Abstract
Objective: The aim of this study was to evaluate the effect of therapeutic ultrasound (TUS) on dermal delivery and therapeutic effect for frostbite of nanogel containing triterpenoids isolated from Ganoderma lucidum (GLT).
Methods: GLT nanosuspension (GLT-NS) was prepared by high pressure homogenization and then suitably gelled to obtain GLT nanogel. The effects of TUS on GLT releasing from GLT nanogel and GLT permeation through the excised rat abdominal skin were evaluated. Moreover, a comparative study was also undertaken between different treatments of frostbite in rats: topical application of GLT nanogel (alone), TUS (alone) and GLT nanogel + TUS (plus).
Results: In the in vitro release study, TUS has no influence on drug release from the nanogel. Results of the in vitro transdermal study indicated that TUS significantly increased the cumulative amount of GLT permeating across and into the skin and reduced the lag time in comparison with passive diffusion (without TUS). As evidenced by the significant increase of wound healing area and the improvement in frostbite, TUS applied with simultaneous treatment method could improve the therapeutic effect of the GLT nanogel for frostbite.
Conclusion: The present study revealed that the TUS can be effectively used to actively enhance topical delivery of GLT from nanogel and improve the therapeutic effect for frostbite in rats.
Introduction
Frostbite is damage to the body as a result of local or systemic effect caused by extreme cold. Inadequate blood circulation when the ambient temperature is below freezing leads to frostbite. In the past, mostly people in military environments were easy to frostbite, but now it can be found in high-altitude mountaineers, skiers, work-related and among homeless people. The body of knowledge regarding the different facets of frostbite injury continues to expand. However, other than local rewarming, local disinfection and symptomatic medications, today no commercial and special drugs can be available for the treatment of frostbite.
In the previous studies, triterpenoids extracted from the Ganoderma lucidum (GLT), which possess the bioactivities of anti-inflammatory (Dudhgaonkar et al., Citation2009), antimicrobial (Karwa et al., Citation2012), antioxidation (Smina et al., Citation2011), immunoregulation (Boh, Citation2013) and antinociception (Koyama et al., Citation1997), were found to have therapeutic effect for frostbite (Han et al., Citation2012a,Citationb). And formulation of GLT as nanogel was developed to ameliorate problems of poor solubility and poor penetration into the skin of GLT (Shen et al., Citation2014). GLT nanogel exhibited higher skin permeation and deposition, and superior therapeutic effect for frostbite compared with the GLT–carbopol gel (Shen et al., Citation2014). On the basis of improved biopharmaceutical characteristics, nanogel is eligible for the use as a suitable nanomedicine for dermal delivery of GLT for frostbite treatment. In this paper, the GLT nanogel was prepared according to our previous study with a modification and then characterized by particle size distribution, zeta potential analysis, SEM, as well as its physical stability after storage for 1, 3 and 6 months.
Therapeutic ultrasound (TUS) established for physiotherapy has become largely used for the treatment of a variety of conditions, including the promotion of wound healing, edema reduction and pain relief, for different etiologies (Maia Filho et al., Citation2010; Silveira et al., Citation2014). In addition, ultrasound, generally referred to as sonophoresis (also known as phonophoresis), was also used for the delivery of drugs to or through the skin (Polat et al., Citation2011; Alexander et al., Citation2012). TUS had been carried out as a viable method for the delivery of drugs for treating localized skin conditions and joint inflammation in the past decades because of its track-record of safety and its ability to increase the epidermal penetration of topical gel (Alfredo et al., Citation2009; Polat et al., Citation2011). In order to enhance the dermal delivery and improve the therapeutic effect for frostbite of GLT nanogel, TUS with simultaneous treatment mode was introduced in this study.
Based on the therapeutic properties of the GLT nanogel and TUS, this study aims to assess the simultaneous application of GLT nanogel and the TUS as an accelerator for frostbite treatment. For this purpose, the effects of TUS on GLT releasing from GLT nanogel and GLT permeation through excised rat abdominal skin were evaluated. A comparative study was also undertaken between different treatments of frostbite in rats: topical application of GLT nanogel (alone), TUS (alone) and GLT nanogel + TUS (plus).
Materials and methods
Materials
Ganoderma lucidum (22.7% ganoderic acid A, 11.3% ganoderic acid B, 9.2% ganoderic acid C2, 7.9% ganoderic acid E, 6.5% ganoderic acid G, 9.8% ganoderic acid B, 9.1% ganoderic acid E, 6.3% lucidenic acid A, 7.3% lucidenic acid H, 5.6% ganederiol F) were prepared in the laboratory of Dr Yuan. Poloxamer F68 was purchased from the BASF Corp. (Ludwigshafen, Germany). Tween 80 was obtained from Sigma Aldrich Chemicals Pvt. Ltd. (St Louis, MO). Carbopol 940 was obtained from Guangzhou Bo Feng Chemical Co., Ltd. (Guangzhou, China).
Preparation of GLT nanogel
Ganoderma lucidum nanosuspension (GLT-NS) was prepared by high pressure homogenization method. Briefly, GLT powder (10%, w/v) was dispersed in a stabilizer solution containing 2% (w/v) Tween 80 and 1% (w/v) Poloxamer F68. Then the dispersion was processed using a high shear homogenizer (Ultra-Turrax® T25, IKA, Germany) at 15 000rpm for 5 min and then homogenized at predetermined process parameters (homogenization pressure and cycle numbers) using a piston-gap high pressure homogenizer (EmulsiFlex-C3, Avestin Inc., Ottawa, Canada). The premilling step was firstly carried out at 500 bar for 5 cycles, and then the GLT-NS were obtained by homogenization at 1000–2500 bar for 10–30 cycles.
Ganoderma lucidum nanogel, which acts as both coupling agent and drug reservoir, was prepared according to our previous study with a modification (Shen et al., Citation2014). Briefly, carbopol 940 (0.5%, w/w) was dispersed in purified water and kept aside for 1 h for swelling. Subsequently, 50 mL GLT-NS and propylene glycol (10%, w/w) were added into the swelled carbopol 940 dispersions using a magnetic stirrer (85-2, Changzhou Guohua Electric Appliance Co., Ltd., Jiangsu, China) at 1200 rpm for 10 min and then adjusted to 100 g with purified water. The GLT nanogel was neutralized with triethanolamine until pH 5.5–6.5 and was allowed to stand 24 h at room temperature to equilibrate.
Characterization of GLT nanogel
Particle size and zeta potential analyses
The volume average particle size and polydispersity index (PI) of GLT-NS during process optimization and reconstituted GLT nanoparticles from the GLT nanogel were determined by Photon Correlation Spectroscopy (PCS) using a laser particle size analyzer (Winner 801, Jinan Winner Particle Instrument Stock Co., Ltd., Jinan, China). Their zeta potential was estimated by the method of electrophoretic mobility using a Zeta-sizer (3000SH, Malvern Instruments Ltd., Malvern, UK). GLT-NS was diluted with distilled water to achieve the required dilution for the analysis. For measurement of reconstituted GLT nanoparticles from the GLT nanogel, the gel network was destroyed by dilution with distilled water and then analyzed at room temperature. In order to evaluate the stability of the GLT nanogel, samples were stored at room temperature and measurements were done on the first day (D1) and at the 1st, 3rd and 6th month (M1, M3, M6).
Scanning electron microscopy
The morphology of GLT and GLT nanogel were observed by scanning electron microscopy (SEM, S-4800, Hitachi Technologies Corporation, Ibaraki-ken, Japan). All samples were fixed on the metal stub using a double-sided adhesive tape and then coated with gold in vacuum by a sputter coater. An excitation voltage of 5 kV was used in the experiments.
Determination of drug content, spreadability and pH
The drug content, spreadability and pH of GLT nanogel were analyzed according to the previously reported method (Shen et al., Citation2014).
In vitro release experiments
In order to assess the effect of simultaneous application of ultrasound on the drug release from the gel and degradation of drug molecules, an in vitro release study was carried out. The release rate of GLT from the nanogel, with and without TUS (1 MHz frequency, 0.5 W/cm2 intensity), was determined by following the procedure described by Bommannan et al. (Citation1992).
In vitro transdermal study
The in vitro permeation studies were conducted using Franz diffusion cells with a surface area of 1.77 cm2 and a receptor compartment capacity of 22.5 mL (Mutalik et al., Citation2012). Membrane used for permeability studies was full thickness skin from the abdominal section of Sprague-Dawley rats of 6–8 weeks old. The rat excised full thickness skin was mounted on the diffusion cell between the donor and the receptor compartments, with the stratum corneum (SC) facing the donor chamber. The receptor cell was filled with phosphate buffer saline (PBS, pH 7.4) containing 20% ethanol to maintain sink condition and the receptor solution was continuously stirred at 500 rpm using magnetic stirrer (SH-2, Beijing Jinbeide Industrial And Trading Co., Ltd., Beijing, China) and kept at 32 ± 0.5 °C to simulate the skin temperature. For permeation studies, 0.5 g GLT nanogel (25 mg of GLT) were accurately weighed and placed onto the skin in the donor compartment. Studies were performed with or without the application of TUS. Ultrasound was applied with the aid of an ultrasonic therapy apparatus (YTS-60-30, Beijing Nava Medical Technology Co., Ltd., Beijing, China), which was equipped with a flat tip probe with an application surface area of 1.13 cm2. The probe was centrally positioned in the donor chamber and submerged into the donor nanogel about 3 mm from the skin surface. Ultrasound with a frequency of 1.0 MHz was applied for 60 min in a continuous mode at intensity of 0.5 W/cm2. Aliquots of 1 mL were withdrawn from the receptor compartment at scheduled intervals 0, 5, 10, 15, 20, 30, 45 and 60 min and were immediately replenished by equal volume of the fresh receptor medium.
At the end of permeation experiments, the skin surface was gently washed several times with PBS to remove any nanogels remaining and the GLT was extracted from the skin layers using absolute ethanol under sonication for 20 min. The amount of GLT in the permeation medium and accumulated in the skin layer sample was assayed by HPLC. All experiments were done in three replicates.
Data analysis
The cumulative amount of GLT permeated across rat skin (μg) divided by the diffusion area (cm2) was plotted versus time (min) and the steady state fluxes (J, μg/cm2/min) were calculated from the slope of the best-fit regression line. The lag time (Tlag) was determined by extrapolating the cumulative mass per unit area versus time profile at a steady state to the abscissa. The enhancement ratio (ER) was also calculated by dividing J with TUS by J without TUS.
HPLC assay
The content of ganoderic acid A (GA, an indexical component found in GLT, 22.7%, w/w) was measured using the HPLC method by following the previously reported method (Shen et al., Citation2014). The HPLC system (Shimadzu Corporation, Kyoto, Japan) was equipped with a pump (LC-20AT), a diode array detector (SPD-M20A), a degassing unit (DGU-20A5R), an auto sampler (SIL-20A) and Shimadzu CBM-20A station for data analysis. The GA was separated on an Alltima C18 column (250 mm × 4.6 mm, 5 μm, Alltech) at 15 °C using a mobile phase composed of (A) acetonitrile and (B) 0.03% formic acid solution. The HPLC system was operated using a gradient elution of 25–35% A at 0–35 min, 35–45% A at 35–55 min, 45–55% A at 55–65 min, 55–25% A at 65–66 min, 25% A at 66–80 min with a flow rate of 1.0 mL/min. The injection volume was 10 μL and the detection wavelength was 254 nm.
Pharmacodynamic efficacy of GLT nanogel using TUS
Male Sprague-Dawley rats (270–300 g) provided by the Experimental Animal Center of Military Medical Sciences (Beijing, China) were housed under standard conditions (12 h/d fluorescent light (06:00–18:00), 23 ± 1 °C room temperature) and given free access to food and water. This experiment was carried out in accordance with the Principles of Laboratory Animal Care and the Guide for the Care and Use of Laboratory Animals. This study was approved by 302 Military Hospital of China Institutional Animal Care and Use Committee.
The rat’s model for frostbite was made as described according to the method described in our previous study (Shen et al., Citation2014) with a modification. The dorsal side of each rat was shaved and cleaned under light ether anesthesia 24 h prior to frostbite. The frostbite was made by exposing the shaved skin of anesthetized animals to cryogenic steel (about −196 °C). For this procedure, a round sheet of steel with a diameter of 2.5 cm and a thickness of 2 mm was completely immersed in liquid nitrogen tanks for at least 15 min to make it fully cooled to −196 °C. The steel sheet was then took out and immediately and tightly attached to the hair free skin of anesthetic rat for 8 s to make topical frostbite.
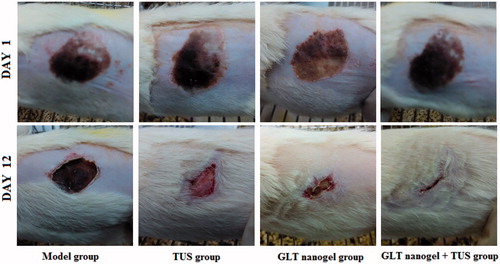
The rats were randomly divided into four groups of six each: (A) Model group (no ultrasound or drug treatment); (B) TUS group (placebo gel without GLT group with TUS); (C) GLT nanogel group (5% GLT nanogel group with no TUS); (D) GLT nanogel + TUS group (5% GLT nanogel group with TUS). The rats of groups A–D were made frostbite injury on skin as described above and the drugs were applied to the injured part after 24 h frostbite. The rats of groups B and D were treated with 1 g placebo gel and 1 g GLT nanogel immediately followed by the application of TUS (1 MHz frequency, 0.5 W/cm2 intensity for 10 min), group C with the 5% GLT nanogel without the application of TUS and groups A with no topical treatment. The ultrasound head was moved over the gel using small, continuous, circular movements in order to make an equal radiation to the whole injured part. All rats were treated once daily for 12 consecutive days. Photographs of the wound surfaces were taken for visual comparison.
The percentage of wound healing area (Pwa) for each animal of every group at 6 and 12 days after frostbite was calculated and used for the evaluation of therapeutic effect of different treatments for frostbite. According to the literature method (Srivastava & Durgaprasad, Citation2012; Venkataraman & Nagarsenker, Citation2013), the area of frostbite wound was traced on a transparent paper throughout the monitoring period and then the tracing was transferred to 1 mm2 graph sheet from which the wound surface area was evaluated. The evaluated surface area was then employed to calculate the percentage of wound healing area, taking the initial size of the wound as 100% by using the following equation:
where S0 is the initial wound area of rats’ skin after frostbite, St is the wound area of rats’ skin on a specific day. 24 h after last treatment, the rats were anesthetized and sacrificed. The rats’ skin were removed, fixed in 10% formalin, dehydrated in a graded ethanol solution, vitrication by dimethylbenzene, embedded in paraffin and cut into 4 μm thickness sections. The sections were then stained by hematoxylin and eosin (H&E) for histological analysis. A normal rat’s skin taking from another side (no frostbite) was used as normal control group.
Statistical analysis
All data were expressed as the mean ± standard deviation (SD). The data were analyzed by the one-way analysis of variance (ANOVA) followed by the least significant difference post-hoc test using SPSS 19.0 software (Chicago, IL). In both sets of statistical analyses, p value less than 0.05 was considered statistically significant.
Results and discussions
Preparation of GLT nanogel
Drug nanosuspensions can be regarded as pure drug particles stabilized by stabilizer with theoretical encapsulation of 100% (Xu et al., Citation2012). It can be produced by top-down process (breaking large drug particles as in media milling and high pressure homogenization), bottom-up process (building up nanoparticles from drug molecules via precipitation), or a combination of both (Pu et al., Citation2009; Ali et al., Citation2011). In previous study, 1% (w/v) GLT-NS with particle size of 283 ± 7 nm was successfully prepared by high pressure homogenization technique and the GLT-NS was firstly freeze-dried and then gelled with swelled carbopol 940P, so as to obtain GLT nanogel with drug loading of about 5% (w/w) (Shen et al., Citation2014).
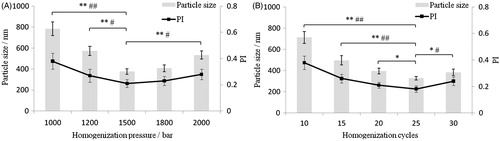
In this paper, a highly concentrated GLT-NS (10%, w/v) was prepared by high pressure homogenization and then directly gelled with swelled carbopol 940. Compared with our previous study, the concentration of GLT and stabilizers were all increased to 10-fold. The process of high pressure homogenization was re-optimized with particle size and PI as index based on our previous study and the results are shown in . The particle size and PI of the GLT-NS significantly decreased with the increase of homogenization pressure and cycles to 1500 bar and 25 cycles (p < 0.05 or p < 0.01), respectively. However, continuous increase in the homogenization pressure and cycles to 2000 bar and 30 cycles resulted in significant increase in particle size and PI of the GLT-NS (p < 0.05 or p < 0.01). This may be because that excessive energy input would probably cause damage to the stabilizer layer and might induce agglomeration (Xu et al., Citation2012). Therefore, 25 cycles of 1500 bar pressure is the optimum pressure cycle required to produce 10% GLT-NS.
Figure 1. Effects of homogenization pressure (A) and cycles (B) on particle size and PI value of GLT nanosuspension (*p < 0.05, **p < 0.01 for particle size; #p < 0.05, ##p < 0.01 for PI value).
In a previous study, freeze drying was used for solidification of GLT-NS before formulation of GLT nanogel. But the freeze drying of NS is time-consuming and agglomeration often occurs during freeze-drying of NS (Yue et al., Citation2014). In addition, the dry powders and swelled carbopol 940 are difficult to mix evenly for forming gel. In this study, the obtained GLT-NS was directly gelled with swelled carbopol 940 for forming GLT nanogel and the freeze drying process was not used, which is a simple and cost-effective manner.
Characterization of GLT nanogel
The particle size distribution and zeta potential analysis of GLT nanogel were assessed on the first day (D1) and the 1st, 3rd and 6th month, respectively. Before the incorporation of gel, the particle size and PI of freshly prepared GLT-NS were 328 ± 16 nm and 0.182 ± 0.024, which was just over the results of our previous study. As shown in , the particle size and PI of the GLT nanogel were 314 ± 23 nm and 0.197 ± 0.018 on the first day of production and no significant change in particle size were observed after 6 months of storage at room temperature. This indicated that the GLT-NS was still stably kept their particle size after it was entrapped in the gel and no particle aggregation occurred during storage at room temperature. The zeta potential of GLT-NS was −31.4 ± 2.8 mV and increased to −42.3 ± 3.7 mV after entrapment in the gel, indicating high stability in storage because of electrostatic repulsion.
Table 1. Stability of GLT nanogel at room temperature (25 ± 1°C) (means ± SD, n = 3).
Morphology of raw GLT and prepared GLT nanogel are compared in . Raw GLT performs irregular shape with the diameters in the range of 1–10 μm (). While well-dispersed GLT nanoparticles with particle size ranging from 200 to 400 nm were observed in GLT nanogel () and no significant particle agglomeration can be observed in the GLT nanogel.
The drug loading of GLT nanogel was 5% with relative drug content of 100.3 ± 2.9% and the pH of GLT nanogel was found to be 6.12 ± 0.1, which was within acceptable limits. The value of spreadability was 26.93 ± 2.75 (g·cm)/s, which indicated that the gel was easily spreadable by small amount of shear and possessed acceptable bioadhesion.
In vitro release experiments
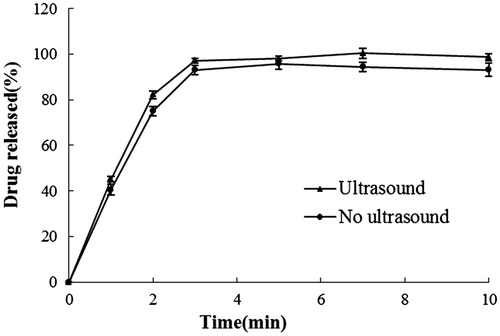
shows GA release profiles from the nanogel under the influence of TUS. There is no statistically significant difference between the release profiles, indicating that ultrasound has no influence on drug release from the nanogel.
In vitro transdermal study
In vitro transdermal permeation GA across rat skin was investigated under different experimental conditions. The data on skin permeation parameters (J, Tlag, ER and drug content in skin) of GA are listed in . The permeation profiles are shown in . As seen in this figure, the amounts of GA in the initial 20 min that permeated by means of TUS were significantly (p < 0.05) higher than that of passive diffusion (no TUS) that no permeation was observed. At the end of 60 min, the permeated amount of GA from GLT nanogel with TUS (10.60 μg/cm2) was about nine times enhancement over that of without TUS (1.14 μg/cm2) and there was a 4.5-fold increase in skin accumulation of GA from nanogel plus TUS (23.81 μg) compared with passive diffusion (5.28 μg). Higher values of flux and drug content in the skin were obtained by the application of TUS and indicated that TUS has a pronounced enhancement effect on drug across and into the skin. GA was detected in the receptor medium much sooner with the application of TUS (Tlag = 3.34 versus 21.12, as shown in ), indicating that the lag time for diffusion with TUS was reduced.
Figure 4. Amount of GA from nanogel penetrate through rat skin under the influence of TUS application.
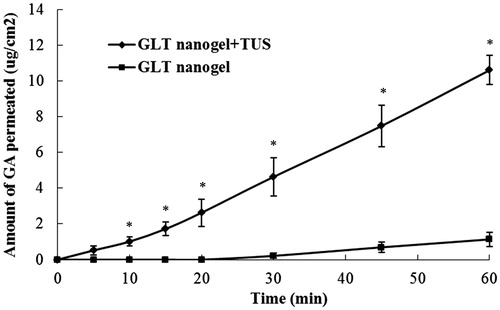
Table 2. Permeation parameters for GA from nanogel through rat skin with and without TUS (means ± SD, n = 3).
The use of ultrasound for the transdermal delivery of drugs has been investigated extensively and its mechanisms of skin permeability enhancement may include cavitation (generation and oscillation of gas bubbles), thermal effects (temperature increase), induction of convective transport and mechanical effects (occurrence of stresses due to pressure variation induced by ultrasound) (Tiwari et al., Citation2004; Polat et al., Citation2011; Alexander et al., Citation2012). However, the mechanisms of skin permeabilization induced by ultrasound with different frequency are different. With high-frequency ultrasound (high-frequency sonophoresis, HFS) which includes frequencies in the range of 0.7–16 MHz, non-inertial cavitation within the skin, either in skin appendages or at locations near the keratinocytes of the stratum corneum, is the main contributor to skin permeability enhancement (Polat et al., Citation2011). In addition, convection-related mechanisms occur only when ultrasound is applied and attenuation of an ultrasound wave leads to heating of the medium that the wave traverses, which also play minor roles on skin’s permeability to drugs. TUS with a frequency of 1 MHz was applied with simultaneous treatment method in this study. Therefore, non-inertial cavitation may be mainly responsible for skin permeability enhancement of GA and convection and thermal effects also play a major role in increasing permeant transport of GA. The specific mechanism of skin’s permeability to GA was not investigated because it was not the focus of the present study. But it deserves further investigation.
Pharmacodynamic efficacy of GLT nanogel using TUS
To address the effectiveness of TUS in the delivery of drugs for treating frostbite, pharmacodynamic trials to evaluate the efficacy of topical application of GLT nanogel (alone), TUS (alone) and GLT nanogel + TUS (plus) were performed in rats. Comparison among groups was done on the basis of Pwa on the particular day. The initial wound size before the application of drugs was taken as 100% and the results of the Pwa on days 6 and 12 are as shown in . On day 6, the mean Pwa of rats’ skin in the model group was 9.11%, whereas in the GLT nanogel group and GLT nanogel + TUS group, the mean Pwa of rats’ skin was 20.30 and 30.95%, respectively, and was significantly higher than that of the model group (p < 0.01). The mean Pwa of rats’ skin in the ultrasound group was 14.38% and was higher compared to the model group but not statistically different. On day 12, there was a statistically significant difference in the Pwa among all groups. Moreover, the animals treated with GLT nanogel associated to TUS exhibited better activity compared to other groups, indicating that ultrasound with a frequency of 1 MHz could improve the therapeutic effect for frostbite of the GLT nanogel. Representative photographs of wounds of different groups on day 1 and 12 are shown in .
Table 3. Therapeutic effect of GLT nanogel (alone), TUS (alone) and GLT nanogel + TUS (plus) on frostbite of rats’ skin (Means ± SD, n = 6).
Histopathological examination was performed to further support the results obtained from Pwa analysis. As displayed in , the skin of rats in the normal control group () showed clear structure with delineation between epidermis and dermis. The skin epithelial cells arranged neatly, and hair follicles and sebaceous glands can be observed in dermis. No inflammation and cell swelling were observed, and subcutaneous connective tissues were normal. Compared with the normal control group, typical pathological characteristics including massive epidermal cells diabrosis and defect with invisible delineation and cell necrosis, hemorrhage and hyperemia of capillary vessels, subcutaneously visible congestion, inflammation and cell swelling confirmed the successful establishment of frostbite (). The skins of rats treated with topical TUS, GLT nanogel and GLT nanogel plus TUS exhibited significant and varying degrees of improvement in frostbite. In the Ultrasound group and the GLT nanogel group, the epidermal diabrosis and defect were not fully healed and vascular congestion, edema and inflammatory cell infiltration were still visible in dermis (). While there were only subcutaneous edema and inflammatory cell infiltration in the GLT nanogel + TUS group, skin appendages showed no obvious morphological changes and the epidermal diabrosis and defect were fully healed (). The results of Pwa and histopathological examination showed that GLT nanogel and ultrasound with a frequency of 1 MHz were all effective in treatment of frostbite and ultrasound could improve the therapeutic effect for frostbite of the GLT nanogel.
Figure 6. Histological photomicrographs of rats’ skin (H&E stain × 20, N. Normal control group; (A) Model group; (B) Ultrasound group; (C) GLT nanogel group; (D) GLT nanogel + TUS group).
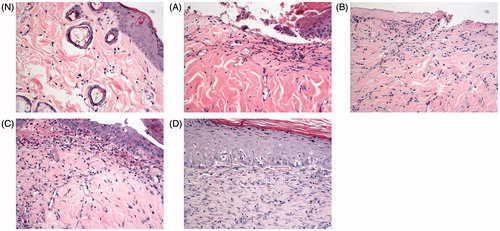
Possible explanation for the improvement of therapeutic effect for frostbite of the GLT nanogel caused by TUS could be as follows: (i) the biological changes made by the mechanical and thermal effects of TUS including the promotion of blood circulation, an increase in the tissues’ regenerative power, reduction of edema and modifying the formation of scar tissue (Cagnie et al., Citation2003; Merino et al., Citation2003;Yang et al., Citation2005) could promote the healing of frostbite tissue; (ii) ultrasound could improve the diffusion of the GLT nanogel through and into the skin and then enhanced its therapeutic effect for frostbite.
Conclusion
Considering results of this study and the previous results from our group, it was concluded that TUS can be effectively used to actively enhance topical delivery of GLT from nanogel and improve the therapeutic effect for frostbite in rats. GLT nanogel + TUS has beneficial effects on the frostbite healing process by increasing survival area and improving the degree of pathological change of skin tissue of the rats’ skin with frostbite.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
This study was supported by the Beijing Natural Science Foundation of China (No. 7122176), the National Natural Science Foundation of China (No. 81102821) and the National Key New Drugs Innovation Foundation (No. 2014ZX09J14106-01A, CWS11J165).
References
- Alexander A, Dwivedi S, Ajazuddin, et al. (2012). Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J Control Release 164:26–40
- Alfredo PP, Anaruma CA, Pião AC, et al. (2009). Effects of phonophoresis with Arnica montana onto acute inflammatory process in rat skeletal muscles: an experimental study. Ultrasonics 49:466–71
- Ali HS, York P, Ali AM, Blagden N. (2011). Hydrocortisone nanosuspensions for ophthalmic delivery: a comparative study between microfluidic nanoprecipitation and wet milling. J Control Release 149:175–81
- Boh B. (2013). Ganoderma lucidum: a potential for biotechnological production of anti-cancer and immunomodulatory drugs. Recent Pat Anticancer Drug Discov 8:255–87
- Bommannan D, Okuyama H, Stauffer P, Guy RH. (1992). Sonophoresis. I. The use of high-frequency ultrasound to enhance transdermal drug delivery. Pharm Res 9:559–64
- Cagnie B, Vinck E, Rimbaut S, Vanderstraeten G. (2003). Phonophoresis versus topical application of ketoprofen: comparison between tissue and plasma levels. Phys Ther 83:707–12
- Dudhgaonkar S, Thyagarajan A, Sliva D. (2009). Suppression of the inflammatory response by triterpenes isolated from the mushroom Ganoderma lucidum. Int Immunopharmacol 9:1272–80
- Han F, Yin R, Che X, et al. (2012a). Nanostructured lipid carriers (NLC) based topical gel of flurbiprofen: design, characterization and in vivo evaluation. Int J Pharm 15;439:349–57
- Han J, Zhou X, Yuan HL, et al. (2012b). Research on the therapertic effect of compound Lingzhi cream for mild frostbite on rabbit ears. Chin Pharm J 47:689–92
- Karwa AS, Rai MK. (2012). Naturally occurring medicinal mushroom-derived antimicrobials: a case-study using Lingzhi or Reishi Ganoderma lucidum (W. Curt.:Fr.) P. Karst. (higher Basidiomycetes). Int J Med Mushrooms 14:481–90
- Koyama K, Imaizumi T, Akiba M, et al. (1997). Antinociceptive components of Ganoderma lucidum. Planta Med 63:224–7
- Maia Filho AL, Villaverde AB, Munin E, et al. (2010). Comparative study of the topical application of Aloe vera gel, therapeutic ultrasound and phonophoresis on the tissue repair in collagenase-induced rat tendinitis. Ultrasound Med Biol 36:1682–90
- Merino G, Kalia YN, Guy RH. (2003). Ultrasound-enhanced transdermal transport. J Pharm Sci 92:1125–37
- Mutalik S, Nayak UY, Kalra R, et al. (2012). Sonophoresis-mediated permeation and retention of peptide dendrimers across human epidermis. Skin Res Technol 18:101–7
- Polat BE, Hart D, Langer R, Blankschtein D. (2011). Ultrasound-mediated transdermal drug delivery: mechanisms, scope, and emerging trends. J Control Release 152:330–48
- Pu X, Sun J, Wang Y, et al. (2009). Development of a chemically stable 10-hydroxycamptothecin nanosuspensions, Int J Pharm 379:167–73
- Shen CY, Xu PH, Shen BD, et al. (2014). Nanogel for dermal application of the triterpenoids isolated from Ganoderma lucidum (GLT) for frostbite treatment. Drug Deliv 25:1–9
- Silveira PC, Victor EG, Notoya FD, et al. (2014). Effects of phonophoresis with gold nanoparticles on oxidative stress parameters in a traumatic muscle injury model. Drug Deliv 17:1–7
- Smina TP, Mathew J, Janardhanan KK, Devasagayam TP. (2011). Antioxidant activity and toxicity profile of total triterpenes isolated from Ganoderma lucidum (Fr.) P. Karst occurring in South India. Environ Toxicol Pharmacol 32:438–46
- Srivastava P, Durgaprasad S. (2012). Burn wound healing property of Cocos nucifera: an appraisal. Indian J Pharmacol 40:144–6
- Tiwari SB, Pai RM, Udupa N. (2004). Influence of ultrasound on the percutaneous absorption of ketorolac tromethamine in vitro across rat skin. Drug Deliv 11:47–51
- Venkataraman M, Nagarsenker M. (2013). Silver sulfadiazine nanosystems for burn therapy. AAPS PharmSciTech 14:254–64
- Xu Y, Liu X, Lian R, et al. (2012). Enhanced dissolution and oral bioavailability of aripiprazole nanosuspensions prepared by nanoprecipitation/homogenization based on acid-base neutralization. Int J Pharm 438:287–95
- Yang JH, Kim DK, Kim TY, et al. (2005). Anti-inflammatory effects by transdermal application of triamcinolone acetonide gel using phonophoresis in rats. Int J Pharm 302:39–46
- Yue PF, Li G, Dan JX, et al. (2014). Study on formability of solid nanosuspensions during solidification: II novel roles of freezing stress and cryoprotectant property. Int J Pharm 475:35–48