Abstract
Objective: Insulin is a hormone used in the treatment of diabetes mellitus. Multiple injections of insulin every day may causes pain, allergic reactions at injection site, which lead to low patient compliance. The aim of this work was to develop and evaluate an efficient solid lipid nanoparticle (SLN) carrier for oral delivery of insulin.
Methods: SLNs were prepared by double emulsion solvent evaporation (w/o/w) technique, employing glyceryltrimyristate (Dynasan 114) as lipid phase and soy lecithin and polyvinyl alcohol as primary and secondary emulsifier, respectively, and evaluated in vitro for particle size, polydispersity index (PDI) and drug entrapment.
Results: Among the eight different developed formulae (F1–F8), F7 showed an average particle size (99 nm), PDI (0.021), high entrapment of drug (56.5%). The optimized formulation (F7) was further evaluated by FT-IR, DSC, XRD, in vitro release, permeation, stability, bioavailability and pharmacological studies. Insulin-loaded SLNs showed better protection from gastrointestinal environment as evident from the relative bioavailability, which was enhanced five times as compared to the insulin solution. A significant enhancement of relative bioavailability of insulin was observed, i.e. approximately five times of pure insulin solution when loaded in SLN (8.26% versus 1.7% only).
Introduction
Insulin is usually administered to diabetic patients through subcutaneous injection. Patients with Type 1 diabetes are completely dependent on insulin injections. However, long-term insulin injections have many disadvantages like pain, allergic reactions, hyper-insulinemia and insulin lipodystrophy around the injection site (Gowthamarajan & Kulkarni, Citation2003; Yu et al., Citation2015). Fear of insulin injection is associated with poor glycemic control, clinical complications, psychological co-morbidities, poor general well-being and health status and increased risk of mortality for diabetes patients. Moreover, fear of injection greatly reduces compliance and adherence to the treatment. Insulin administered via the oral route will help eliminate the pain caused by injection, psychological barriers associated with multiple daily injections, such as needle anxiety (Korytkowski, Citation2002) and possible infections (Lin et al., Citation2007). However, being a protein, it undergoes rapid enzymatic degradation in the stomach, inactivation and digestion by proteolytic enzymes in the intestinal lumen (Patki & Jagasia, Citation1996; Agarwal & Khan, Citation2001, Nakamura et al., Citation2004; Jain et al., Citation2005; Sajeesh & Sharma Citation2006). Another major barrier to the absorption of hydrophilic macromolecules like insulin is that they cannot diffuse across epithelial cells through lipid-bilayer cell membranes to the blood stream (Lin et al., Citation2007). In other words, insulin has low permeability through the intestinal mucosa (Toorisaka et al., Citation2005). High molecular weight and lack of lipophilicity of insulin causes poor permeability across intestinal epithelium. Due to such inherent problems, the oral bioavailability of insulin is less than 1% (Lee, Citation1991).
Several approaches have been proposed to increases the oral bioavailability of insulin including the inhibition of proteolytic enzymes (Ziv et al., Citation1987; Liu et al., Citation2003) and use of penetration enhancers (Soltero & Ekwuribe, Citation2001; Eaimtrakarn et al., Citation2002; Li & Deng, Citation2004; Liang & Yang Citation2005). The use of enzyme inhibitors in long-term therapy however remains questionable because of possible absorption of unwanted proteins, disturbance of digestion of nutritive proteins and stimulation of protease secretion (Shah et al., Citation2002; Soares et al., Citation2013). The drawbacks with penetration enhancers include lack of specificity, i.e. they allow all contents of the intestinal tracts including toxins and pathogens the same access to the systemic bloodstream (Rieux et al., Citation2006). Risk to mucous membranes by surfactants and damage of cell membrane by chelators (Gowthamarajan & Kulkarni, Citation2003) are other drawback.
Several drug carriers have been proposed to increase the oral bioavailability of insulin including microemulsions (Cho & Flynn Citation1989), liposomes (Spangler, Citation1990), microspheres (Morishita et al., Citation1993; Timmy, Citation2002) and polymeric nanoparticles (Lin et al., Citation2007; Zhang et al., Citation2009; Zhao et al., Citation2009; Fonte et al., Citation2015). However, such carrier systems have disadvantages like low stability, low drug carrying capacity, leakage of entrapped drug and toxicity of the residual solvents and surfactants used (Gowthamarajan & Kulkarni, Citation2003).
Solid lipid nanoparticles (SLNs) have been proposed as an alternative drug carrier system to other novel delivery approaches, such as microemulsions, microsphere, liposomes and polymeric nanoparticles, due to various advantages, including feasibility of incorporation of lipophilic and hydrophilic drugs, improved physical stability, low cost, ease of scale-up and manufacturing (Peters & Müller, Citation1998; Ravi Kumar, Citation2000). Several studies of insulin-loaded solid lipid-based nanoparticles have recently been published however many of these either did not report bioavailability (Liu et al., Citation2007; Fonte et al., Citation2012) or reported pharmacological activity only (Sarmento et al., Citation2007; Zhang et al., Citation2009).
The objective of the proposed research is to develop and optimize successful insulin-loaded SLNs in order to overcome physiologic and morphologic barriers to insulin absorption, by preventing acidic and enzymatic degradation and enhancing intestinal permeability that would eventually increase the oral bioavailability of insulin.
Materials and methods
Chemicals and reagents
Recombinant human insulin was purchased from Sigma-Aldrich Chemical Company (St. Louis, MO). Dynasan™ 114 was purchased from Sasol Germany GmbH (Witten, Germany). Soya lecithin was purchased from AppliChem (Darmstadt, Germany). Polyvinyl alcohol (PVA), M.W. 22 000 was obtained from BDH Laboratories (Poole, England). All other reagents and chemicals were of analytical grade.
Preparation of SLNs
The insulin-loaded SLN was prepared by a w/o/w double emulsion–solvent evaporation technique as described previously (Garcia-Fuentes et al., Citation2005; Sarmento et al., Citation2007). Briefly, weighed amount (5 mg) of insulin was dissolved in 0.1 M hydrochloric acid. The insulin solution was emulsified in dichloromethane containing varying amount of lipid and emulsifier () by probe sonication (Bandelin, Germany) for 60 s at 40% voltage efficiency over ice bath (Yassin et al., Citation2010). The produced primary emulsion (w/o) was emulsified immediately with 20 ml aqueous solution of PVA (1% w/v) by mechanical stirring at 1100 rpm for 30 min over ice bath (IKA T-25 Ultra Turrax, Germany). The temperature was allowed to increase gradually between 15 and 18 °C during stirring for optimum solvent evaporation. Lipid nanoparticles were separated from bulk aqueous phase by centrifugation at 14 000 rpm for 10 min (2015 Centurion scientific, UK) followed by subsequent washing with cold distilled water and freeze drying (Martin Christ Alpha-1-4LD freeze-drier, Osterode, Germany).
Table 1. Formulation composition of insulin-loaded SLNs.
Measurement of particle size, polydispersity index (PDI)
Laser diffraction technique was used for particle characterization. Photon correlation spectroscopy was done for all formulations in triplicate using 90 Plus particle size analyzer, Brookhaven Instruments Corporation (Holtsville, NY). Briefly, SLN dispersions were diluted 1:200 with distilled water and analyzed at 25 °C with an angle of detection of 90°. Mean particle size and PDI for each formula were measured in triplicate ().
Determination of % entrapment efficiency (EE)
Drug EE of insulin SLN was determined by centrifugation of the colloidal samples at 14 000 rpm at 25 °C for 10 min. The amount of insulin entrapped within nanoparticle was calculated by difference between the total amount used and the amount present in the aqueous phase of supernatant. The non-entrapped insulin in the supernatant obtained after ultracentrifugation of SLN was determined by HPLC method (Ansari et al., Citation2014). The obtained amount was deducted from the actual amount of insulin added to calculate the difference. The %EE was then calculated by dividing the difference by actual amount of insulin and by total amount of lipids with insulin in SLN minus free amount of insulin observed in the aqueous phase of supernatant, respectively.
where W = theoretical amount of insulin; w = observed amount of insulin.
Characterization of insulin-loaded SLN
Insulin-loaded SLN with promising results based on results of particle size, PDI and drug entrapment studies, was further to be evaluated by differential scanning calorimetry (DSC), Fourier transform infra-red spectroscopy (FT-IR), powder X-ray diffraction, scanning electron microscopy (SEM), in vitro release and ex vivo permeation, stability and pharmacokinetic studies.
Differential scanning calorimetry
The physical state of the insulin-loaded SLN was determined by differential scanning calorimetry (DSC) (DSC-60, Shimadzu, Kyoto, Japan). For the investigation of bulk material, about 5 mg of the sample was used. Each sample was heated from 50 °C at heating 10 °C/min to 300 °C.
Fourier transform infra-red spectroscopy
The Fourier transform infra-red spectroscopy (FT-IR) spectra of SLN was recorded on the FT-IR (Alpha, Germany) using the potassium bromide (KBr) disc technique. SLN equivalent to 2 mg of insulin was mixed with potassium bromide (about 100 mg) in a clean glass mortar and pestle and compressed to get a pellet.
Powder X-ray diffractometry (X-RD)
Powder X-ray diffraction pattern of insulin, lipid, physical mixture and insulin-loaded SLN was recorded in X-ray diffractometer (Altima IV, Rigaku, Japan); Cu radiation, voltage of 40 kV and current of 40 mA. Patterns were obtained by scanning from 3° to 120° 2θ at a step size of 0.02° with step time of 0.5 s.
Scanning electron microscopy
The morphology of the insulin-loaded SLN was examined by scanning electron microscopy (SEM) (H-600, Hitachi, Japan). The samples were stained with 2% (w/v) phosphotungstic acid for 30 s and placed on copper grid with film for viewing.
In vitro release studies
In vitro release of SLN were performed by suspending 10 mg of insulin-loaded SLN in 1 ml of 0.1 N HCl (pH 1.2) in a 1.5 ml micro-tube and incubated at 37 °C with shaking at 150 rpm in an incubator (LBS-030S-Lab Tech, Namyangju, Korea) (Yang et al., Citation2010). At time interval 0, 1, 2, 3, 6, 12, 24 and 48 h, 0.25 ml aliquots were withdrawn, transferred to a 1.5 ml micro-tube and centrifuged at 15 000 rpm (2015 Centurion scientific, UK) for 10 min. The supernatant was taken for insulin quantitation by HPLC method (Ansari et al., Citation2014).
Drug permeation studies across rat everted intestinal sac
Permeation study was carried out using rat everted intestinal sac (Patil et al., Citation2010). Male Wistar rats weighing 200–300 g were used for the study. Prior to surgical procedure, the rats were fasted overnight (16–20 h) with water ad libitum. The rats were anaesthetized with phenobarbital sodium (60 mg/kg, i.p). The intestine of rats were exposed by a middle abdominal incision and a 20–25 cm segment of the proximal rat jejunum was excised and placed in oxygenated tissue culture media (TC 199). The intestine was gently everted over a glass rod, divided into segments of length of approximately 5 cm each. Sac was filled with TC 199 medium and placed in tubes containing 25 ml of TC 199 medium maintained in a biological shaker at 37 °C at a speed of 50 rpm (LBS-030S-Lab Tech, Korea). Either pure insulin alone or insulin-loaded SLN with equivalent amount was dispersed in this tube and then 0.5 ml of samples were withdrawn from serosal side at time interval 0, 15, 30, 60, 90 and 120 minu. The samples were centrifuged for 5 min at 2000 rpm, filtered through 0.22 µm membrane filter and analyzed by HPLC method. The apparent permeability was then calculated based on the following equations:
To calculate mucosal surface area, the intestine was considered as a cylinder and the following equation was used:
where h = length and r = radius of intestinal sac.
Stability studies
Stability studies were carried out in pepsin solution (0.05 mg/ml, Tris-HCl buffer, pH 2) and trypsin solution (0.36 mg/ml, phosphate buffer, pH 7.4) (Wu et al., Citation2003). A 1.5 ml of proteolytic enzyme solution was added either into 1.5 ml of insulin-loaded SLN or insulin solution (each formulation containing insulin equivalent to 1 mg). The mixture was immediately incubated at 37 °C. Two hundred microliters of sample were withdrawn at selected period of time. The pepsin digestion reaction was stopped by adding 100 µl of 0.05 mol/l NaOH, while the trypsin digestion reaction was stopped by adding 100 µl of 0.1 mol/l HCl. The remaining concentration of insulin in the samples was determined by HPLC.
Pharmacological and bioavailability evaluations
Diabetes was induced in male Wistar rats by intraperitoneal injection of alloxan at a dose of 150 mg/kg body weight. Rats were considered diabetic when their fasting blood glucose levels were higher than 300 mg/dl, two weeks after the alloxan treatment. Diabetic rats were selected in the study. The first group was given normal saline (untreated) as control for the experiment. Second group was injected with insulin subcutaneously (insulin 2 IU/kg). The third and fourth groups received oral insulin and insulin-loaded SLN, respectively at a dose level of 30 IU/kg body weight. Prior to dosing blood glucose level were checked for all the groups. After the rats were anaesthetized by either inhalation, approximately 1 ml of blood was withdrawn from the retro orbital puncture at each time interval. 15 µl of blood was taken to determine the blood glucose level using glucometer and rest of the blood was kept at room temperature for clotting followed by centrifugation (10 000 rpm for 10 min) to separate serum. A 100 µl of serum was separated to determine the insulin concentrations using human insulin ELISA kit (Invitrogen, Carlsbad, CA). The relative bioavailability was calculated by area under the curve of serum insulin.
Results and discussion
Particles characterization and EE
Mean particle size and PDI for all the prepared formulae are shown in . In all formulations, particle size ranged from 91 ± 5.12 to 250 ± 6.12 nm. Increase in the concentration of surfactant in SLN formulation could reduce the interfacial tension between lipid matrix and aqueous phase, consequently favor the formation of SLN with lower particle size. The PDI is a measure of width of dispersion of particles. Narrow dispersion comprises PDI values between 0.1 and 0.2, therefore F1, F2 and F7 can be considered as narrow disperse, whereas other formulations were found to have slightly higher polydispersity indices.
In order to optimize the lipid to drug ratio, three different amount of Dynasan 114 (50, 60 and 70 mg) were tried with fixed amount of insulin (5 mg) and soya lecithin (10 mg). It was observed that as the amount of Dynasan increased particle size decreased. The result indicated that increasing the lipid concentration also increases the drug entrapment (). This may be because higher concentration of lipid would provide more space to increase the increment of lipid content and also reduce the escaping of drug into external phase, thus ensuring highest drug entrapment.
The effect of PVA was determined by taking 0.5%, 1% and 2% concentrations. Empty SLN containing 0.5%, 1% and 2% of PVA showed the mean particle size 1865 ± 0.05, 250 ± 0.02 and 2654 ± 0.05 nm, respectively. This may be due to the fact that low PVA concentration (0.5%), an optimal surface coverage may not be achieved resulting in less optimal stabilization of the particle dispersion. The result suggested that relatively high concentration of PVA was needed to prevent particle aggregation (Yassin et al., Citation2010), however, high concentration of (2%) did not produce smaller particle further that might be due to the fact that the increase in PVA concentration from 1% to 2% increased the viscosity of the external aqueous phase (Abdelwahed et al., Citation2006). This resulted in a decrease in the net shear stress, reducing the diffusion speed and therefore increasing the particle size.
Soya lecithin was used to decrease interactions between the aqueous and organic phases. Higher amount of lecithin produced stable emulsion with less PDI. PLGA polymer used in the formulation significantly increased the entrapment of insulin inside SLN from 82.26% (F5) to 89.70% (F7). However, formula F7 was optimized among all formulations and further evaluation was done.
Characterization of insulin-loaded SLN
SLN with promising results based on particle size, PDI and drug entrapment was evaluated by FT-IR, DSC, XRD and SEM.
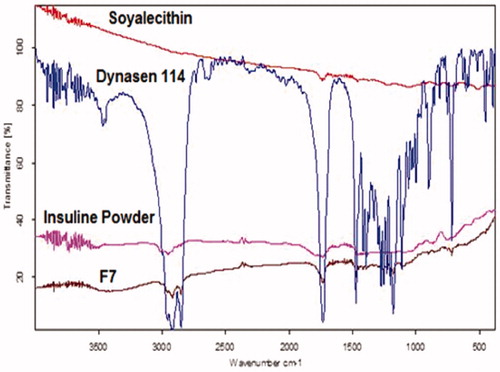
FT-IR spectral analysis
FT-IR spectra of insulin showed a characteristic peak in the region of 3307, 1656 and 624 cm−1. Significant changes were observed in the spectra of formulations as is illustrated in . All the characteristic absorption bands of insulin diminished significantly in the finger print region of drug, which revealed that the encapsulated drug inside the lipid core material existed in an amorphous state (Illing & Unruh, Citation2004).
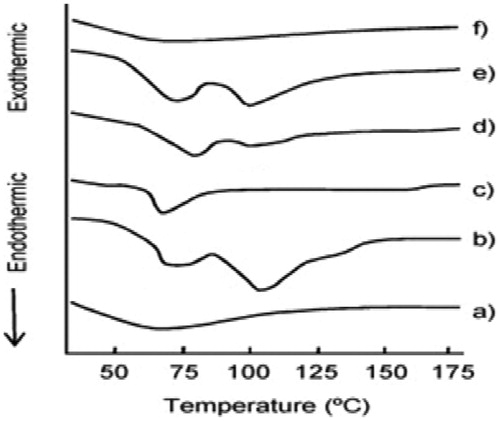
DSC thermal analysis
Thermal behavior of the insulin, Dynasan 114, soya lecithin, physical mixture and formulation (F7) is shown in . DSC curve of insulin (a) showed that, an endothermic event at 62 °C was characteristic process of that corresponding to denaturation. The exothermic events were characteristics of insulin degradation that may occur by chemical or physical process, influenced by temperature conditions. This thermal profile of insulin is in agreement as reported in literature (Sarmento et al., Citation2007). Thermogram of soya lecithin (b) depicted two blunt endotherms at 70 and 105 °C, whereas that of Dynasan 114 (c) showed a characteristic sharp peak at 58 °C corresponding to the melting of the lipid. Thermogram of physical mixture (e) exhibited pattern of all component revealing no or lesser degree of interactions among components. Thermogram of the prepared SLN (f) depicted blunt endotherm that can be attributed to semicrystalline state of the lipid inside the prepared SLN.
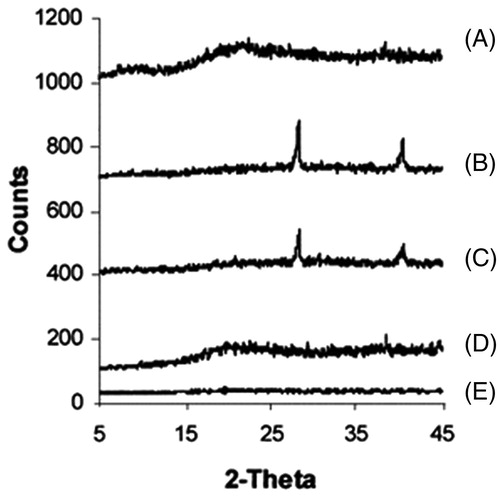
XRD analysis
All diffractograms displayed partial sharp crystalline peaks, which is the characteristic of some crystallinity where SLN (F7) tends to be amorphous in nature. This was in agreement with DSC results ().
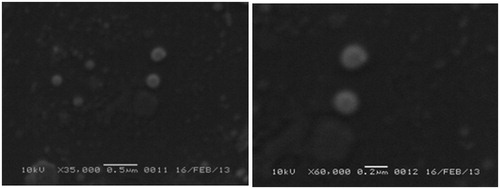
Particle morphology
SEM images of SLNs F7 are presented in . It was clear from all the images that insulin-loaded SLNs were spherical in shape with smooth regular surfaces. The sizes observed from SEM micrographs were similar to those obtained from particle size analyzer
In vitro release studies
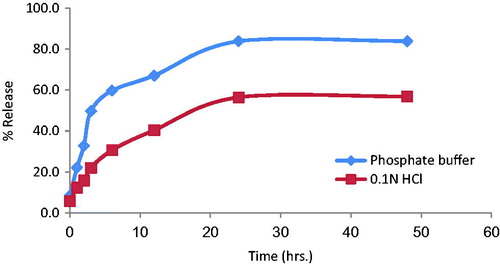
In vitro release of insulin from optimized SLN (F7) was studied in 0.1 N HCl and phosphate buffer (pH 7.4). The release of insulin from SLN was considerably slow, after 12 h approximately 40% of insulin was released in phosphate buffer, whereas at the same time 67% of insulin was released in 0.1 N HCl (). The rapid burst effect was observed between 2 and 3 h, releasing about 22% and 50% in phosphate buffer and acidic media, respectively. These data of burst effect in SLNs were supported by the results reported in the literature (Rahman et al., Citation2010). The biphasic drug release pattern is very common with SLNs (Liu et al., Citation2007).
Drug permeation studies across rat everted intestinal sac
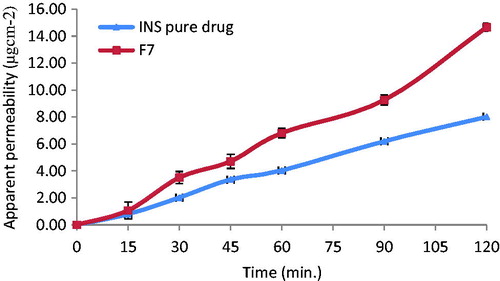
The apparent permeability of insulin from formulation F7 using everted sac technique is shown in . The permeability of insulin from F7 was greatly enhanced compared with pure insulin. Permeability of insulin after 2 h was calculated as 8.02 and 14.76 from pure insulin and insulin-loaded SLN (F7), respectively (). This indicates that incorporation of insulin into SLN resulted in approximately twofold increase in permeability of insulin. This can be possibly attributed to the marked decrease in particle size and enhancement of surface area leading to a higher rate of drug dissolution and diffusion. In addition, the SLN can diffuse through the mucus layer and release the drug directly on the surface of the cell membrane.
Stability studies
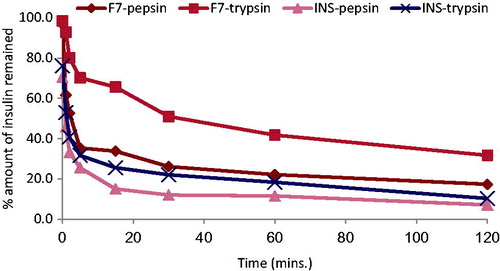
shows the percentage of remaining insulin after incubation of free insulin solution and insulin-loaded SLN (F7) in pepsin and trypsin solutions. Insulin was degraded rapidly in both the solution and at 1 h remaining insulin was approximately 45% in pepsin and 53% in trypsin. Thus, free insulin was degraded more slowly by trypsin compared to pepsin as reported in literature (Zhang et al., Citation2012). Compared to the free insulin solution, about 61.6% and 92.9% of insulin remained at 1 h incubation of F7 formulation with pepsin and trypsin, respectively. This result proved that the SLNs had a certain effect in protecting insulin from degradation with pepsin and trypsin enzymes.
Pharmacological and bioavailability evaluations
Insulin-loaded SLN was administered orally to overnight fasted diabetic rats. The serum insulin levels after intragastric insulin-loaded SLN (F7), insulin solution and subcutaneous administration of insulin in each group were calculated using human insulin ELISA kit. Calibration curve was constructed to calculate the corresponding insulin concentrations in serum sample. Serum insulin concentration versus time curve for all treatments is depicted in . Insulin-loaded SLN (F7) decreased glycemia by comparison with rats treated with oral insulin solution. Insulin released from SLN in the intestinal lumen is able to be directly internalized, being the first responsible for the physiological effect. Then, after arrival to the appropriate sites for nanoparticle uptake in the posterior ileum (Jung et al., Citation2000), SLN is able to be absorbed (Garcia-Fuentes et al., Citation2005), resulting in significant hypoglycemic effect compared with oral insulin solution administration. Afterwards, it can be postulated that SLN undergoes physiological degradation and the insulin enters into the blood circulation. It is generally accepted that nanoparticles with hydrophobic surfaces, such as SLN, are taken up more extensively by the intestinal epithelium than those with hydrophilic surfaces (Eldridge et al., Citation1990). Thus, the uptake of nanoparticles with lipid matrix is potentially facilitated. Also, the bioadhesive properties of lipids can lead to a gradient diffusion of insulin from the high concentrations in the SLN matrix towards the intestinal cells. The association of both mechanisms has been related with the prolonged physiologic effect of insulin after oral administration (Damge et al., Citation1997).
Table 2. Pharmacokinetic parameters of serum insulin.
As shown in , insulin-loaded SLN showed better protection of insulin from harsh gastro intestinal environment than the insulin solution as evident from the data (Cmax 196.4 versus 16.4 only). The relative pharmacological bioavailability of insulin was enhanced approximately five times of pure insulin solution when loaded in SLN (8.26% versus 1.7% only). These overall results suggested that SLN could protect insulin from degradation and enhance intestinal absorption of insulin.
Statistical analysis
The t-test and the one-way analysis of variance (ANOVA) with the pairwise multiple comparison procedures (Student–Newman–Keuls method) were performed to compare two or multiple groups, respectively. All analyses were run using the SPSS program, Version 14.0 (SPSS Inc., Chicago, IL) and differences were considered to be significant at a level of p < 0.05.
Conclusion
SLN was successfully developed by a modified solvent emulsification–evaporation method based on a w/o/w double emulsion. They are considered to be stable carriers for oral administration. PVA (1% w/v) was used as a secondary emulsifier in the aqueous phase when preparing the insulin-loaded SLN to increase their stability. The mean diameters confirmed that the SLN produced is submicron colloidal carrier, suitable for enabling gastrointestinal absorption by M-cells on Peyer's patches. The microscopic appearance and the structural characterization of Dynasan 114-based SLNs with insulin were performed using SEM. The particle size of insulin-loaded SLN depicted in SEM images is in agreement with the results obtained with PCS. The relative pharmacological bioavailability of insulin was enhanced approximately five times of pure insulin solution when loaded in SLN (8.26% versus 1.7% only).
The attempts to develop an insulin-loaded SLN formulation for oral administration produced nanoparticles with spherical shape, and good EE. The plasma glucose levels of rats after oral administration of insulin-loaded SLN were lower than those obtain after administration of oral insulin solution. The solid matrix of SLN was able to partially protect insulin against chemical degradation in the gastrointestinal tract and to promote the intestinal absorption of insulin. In conclusion, SLNs were found to be suitable carrier systems for the administration of insulin through the oral route.
Declaration of interest
We declare that we have no conflict of interest.
This project was supported by Deanship of Scientific Research, Salman Bin Abdulaziz University, Al-kharj, Saudi Arabia (Project No. 8H/1432).
References
- Abdelwahed W, Degobert G, Stainmesse S, Fessi H. (2006). Freeze-drying of nanoparticles: Formulation, process and storage considerations. Adv Drug Deliv Rev 58:1688–713
- Agarwal V, Khan MA. (2001). Current status of the oral delivery of insulin. Pharma Tech 25:76–90
- Ansari MJ, Jamil S, Anwer MK, et al. (2014). Development and validation of simple and rapid high performance liquid chromatographic method for routine analysis of human insulin in formulations. Afr J Pharm Pharmacol 8:1018–24
- Cho YW, Flynn M. (1989). Oral delivery of insulin. Lancet 2:1518–9
- Damge C, Vrancks H, Balschmidt P, et al. (1997). Poly(alkyl cyanoacrylate) nanospheres for oral administration of insulin. J Pharm Sci 86:1407–500
- Eaimtrakarn S, Ramaprasad YV, Ohno T, et al. (2002). Absorption-enhancing effect of labrasol on the intestinal absorption of insulin in rats. J Drug Target 10:255–60
- Eldridge JH, Hammond CJ, Meulbroek JA, et al. (1990). Controlled vaccine release in the gut-associated lymphoid tissues. I. Orally administered biodegradable microspheres target the Peyer’s patches. J Control Release 11:205–14
- Fonte P, Andrade F, Araujo F, et al. (2012). Chitosan-coated solid lipid nanoparticles for insulin delivery. Methods Enzymol 508:295--314
- Fonte P, Araújo F, Silva C, et al. (2015). Polymer-based nanoparticles for oral insulin delivery: revisited approaches. Biotechnol Adv (in press). doi:10.1016/j.biotechadv.2015.02.010
- Garcia-Fuentes M, Torres D, Alonso MJ. (2005). New surface-modified lipid nanoparticles as delivery vehicles for salmon calcitonin. Int J Pharm 296:122–32
- Gowthamarajan K, Kulkarni GT. (2003). Oral insulin—fact or fiction? Resonance 8:38–46
- Illing A, Unruh T. (2004). Investigations on the flow behavior of dispersions of solid triglyceride nanoparticles. Int J Pharm 284:123–31
- Jain D, Panda AK, Majumdar DK. (2005). Eudragit S100 entrapped insulin microspheres for oral delivery. AAPS PharmSciTech 6:E100–7
- Jung T, Kamm W, Breitenbach A, et al. (2000). Biodegradable nanoparticles for oral delivery of peptides: is there a role for polymers to affect mucosal uptake. Eur J Pharm Biopharm 50:147–60
- Korytkowski M. (2002). When oral agents fail: practical barriers to starting insulin. Int J Obesity 26:S18–24
- Lee VH. (1991). Oral route of peptide and protein drug delivery, Chapter 16. In: Vincent HL Lee, ed. Peptide and protein drug delivery. New York: Marcel Dekker Inc., 691–738
- Li CL, Deng YJ. (2004). Oil-based formulations for oral delivery of insulin. J Pharm Pharmacol 56:1101–7
- Liang JF, Yang VC. (2005). Insulin-cell penetrating peptide hybrids with improved intestinal absorption efficiency. Biochem Biophys Res Commun 335:734–8
- Lin YH, Chen CT, Liang HF, et al. (2007). Novel nanoparticles for oral insulin delivery via the paracellular pathway. Nanotechnology 18:1–10
- Liu H, Tang R, Pan WS, et al. (2003). Potential utility of various protease inhibitors for improving the intestinal absorption of insulin in rats. J Pharm Pharmacol 55:1523–9
- Liu J, Gong T, Wang C, Zhong Z, Zhang Z. (2007). Solid lipid nanoparticles loaded with insulin by sodium cholate-phosphatidylcholine-based mixed micelles: preparation and characterization. Int J Pharm 340:153–62
- Morishita M, Takayama K, Machida Y, Nagai T. (1993). Enteral insulin delivery by microspheres in three different formulations using Eudragit L-100 and S-100. Int J Pharm 91:29–37
- Nakamura K, Murray RJ, Joseph JI, et al. (2004). Oral insulin delivery using P(MAA-g-EG) hydrogels: effects of network morphology on insulin delivery characteristics. J Control Release 95:589–99
- Patil A, Raheja V, Damre A. (2010). Simultaneous analysis of intestinal permeability markers, caffeine, paracetamol and sulfasalazine by reverse phase liquid chromatography: a tool for the standardization of rat everted gut sac model. Asian J Pharm Clin Res 3:204–7
- Patki VP, Jagasia SH. (1996). Progress made in noninvasive insulin delivery. Ind J Pharmacol 28:143–51
- Peters K, Müller RH. (1998). Nanosuspensions – a novel formulation of poorly soluble drugs. In: Diederichs JE, Müller RH, eds. Future strategies for drug delivery with particulate systems. Boca Raton: CRC Press, 101–8
- Rahman Z, Zidan S, Khan MA. (2010). Non-destructive methods of characterization of risperidone solid lipid nanoparticles. Eur J Pharm Biopharm 76:127–37
- Ravi Kumar MNV. (2000). Nano and microparticles as controlled drug delivery devices. J Pharm Pharm Sci 3:234–58
- Rieux A, Fievez V, Garinot M, et al. (2006). Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release 116:1–27
- Sajeesh S, Sharma CP. (2006). Cyclodextrin-insulin complex encapsulated polymethacrylic acid based nano-particles for oral insulin delivery. Int J Pharm 325:147–54
- Sarmento B, Martins S, Ferreira D, Souto EB. (2007). Oral insulin delivery by means of solid lipid nanoparticles. Int J Nanomedicine 2:743–9
- Shah RB, Ahsan F, Khan MA. (2002). Oral delivery of proteins: Progress and prognostication. Crit Rev Ther Drug Carrier Syst 19:135–69
- Soares S, Fonte P, Costa A, et al. (2013). Effect of freeze-drying, cryoprotectants and storage conditions on the stability of secondary structure of insulin-loaded solid lipid nanoparticles. Int J Pharm 456:370–81
- Soltero R, Ekwuribe N. (2001). The oral delivery of protein and peptide drugs – a report. Innovat Pharmaceut Technol 1:106–10
- Spangler RS. (1990). Insulin administration via liposomes. Diabetes Care 13:911–22
- Timmy SA, Victor SP, Sharma CP, Kumari VJ. (2002). Betacyclodextrincomplexed insulin loaded alginate microspheres – oral delivery system. Trends Biomater Artif Organs 15:48–53
- Toorisaka E, Hashida M, Kamiya N, et al. (2005). An enteric-coated dry emulsion formulation for oral insulin delivery. J Control Release 107:91–6
- Wu ZH, Ping QN, Lai JM, Wei Y. (2003). Hypoglycemic effect of polysaccharide-coated insulin liposomes after oral administration in mice. Acta Pharm Sin 38:138–42
- Yang R, Gao RC, Cai CF, et al. (2010). Preparation of gel-core-solid lipid nanoparticle: a novel way to improve the encapsulation of protein and peptide. Chem Pharm Bull 58:1195–202
- Yassin AB, Anwer MK, Mowafy HA, et al. (2010). Optimization of 5-flurouracil solid-lipid nanoparticles: a preliminary study to treat colon cancer. Int J Med Sci 7:398–408
- Yu F, Li Y, Liu CS, et al. (2015). Enteric-coated capsules filled with mono-disperse micro-particles containing PLGA-lipid-PEG nanoparticles for oral delivery of insulin. Int J Pharm 484:181–91
- Zhang J, Fan Y, Smith E. (2009). Experimental design for the optimization of lipid nanoparticles. J Pharm Sci 98:1813–19
- Zhang ZH, Zhang YL, Zhou JP, Lv HX. (2012). Solid lipid nanoparticles modified with stearic acid–octaarginine for oral administration of insulin. Int J Nanomedicine 7:3333–9
- Zhao Y, Trewyn BG, Slowing II, Lin VS. (2009). Mesoporous silica nanoparticle-based double drug delivery system for glucose-responsive controlled release of insulin and cyclic AMP. J Am Chem Soc 131:8398–400
- Ziv E, Lior O, Kidron M. (1987). Absorption of protein via the intestinal walls: a quantitative model. Biochem Pharmacol 39:1035–9