- Singh K, Arora N, Garg T. (2012a). RFID: a trustable security tool in pharmaceutical industry. Am J Pharm Tech Res 2:113–27
- Singh K, Arora N, Garg T. (2012b). Superbug: antimicrobial resistance due to NDM-1. Int J Inst Pharmacy Life Sci 2:58–66
- Singh O, Garg T, Rath G, Goyal AK. (2014c). Microbicides for the treatment of sexually transmitted HIV infections. J Pharm 1–18. DOI: org/10.1155/2014/352425
- Sprott GD, Tolson DL, Patel GB. (1997). Archaeosomes as novel antigen delivery systems 1. FEMS Microbiol Lett 154:17–22
- Szoka FJr, Papahadjopoulos D. (1980). Comparative properties and methods of preparation of lipid vesicles (liposomes). Annu Rev Biophys Bioeng 9:467–508
- Vossenberg JL, Ubbink-Kok T, Elferink MG, et al. (1995). Ion permeability of the cytoplasmic membrane limits the maximum growth temperature of bacteria and archaea. Mol Microbiol 18:925–932
- Whitcomb DC, Lowe ME. (2007). Human pancreatic digestive enzymes. Digest Dis Sci 52:1–17
- Woodle MC, Lasic DD. (1992). Sterically stabilized liposomes. Biochim Biophys Acta Rev Biomembr 1113:171–99
- Yamauchi K, Doi K, Kinoshita M, et al. (1992). Archaebacterial lipid models: highly salt-tolerant membranes from 1,2-diphytanylglycero-3-phosphocholine. Biochim Biophys Acta Biomembr 1110:171–7
- Zavec AB, Ota A, Zupancic T, et al. (2014). Archaeosomes can efficiently deliver different types of cargo into epithelial cells grown in vitro. J Biotechnol 192 A:130–5
- Zuidam NJ, Lee SS, Crommelin DJ. (1993). Sterilization of liposomes by heat treatment. Pharm Res, 10:1591–6