?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In present study, two types of micelles based on sodium cholate (NaC) were prepared through non-covalent bonding interaction and the potential of micelles as oral drug delivery systems for paclitaxel (PTX) was evaluated. Pluronic–chitosan (F127–CS) and Pluronic–poly (acrylic acid) (F127–PAA) copolymers were synthesized. Electrostatic interaction and hydrogen bond were used to prepare F127–CS/NaC micelles and F127–PAA/NaC micelles, respectively. The physicochemical characteristics of micelles were determined. An average diameter of 67.5 nm and unimodal pattern of size distribution were observed for F127–CS/NaC micelles. While for F127–PAA/NaC micelles, an average diameter of 85.89 nm and non-unimodal pattern of size distribution were observed. The results revealed that F127–CS/NaC micelles were more integrated than F127–PAA/NaC micelles. Further experiments showed that the F127–CS/NaC micelles had a higher drug-loading content of 12.8% and a lower critical micelle concentration (CMC) of 2.5 × 10−3 mol/L compared with F127–PAA/NaC micelles. In vitro cytotoxicity analysis demonstrated that the PTX-loaded F127–CS/NaC micelles were of great efficiency in inhibiting the growth of drug-resistant breast cancer MCF-7 cells (MCF-7/Adr). The intragastric administration of the PTX-loaded F127–CS/NaC micelles in rats provided a 4.33-fold higher absolute bioavailability compared to commercial Taxol®, indicating an efficient oral absorption of PTX delivered by micelles. These findings signify that F127–CS/NaC micelle may be a promising carrier for the delivery of PTX.
Introduction
Paclitaxel (PTX) is a naturally derived drug that acts against a wide range of cancers, especially against non-small-cell lung cancer, metastatic breast cancer and refractory ovarian cancer (Pandey et al., Citation2015). However, its poor solubility and the efflux pump function of multidrug resistance transporter P-glycoprotein (P-gp) limited the application of PTX (Kumar et al., Citation2010; Wang et al., Citation2014). For example, clinically, Taxol is a commercial preparation of PTX for intravenous administration and is composed of 50:50 (v/v) mixtures of Cremophor EL and dehydrated alcohol. Unfortunately, Cremophor EL causes severe adverse effects, such as hypersensitivity, nephrotoxicity and neurotoxicity (Mo et al., Citation2011; Vader et al., Citation2013). Thus, many alternative delivery systems for PTX have been developed.
Theoretically, oral administration is a much more preferable choice for PTX. Compared with injection, it shows more convenience, better patient compliance, less cost and more chronic treatment regimen (Russell-Jones, Citation2004; Simoes et al., Citation2015). However, as the result of the poor solubility (0.25 mg/mL), the action of the multidrug efflux transporter P-gp, highly expressed in intestinal tract, as well as the extensive first-pass metabolisms by either the intestinal or liver cytochrome P450 enzymes like CYP3A4, the oral bioavailability of PTX is even less than 10% (Mo et al., Citation2011).
Due to these problems, many efforts have been taken to develop an alternative oral delivery system for PTX to improve its poor solubility and low permeability across the intestinal barrier as well as overcome the multidrug resistance against it (Nornoo et al., Citation2009; Agueros et al., Citation2010; Mo et al., Citation2011; Yao et al., Citation2011). Among these drug delivery systems, polymeric micelles have gained considerable attention owing to their unique core/shell structure and high loading capacity for drugs (Kataoka et al., Citation2001; Nishiyama & Kataoka, Citation2006). The inner core formed by hydrophobic moieties of copolymers encapsulates the poorly soluble drugs and outer shell formed by hydrophilic moieties protects the loaded drug from non-specific uptake by reticuloendothelial system (RES) (Gong et al., Citation2012; Yang et al., Citation2012). Pluronic block copolymer is one of the most widely used materials to construct micelles. Pluronic block copolymers are benign and some of them have been approved by FDA as pharmaceutical excipients (Cha et al., Citation2011). These macromolecules are surface active and self-assemble into micelles above critical micelle concentrations (CMCs) and certain temperatures. Interestingly, Pluronic unimers incorporate themselves into the cell membranes altering the membrane microviscosity and inhibiting functioning of some membrane proteins, such as P-gp, responsible for pumping chemotherapeutic drugs out of the cell (Liu et al., Citation2014). A problem encountered with the use of Pluronic micelles for the delivery of PTX is its lower drug loading.
Different from conventional surfactants, sodium cholate (NaC) has a large, rigid and hydrophobic steroid nucleus with hydrophilic moieties of three hydroxyl groups and an ionic head of a carboxyl group (Subuddhi & Mishra, Citation2007). As biosurfactants, bile salts act as solubilizer and emulsifier for cholesterol and dietary lipids in the gastrointestinal tract. And they also have been studied as delivery system for medicines owing to their unusual solubilizing and emulsifying capabilities (Sugioka & Moroi, Citation1998). However, their solubilization capacity is limited because dilute bile salt solutions present small micelles with low aggregation number due to the steric hindrance of the large steroidal skeleton. Many strategies have been contributed to increase the aggregation number of bile salts. Most of the investigations studied the application of mixed micelles, such as phospholipids/bile salts mixed micelles as drug delivery systems (Maswal & Dar, Citation2013).
During the last decades diverse materials have been synthesized through non-covalent interactions including hydrogen-bond and electrostatic interaction. For example, by reacting the block ionomers with oppositely charged molecules, such as surfactants and proteins, a type of complex materials have been synthesized. These materials can form micelle-like nanoparticles in aqueous solution with the core–corona structure, called “polyion complex micelles”. Surrounded by a hydrophilic corona, the core is formed by the aggregation of surfactant molecules electrostatically bound to the polyion chain. And the pharmaceutical agents can be incorporated into the core through a combination of electrostatic, hydrophobic and hydrogen bonding interactions. The size, structure and loading capacity of the core can be altered by changing the ratio of the polyion and surfactant components in the mixture. As a result, these systems have a potential as drug release systems, especially for hydrophobic drugs (Luo et al., Citation2010). In addition, hydrogen-bond is also used to prepare nanomaterials. A physical micelle is synthesized, where the core and shell are reversibly connected by multiple triple hydrogen-bonds. The micelles can undergo shell disruption upon the encountering of specific chemicals or changing of pH, and aggregation and precipitation can occur during shell disruption due to the sharply decreased CMC (Kuang et al., Citation2012).
In this paper, Pluronic–chitosan (F127–CS) and Pluronic–poly (acrylic acid) (F127–PAA) copolymers were synthesized and two types of micelles were prepared. At certain pH, anionic NaC reacted with cationic F127–CS copolymer to form polyion complex micelles, F127–CS/NaC micelles. F127–PAA formed hydrogen bonds with the protonated NaC to prepare F127–PAA/NaC micelles. The potential of the micelles as oral drug delivery systems for PTX was evaluated. Systematic studies on physicochemical properties including size distribution, zeta-potential and morphology were conducted to validate the formation of micelle. CMC, drug-loading content and release behavior were also studied. The pharmacokinetic study in rats was conducted. The oral bioavailabilities of the micelles and commercial Taxol® were compared.
Materials and methods
Materials
PTX was purchased from Xian Haoxuan Biological Technology Co. Ltd. (Xian, China). NaC, N-hydroxysuccinimide (NHS) and succinic anhydride were purchased from Aladdin Reagent Database Inc. (Shanghai, China). Pluronic F127 (F127), 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) and rhodamine 123 (Rh-123) were purchased from Sigma-Aldrich Co. Ltd. (St. Louis, MO). Chitosan (Mw ≤ 3000) was purchased from Jinan Haidebei Marine Bioengineering Co. Ltd. (Jinan, China). 3-(4, 5-Dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) was purchased from Beijing Solarbio Biological Technology Co. Ltd. (Beijing, China). All other chemicals were of analytical grade.
Drug resistance human breast cancer MCF-7/Adr cell lines were obtained from the Institute of Immunopharmacology and Immunotherapy, the School of Pharmaceutical Sciences, Shandong University (Jinan, China).
Synthesis of F127–PAA copolymer
Poly(acrylic acid) (PAA) was conjugated on both termini of F127 copolymer to produce F127–PAA (Lin et al., Citation2009). First, PAA (20 mg, 0.01 mmol) and EDC (40 mg, 0.21 mmol) were dissolved in deionized water (20 mL) at pH 7 regulated with 1 M NaOH solution, and stirred at room temperature. After 24 h, F127 (20 mg, 1.59 × 10−3 mmol) was added into the crude solution and the further reaction was performed for another 48 h. Then the reaction mixture was respectively dialyzed against 1% NaCl solution and distilled water using a membrane (Molecular weight Cut Off: 8000–14 000) for 3 days and lyophilized to give F127–PAA. The chemical structures of the synthesized polymers were confirmed by FT-IR spectrum and proton nuclear magnetic resonance (1H NMR) analysis.
Synthesis of F127–CS copolymer
The F127–CS copolymers were synthesized using succinic anhydride as the bridge molecules between F127 and chitosan (Park et al., Citation2008). F127 (3.81 g, 0.3 mmol), succinic anhydride (45 mg, 0.45 mmol), DMAP (55 mg, 0.45 mmol) and triethylamine (60 mg, 0.6 mmol) were dissolved in anhydrous dioxane and stirred for 24 h at room temperature. And then the organic solvent was subsequently removed by rotary vacuum evaporation and the residue was filtered and precipitated in diethyl ether thrice, followed by vacuum drying overnight. The monocarboxylated F127 obtained above, EDC (87 mg, 0.45 mmol), NHS (52 mg, 0.45 mmol) and chitosan (1.35 g, 0.45 mmol) were, respectively, dissolved in PBS buffer (pH 5) and stirred at room temperature. After 24 h, the resulting mixture was dialyzed against distilled water using a membrane (Molecular weight Cut Off: 8000–14 000) for 3 days and lyophilized to give F127–CS. The chemical structures of the synthesized polymers were confirmed by FT-IR spectrum and proton nuclear magnetic resonance (1H NMR) analysis.
Nuclear magnetic resonance spectroscopy (NMR)
The proton nuclear magnetic resonance (1H NMR) analysis was performed using 1H NMR spectroscopy (1H NMR; 600 MHz Inova-600, Varian, CA). All spectra were obtained at room temperature with 15% (w/v) DMSO as a solvent.
FT-IR analysis
Functional group characterization was carried out by FT-IR analysis using a FT-IR spectrometer (Vertex70, Bruker, Germany). Dried samples were pressed with potassium bromide power into pellets.
Preparation of PTX-loaded micelles
The preparation of PTX-loaded F127–PAA/NaC micelles
PTX-loaded polymeric micelles were prepared by the thin-film hydration method. Briefly, 6 mg of PTX and 34 mg of NaC were dissolved in methanol in a round-bottom flask and the organic solvent was subsequently removed by rotary vacuum evaporation. The resulting solid film was stored under vacuum overnight to remove the residual methanol and hydrated with 2 mL distilled water (pH 4) containing 17 mg of F127–PAA. The mixture was stirred for 30 min at 25 °C to obtain a micelle solution, which was then centrifuged at 10 000 rpm for 15 min to remove the unentrapped PTX.
The preparation of PTX-loaded F127–CS/NaC micelles
PTX-loaded polyion complex micelles were also prepared by the thin-film hydration method. To prepare the polyion complex micelles, 20 mg of NaC and 6 mg of PTX were dissolved in methanol in a round-bottom flask and the organic solvent was subsequently removed by rotary vacuum evaporation. The resulting solid film was stored under vacuum at room temperature overnight and then hydrated with 6 mL distilled water (pH 6.5) containing 20 mg of F127–CS. The mixture was mixed ultrasonically at 25 °C for 5 min to obtain the micelle solution, which was then centrifuged to remove the unentrapped PTX.
Determination of drug-loading content of micelles
The drug-loading content (DL%) and encapsulation efficiency (EE%) were determined by the high performance liquid chromatography (HPLC). The HPLC analysis was carried out using Agilent 1200 HPLC system equipped with a reverse phase (RP) column (C18, 5 µm pore size, 250 mm× 4.6 mm, Dikma, China). Firstly, 200 mL micelle solution was diluted with 800 mL acetonitrile to destroy micelle structure and release PTX. Mixed solution was then filtered by 0.22 µm nylon filter. The mobile phase was acetonitrile/water (50:50, v/v) and the detection wavelength was 227 nm. The standard regression equation was A = 43.939C + 12.441 (r2 = 0.9999). DL% and EE% were calculated by the following equations:
Size distribution and zeta-potential measurement
Size distribution and zeta-potential of the micelles were measured using a dynamic light scattering (DLS) instrument (Zetasizer-3000, Malvern Co., Malvern, UK) equipped with a He–Ne laser of 633 nm at a fixed angle of 90° at 25 °C. These two measurements were performed in triplicate at a polymer concentration of 6 mmol/L.
Transmission electron microscopy (TEM) for morphology
The morphology was observed by transmission electron microscope (TEM) (JEM-100SX, JEOL, Tokyo, Japan). A drop of sample after dilution was placed on the copper grid and the excess solution was removed with a piece of filter paper. The samples were stained with 1.5% phosphotungstic acid (w/v) and then dried in air.
CMC determination
The CMC was determined by the method of pyrene fluorescence probe spectrometry using a fluorescence spectrometer (F-7000, Hitachi, Tokyo, Japan). The prepared micelles were diluted to a series of concentrations which were then added separately to the centrifuge tubes containing pyrene to get a final concentration of pyrene of 2.0 × 10−6 M. The mixed solutions were allowed to stand to equilibrate overnight at room temperature. The intensity ratio of excitation spectra at I1 (intensity of first peak) to I3 (intensity of third peak) (emission 350–500 nm) was determined and plotted as a function of polymer concentration. CMC value for each polymer solution was identified at the inflection point of the plot.
In vitro release of PTX-loaded micelles
The in vitro release behavior of PTX from micelles was investigated by the dialysis method as reported previously (Yao et al., Citation2011). PTX-loaded micelle solution (containing 250 µg PTX) was added into the dialysis bag (MWCO = 3500 Da) and then the end-sealed dialysis bag was immersed fully into 50 mL incubation media in a shaking bed at 37 °C and 100 rpm. Simulated gastric fluid (SGF, pH 1.2), phosphate buffered saline (PBS, pH 5) simulating the environment of upper small intestine and simulated intestinal fluid (SIF, pH 6.8) containing 1% w/v Tween 80 was used as release media, respectively. At specified time intervals (0.25, 0.5, 1, 2, 4, 6, 8, 10, 12, 24, 36, 48 h), 2 mL of samples were removed and replaced with an equal volume of fresh release media. The PTX concentrations of the samples were determined by HPLC analysis as described above.
In vitro cytotoxicity assay
The cytotoxicity studies were evaluated by the MTT method to investigate whether the copolymers affect cell proliferation of MCF-7/Adr cells. Drug resistance human breast cancer MCF-7/Adr cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% (v/v) fetal bovine serum, 1% non-essential amino acid and 1% penicillin–streptomycin. The cells were seeded into 96-well plates at the density of 5 × 103 cells per well and incubated for 24 h in a humidified atmosphere containing 5% CO2 at 37 °C. When the cells reached 80% confluence, the samples were added to each well. After 48 h incubation, 20 μL of MTT (5 mg/mL) solution was added to each well and incubated for 4 h. Then medium was removed and 150 μL of DMSO was added to each well to dissolve the purple formazan crystals with vigorous shake for 15 min. The absorbance at 570 nm was measured with a microplate reader (680 BIO-RAD, Hercules, CA).
In vitro cellular uptake of Rh-123-loaded micelles
The cell uptake was evaluated by fluorescence microscopy (Yang et al., Citation2007). Rh-123 (a poorly water-soluble fluorescent substance) was loaded into the micelles using the same method with preparation of PTX-loaded micelles. The MCF-7/Adr cells were seeded at the density of 2 × 105 cells per well in 6-well plates and incubated in a humidified atmosphere containing 5% CO2 at 37 °C. When the cells were grown to confluence, the different preparations of Rh-123 were added to each well (5 μmol/L of Rh-123). After additional 4 h incubation, the medium was removed and the plate was rinsed with PBS to eliminate excess Rh-123 not entrapped by cells. PBS (500 μL) was added to each well and the cells were observed under the inverted fluorescence microscopy (IX-71, Olympus, Tokyo, Japan).
Pharmacokinetic studies in rats
Animal experiments were conducted in compliance with the national regulations and approved by the local ethical committee for animal experimentation. Pharmacokinetic studies were performed in male Wistar rats (Experimental Animal Center of Shandong University, Jinan, China) weighting 200–250 g. The rats were randomly divided into four groups. The first group received intravenously a dose (6 mg/kg) of commercial Taxol. Besides, Taxol, PTX-loaded NaC micelles and PTX-loaded F127–CS/NaC micelles were intragastrically administered to the other groups at a dose of 20 mg/kg, respectively. Blood samples (0.5 mL) were collected at different times in heparinized tubes. The plasma was obtained by centrifugation at 4000 rpm for 15 min immediately and the supernatant plasma fractions were stored at −20 °C until HPLC analysis.
Quantification of PTX in plasma samples by HPLC
The concentration of PTX in rat plasma was determined by HPLC method as described above. Briefly, 0.8 mL of methanol–acetonitrile (1:1, v/v) was added to 0.2 mL plasma samples, vortexed for 5 min and then centrifuged for 15 min at 10 000 rpm. The upper organic layers were evaporated to dryness under a gentle flow of nitrogen at 45 °C and then the residue was reconstituted in 100 μL acetonitrile and vortexed for 5 min before filtered by 0.22 µm nylon filter. A 20 μL aliquot of each sample was injected into the HPLC column for analysis. Calibration curve for the concentration range of 0.01–2 μg/mL was A = 87.556 C − 0.0922 (r2 = 0.9994). The average recovery was (75.57 ± 5.75)% and the coefficients of variation (CV) for within and between days were 1.92% and 2.46%, respectively. The absolute bioavailability (F%) of PTX from different oral formulations was examined as the following equation:
where AUCoral and AUCi.v. were the areas under the plasma concentration–time curve of PTX for the oral and intravenous administration, respectively; Di.v. and Doral were the dosages of PTX for the intravenous and oral administration, respectively.
Statistical analysis
Results are given as mean ± S.D. Statistical analysis was performed using Student’s t-test. Pharmacokinetic parameters were calculated using a non-compartmental model by the software of Drug and Statistics (Chinese Pharmacological Society, Beijing, China), version 2.0 (DAS). Statistical significance was tested by two-tailed Student's t-test. Statistical significance was set at p < 0.05.
Results and discussions
Characterization of synthetic polymers
Poly (acrylic acid) (PAA) was conjugated on both termini of F127 copolymer to produce F127–PAA as schematically presented in . presents the FT-IR spectra of PAA, F127 and F127–PAA. The spectrum of F127 clearly displayed peaks at 1112.10 cm−1 which was assigned to C–O–C stretching. The typical spectrum of PAA revealed the broad C = O stretching at 1709.76 cm−1. After grafting PAA onto F127, the characteristic peaks of both F127 and PAA for C–O–C stretching and C = O stretching were at 1109.84 and 1723.89 cm−1, respectively.
Figure 1. Schematic diagram of the synthesis routes of F127–PAA copolymer (A) and F127–CS copolymer (B).
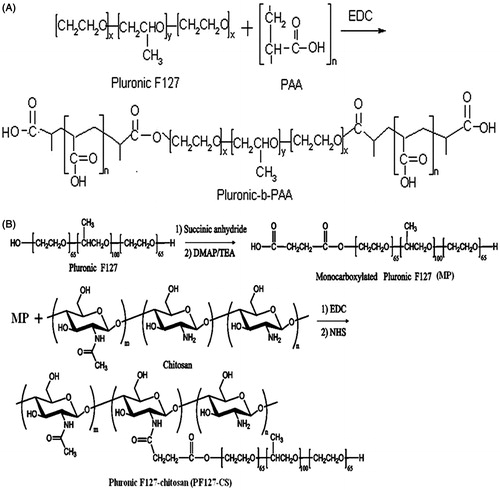
Figure 2. FT-IR spectra of F127, PAA and F127–PAA (A) and FT-IR spectra of F127, CS and F127–CS (B).
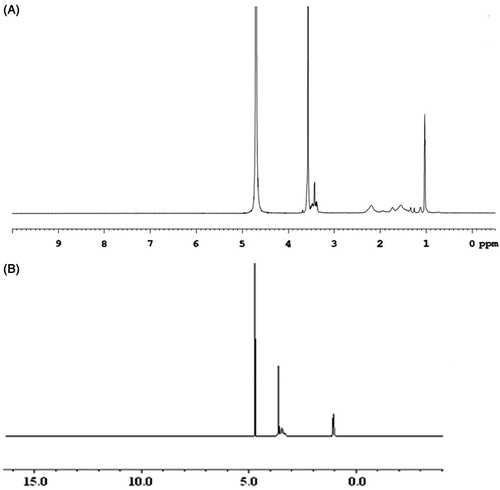
presents the 1H NMR spectra of F127–PAA in DMSO. Peaks at 1.13 ppm belong to the protons of PPO unit in F127. The sharp peak at 3.36 ppm is attributed to the protons of PEO unit in F127. Peak at 2.19 ppm is assigned to methylene protons of PAA. The results confirmed the successful grafting of PAA onto F127 (Lin et al., Citation2009).
As shown in , the F127–CS copolymers were synthesized using succinic anhydride as the bridge molecules between F127 and chitosan.
As shown in , the FT-IR spectrum of F127–CS displayed the peak at 1736.16 cm−1 which was assigned to C = O stretching vibration of carbonyl group. The results demonstrated the conjugation of Pluronic onto chitosan backbone by the formation of amide bond compared with spectra of chitosan and Pluronic. The 1H NMR spectra in DMSO were also used to study the chemical structure of F127–CS copolymer. showed the peaks at δ (ppm) = 1.12 (d, 3H, –CH3 of PPO), 3.41–3.65 (m, 3H, 4H, –CH2CHO– of PPO and –CH2CH2O– of PEO), 2.12 and 3.11 (–CH2NH of CS). These results indicated that the F127–CS copolymer was synthesized (Park et al., Citation2008).
Characterization of PTX-loaded micelles
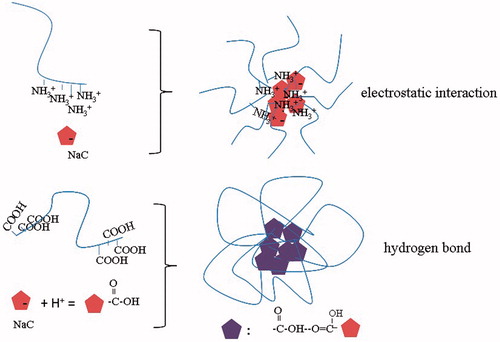
Cholate is a very useful building block for synthesizing biocompatible polymers due to its unique facial structure and amphiphilicity. Oligomers of cholate have been employed as functional molecular containers for caging hydrophobic molecules; however, their application in drug delivery is very limited mainly because of low aggregation number induced by the steric hindrance of the large steroidal skeleton. To solve this problem, in present work two types of polyion complex micelles were constructed through electrostatic interaction and hydrogen-bond ().
The TEM photographs of PTX-loaded micelles under different pH values are shown in . As shown in , PTX-loaded F127–PAA/NaC micelles were disintegrated at pH 1.2 and pH 6.8 due to the poor interactions between NaC and F127–PAA and there were no apparent differences in morphology under different pH values. However, PTX-loaded F127–CS/NaC micelles at pH 6.8 revealed better morphology with spherical shape and smooth surface compared with those at pH 1.2. These differences can be explained by the difference of non-covalent interaction between NaC and F127–CS or F127–PAA. In acidic condition (pH 1.2), the structure of PTX-loaded F127–CS/NaC micelles was loose because of the poor electrostatic interaction between protonated NaC and positively charged F127–CS. At pH 6.8, the electrostatic attraction between negatively charged NaC and positively charged F127-CS made the micellar cores compact. For F127-PAA/NaC micelles, at pH 1.2, the hydrogen bonding interaction between protonated PAA and NaC was weak, thus the micelles formed were loose. At pH 6.8, the PAA chains were deprotonated and the electrostatic repulsion between them caused chain stretching.
Figure 5. TEM images of PTX-loaded F127–PAA/NaC micelles at pH 1.2 (A) and pH 6.8 (B); PTX-loaded F127–CS/NaC micelles at pH 1.2 (C) and pH 6.8 (D).

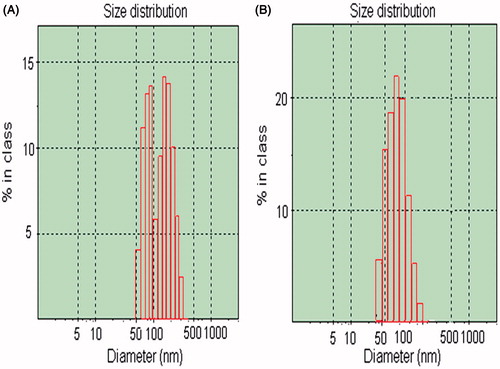
Particle diameters of the micelles were measured using DLS. As shown in the average diameter of F127–CS/NaC micelles was about 67.5 nm and size distribution exhibited a unimodal pattern at pH 6.5. However, for PTX-loaded F127–PAA/NaC micelles, the average diameter was about 85.89 nm and size distribution exhibited a non-unimodal pattern at pH 4 (). These differences were perhaps due to the difference of non-covalent interaction between NaC and F127–CS or NaC and F127–PAA. In the case of F127–CS/NaC micelles, the electrostatic interaction was strong enough to form F127–CS/NaC complex material and the polyion complex micelles were formed in aqueous solution. But the hydrogen-bond between F127–PAA and NaC was too weak and the micelle structure formed was loose in aqueous solution, leading to non-unimodal pattern of size distribution.
Figure 6. The size and size distribution of PTX-loaded F127–PAA/NaC micelles (A); PTX-loaded F127–CS/NaC micelles (B).
Pyrene is highly hydrophobic fluorescence marker and could preferentially migrate into the hydrophobic micelle core in aqueous solution. When the surrounding environment of pyrene changes from the polar aqueous solution to the hydrophobic micelle core, the intensities of the first and third bands change significantly in the pyrene fluorescence spectrum (Kim et al., Citation2000). The CMC values of micelles were obtained from the inflection point in the plot of pyrene fluorescence intensity versus copolymer concentration. The CMC values of F127–CS/NaC, NaC and F127–PAA/NaC micelles were 2.5 × 10−3 M, 1.8 × 10−2 M and 2.4 × 10−2 M. The lower CMC value of F127–CS/NaC micelles compared with that of NaC micelles suggested the higher stability of guaranteeing the micelles to retain a good stabilization under very dilute aqueous milieu, such as blood and body fluids before reaching the targeting site. However, the CMC value of F127–PAA/NaC micelles was almost the same as that of NaC micelles revealing the loose formation of F127–PAA/NaC micelles.
The drug-loading contents in the micelles are shown in and . The results showed that the drug-loading content of PTX in F127–CS/NaC micelles was increased by increasing NaC ratio in micelles. When the ratio was 1:1 the optimal drug-loading content was obtained (12.77%), displaying superior loading content of PTX (). In addition, at this ratio, the zeta-potential was approximately zero (−0.27 mV) () indicating the formation of micelles by the charge neutralization.
Table 1. Characteristics and drug-loading content of F127–CS/NaC micelles (n = 3).
Table 2. Characteristics and drug-loading content of F127–PAA/NaC micelles (n = 3).
For F127–PAA/NaC micelles, with the increase of NaC ratio the drug-loading content was only increased slightly, indicating that not too much NaC was incorporated into micelles due to weak hydrogen-bonds between F127–PAA and NaC.
In general, F127–CS/NaC micelles may be a promising carrier for delivery of PTX. So, the following experiments focus on F127–CS/NaC micelles.
In vitro release of PTX-loaded micelles
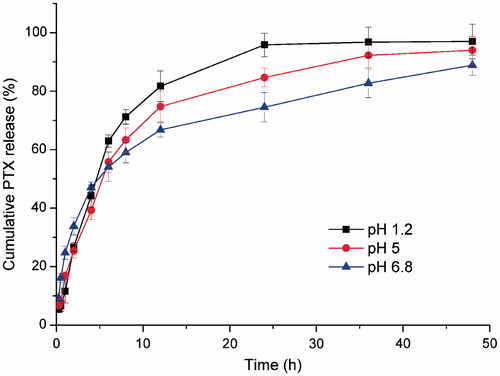
Drug release from its dosage forms is commonly evaluated under sink conditions and it has been recommended as a rule of thumb that the drug concentration in a release medium must be kept below 10% of saturation to maintain a sink condition (Cho et al., Citation2004). The solubility of PTX could be increased prominently in the release medium with the presence of 1% w/v Tween 80 (24.1 μg/mL). A 50 mL volume of the release medium can dissolve up to 1205 μg of PTX, which is significantly greater than the amount of the drug (250 μg) in formulations under examination. Therefore, the sink condition was maintained throughout the release experiments. In vitro release behavior of PTX from micelles was performed in SGF (pH 1.2), SIF (pH 6.8) and PBS (pH 5) used to simulate the environment of upper small intestine. As shown in , only 66.8% of PTX was released in SIF media, while the released PTX from F127–CS/NaC micelles in 12 h in SGF media and PBS (pH 5) were increased to 81.7% and 74.8%, respectively. The difference can be explained by the electrostatic interaction between F127–CS and NaC. In acidic condition (pH 1.2 and pH 5), the protonated NaC has a weaker interaction with the positively charged F127–CS and subsequently the disassembly of the micelles accelerates the release of PTX from the micelles. At basic condition (pH 6.8), NaC is deprotonated and negatively charged, whereas F127–CS segments remain positively charged. The electrostatic attraction between F127–CS and NaC makes the micellar cores compact and thus retards the drug release. This fact suggested that F127–CS/NaC micelles were more stable in SIF media and could transport the drug across the mucus layer and get to the enterocytes.
In vitro cytotoxicity
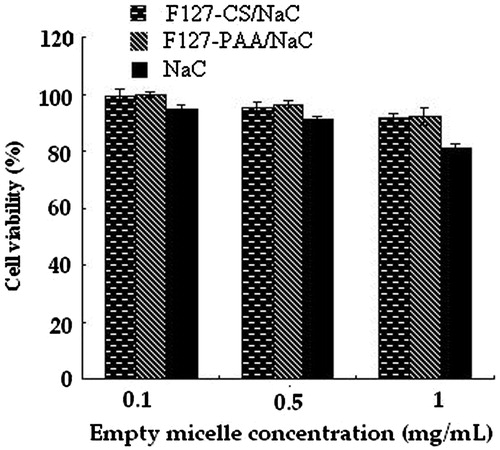
The in vitro cytotoxicity of blank F127–CS/NaC polyion complex micelles and F127–PAA/NaC micelles were investigated compared with NaC micelles with different concentrations on MCF-7/Adr cells. The viability of the cells was quantified using MTT assay. As shown in , a dose-dependent cytotoxicity was observed and the viabilities of MCF-7/Adr cells after 48 h incubation with F127–CS/NaC and F127–PAA/NaC micelles have less change compared with those of NaC micelles and above 90% of the MCF-7/Adr cells were still alive at the concentration of 1 mg/mL. These results indicated that blank F127–CS/NaC and F127–PAA/NaC micelles do not exhibit apparent toxicity, and are in accordance with the previous research (Godbey & Mikos, Citation2001). The cytotoxicity of NaC was decreased significantly by modification of F127 and formation of micelles.
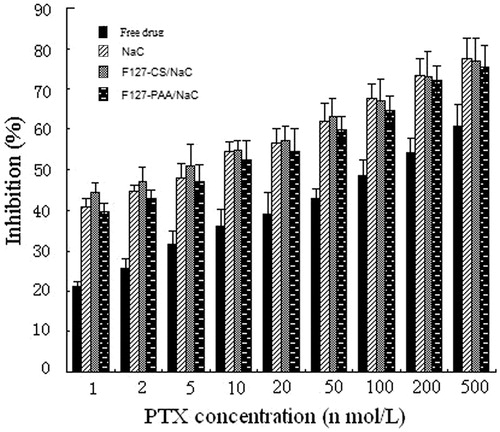
The anti-tumor activity of the PTX-loaded micelles was evaluated by the MTT method using MCF-7/Adr cells. For comparison, the cytotoxicity of free PTX (dissolved in DMSO and diluted by distilled water, DMSO < 1%, v/v) was also evaluated. The IC50 values for free PTX, NaC micelles, F127–PAA/NaC micelles and F127–CS/NaC micelles were found to be 129, 7.1, 9.8 and 4.6 nmol/L, respectively and suggested that PTX-loaded micelles displayed higher cytotoxicity compared with that of the free drug. This could be explained by the enhanced solubility of PTX in the micelle solution and inhibition of P-gp efflux due to the presence of Pluronic in micelles.
In addition, compared with NaC and F127–PAA/NaC micelles, the PTX-loaded F127–CS/NaC micelles demonstrated a highest cytotoxicity in low concentrations, while in high concentrations there was no significantly difference among F127–CS/NaC, F127–PAA/NaC and NaC micelles (). This phenomenon was perhaps due to the different stability of micelles. F127–CS/NaC micelles had a lower CMC value and could still remain the integrity in low concentration compared with F127–PAA/NaC and NaC micelles. PTX in F127–CS/NaC micelles could be taken into MCF-7/Adr cells better than that in F127–PAA/NaC and NaC micelles, thus made more drugs into the tumor cells and performed a better anti-cancer effect in low concentration.
In vitro cellular uptake of Rh-123-loaded micelles
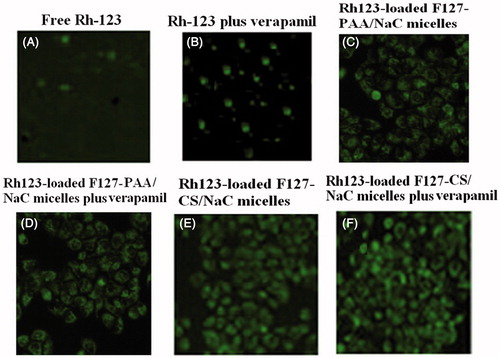
Rh-123, a fluorescent probe, is a substrate for P-gp and can, therefore, be used as a marker for P-gp activity in cells (Dabholkar et al., Citation2006). Fluorescence microscopy (FM) was employed to qualitatively observe the internalization process as a function of time in MCF-7/Adr cells cultured with free Rh-123, Rh-123 plus verapamil, Rh-123 loaded F127–PAA/NaC micelles, Rh-123 loaded F127–PAA/NaC plus verapamil, Rh-123 loaded F127–CS/NaC micelles and Rh-123 loaded F127–CS/NaC micelles plus verapamil (100 μM) (). As can be seen, the fluorescence intensity of free Rh-123 was higher in the existence of verapamil (nonspecific P-gp inhibition) than that without verapamil due to the P-gp efflux pump inhibition effect of verapamil. And cellular uptake of Rh-123 loaded in F127–PAA/NaC and F127–CS/NaC micelles also increased compared with free Rh-123 indicating that incorporating into micelles could enhance the drug accumulation in cells. More importantly, no difference in cellular uptake of Rh-123 loaded in F127–PAA/NaC and F127–CS/NaC micelle with or without verapamil was observed. This suggested that micelles modified by F127 exerted a comparable P-gp inhibition in contrast to verapamil. Moreover, compared with F127–PAA/NaC micelles, F127–CS/NaC micelles showed a marked increase of cellular accumulation. Therefore, F127–CS/NaC micelles may be a promising carrier for oral delivery of PTX for cancer therapy.
Figure 10. Fluorescence images of MCF-7/Adr cells after incubation for 2 h with Rh-123 solution (A), Rh-123 solution with verapamil (B), Rh-123-loaded F127–PAA/NaC micelles (C), Rh-123-loaded F127–PAA/NaC micelles with verapamil (D), Rh-123-loaded F127–CS/NaC micelles (E), Rh-123-loaded F127–CS/NaC micelles with verapamil (F).
In vivo pharmacokinetic studies in rats
For the pharmacokinetic study, Taxol® and PTX-loaded micelles were intravenously or intragastrically administered to rats. Plasma concentration–time profiles are shown in and the main pharmacokinetic parameters are summarized in .
Figure 11. PTX plasmatic levels after intravenous administration (6 mg/kg) of Taxol® (A) and intragastric administration (20 mg/kg) of Taxol® and PTX-loaded micelles (B) (n = 3).
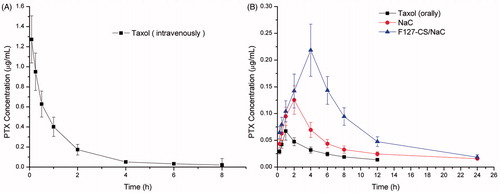
Table 3. Plasma pharmacokinetic parameters of PTX in rats following a single i.v. administration of Taxol (6 mg/kg) and intragastric administration of Taxol and PTX-loaded micelles (20 mg/kg).
When commercial Taxol® was administered to rats by the intragastric route, the area under the concentration–time curve of PTX, AUC was 0.429 mg/L h. While the AUC of PTX-loaded NaC micelles and F127–CS/NaC micelles treated groups increased to about 1.262 and 1.859 mg/L h, and the absolute bioavailability (F%) was increased to 25.74 and 37.91, about 2.94-fold and 4.33-fold higher than that of the Taxol® group after intragastric administration, respectively. Further, PTX-loaded F127–CS/NaC micelles demonstrated higher oral bioavailability in comparison with PTX-loaded NaC micelles. In our opinion, the efficient oral absorption of PTX is associated with the properties of the stabilized micelles. Based on the result from the anti-tumor activity of the PTX-loaded micelles, it could be considered that the lower CMC value of F127–CS/NaC micelles enables micelle penetration through the mucus layer, facilitating their transport to the absorptive surface of the enterocytes. On the other hand, Pluronics inhibit functioning of P-gp responsible for pumping chemotherapeutic drugs out of the enterocytes, thus enhancing the transportation of drug across the intestinal epithelium and improving the oral absorption of drug.
Conclusion
We have successfully synthesized Pluronic–chitosan (F127–CS) and Pluronic–poly (acrylic acid) (F127–PAA) copolymers and prepared two types of PTX-loaded micelles through ionic interactions and hydrogen-bonding, respectively. The F127–CS/NaC micelle has a higher drug-loading capacity and lower CMC value than F127–PAA/NaC micelles. And F127-CS/NaC micelles were more stable in SIF media. Moreover PTX-loaded F127-CS/NaC micelles displayed a great efficiency in inhibiting the growth of drug-resistant breast cancer MCF-7 cells and the polymer was biocompatible. The oral bioavailability of PTX-loaded F127–CS/NaC micelles was greatly improved. Therefore, F127–CS/NaC micelle may be a promising carrier for oral delivery of PTX for cancer therapy.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by Natural Science Foundation for Young Scholar of Shandong Province (ZR2013HQ011) and Scientific Research Foundation for Returned Scholars, Ministry of Education of China.
References
- Agueros M, Zabaleta V, Espuelas S, et al. (2010). Increased oral bioavailability of paclitaxel by its encapsulation through complex formation with cyclodextrins in poly(anhydride) nanoparticles. J Control Release 145:2–8
- Cha M-H, Choi J, Choi BG, et al. (2011). Synthesis and characterization of novel thermo-responsive F68 block copolymers with cell-adhesive RGD peptide. J Colloid Interf Sci 360:78–85
- Cho YW, Lee J, Lee SC, et al. (2004). Hydrotropic agents for study of in vitro paclitaxel release from polymeric micelles. J Control Release 97:249–57
- Dabholkar RD, Sawant RM, Mongayt DA, et al. (2006). Polyethylene glycol–phosphatidylethanolamine conjugate (PEG–PE)-based mixed micelles: some properties, loading with paclitaxel, and modulation of P-glycoprotein-mediated efflux. Int J Pharm 315:148–57
- Godbey WT, Mikos AG. (2001). Recent progress in gene delivery using non-viral transfer complexes. J Control Release 72:115–25
- Gong J, Chen M, Zheng Y, et al. (2012). Polymeric micelles drug delivery system in oncology. J Control Release 159:312–23
- Kataoka K, Harada A, Nagasaki Y. (2001). Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev 47:113–31
- Kim JH, Domach MM, Tilton RD. (2000). Effect of electrolytes on the pyrene solubilization capacity of dodecyl sulfate micelles. Langmuir 16:10037–43
- Kuang H, Wu S, Xie Z, et al. (2012). Biodegradable amphiphilic copolymer containing nucleobase: synthesis, self-assembly in aqueous solutions, and potential use in controlled drug delivery. Biomacromolecules 13:3004–12
- Kumar S, Mahdi H, Bryant C, et al. (2010). Clinical trials and progress with paclitaxel in ovarian cancer. Int J Women Health 2:411–27
- Lin J-J, Chen J-S, Huang S-J, et al. (2009). Folic acid–Pluronic F127 magnetic nanoparticle clusters for combined targeting, diagnosis, and therapy applications. Biomaterials 30:5114–24
- Liu J-S, Wang J-H, Zhou J, et al. (2014). Enhanced brain delivery of lamotrigine with Pluronic (R) PI23-based nanocarrier. Int J Nanomed 9:3923–35
- Luo Y-L, Yuan J-F, Shi J-H, Gao Q-Y. (2010). Synthesis and characterization of polyion complex micelles and their controlled release of folic acid. J Colloid Interf Sci 350:140–7
- Maswal M, Dar AA. (2013). Mixed micelles of sodium cholate and Brij30: their rheological behaviour and capability towards solubilization and stabilization of rifampicin. Colloids Surf A 436:704–13
- Mo R, Jin X, Li N, et al. (2011). The mechanism of enhancement on oral absorption of paclitaxel by N-octyl-O-sulfate chitosan micelles. Biomaterials 32:4609–20
- Nishiyama N, Kataoka K. (2006). Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery. Pharmacol Ther 112:630–48
- Nornoo AO, Zheng H, Lopes LB, et al. (2009). Oral microemulsions of paclitaxel: in situ and pharmacokinetic studies. Eur J Pharm Biopharm 71:310–17
- Pandey V, Gajbhiye KR, Soni V. (2015). Lactoferrin-appended solid lipid nanoparticles of paclitaxel for effective management of bronchogenic carcinoma. Drug Deliv 22:199–205
- Park KM, Bae JW, Joung YK, et al. (2008). Nanoaggregate of thermosensitive chitosan–pluronic for sustained release of hydrophobic drug. Colloids Surf B Biointerfaces 63:1–6
- Russell-Jones GJ. (2004). Use of targeting agents to increase uptake and localization of drugs to the intestinal epithelium. J Drug Target 12:113–23
- Simoes SMN, Figueiras AR, Veiga F, et al. (2015). Polymeric micelles for oral drug administration enabling locoregional and systemic treatments. Expert Opin Drug Deliv 12:297–318
- Subuddhi U, Mishra AK. (2007). Micellization of bile salts in aqueous medium: a fluorescence study. Colloids Surf B Biointerfaces 57:102–7
- Sugioka H, Moroi Y. (1998). Micelle formation of sodium cholate and solubilization into the micelle. Biochim Biophys Acta 1394:99–110
- Vader P, Fens MHAM, Sachini N, et al. (2013). Taxol (R)-induced phosphatidylserine exposure and microvesicle formation in red blood cells is mediated by its vehicle Cremophor (R) EL. Nanomedicine 8:1127–35
- Wang X, Chen Y, Dahmani FZ, et al. (2014). Amphiphilic carboxymethyl chitosan-quercetin conjugate with P-gp inhibitory properties for oral delivery of paclitaxel. Biomaterials 35:7654–65
- Yang C, Attia ABE, Tan JPK, et al. (2012). The role of non-covalent interactions in anticancer drug loading and kinetic stability of polymeric micelles. Biomaterials 33:2971–9
- Yang T-F, Chen C-N, Chen M-C, et al. (2007). Shell-crosslinked Pluronic L121 micelles as a drug delivery vehicle. Biomaterials 28:725–34
- Yao H-J, Ju R-J, Wang X-X, et al. (2011). The antitumor efficacy of functional paclitaxel nanomicelles in treating resistant breast cancers by oral delivery. Biomaterials 32:3285–302