Abstract
Bupropion HCl is an atypical antidepressant drug with rapid and high first-pass metabolism. Sustained release dosage form of this drug is suggested for reducing its side effects which are mainly seizures. The aim of the present study was to design pulmonary agar nanospheres of bupropion HCl with effective systemic absorption and extended release properties. Bupropion HCl was encapsulated in agar nanospheres by ionic gelation, and characterized for physical and release properties. Pharmacokinetic studies on nanospheres were performed on rats by intratracheal spraying of 5 mg/kg of drug in form of nanospheres compared to intravenous and pulmonary delivery of the same dose as simple solution of the drug. The optimized nanoparticles showed particle size of 320 ± 90 nm with polydispersity index of 0.85, the zeta potential of −29.6 mV, drug loading efficiency of 43.1 ± 0.28% and release efficiency of 66.7 ± 2%. The area under the serum concentration–time profile for the pulmonary nanospheres versus simple solution was 10 237.84 versus 28.8 µg/ml min, Tmax of 360 versus 60 min and the Cmax of 1927.93 versus9.93 ng/ml, respectively. The absolute bioavailability of the drug was 86.69% for nanospheres and 0.25% for pulmonary simple solution. Our results indicate that pulmonary delivery of bupropion loaded agar nanospheres achieves systemic exposure and extends serum levels of the drug.
Introduction
Bupropion HCl is an antidepressant of the aminoketone class which inhibits the reuptake of dopamine and norepinephrine (Laizure et al., Citation1985; Stahl et al., Citation2004; Brunton et al., Citation2006). Bupropion is among short-acting antidepressants and is used in slow-release preparations to allow less frequent dosing and potentially to temper side effects related to agitation and gastrointestinal disturbances (Brunton et al., Citation2006).
Depression (Zung et al., Citation1983), smoking cessation (Jorenby et al., Citation2006), sexual dysfunction (Labbate et al., Citation1997), obesity (Plodkowski et al., Citation2009), attention deficit hyperactivity disorder (Cantwell, Citation1997) and seasonal affective disorder (Modell et al., Citation2005) are among therapeutic indications ascribed to bupropion. It has also been shown to have anti-inflammatory properties (Brustolim et al., Citation2006).
Bupropion has some shortcomings, especially when administered orally. It undergoes extensive first-pass metabolism resulting in poor bioavailability (5–20%) and accumulation of fatal metabolites (erythroamino alcohol and hydroxy metabolites) in the liver (Laizure et al., Citation1985; Suckow et al., Citation1986; Sweet et al., Citation1995).
Metabolites of bupropion have longer half-lives and are pharmacologically active but much less potent than the parent drug, and achieve higher plasma concentrations. Hydroxybupropion is the major metabolite produced by the metabolism of bupropion by the cytochrome P450 isoenzyme CYP2B6; in animal studies hydroxybupropion was one-half as potent as bupropion. Threohydrobupropion and erythrohydrobupropion are produced by reduction and have the potency of about one-fifth of the parent compound (Sweet et al., Citation1995). These metabolites are also more convulsion inducing than the parent molecule. Convulsive dose (CD50) values have been reported as 35, 50, 61 and 82 mg/kg, for hydroxybupropion, threohydrobupropion, erythrohydrobupropion and bupropion, respectively (Sweet et al., Citation1995; Silverstone et al., Citation2008). In addition, bupropion causes gastric irritation when administered orally (Brunton et al., Citation2006; Hays et al., Citation2009).
To overcome these shortcomings, which are specific to oral administration of bupropion, several parenteral methods have been tried and mentioned in the literature.
Gondaliya & Pundarikakshudu (Citation2003a,Citationb), Kiptoo et al. (Citation2009) and Nicoli et al. (Citation2008) tried transdermal delivery of bupropion. Injection routes have also been tried, apparently as simple solution. Intra-hippocompal injection has been examined by Ghaderpour et al. (Citation2010) to study active avoidance learning in rats. Mokhtari et al. (Citation2010) have also administered intra-ventral tegmental area injection of bupropion to evaluate its effect on aggressive behavior. Nasal route has been mentioned in the literature rather as cases of abuse (Langguth et al., Citation2009; Kim & Steinhart, Citation2010). Beside other parenteral routes, pulmonary delivery can also be attempted in which bypssing the first-pass effect provides the opportunity for the drug to be distributed into body tissues before being metabolized in the liver, leading to more prolonged effect and reduction in the administered dose.
Pulmonary drug delivery system is a needle free technique (Shaikh et al., Citation2011). Compared to the oral route, lungs are more attractive for drug delivery due to the lack of hepatic first-pass effect and lower enzymatic activity (Patton, Citation1996). Because of the high permeability and large absorptive surface area of lungs (approximately 70–140 m2 in adult humans having extremely thin absorptive mucosal membrane), and good blood supply, growing attention has been given to the pulmonary route as a non-invasive administration for systemic and local delivery of therapeutic agents (Patil & Sarasija, Citation2012).
An important aspect of pulmonary delivery is controlled delivery of drugs to lungs. Most inhaled drugs require to be taken 3–4 times a day because of their short duration of action. Sustained release of drugs in lungs can prolong the residence of an administered drug in the airways or alveolar region, and increase patient compliance by reducing dosing frequency (Shaikh et al., Citation2011). Introducing of the drug molecule into the lungs in matrix of sustained release mucoadhesive biodegradable nanospheres can result in more delayed absorption period of the drug molecule due to the prolonged and intimate contact of the nanospheres with the epithelial tissue of the lungs. This can also cause the escape of the drug from the phagocytic action of reticuloendothelial system present in the respiratory tree thanks to their nanoscale.
In the present study, we have tried to deliver bupropion HCl to the lungs of rats via nanospheres composed of agar, a biodegradable/bioadhesive natural polymer. The designed nanospheres also contained hydroxypropyl beta cyclodextrin (HPβCD) as a permeability enhancer. HPβCD shows the lowest cell toxicity on pulmonary epithelial cell lines and is the only modified βCD cited in the FDA’s list of Inactive Pharmaceutical Ingredients (Tewes et al., Citation2011).
To the best of our knowledge no work on pulmonary administration of bupropion HCl has been published in the literature so far.
Materials and methods
Materials
Agar was obtained from Narico (Siegburg, Germany), liquid paraffin from Golnoosh Company (Tehran, Iran), HPβCD was purchased from Sigma, (Ontario, Canada), bupropion HCl from Dipharma (Baranzate, Italy), calcium chloride and methanol HPLC grade from Merck Chemical Company (Darmstadt, Germany). Timolol maleate was provided by Excella Company (Nürnberger, Germany). All other reagents were of analytical grades.
Preparation of nanospheres
Agar (100, 150 and 200 mg) was added to 10 ml of water. The resultant suspension was heated up to boiling temperature to dissolve the agar and then cooled down to 45 °C, keeping the vessel covered meanwhile to prevent the loss of water. Calcium chloride, HPβCD and bupropion HCl previously dissolved in appropriate amounts of water () were added at this temperature and mixed well. The resultant solution was added under homogenization to 40 ml of liquid paraffin previously warmed up to 40 °C. After homogenizing at predetermined speeds by homogenizer (Ika T25 basic, Janken & Kunkel GmbH, Staufen, Germany) for 2 min the suspension was cooled down below 20 °C by immersing the vessel in ice water bath. Centrifugation at 8000 rpm for 5 min was performed to settle the nanospheres. The sediment was washed three times by re-dispersing in 5 ml of methylene chloride. The final sediment was dispersed in 3 ml of ethanol, added to 3 g of mannitol, mixed thoroughly to get a homogenized paste and left overnight to get air dried.
Table 1. Design of the experiment according to a D-optimal design for formulating agar nanosphres loaded with bupropion HCl.
Morphological study of nanospheres
The scanning electron microscopy (SEM) studies were conducted on a Philips (Oregon 97045, US) XL 30 instrument operating at 26 keV.
Zeta potential, particle size and size distribution measurement
Particle size and zeta potential measurements were performed by Malvern ZetaSizer (Model 3000 NS, Worcestershire, UK).
Drug loading efficiency determination
Accurately weighted 40 mg of nanoparticles were dispersed in 20 ml of phosphate buffer (pH 7.2), shaken for 24 h and assayed after filtration through 0.22 µm syringe filter by HPLC method according to Loboz et al. (Citation2005) method.
Loading efficiency (LE) was calculated according to the following equation:
(1)
In vitro release study
Nanospheres equivalent to 2.5 mg of bupropion HCl were dispersed in 3 ml of phosphate buffer (pH 7.4), decanted in dialysis bag and placed in 200 ml of 37 °C buffer. At predetermined time intervals 3 ml of release medium was withdrawn and analyzed spectrophotometrically at 298 nm to determine the released amount of bupropion HCl. The withdrawn samples were replaced by 3 ml of fresh 37 °C buffer.
Release efficiency (RE) at the 300th minute was calculated according to the following equation for each formulation (Khan & Rhodes, Citation1972; Ammar et al., Citation2009):
(2)
where y is the released percent at time t.
Optimization of nanospheres
Genetic algorithms (GAs) are optimization techniques. Starting with a randomly generated population of chromosomes, a GA carries out a process of fitness-based selection and recombination to produce the next generation. During recombination, parent chromosomes are selected and their genetic material is recombined to produce child chromosomes. These then pass into the next generation. As this process is iterated, a sequence of successive generations evolves and the average fitness of the chromosomes tends to increase until some stopping criterion is reached. In this way, a GA “evolves” a best solution to a given problem (McCall, Citation2005).
The individual chromosomes in the GA population were evaluated using the developed artificial neural network (ANN) prediction model. The ANN model was used to predict the particle size, polydispersity index (PdI), zeta potential, drug loading efficiency and release efficiency under the defined constraints. The fitness of a chromosome in the population was evaluated over minimizing the following fitness function (Cazacu & Grama, Citation2014):
where O1, O2, O3, O4 and O5 are particle size, PdI, zeta potential, drug loading efficiency and release efficiency values of ANN output, respectively. The GA optimizations were coded and run in MATLAB software (version 7, The MathWorks, Inc., California 90502, US).
ANNs are computer programs that are designed to simulate some functions of the human brain using different learning algorithms from experience (Sun et al., Citation2003). An ANN is a biologically inspired computational model formed from hundreds of computational units known as artificial neurons, connected with coefficients (weights) which constitute the neural structure. Each processing element (except input neurons) has connecting weight, transfer function and an output. The arriving signals (inputs) are multiplied by the connection weights, summed and then passed through a transfer function to produce the output for that neuron.
The ANN reads the input and output values in the training data set and changes the value of the weighted links to reduce the difference between the predicted and target values. The error in prediction is minimized across many training cycles until network reaches specified level of accuracy.
In this study, collected data were divided into three groups, namely training (60%), evaluating (20%) and testing (20%). The later data set which has never been used during training was applied to test prediction ability of ANN. Data were first normalized within [−1 +1] and introduced to ANN with hyperbolic tangent function. The number of hidden neurons was optimized using GA. Several statistical indicators [i.e. mean squared error (MSE), normalized mean-squared error (NMSE) and the mean of absolute error (MAE) were used according to following equations to evaluate performance of the networks (Fathi et al., Citation2011a,Citationb):
(3)
(4)
(5)
To optimize ANN using GA, an initial population of 60 chromosomes was randomly generated for 60 generations (Heckerling et al., Citation2004; Izadifar & Jahromi Citation2007; Fathi et al., Citation2011b). The roulette wheel selection based on ranking algorithm was applied for the selection operator. Uniform crossover and mutation operators with mixing ratio of 0.5 were used and the probabilities of the crossover and mutation operators were adjusted at 0.9 and 0.01, respectively.
Drug administration to animals
Adult male Wistar rats (200–205 g) were housed (2 rats in each cage) under 12-h light and 12-h dark cycles with free access to food and water for 1 week prior to experiment to get acclimated to the lab condition. The whole animal experiments were performed in accordance with Ethics Committee guidelines for research on laboratory animals of Isfahan University of Medical Sciences, Isfahan, Iran. The animals were divided into three groups (6 rats in each group). The first group received bupropion HCl simple aqueous solution via the tail vein. The second group was administered 100 µl of simple aqueous solution of bupropion HCl through the pulmonary route and the third group received 100 µl of aqueous dispersion of the optimized formulation of agar nanospheres loaded with bupropion HCl through the pulmonary route. The administered dose of the drug in all three groups was 5 mg/kg of body weight. Animals in groups 2 and 3 were lightly anesthetized by diethyl ether prior to pulmonary administration of the drug. Pulmonary administration was performed by microsprayer (Penncentury, Wyndmoor, PA 19038, US). The tip of the device was gently inserted down the trachea of the lightly anesthetized animal – near to the carina – and the predetermined dose (either solution or suspension) was administered intratracheally in the form of microdroplets. Blood samples of 700 µl were taken from the tail vein at 10, 30, 60, 120, 360, 720 and 1440 min after administration. Plasma samples were stored at −20 °C before analysis.
Sample preparation and HPLC analysis
The method developed by Loboz et al. (Citation2005) was used with slight modifications to analyze plasma samples. Briefly, to 300 µl of plasma 10 µl of internal standard solution (timolol maleate 100 µg/ml) was added. Extraction was performed using 200 µl of 0.5 M carbonate buffer pH 10.8 and 3 ml of 1.5% isoamyl alcohol in heptane. The samples were vortexed for 1 min followed by centrifugation at 1500g for 15 min. The organic layer was transferred to a tube containing 0.1 M HCl (250 µl), vortexed and mixed as mentioned earlier. After centrifugation the organic layer was discarded and the aqueous layer was dried under a stream of nitrogen at 37 °C. The residue was reconstituted with 80 µl of mobile phase and 50 µl was injected onto the column.
The analysis was performed by a Knauer HPLC system with Chromgate software version 3.1 equipped with a binary pump, Smartline-1000-1 and Smartline-1000-2, a UV detector (Smartline-UV-2500, variable wavelength, programmable, Berlin, Germany), an online solvent vacuum degasser and a manual sample injector. Analysis was carried out on a C18 column (25 cm × 4.6 mm, particle size of 5 µm) from Agilent (Wyndmoor, PA 19038, US). The mobile phase consisted of methanol and 0.05 M phosphate buffer adjusted to pH 5.5 with phosphoric acid (85%) before addition of methanol (45:55 v/v). The flow rate was maintained at 1.0 ml/min at ambient temperature.
Results
Physicochemical and morphologic properties of bupropione HCl loaded agar nanospheres
shows the physicochemical properties of the nanospheres. As this table shows the range of particle size of nanospheres varied between 310.3 ± 10.4 and 807.2 ± 86.0 nm. The changes of zeta potential were between −21.7 and −37.1 mV, loading efficiency between 18.9 ± 2.0% and 45.6 ± 2.2%, the release efficiency until 5 h between 65 ± 4% and 91 ± 6% and the PdI between 0.21 and 0.99.
In this study, five features of developed nanospheres (i.e. size, PdI, zeta potential, loading efficiency and release efficiency) were modeled using ANN in combination with GA. The prediction efficiency of developed models for different features and architectures are tabulated in .
Table 2. Performance of developed neural network against prediction of nanocarrier features.
The process optimization was also performed using GA for minimizing the particle size, release efficiency and PdI and maximizing the absolute value of zeta potential and loading efficiency. The optimized values of 6.37%, 9616 rpm, 1.69% and 0.99% were obtained for CaCl2 concentration, homogenizer speed, agar concentration and HPβCD concentration. GA optimization using the aforementioned process parameters led to formation of nanospheres with size of 320 ± 90 nm with PdI of 0.85, the zeta potential of −29.6 mV, drug loading efficiency of 43.1 ± 0.28% and release efficiency of 66.7 ± 2%. These nanospheres showed spherical morphology as can be seen in . The SEM of the nanospheres confirmed the particle size analysis results obtained by Malvern zetasizer. shows the relationship between the predicted and actual values of the studied responses.
Table 3. Predicted versus real values of the studied responses of the optimum formulation predicted by the GA methodology.
Pharmacokinetic studies
Pharmacokinetic data analysis was accomplished using pksolver “an add-in program for pharmacokinetic analysis” developed by Zhang et al. (Citation2010). Pharmacokinetic parameters calculated for bupropion HCl following drug administration in the three animal groups mentioned in the Methods section are summarized in .
Table 4. Pharmacokinetic parameters of bupropion HCl after i.v. and pulmonary administration of the simple solution and agar nanospheres of the drug (n = 6).
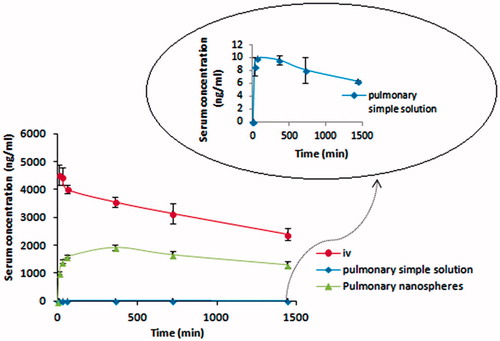
As could be seen in plasma concentration–time profiles (), the maximum plasma concentration or Cmax obtained from pulmonary administration of nanospheres (1927.93 ± 103.07 ng/ml) is far greater than that seen in pulmonary administration of simple solution of bupropion HCl (9.93 ± 0.1 ng/ml) (). Administration of bupropion HCl in matrix of nanospheres has also led to a greater Tmax of −360 min versus 60 min in simple solution group. A much greater absolute bioavailability (F) was also attained from nanospheres administration (86.69% versus0.253%) (). Following intravenous (i.v.) administration of bupropion HCl to rats, a short distribution phase (t1/2α = 31.23 min) was seen followed by a long elimination phase (t1/2β = 33.8 h) ().
Discussion
As indicates, the developed model was able to estimate outputs with acceptable accuracy in which all of the features had NMSE values of lower than 0.5. High correlation coefficients revealed good agreement between predicted and obtained data. Correlation coefficients of 0.83, 0.91, 0.91, 0.95 and 0.85 obtained for particle size, PdI, zeta potential, loading efficiency and release efficiency, respectively, which shows potential application of ANN-GA model for estimation of physicochemical properties of nanospheres.
From the results shown in , it could be concluded that optimum values proposed by GA are near to the desired states for all responses (greater absolute extremums) except PdI and this is in agreement with what is ascribed to it: not getting stuck in local minimums (Xin, Citation1999).
There is a great controversy about pharmacokinetic parameters of bupropion in rats in the literature; for example, elimination half-life has been reported to vary from 0.83 h by Suckow et al. (Citation1986) to 11.3 h by Lufeng et al. (Citation2011). What has been reported as volume of distribution is much greater than that found in our study which seems to be the ratio of Vd/F instead of Vd itself; as bupropion administered via i.p. or oral routes (the main routes of administration in the literature) undergoes extensive first-pass metabolism, F will be considerably low and pure Vd will be much lower than the reported values. However, elimination half-life of 26.6 h has been reported by Kiptoo et al. (Citation2009) following i.v. administration of bupropion in guinea pigs which is near to the value found in our study (33.8 h) (). As i.v. administration to rats was not found anywhere in the literature there is no reference to compare with the i.v. results.
The positive effect of making use of the above-mentioned excipients in context of nanospheres is obvious. Increase in Cmax () could be ascribed to (1) the bioadhesive properties of agar used in production of nanospheres which provides an intimate and prolonged contact with the epithelium of respiratory tract and facilitates the passage of drug molecules into the blood circulation, (2) the nano-dimension of nanospheres which help them to escape phagocytic activity of wandering phagocytes present here and there and (3) to the presence of HPβCD which was used as permeation enhancer in the nanoparticles.
The increased Tmax () could solely be the result of the sustained release of drug molecules from the nanospheres (). In pulmonary delivery, the absence of rapid removal of the drug from the blood stream by the first-pass effect causes a delayed Tmax. This effect is much important in providing a fairly large Tmax by nanosphere compared to simple solution (). The values for Tmax were directly extracted from the plasma concentration–time graphs () which were 360 min for all 6 rats received bupropion as nanospheres.
Suckow et al. (Citation1986) showed that rats do not metabolize bupropion extensively and as this study was not aimed at pursuing the plasma profile of the metabolites, it is beyond the scope of this study to judge the effect of pulmonary delivery on the extent of metabolism when the drug is administered as nanospheres; although the analysis method is capable of detecting simultaneously the metabolites in plasma (Loboz et al., Citation2005). This can be evaluated in another study by administering the nanospheres to an animal like guinea pig which extensively metabolizes the bupropion. It is highly expected that this route of delivery will lead to much lower plasma levels of drug metabolites.
Increasing the absolute bioavailability by formulating the Active Pharmaceutical Ingredients using appropriate excipients and proper dosage form is not surprising, though is rare, but by a precise review of the literature we may find many examples. For example, in one study, a nanoscale formulation of danazol based on the complex of nanoparticles of danazol with cyclodextrin was prepared, which improved the absolute bioavailability of the drug from 5.1% (obtained from a conventional suspension) to 106.7% (Liversidge & Cundy, Citation1995). This was an example of extreme conditions; other examples of high increases in absolute bioavalibility, though not of such huge scale could be found in the literature.
In our case, the most probable explanation – as previously mentioned – is the presence of HPβCD, which is a strong permeation enhancer according to Loftsson & Brewster (Citation2011), a comprehensive review of the role of cyclodextrins as permeation enhancer in the literature is provided by these authors. HPβCD is more readily absorbed from the lungs compared to the other routes of delivery which means that CDs are more effective when used by pulmonary route. Among the mechanisms of increasing the permeability mentioned in the literature, modulation of tight junctions that exist in lungs is of special importance (Deli, Citation2009).
Another participating factor in the increased bioavailability is the presence of agar. According to our own experiments, agar is a strong bioadhesive polymer which impedes the expectoration of the nanosphere bearing droplets by sweeping mechanism of respiratory tract.
Using the microsprayer device provides sufficient assurance of gaining a proper respirable fraction which means readily access of the aerosolized droplets, in which the nanospheres are suspended, to the deepest part, i.e. alveoli of the lungs.
Conclusion
Pulmonary delivery of bupropion HCl loaded in agar nanospheres to Wistar rats caused a great increase in the Cmax (1927.93 versus 9.93 ng/ml) and AUC (10 237.84 versus 28.8 µgmin/ml) and consequently increased the absolute bioavailability compared to pulmonary administration of the drug as a simple aqueous solution (86.69% versus 0.25%). Tmax was also increased from 60 min in the pulmonary simple solution to 360 min in the nanospheres. It can be concluded that agar nanospheres loaded with bupropion HCl are excellent vehicles to deliver the drug via pulmonary route. It is highly probable that this kind of administration will delay the transformation of the parent molecule to less potent and more convulsion producing metabolites, although this needs to be further demonstrated in other animal models as it is reported that rats can rarely metabolize bupropion.
Declaration of interest
The authors acknowledge the financial support of Isfahan University of Medical Sciences. The authors declare no conflict of interest.
References
- Ammar HO, Salama HA, Ghorab M, Mahmoud AA. (2009). Nanoemulsion as a potential ophthalmic delivery system for dorzolamide hydrochloride. Aaps Pharmscitech 10:808–19
- Brunton L, Lazo S, Parker L. (2006). Goodman & Gilman’s the pharmacological basis of therapeutics. 11th ed. New York: McGraw-Hill Professional Publishing
- Brustolim D, Ribeiro-dos-Santos R, Kast RE, et al. (2006). A new chapter opens in anti-inflammatory treatments: the antidepressant bupropion lowers production of tumor necrosis factor-alpha and interferon-gamma in mice. Int Immunopharmacol 6:903–7
- Cantwell DP. (1997). ADHD through the life span: the role of bupropion in treatment. J Clin Psychiatry 59:92–4
- Cazacu R, Grama L. (2014). Steel truss optimization using genetic algorithms and FEA. Procedia Technol 12:339–46
- Deli MA. (2009). Potential use of tight junction modulators to reversibly open membranous barriers and improve drug delivery. Biochim Biophys Acta Biomembr 1788:892–910
- Fathi M, Mohebbi M, Razavi S. (2011a). Application of image analysis and artificial neural network to predict mass transfer kinetics and color changes of osmotically dehydrated kiwifruit. Food Bioprocess Technol 4:1357–66
- Fathi M, Mohebbi M, Razavi SMA. (2011b). Application of fractal theory for prediction of shrinkage of dried kiwifruit using artificial neural network and genetic algorithm. Drying Technol 29:918–25
- Ghaderpour S, Zare S, Ghaderi Pakdel F. (2010). Effects of acute intra-hippocompal injection of bupropion on active avoidance learning in rats. Physiol Pharmacol 14:293–301
- Gondaliya DP, Pundarikakshudu K. (2003a). Enhanced transdermal permeation of bupropion hydrochloride by chemical modification. Ind J Pharm Sci 65:671–4
- Gondaliya DP, Pundarikakshudu K. (2003b). Studies in formulation and pharmacotechnical evaluation of controlled release transdermal delivery system of bupropion. AAPS PharmSciTech 4:18–26
- Hays JT, Hurt RD, Decker PA, et al. (2009). A randomized, controlled trial of bupropion sustained-release for preventing tobacco relapse in recovering alcoholics. Nicotine Tob Res 11:859–67
- Heckerling PS, Gerber BS, Tape TG, Wigton RS. (2004). Use of genetic algorithms for neural networks to predict community-acquired pneumonia. Artif Intel Med 30:71–84
- Izadifar M, Jahromi MZ. (2007). Application of genetic algorithm for optimization of vegetable oil hydrogenation process. J Food Eng 78:1–8
- Jorenby DE, Hays JT, Rigotti NA, et al. Varenicline Phase 3 Study Group (2006). Efficacy of varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA 296:56–63
- Khan KA, Rhodes CT. (1972). Effect of compaction pressure on the dissolution efficiency of some direct compression systems. Pharm Acta Helv 47:594–607
- Kim D, Steinhart B. (2010). Seizures induced by recreational abuse of bupropion tablets via nasal insufflation. Canad J Emerg Med Care 12:158–61
- Kiptoo PK, Paudel KS, Hammell DC, et al. (2009). Transdermal delivery of bupropion and its active metabolite, hydroxybupropion: a prodrug strategy as an alternative approach. J Pharm Sci 98:583–94
- Labbate LA, Grimes JB, Hines A, Pollack MH. (1997). Bupropion treatment of serotonin reuptake antidepressant-associated sexual dysfunction. Ann Clin Psychiatry 9:241–5
- Laizure SC, DeVane CL, Stewart JT, et al. (1985). Pharmacokinetics of bupropion and its major basic metabolites in normal subjects after a single dose. Clin Pharmacol Ther 38:586–9
- Langguth B, Hajak G, Landgrebe M, Unglaub W. (2009). Abuse potential of bupropion nasal insufflation: a case report. J Clin Psychopharmacol 29:618–19
- Liversidge GG, Cundy KC. (1995). Particle size reduction for improvement of oral bioavailability of hydrophobic drugs: I. Absolute oral bioavailability of nanocrystalline danazol in beagle dogs. Int J Pharm 125:91–7
- Loboz KK, Gross AS, Ray J, McLachlan AJ. (2005). HPLC assay for bupropion and its major metabolites in human plasma. J Chromgr B Anal Technol Biomed Life Sci 823:115–21
- Loftsson T, Brewster ME. (2011). Pharmaceutical applications of cyclodextrins: effects on drug permeation through biological membranes. J Pharm Pharmacol 63:1119–35
- Lufeng H, Wang Z, Xu R, et al. (2011). Determination of bupropion and its main metabolite in rat plasma by LC–MS and its application to pharmacokinetics. Die Pharm Int J Pharm Sci 66:924–8
- McCall J. 2005. Genetic algorithms for modelling and optimisation. J Comput Appl Math 184:205–22
- Modell JG, Rosenthal NE, Harriett AE, et al. (2005). Seasonal affective disorder and its prevention by anticipatory treatment with bupropion XL. Biol Psychiatry 58:658–67
- Mokhtari Hashtjin M, Zare S, Ghaderi Pakdel F, Heysieattalab S. (2010). The effect of intra-VTA injection of Bupropion on submissive defensive aggressive behavior induced by electrical foot shock of rat. Pharm Sci 16:125–30
- Nicoli S, Cella S, Aversa V, Santi P. (2008). Transdermal film containing nicotine and bupropion for combined smoking cessation therapy. PharmTech Eur 20:22–8
- Patil JS, Sarasija S. (2012). Pulmonary drug delivery strategies: a concise, systematic review. Lung India Office Organ Indian Chest Soc 29:44–9
- Patton JS. (1996). Mechanisms of macromolecule absorption by the lungs. Adv Drug Deliv Rev 19:3–36
- Plodkowski RA, Nguyen Q, Sundaram U, et al. (2009). Bupropion and naltrexone: a review of their use individually and in combination for the treatment of obesity. Expert Opin Pharmacother 10:1069–81
- Shaikh S, Nazim S, Shaikh A, et al. (2011). Recent trends in applications of pulmonary drug delivery: a review. Int J Pharm Res Dev 2:171–80
- Silverstone PH, Williams R, McMahon L, et al. (2008). Convulsive liability of bupropion hydrochloride metabolites in Swiss albino mice. Ann Gen Psychiatry 7:19–26
- Stahl SM, Pradko JF, Haight BR, et al. (2004). A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion J Clin Psychiatry 6:159–66
- Suckow RF, Smith TM, Perumal AS, Cooper TB. (1986). Pharmacokinetics of bupropion and metabolites in plasma and brain of rats, mice, and guinea pigs. Drug Metab Dispos 14:692–7
- Sun Y, Peng Y, Chen Y, Shukla AJ. (2003). Application of artificial neural networks in the design of controlled release drug delivery systems. Adv Drug Deliv Rev 55:1201–15
- Sweet RA, Pollock BG, Kirshner M, et al. (1995). Pharmacokinetics of single- and multiple-dose bupropion in elderly patients with depression. J Clin Pharmacol 35:876–84
- Tewes F, Gobbo OL, Amaro MI, et al. (2011). Evaluation of HPβCD–PEG microparticles for salmon calcitonin administration via pulmonary delivery. Mol Pharm 8:1887–98
- Xin Y. (1999). Evolving artificial neural networks. Proc IEEE 87:1423–47
- Zhang Y, Huo M, Zhou J, Xie S. (2010). PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed 99:306–14
- Zung WW, Brodie HK, Fabre L, et al. (1983). Comparative efficacy and safety of bupropion and placebo in the treatment of depression. Psychopharmacology 79:343–7