Abstract
Using nanoparticle delivery for anticancer therapy is a potential new drug modality. We developed a novel gelatinase–stimuli nanoparticle. In this study, we studied the antitumor and antimetastasis effect of pemetrexed-loaded targeted nanoparticles and evaluated the correlation between E-cadherin expression and lung metastasis in subcutaneous xenograft model. Compared with free pemetrexed, pemetrexed-loaded targeted nanoparticles exhibited the best antitumor and antimetastasis efficacy among the four therapeutic groups. The study also indicated that there was an inverse correlation between lung metastasis and E-cadherin expression. These results showed pemetrexed-loaded targeted nanoparticles may be a potent drug for tumor therapy and our preclinical data could provide new direction for clinical therapy of malignant melanoma.
Introduction
Active targeting nanoparticle delivery systems have become a potential new drug modality with the opportunity of enhanced water solubility, constant and stable release of the drug, tumor-specific accumulation, improved antitumor efficacy and reduced non-specific toxicity (Chen, Citation2010).
Metastasis is still the primary cause of severe morbidity and mortality in cancer. Matrix metalloproteinases (MMPs) play an important role in cancer progression, including tumor growth, invasion, metastasis and angiogenesis (Gialeli et al., Citation2011). For this reason, treatment methods aiming at MMPs have great significance (Coussens et al., Citation2002). However, traditional MMP inhibitors (MMPIs) do not obtain ideal effects in clinical experiments whether as a single treatment or combined with other chemotherapeutics (Brown, Citation2000; Bramhall et al., Citation2001). More effort must be made to identify novel MMPIs and evaluate the antitumor effect of these new methods. Therefore, gelatinases may be suitable candidates for stimuli-responsive targeted strategies.
In the previous work, we developed the gelatinase–stimuli nanoparticles for the improvement of anticancer efficacy. The new nanoparticle consisted of gelatinase-cleavage peptide and poly(ethylene glycol) (PEG) and poly(ɛ-caprolactone) (PCL)-based structure (Lu et al., Citation2012; Liu et al., Citation2012, Citation2013; Li et al., Citation2013; Cui et al., Citation2014a,b; Wu et al., Citation2015). Nanoparticles characterization, in vitro cellular uptake, real-time biodistribution of nanoparticles and in vivo antitumor efficacy of docetaxel-loaded nanoparticles were systemically studied (Lu et al., Citation2012; Liu et al., Citation2012, Citation2013; Li et al., Citation2013; Cui et al., Citation2014a,b; Wu et al., Citation2015). Compared with PEG–PCL nanoparticles, the targeted nanoparticles showed higher intracellular uptake efficiency, higher local tumor accumulation and retention for long observation, in vivo antitumor superiority (Lu et al., Citation2012; Liu et al., Citation2012, Citation2013; Li et al., Citation2013; Cui et al., Citation2014a,b; Wu et al., Citation2015).
Pemetrexed disodium is a novel antifolate medicine with multiple enzyme targets and shows encouraging activity in a wide range of tumors. However, therapeutic effect of pemetrexed is limited because of the side effects such as anemia, nausea, fatigue, diarrhea and stomatitis (Manegold et al., Citation2000; Miles et al., Citation2001; Hughes et al., Citation2002). To avoid toxicity, we established the pemetrexed-loaded targeted nanoparticles and evaluated the characterizations, drug loading content and encapsulation efficiency, security and release of the pemetrexed-loaded nanoparticles (Lu et al., Citation2012). In the present studies, the pemetrexed-loaded PEG–Peptide–PCL nanoparticles showed superior antimetastatic effect in experimental pulmonary model in vivo (Lu et al., Citation2012). In this study, we assessed the anticancer and antimetastasis effect of pemetrexed-loaded nanoparticles in subcutaneous xenograft and spontaneous pulmonary metastasis model and further examined the correlation between E-cadherin expression and lung metastasis.
Materials and methods
Materials
The pemetrexed disodium in the experiments was provided by Eli Lilly Company (Indianapolis, IN). Methoxy-polyethyleneglycol–NHS (mPEG–NHS) was purchased from Beijing Jiankai Technology Co. (Beijing, China). The gelatinase-cleavable peptide (H2N-PVGLIG-COOH) was purchased from Shanghai HD Biosciences Co. (Shanghai, China). ɛ-Caprolactone (ɛ-CL, Aldrich, Saint Louis, MO) and dimethyl formamide (DMF) was purified by drying over CaH2 at room temperature and extracted under reduced pressure. Gelatinase was purchased from Sigma–Aldrich (Saint Louis, MO), DMEM culture from Gibco (Carlsbad, CA) and Cell Counting Kit-8 from Dojindo (Dojindo, Japan). All the other chemicals were of analytical grade and used without further purification. Murine melanoma cell line B16 was obtained from Shanghai Institute of Cell Biology (Shanghai, China). Male C57B6 mice were purchased from the animal center of Drum Tower Hospital, Medical School of Nanjing University (Nanjing, China).
Synthesis of PEG–Peptide–PCL nanoparticles and PEG–PCL nanoparticles
Synthesis of PEG–Peptide conjugate
The PEG–Peptide–PCL nanoparticles were synthesized for pemetrexed-loaded PEG–Peptide–PCL nanoparticles as follows (Lu et al., Citation2012): predetermined amounts of mPEG–NHS and peptide were dissolved in dimethylformamide (DMF) containing 3% triethanolamine and the mixture was stirred for 3 h at room temperature. The obtained solution was filtered by filter membrane (3500 molecular weight cutoff, Sigma, USA) for 24 h to remove the non-reacted peptide.
Amination of PCL–COOH
Predetermined amount of CL and l-leucine were added into a polymerization tube and the tube was sealed off and placed in an oil bath at 160 °C for 24 h. The complex was dissolved with dichloromethane (DCM) and was precipitated with a large amount of cold ethyl ether to get PCL–COOH. The DMF solution containing PCL–COOH, 4-dimethylaminopryidine (DMAP), was stirred in a water bath at 37 °C for 18 h to get PCL–NH2. The solution was precipitated with ethanol and then filtered before dried in vacuum.
Synthesis of PEG–Peptide–PCL nanoparticles and PEG–PCL nanoparticles
The predetermined amount of PCL–NH2, PEG–Peptide, with the 1-ethyl-3-(3-dimethylaminopropy1) carbodiimide hydrochloride and N-hydroxysulfosuccinimide sodium salt (NHS) were mixed with DMF at 32 °C overnight. The obtained solution was filtered by filter membrane (13 000 molecular weight cutoff, Sigma, USA) to remove the non-reacted monomer and oligomer. The resulting solution was freeze dried (FreeZone6, Labconcon, Kansas City, MO) and stored at 4 °C. The PEG–PCL copolymers were synthesized for pemetrexed-loaded PEG–PCL nanoparticles by the method previously described (Li et al., Citation2009; Lu et al., Citation2012).
Preparation of pemetrexed-loaded nanoparticles
Pemetrexed-loaded nanoparticles were prepared according to a previously described method (Lu et al., Citation2012). Briefly, a certain amount of vehicle and pemetrexed were dissolved in 1 ml of DCM. The mixture was emulsified in 3 ml of 5% PVA solution (w/v) by sonication (XL2000, Misonix, Farmingdale, NY) for 30 s and was converted into an o/w emulsion. The obtained emulsion was added into 8 ml of 1% PVA solution (w/v) and handled by sonication again, then stirred to remove DCM at room temperature in a fume cupboard. The resulting solution was filtered through filter membrane to remove non-incorporated drugs and copolymer aggregates. The nanoparticles solution was freeze dried with 3% mannitol and saved at 4 °C. Drug-free nanoparticles were produced in a similar way without adding pemetrexed.
In vivo antitumor effect in B16 bearing mice
The animal experiments were performed following the guidelines in the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No 85-23, revised 1985) and was approved by the Ethics Review Board for Animal Studies of Drum Tower Hospital, Medical School of Nanjing University (DTH ERMA 66.01/210 A/2011).
The six-week-old male C57/B6 mice were raised under specific pathogen-free circumstances. About 1 × 106 cells were implanted in the right armpit of each mouse and the day was designated as “Day 0”. At day 3, mice were randomized into five groups and each group included six mice. The mice were treated i.v. with saline, empty nanoparticles, free pemetrexed, pemetrexed-loaded PEG–PCL nanoparticles (common nanoparticles) and pemetrexed-loaded PEG–Peptide–PCL nanoparticles (targeted nanoparticles) twice on days 3 and 6, respectively. The pemetrexed solution was administered at the dose of 10 mg/kg. The pemetrexed-loaded nanoparticles were administered as a saline solution at the equivalent pemetrexed dose of 10 mg/kg. Tumor volume was measured every day and calculated by the formula: 0.52 × length × width2. Mice also were weighed every day during the experiment.
In vivo antimetastatic study in subcutaneous xenograft model
About 1 × 106 cells were implanted in the right armpit of each C57/B6 mouse and the day was designated as “Day 0”. At day 9, mice were randomized into five groups and each group included six mice. The mice were treated i.v. with saline, empty nanoparticles, free pemetrexed, pemetrexed-loaded PEG–PCL nanoparticles and pemetrexed-loaded PEG–Peptide–PCL nanoparticles twice on days 9 and 13, respectively. The pemetrexed solution was administered at dose of 10 mg/kg. The pemetrexed-loaded nanoparticles were administered as a saline solution at the equivalent pemetrexed dose of 10 mg/kg. All the mice were euthanized on day 16 and the lung and liver tissues were removed for histologic examination.
Hematoxylin and eosin (H&E) staining
The lung and liver tissues were obtained after the animal experiment ended. The tissues were fixed in buffered formalin overnight followed by dehydration with tissue processor for 16 h. The tissues were embedded by paraffin. The 7 µm sections were prepared for H&E staining.
Immunohistochemistry
After B16 tumor samples were fixed by formalin and embedded using embedding equipment, 4 mm sections were prepared. The sections were baked at 65 °C for 60 min, followed by 10-min washes with xylene. The rehydration of tissues was performed by 5-min washes in 100%, 95%, 85% and 70% ethanol and distilled water, respectively. Antigen retrieval was performed by heating the samples at 100 °C for 20 min in 10 mmol/l sodium citrate (pH 6.0). Endogenous peroxidase activity of the tissue was blocked by incubation in 3% hydrogen peroxide in methylalcohol for 15 min at 37 °C. The sections were incubated with rabbit anti-mouse polyclonal antibody (Wuhan Boster Biotechnology, Wuhan, China) at 4 °C overnight. The sections were then incubated for 30 min with the secondary antibody (PV-6000 HRP DAB Detection Kit, ZSGB-BIO Biotechnology, Beijing, China). Subsequently, the samples were redyed by hematoxylin. Dehydration was then performed following a standard procedure, and the slides were sealed with cover slips.
RNA extraction and RT-PCR analysis
The RNA of lung and liver sections was extracted according to a previously described method (Specht et al., Citation2001). The samples were resuspended in 300 µl RNA lysate [10 mM Tris–HCl (pH 8.0), 0.1 mM EDTA (pH 8.0), 2% SDS (pH 7.3) and 500 µg/ml proteinase K] for 16 h at 60 °C. The RNA was extracted by chloroform and phenol. The siopropanol and sodium acetate was used for precipitation at −20 °C for 1 h. The RNA was washed with 70% ethanol and dissolved in 50 µl of RNase-free water. The RNA was reverse-transcribed to cDNA with reverse-transcriptase (Takara, Japan). Quantitative PCR was performed by SYBR green qPCR kit (Takara, Japan) by a fluorescent temperature cycler (Mx3000P Real-Time PCR System, Stratagene). The target cDNA sequences were amplified with the following primers: GAPDH 5′-AAATGGTGAAGGTCGGTGTG-3′ and 5′-TGAAGGGGTCGTTGATGG-3′; E-cadherin 5′-GCACTCTTCTCCTGGTCCTG-3′ and 5′-TATGAGGCTGTGGGTTCCTC-3′. The following PCR conditions were used: denaturation at 95 °C for 1 min, followed by 40 cycles of denaturation at 95 °C for 30 s, annealing and extension at 60 °C for 1 min. Each real-time PCR was repeated 3 times and the level of mRNA expressions were calculated according to the comparative Ct method using GAPDH as an internal standard. The results were analyzed by the 2−ΔΔCt method (Livak & Schmittgen, Citation2001).
Statistical analysis
Analysis was carried out as means ± standard differentiation (SD). Differences within the treatment groups were presented by two-factor analysis of variance methods and T-test. Differences were considered significant when p < 0.05.
Results
Pemetrexed-loaded PEG–Peptide–PCL nanoparticles suppress subcutaneous tumor growth
depicts the experimental design of the subcutaneous transplantation tumor model. depicts the changes of tumor volume by i.v. administration in B16 tumor-bearing mice. Growth of tumor mass was rapid and no antitumor effect was observed in the empty nanoparticles group and saline group. Significant difference was not observed between the group of free pemetrexed and saline. As shown in , two kinds of pemetrexed-loaded nanoparticles effectively inhibited tumor growth and pemetrexed-loaded PEG–Peptide–PCL nanoparticles exhibited more efficient antitumor efficacy than pemetrexed-loaded PEG–PCL nanoparticles by delaying tumor growth. The difference of tumor volume between the groups of pemetrexed-loaded PEG–PCL nanoparticles and saline was significant (p < 0.05). The difference of tumor volume between the group of pemetrexed-loaded PEG–Peptide–PCL nanoparticles and saline was highly significant (p < 0.01) at the equivalent dose.
Figure 1. Pemetrexed-loaded PEG–Peptide–PCL nanoparticles inhibited subcutaneous tumor growth. (A) Experimental design of the subcutaneous transplantation tumor model. (B) Tumor volume of established B16 xenografts in C57/B6 mice during therapy under different treatments. Empty np: empty nanoparticles; common np: pemetrexed-loaded PEG–PCL nanoparticles; targeted np: pemetrexed-loaded PEG–Peptide–PCL nanoparticles. Data were presented as mean ± SD (n = 6). *p < 0.05, **p < 0.01 versus the saline group. (C) Body weight development was monitored during treatment period. Data were displayed with SD shown (n = 6). (D) Hematoxylin and eosin (H&E) staining pictures of B16 tumors (a: Magnification 40×; b: Magnification 200×).
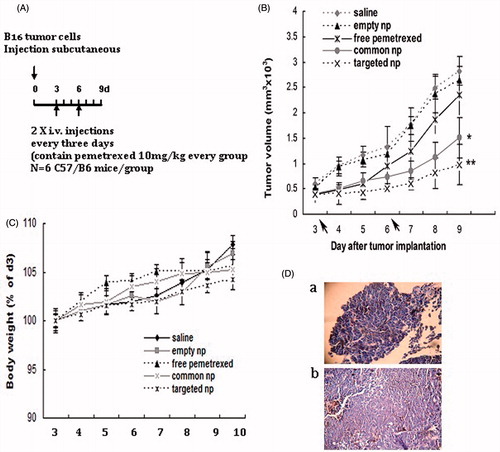
An analysis of body weight variations evaluated the adverse effects of the different therapy regiments (). For i.v. administration, pemetrexed-loaded nanoparticles and empty nanoparticles showed favorable results without any obvious body weight. These results indicated that the security of the pemetrexed-loaded nanoparticles was acceptable. shows the hematoxylin and eosin (H&E) staining pictures of B16 tumors.
Pemetrexed-loaded PEG–Peptide–PCL nanoparticles suppresses lung metastasis in subcutaneous xenograft model
Macroscopic pictures of lung tissues after treatment were shown from four treatment groups (, top panel). The mice in the saline and free pemetrexed group displayed several metastatic nodules (). Pemetrexed-loaded PEG–PCL nanoparticles treated group showed fewer visible tumor nodules by microscopic examining (). The number of metastatic nodules in the group treated with pemetrexed-loaded PEG–Peptide–PCL nanoparticles was fewest in all the treatment groups. Lung metastasis burden was calculated by counting the number of tumor nodules on the lung surface (). Significant difference was not observed between the groups of free pemetrexed and saline. The difference of metastatic nodules between the groups of pemetrexed-loaded nanoparticles and saline was highly significant (p < 0.01). The difference of metastatic nodules between the two pemetrexed-loaded nanoparticles groups was significant (p < 0.05) at the equivalent dose.
Figure 2. Pemetrexed-loaded PEG–Peptide–PCL nanoparticles inhibited pulmonary metastasis. (A) Macroscopic pictures of cancer cell affected lungs from five treatment groups (top panel) (n = 6). Tumor cells were visualized by H&E staining on paraffin-embedded tissue sections (bottom panel, black arrows indicated tumor cells; amplification: 40×). Common np: pemetrexed-loaded PEG–PCL nanoparticles; targeted np: pemetrexed-loaded PEG–Peptide–PCL nanoparticles. (B) Lung metastasis burden was calculated by counting the number of tumor nodules on the lung surface (n = 6). **p < 0.01 versus the saline group, ap < 0.05 versus the common nanoparticles group.
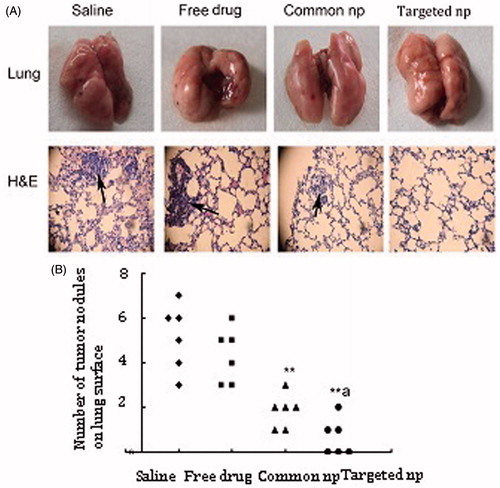
Lung microstructure was examined by hematoxylin and eosin staining (, bottom panel). Disorder of lung structure, alveolar septal thickening, inflammatory cell and red blood cell infiltration was observed in the saline group. The group treated with free pemetrexed showed slight inflammation and exudation of red blood cells in the pulmonary interstitial by histological evaluation. Additionally, the structure of pulmonary alveoli was intact and no obvious inflammation and fibrosis was observed in the pemetrexed-loaded nanoparticles groups ().
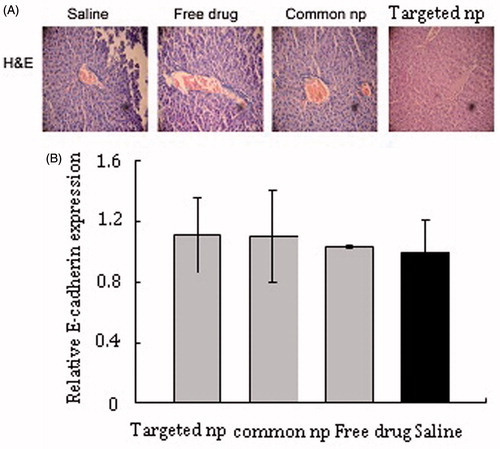
Metastatic and micrometastatic nodules were not found in liver tissues in all of the animals examined. Microstructure also was intact by histological evaluation ().
Immunohistochemical analysis
represents the expression of E-cadherin in the normal lung tissues and metastatic tumor cells. In the normal lung tissues, E-cadherin was expressed at the membrane of epithelial cells and the cell membrane was dyed pale brown. The obvious tumor cells were observed and lung metastatic tumor cells showed reduced and aberrant expression of E-cadherin between the groups of free pemetrexed and saline (). Additionally, a few metastatic tumor cells were observed in the pemetrexed-loaded targeted nanoparticles group and the targeted group showed the highest level of E-cadherin in the four treatment groups ().
Figure 3. The E-cadherin expression of lung metastatic tissues was analyzed by immunohistochemistry and RT-PCR. (A) Immunohistochemistry panel representing the expression of E-cadherin in the normal lung tissues and lung metastatic tissues from different treatment groups (n = 6, the black arrows indicated tumor cells; amplification: 400×). Common np: pemetrexed-loaded PEG–PCL nanoparticles; targeted np: pemetrexed-loaded PEG–Peptide–PCL nanoparticles. (B) The expression levels of E-cadherin of lung metastatic tissues were analyzed by RT-PCR and normalized to GAPDH in transplanted tumor model (n = 6). All data from six samples were carried out as mean and standard deviation.
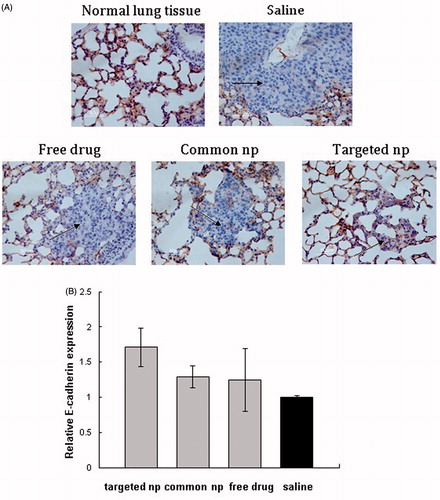
Figure 4. HE pictures and mRNA expression of E-cadherin in livers tissue samples. (A) HE pictures of livers tissue samples from corresponding treatment groups. Common np: pemetrexed-loaded PEG–PCL nanoparticles; targeted np: pemetrexed-loaded PEG–Peptide–PCL nanoparticles. (B) The expression levels of E-cadherin of liver tissues were analyzed by RT-PCR and normalized to GAPDH in transplanted tumor model (n = 6). All data from six samples were carried out as mean and standard deviation.
Molecule alterations in vivo experiment
shows the E-cadherin expression of lung tissues from different treatment groups in transplanted tumor model. The mice treated with PEG–Peptide–PCL nanoparticles showed the highest level of E-cadherin mRNA and the E-cadherin expression in the saline group was lowest of all the experiment groups, but the difference of E-cadherin expression between the PEG–Peptide–PCL nanoparticles group and the saline group was not highly significant (p > 0.05). lists the E-cadherin expression of liver tissues under different treatments in transplanted tumor model. Among the four therapeutic groups, pemetrexed-loaded nanoparticles groups produced the slightly higher expression level of E-cadherin and no significant differences in E-cadherin expression were observed among the four therapeutic groups.
Discussion
In the previous study, we established the pemetrexed-loaded targeted nanoparticles and evaluated the characterizations, drug loading content and encapsulation efficiency, security and release of nanoparticles of the pemetrexed-loaded nanoparticles (Lu et al., Citation2012). In this study, we provided novel insights into the anticancer and antimetastasis effect of pemetrexed-loaded nanoparticles in subcutaneous xenograft and spontaneous pulmonary metastasis model and further examined the correlation between E-cadherin expression and lung metastasis.
In vivo antitumor test, pemetrexed-loaded PEG–Peptide–PCL nanoparticles exhibited best antitumor efficacy in the all the treatment groups. Similar results were observed from the studies of Liu et al. (Citation2012, Citation2013), Li et al. (Citation2013). Liu et al., examined the antitumor effect of docetaxel-loaded PEG–Peptide–PCL nanoparticles through intravenous administration and the docetaxel-loaded nanoparticles formed by PEG–Peptide–PCL showed more efficient antitumor efficacy over PEG–PCL nanoparticles on H22 tumor-bearing mice (Liu et al., Citation2012, Citation2013; Li et al., Citation2013). PEG–Peptide–PCL nanoparticles contained the active peptide substrates of gelatinases and the gelatinases-responsive PEG–Peptide–PCL nanoparticles effectively targeted to the tumor tissues and cells, and exhibited distinguished antitumor potency when they were used to load chemotherapeutic agent pemetrexed and docetaxel (Liu et al., Citation2012, Citation2013; Lu et al., Citation2012; Li et al., Citation2013).
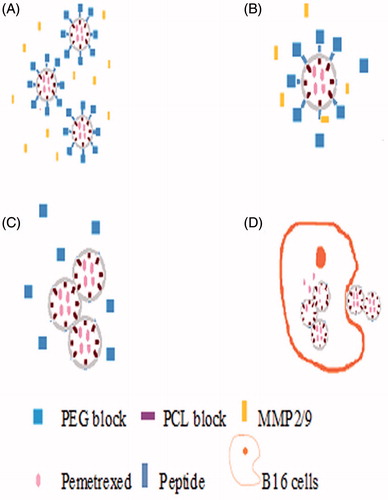
In spontaneous pulmonary metastasis model, the antimetastatic effects of targeted nanoparticles were evaluated. The present results with targeted nanoparticles in mouse models suggested a therapeutic benefit in preventing metastasis to the lung. Reasonable explanation could be deduced from the release mechanism of PEG–Peptide–PCL nanoparticles (). Many studies revealed that tumor cells secreted gelatinase and our experiments also obtained similar results by measuring the MMPs activities of B16 cells (Bai et al., Citation2010; Lu et al., Citation2012; Liu et al., Citation2012, Citation2013). The release mechanism of PEG–Peptide–PCL nanoparticles was triggered when gelatinase that B16 cells secreted encountered with vehicle. Then, peptide occurred hydrolysis as ablating of PEG fragment and exposing of PCL fragment and the vehicle continued to deform. Finally, the intake of nanoparticles was done by tumor cells and pemetrexed was released from nanoparticles, the tumor cells was killed (Lu et al., Citation2012; Liu et al., Citation2012, Citation2013; Li et al., Citation2013). The targeted nanoparticles can deliver more pemetrexed to tumor cells and exhibit more antitumor effect. Sustained release and constant plasma concentration of pemetrexed might explain the superiority of the pemetrexed-loaded nanoparticles against the free pemetrexed.
Figure 5. The release mechanism of PEG–Peptide–PCL nanoparticles. (A/B) After release from tumor capillaries, the PEG–Peptide conjugates were cleaved by gelatinases, which were specifically secreted in the tumor microenvironment. The gelatinase–stimuli strategy enhanced nanoparticles interactions with cancer cells in the tumor tissues. (C/D)The remained PCL blocks aggregated and the PEG-uncoated PCL nanoparticles interacted efficiently with cancer cells, resulting in fast drug release and effective therapeutics. PEG, poly(ethylene glycol); PCL, poly(ɛ-caprolactone).
The first description on E-cadherin was a member of a family of transmembrane glycoproteins responsible for a family of transmembrane glycoproteins responsible for calcium-dependent cell-to-cell adhesion (Thiery, Citation2002). It was known that the expression of E-cadherin was correlated with tumor grade and stage and E-cadherin played an important role as an invasion suppressor gene (Berx et al., Citation1995; Berx & Van Roy, Citation2001). Loss of E-cadherin, being correlated with tumor grade and stage, increased tumor cell metastasis (Kin et al., Citation2008; Saad et al., Citation2010). E-cadherin gene loss expression led neoplastic cells to invade neighboring tissues, as show in several models of human cancer, including breast cancer and gastric cancer (Mahler-Araujo et al., Citation2008; Becker et al., Citation1999; Carneiro et al., Citation1999; Pinheiro et al., Citation2010; Reis-Filho et al., Citation2002). Recent reports found that there seemed to be an inverse correlation between E-cadherin levels and cancer grade or patient survival, including carcinomas of the head and neck, skin, lung, liver, colon and prostate (Hirohashi, Citation1998). In our study, the expression of E-cadherin in lung metastatic tissue among the four groups was analyzed by immunohistochemical analysis and RT-PCR. Our results showed that the saline group with prominent lung metastases had the lowest E-cadherin expression level and the targeted nanoparticles group with mild lung metastases had the highest E-cadherin expression level. The results also indicated that there was an inverse correlation between lung metastasis and E-cadherin expression. Our previous results also supported the point that the downregulation of E-cadherin may be related with tumor metastasis (Liu et al., Citation2013). The group treated with miR-200c/DOC which showed significantly decreased invasive capacity of tumor cells had the highest E-cadherin expression level among all the groups (Liu et al., Citation2013).
Malignant melanoma was one of the most aggressive cancers and can disseminate from a relatively small primary tumor and metastasize to multiple sites, including the lung, liver, brain, bone and lymph nodes (Braeuer et al., Citation2014). The lung and liver were the common metastatic organs in malignant melanoma and the 5-year overall survival rate of patients with distant metastasis dropped to <10% (Balch et al., Citation2009). In this study, lung metastasis was seen differences among four groups. Hepatic metastasis was evaluated in further vivo antimetastatic study. Metastatic and micrometastatic nodules were not found in liver tissues in the experimental groups and the obvious changes of the tissues structure were also not noticed. The expression of E-cadherin in liver tissues was assessed and no significant differences in E-cadherin expression were observed among the four therapeutic groups. Reasonable explanation could be deduced from the theory of “seed and soil” (Fidler, Citation2003; Langley & Fidler, Citation2011). Tumor was described as uncontrolled cell growth that was unstable, thereby resulting in tumor heterogeneity. Recent studies found that melanoma cells generally metastasized to organs in a predictable manner and this had been termed the “seed and soil hypothesis” (Kaplan et al., Citation2006; Fokas et al., Citation2007). Cameron et al., found that there was dramatic differences in metastatic efficiency between lung tissues and liver tissues (>0.1 versus 0.0002) after injecting B16 cells by tail vein (Cameron et al., Citation2000). By injecting melanoma cells, Luzzi et al., found that very few of extravasated cells divided and formed colonies in the liver tissues (Luzzi et al., Citation1998). The research found that the rest remained cancer cells in liver tissues as solitary cancer cells and most of them were dormant, few micrometastases continued to form macroscopic tumors and most of micrometastases disappeared (Luzzi et al., Citation1998).
This article was focused on the advantages of pemetrexed-loaded PEG–Peptide–PCL nanoparticles in the antitumor and antimetastasis effect. Pemetrexed was an antifolate medicine with multiple enzyme targets and showed encouraging antitumor activity in many tumors, including non-small cell lung cancer, breast cancer, carcinoma of the cervix, head and neck cancer, ovarian cancer and gastric cancer (Argiris et al., Citation2011; Schneeweiss et al., Citation2011; Chambers et al., Citation2012; Amadori et al., Citation2013; Miller et al., Citation2014; Zhang et al., Citation2015). As the current platform can be universally applied to almost any malignant tumors, and was adaptable to load a wide range of anticancer drugs. Choosing the most suitable animal models will likely demonstrate the techniques thoroughly. The drug delivery system presented here was proposed to include the following advantages: (1) maintenance of the targeting advantages of nanoparticles, (2) superior inhibition of tumor growth and (3) effectively antimetastatic effect.
Conclusions
In the previous work, we reported new pemetrexed-loaded PEG–Peptide–PCL nanoparticles. In this article, we have provided positive data for antitumor and antimetastasis effect of targeted nanoparticles as a potential strategy. Our results provide a basis for further study of targeted nanoparticles. Our preclinical data could provide new selections and directions for future therapeutic applications.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article. This work was supported by the National Natural Science Foundation of China (No 81472216) and the Anhui Province Natural Science Foundation of China (No 1408085QH187).
References
- Amadori D, Carrasco E, Roesel S, et al. (2013). A randomized phase II non-comparative study of pemetrexed-carboplatin and gemcitabine-vinorelbine in anthracycline- and taxane-pretreated advanced breast cancer patients. Int J Oncol 42:1778–85
- Argiris A, Karamouzis MV, Gooding WE, et al. (2011). Phase II trial of pemetrexed and bevacizumab in patients with recurrent or metastatic head and neck cancer. J Clin Oncol 29:1140–5
- Bai J, Zhang J, Wu J, et al. (2010). JWA regulates melanoma metastasis by integrin alphaVbeta3 signaling. Oncogene 29:1227–37
- Balch CM, Gershenwald JE, Soong SJ, et al. (2009). Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 27:6199–206
- Becker KF, Kremmer E, Eulitz M, et al. (1999). Analysis of E-cadherin in diffuse-type gastric cancer using a mutation-specific monoclonal antibody. Am J Pathol 155:1803–9
- Berx G, Van Roy F. (2001). The E-cadherin/catenin complex: an important gatekeeper in breast cancer tumorigenesis and malignant progression. Breast Cancer Res 3:289–93
- Berx G, Cleton-Jansen AM, Nollet F, et al. (1995). E-cadherin is a tumour/invasion suppressor gene mutated in human lobular breast cancers. EMBO J 14:6107–15
- Braeuer RR, Watson IR, Wu CJ, et al. (2014). Why is melanoma so metastatic? Pigment Cell Melanoma Res 27:19–36
- Bramhall SR, Rosemurgy A, Brown PD, et al. (2001). Marimastat as first-line therapy for patients with unresectable pancreatic cancer: a randomized trial. J Clin Oncol 19:3447–55
- Brown PD. (2000). Ongoing trials with matrix metalloproteinase inhibitors. Expert Opin Investig Drugs 9:2167–77
- Cameron MD, Schmidt EE, Kerkvliet N, et al. (2000). Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Res 60:2541–6
- Carneiro F, Machado JC, Seruca R, et al. (1999). E-cadherin changes in gastric carcimona. Histopathology 35:477–8
- Chambers SK, Chow HH, Janicek MF, et al. (2012). Phase I trial of intraperitoneal pemetrexed, cisplatin, and paclitaxel in optimally debulked ovarian cancer. Clin Cancer Res 18:2668–78
- Chen, ZG. (2010). Small-molecule delivery by nanoparticles for anticancer therapy. Trends Mol Med 16:594–602
- Coussens LM, Fingleton B, Matrisian LM. (2002). Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 295:2387–92
- Cui FB, Li RT, Liu Q, et al. (2014a). Enhancement of radiotherapy efficacy by docetaxel-loaded gelatinase-stimuli PEG-Pep-PCL nanoparticles in gastric cancer. Cancer Lett 346:53–62
- Cui FB, Liu Q, Li RT, et al. (2014b). Enhancement of radiotherapy efficacy by miR-200c-loaded gelatinase-stimuli PEG-Pep-PCL nanoparticles in gastric cancer cells. Int J Nanomed 9:2345–58
- Fidler IJ. (2003). The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 3:453–8
- Fokas E, Engenhar-Cabillic R, Daniilidis K, et al. (2007). Metastasis: the seed and soil theory gains identity. Cancer Metastasis Rev 26:705–15
- Gialeli C, Theocharis AD, Karamanos NK. (2011). Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J 278:16–27
- Hirohashi S. (1998). Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol 153:333–9
- Hughes A, Calvert P, Azzabi A, et al. (2002). Phase I clinical and pharmacokinetic study of pemetrexed and carboplatin in patients with malignant pleural mesothelioma. J Clin Oncol 20:3533–44
- Kaplan RN, Rafii S, Lyden D. (2006). Preparing the “soil”: the premetastatic niche. Cancer Res 66:11089–93
- Kin JH, Park JM, Jung CW, et al. (2008). The significances of lymph node micrometastasis and its correlation with E-cadherin expression in pT1-T3N0 gastric adenocarcinoma. J Surg Oncol 97:125–30
- Langley RR, Fidler IJ. (2011). The seed and soil hypothesis revisited – the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer 128:2527–35
- Li R, Li X, Xie L, et al. (2009). Preparation and evaluation of PEG-PCL nanoparticles for local tetradrine delivery. Int J Pharm 379:158–66
- Li R, Wu W, Liu Q, et al. (2013). Intelligently targeted drug delivery and enhanced antitumor effect by gelatinase-responsive nanoparticles. PLoS One 30:8
- Liu Q, Li R, Qian H, et al. (2012). Gelatinase-stimuli strategy enhances the tumor delivery and therapeutic efficacy of docetaxel-loaded poly(ethylene glycol)-poly(ɛ-caprolactone) nanoparticles. Int J Nanomed 7:281–95
- Liu Q, Li R, Qian H, et al. (2013). Targeted de livery of miR-200c/DOC to inhibit cancer stem cells and cancer cells by the gelatinases-stimuli nanoparticles. Biomaterials 34:7191–203
- Livak KJ, Schmittgen TD. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–8
- Lu N, Liu Q, Li R, et al. (2012). Superior antimetastatic effect of pemetrexed-loaded gelatinase-responsive nanoparticles in a mouse metastasis model. Anticancer Drugs 23:1078–88
- Luzzi KJ, MacDonald IC, Schmidt EE, et al. (1998). Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am J Pathol 153:865–73
- Mahler-Araujo B, Savage K, Parry S, et al. (2008). Reduction of E-cadherin expression is associated with non-lobular breast carcinomas of basal-like and triple negative phenotype. J Clin Pathol 61:615–20
- Manegold C, Gatzmeier U, von Pawel J, et al. (2000). Front-line treatment of advanced non-small-cell lung cancer with MTA (LY231514, pemetrexed disodium, ALIMTA) and cisplatin: a multicenter phase II trial. Ann Oncol 11:435–40
- Miles DW, Smith IE, Coleman RE, et al. (2001). A phase II study of pemetrexed disodium (LY231514) in patients with locally recurrent or metastatic breast cancer. Eur J Cancer 37:1366–71
- Miller DS, Blessing JA, Ramondetta LM, et al. (2014). Pemetrexed and cisplatin for the treatment of advanced, persistent, or recurrent carcinoma of the cervix: a limited access phase II trial of the gynecologic oncology group. J Clin Oncol 32:2744–9
- Pinheiro H, Bordeira-Carriço R, Seixas S, et al. (2010). Allele-specific CDH1 downregulation and hereditary diffuse gastric cancer. Hum Mol Genet 19:943–52
- Reis-Filho JS, Cancela Paredes J, Milanezi F, et al. (2002). Clinicopathologic implications of E-cadherin reactivity in patients with lobular carcinoma in situ of the breast. Cancer 94:2114–15
- Saad AA, Awed NM, Abd Elierim NN, et al. (2010). Prognostic significance of E-cadherin expression and peripheral blood micrometastasis in gastric carcinoma patients. Ann Surg Oncol 17:3059–67
- Schneeweiss A, Marmé F, Ruiz A, et al. (2011). A randomized phase II trial of doxorubicin plus pemetrexed followed by docetaxel versus doxorubicin plus cyclophosphamide followed by docetaxel as neoadjuvant treatment of early breast cancer. Ann Oncol 22:609–17
- Specht K, Richter T, Müller U, et al. (2001). Quantitative gene expression analysis in microdissected archival formalin-fixed and paraffin-embedded tumor tissue. Am J Pathol 158:419–29
- Thiery JP. (2002). Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 20. 2:442–54
- Wu FL, Li RT, Yang M, et al. (2015). Gelatinases-stimuli nanoparticles encapsulating 5-fluorouridine and 5-aza-2′-deoxycytidine enhance the sensitivity of gastric cancer cells to chemical therapeutics. Cancer Lett doi: 10.1016/j.canlet.2015.01.006
- Zhang DS, Jin Y, Luo HY, et al. (2015). Pemetrexed for previously treated patients with metastatic gastric cancer: a prospective phase II study. Br J Cancer 112:266–70
