Abstract
Context: Dexamethasone is the major drug in the treatment of ulcerative colitis (UC). However, the extensive or long-time use of dexamethasone causes many toxic side-effects. Ion exchange resins react with external-ions through their own functional groups and Eudragit S occurs degradation when pH > 7. These features make them suitable for oral delivery system.
Objective: Resin microcapsule (DRM) composed by 717 anion exchange resin and Eudragit S100 was used to target dexamethasone to the colon to improve its treatment effect on UC and reduce its toxic side-effects.
Results: Dexamethasone sodium phosphate (DXSP) was sequentially encapsulated in 717 anion-exchange resin and Eudragit S100 to prepare the DXSP-loaded resin microcapsule (DXSP-DRM). The in vitro release study and in vivo study of pharmacokinetics and the intestinal drug residues in rat demonstrated the good colon-targeting of DXSP-DRM. Moreover, the DXSP-DRM can reduce the toxic side-effects induced by DXSP and have good therapeutic effects on colitis mouse induced by 2,4,6-trinitrobenzenesulfonic acid.
Discussion: Dexamethasone can be targeted to the colon by DRM, thereby enhancing its treatment effect and reducing its toxic side effects.
Conclusion: The resin microcapsule system has good colon-targeting and can be used in the development of colon-targeted preparations.
Introduction
As the representative type of inflammatory bowel disease (IBD), ulcerative colitis (UC) is a chronic disease and the etiology of which is still unknown (Meier & Sturm, Citation2011; Monteleone et al., Citation2011; Freeman, Citation2013; Blonski et al., Citation2014). UC has a long course of disease and is prone to recur, thus seriously affecting the quality of patients’ life. Corticosteroids have played an important role in the treatment of UC in the past 50 years (Truelove & Witts, Citation1954; Lennardjones, Citation1983; Turner et al., Citation2007). Through reducing the permeability of capillaries, stabilizing the membranes of cell and lysosome and preventing the combined arachidonic acid from being converted to the free arachidonic acid in the phospholipids of cell membranes, corticosteroids reduce the proinflammatory cytokines (e.g. leukotrienes, prostaglandins and thromboxane), suppress the immune response and ease the symptoms ultimately (Dial & Lichtenberger, Citation1986; Jewell, Citation1989; Mondino et al., Citation2004; Myou et al., Citation2004; Barnes, Citation2006; Li et al., Citation2007). However, the extensive or long-time use of corticosteroids causes many toxic side-effects, such as the risk of opportunistic infection, diabetes mellitus, osteoporosis and the possible development of steroid-dependent disease (Buchman, Citation2001; Dahl, Citation2006; De Cassan et al., Citation2012). Therefore, using pharmaceutical methods, namely, specific drug carriers (e.g. microcapsule, microsphere and hydrogels), to deliver drugs into lesion sites can prevent the toxic side-effects caused by systemic absorption and these methods have attracted more and more attention (Eroglu et al., Citation2001; Kopacz et al., Citation2003; Zhao et al., Citation2011; Das & Subuddhi, Citation2014; Dong et al., Citation2014).
Ion-exchange resins (IER) are a kind of water insoluble inert polymer materials which have been widely used in several scientific investigations due to their ion-exchange reaction with external-ions by their own functional groups (Amsel & Hinsvark, Citation1984; Anand et al., Citation2001; Guo et al., Citation2009). In recent years, numerous researches have revealed that IER were suitable for a variety of drug-delivery systems, such as controlled-release, transdermal, site-specific, fast dissolving and taste-masked systems (Narisawa et al., Citation1994; Burton et al., Citation1995; Jaskari et al., Citation2000; Sohi et al., Citation2004; Yu et al., Citation2006; Jeong & Park, Citation2008; Samprasit et al., Citation2013; Yewale et al., Citation2013). Among them, the controlled-release drug-delivery systems based on IER have been paid more and more attention due to their improved effectiveness and safety of drugs, reduced side-effects and the prolonged duration of drug action. Moreover, as a kind of methyl acrylic acid-methyl methacrylate copolymer, Eudragit has been widely used in the pharmaceutical industry (Thakral et al., Citation2013). As a kind of Eudragit, Eudragit S100 is stable in the environment below pH 7.0 and degradation occurs above 7.0. This feature makes it usually be used as the coating material in the enteric and colon-targeted preparations (Deeb & Becker, Citation1960; Lilienfield & Zapolski, Citation1983; Chen et al., Citation1992).
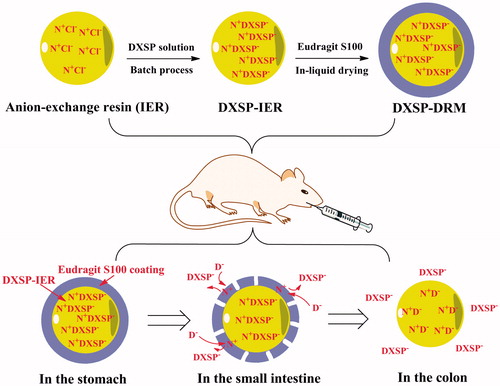
In this study, both IER and Eudragit S100 were used to encapsulate a kind of corticosteroid, dexamethasone (in the form of its sodium salt), to achieve the colon-targeting. Dexamethasone sodium phosphate (DXSP) was sequentially encapsulated in 717 anion-exchange resin and Eudragit S100 to prepare the DXSP-loaded resin (DXSP-IER) and DXSP-load resin microcapsule (DXSP-DRM).The in vitro release study investigated the release behavior of DXSP-IER and DXSP-DRM, respectively. In the in vivo study, the determination of the pharmacokinetics and the intestinal drug residues in rat investigated the colon-targeting of the DXSP-DRM. The improvement of DRM on the toxic side-effects induced by DXSP was also studied in detail. The therapeutic effect of DXSP-DRM on the TNBS induced colitis mouse was tested and the results showed good treatment effect. Consequently, the DXSP-DRM has good colon-targeting and can significantly reduce the toxic side-effects induced by DXSP.
Materials and methods
Materials and reagents
Dexamethasone sodium phosphate (DXSP) was purchased from Henan Rongsheng Chemical, China. In total, 717 anion exchange resin was obtained from Xi’an Lanxiao Technology, China. Eudragit S100 was purchased from Rohm, Germany. Liquid paraffin, Span 80, PEG 400 and PEG 4000 were purchased from Tianjin Kermel Chemical, China. All other reagents were analytical grade and obtained from commercially available sources.
2,4,6-Trinitrobenzenesulfonic acid (TNBS) was purchased from Sigma (St. Louis, MO). O-dianisidine dihydrochloride (ODD) were purchased from Shanghai Aladdin Chemistry, China. Hexadecyl trimethyl ammonium bromide (HTAB) and 30% H2O2 solution were purchased from Tianjin Kermel Chemical, China. Heparin sodium (average molecular weight: 12 000) was purchased from Guangdong Guanghua Chemical, China. All other chemicals were analytical grade, commercially available products.
Animals
Male Sprague–Dawley rats weighing 200 ± 10 g were used for the pharmacokinetics and the intestinal drug residues evaluations. Male BALB/c mice weighing 20 ± 2 g (mean ± SD) were used for the improvement of the adverse reactions induced by DXSP and the treatment of the TNBS-induced colitis. These animals were purchased from the Laboratory Animal Center of Xi’an Jiaotong University and housed under controlled temperature and relative humidity conditions of 20–22 °C, and 50–60%, respectively, and under a 12/12 h light/dark cycle. All animals had ad libitum access to water and food. Meanwhile, they were quarantined for 1 week prior to treatment.
Preparation of drug-resin microcapsule (DXSP-DRM)
Pretreatment of ion-exchange resin (IER)
A certain quality of 717 anion-exchange resin was immersed and washed in 50 °C deionized water to remove the water-soluble impurities. Then, it was transferred into 95% ethanol with stirring to remove the organic impurities. After washed with deionized water until no residual ethanol existed, the IER was dried under a vacuum at 50 °C. The pre-dried IER was immersed in 0.1 mol/l hydrochloric acid solution with constant stirring for 24 h, washed with deionized water until neutral and dried to obtain the anion(Cl−) exchange resin.
Preparation of DXSP–IER complex
The DXSP–IER complex was prepared through a batch process. The purified IER (0.50 g) was suspended in a 6.0 g/l DXSP aqueous solution under magnetic stirring at 30 °C. Samples which were determined by high-performance liquid chromatography (HPLC; Chromatographic conditions: C18 ODS chromatographic column, mobile phase: pH = 3.0 triethylamine aqueous solution:acetonitrile:methanol 55:40:5, v/v/v, column temperature 30 °C, flow rate 1 ml/min, detection wavelength 242 nm) were collected from DXSP aqueous solution at each pre-determined time internal, and the drug-loading capacity (Q) of IER was determined by the measurement of the residual DXSP in solution, which was calculated according to the following equation:
where Qt was the drug-loading capacity of resin at time t. C0 was the initial drug concentration and Ct was the drug concentration at time t. V was the volume of drug solution and WR was the quality of resin.
Preparation of drug-resin microcapsule (DXSP-DRM)
In this study, the water insoluble coating material Eudragit S100 which was easily dissolved in the alkaline environment (pH > 7) was used to encapsulate the DXSP-IER to prepare the colon-targeted drug-resin microcapsule (DXSP-DRM) through an in-liquid drying process. Specifically, 20 ml liquid paraffin and 2.5 ml Span 80 were mixed together and stirred evenly to form the continuous phase. About 0.1 g Eudragit S100 and 0.01 g PEG 400 were dissolved in 7.5 ml acetone to form the dispersed phase following 0.5 g DXSP-IER which was firstly immersed in 20% (w/w) PEG 4000 solution added in the dispersed phase with stirring. The prepared dispersed phase was dropped into the continuous phase to form the emulsion with constant stirring. Then, the emulsion was stirred for 6 h at 40 °C to remove acetone. Finally, the DXSP-DRM was obtained after filtered and washed by petroleum ether and dried at 40 °C.
In vitro release study
In this part, two in vitro release experiments was investigated according to the paddle method of the dissolution determination of Chinese Pharmacopoeia (2010 edition), respectively. First of all, to investigate the release behavior of DXSP-IER in physiological saline (PS), 0.5 g DXSP-IER was added in 900 ml PS and the operation was carried under the condition of 37 ± 0.5 °C and 50 rpm. Five milliliters samples were collected and the same volume of release medium was added at the pre-determined time internal. The HPLC method was used to determine the DXSP content in the release medium. Second, the release behavior of DXSP-DRM was investigated through simulating the in vivo environment of the gastrointestinal tract. Specifically, 0.5 g DXSP-DRM was successively added into the artificial gastric juice (pH = 1.2) for 2 h, artificial small intestinal juice (pH = 6.8) for 4 h and artificial colon juice (pH = 7.4) for 6 h. And, the operation was carried under the condition of 37 ± 0.5 °C and 50 rpm. Five milliliters samples were collected and the same volume of release medium was added at the pre-determined time internal (1 h). After filtered by 0.45 μm filter membrane, samples were determined by HPLC to calculate the cumulative release rate (%). In this part, the method of HPLC was determined according to the “Preparation of DXSP–IER complex” section.
Colon-targeting evaluation of DXSP-DRM
Pharmacokinetic study
In this study, rats were randomly divided into two groups (n = 6 per group) according to the forms of DXSP administered (p.o. single dose of 5 mg/kg): DXSP solution group and DXSP-DRM group (suspended with 0.5% CMC-Na solution). The blood samples were drawn from the tail vein at 10 min, 40 min, 70 min, 100 min, 2 h, 2.5 h, 3 h, 3.5 h, 4 h, 5 h, 6 h, 7 h and 8 h post-dosing. Each blood sample was immediately centrifuged at approximately 12 000 rpm for 10 min followed collecting 100 μl supernatant plasma. Then 400 μl of methanol was added to the collected plasma sample and stirred vigorously for 1 min to precipitate residual proteins. The mixture was centrifuged at 12 000 rpm for 10 min followed transferring a 400 μl aliquot of the upper organic phase to another tube and evaporating to dryness at 40 °C with nitrogen. The resulting extract was dissolved in 150 μl of methanol and mixed. After centrifugation at 12 000 rpm for 10 min, 100 μl of the supernatant was taken for high-performance liquid chromatography (HPLC) analysis.
The methodological HPLC study was performed according to the “Preparation of DXSP–IER complex” section and the sample injection volume was 100 μl. The linearity of DXSP was assessed by a calibration that was obtained by plotting the peak area of each analyte versus DXSP concentration. The detected concentrations were in the range of 0.15–40 μg/ml. The absolute recovery of DXSP was determined at different standard concentrations (0.15, 5 and 40 μg/ml) by spiking the corresponding fresh blank plasma. The percentage recovery was obtained by comparing the peak area of the extracted samples with samples in which the same amounts of compounds were diluted with mobile phase and injected directly. The recoveries for three standard concentration levels of DXSP were tested at least five times. To evaluate the precision, these extracted samples at three concentrations (0.15, 5 and 40 μg/ml) were processed and injected five times within 1 day (intraday) and within 5 days (interday) for calculating the relative standard deviation (RSD).
Determination of the intestinal drug residues
Fifteen rats were orally administered with DXSP-DRM after fasted for 12 h (p.o. single dose of 5 mg//kg). Three of them were sacrificed per hour at the 1st, 2nd, 3rd, 4th and 6th h, respectively. Then the contents of the stomach, small intestine and cecum were immediately separated and collected. After washed by distilled water, the free DXSP was extracted from these contents through ultrasonic processing. The contents of the free DXSP was determined by HPLC method after filtered by 0.45 μm filter membrane and the measurement was the same as in the “Preparation of DXSP–IER complex” section.
Improvement of DXSP-DRM on the adverse reaction induced by DXSP
Thirty six mice were rats were randomly divided into three groups (n = 12 per group): control group, DXSP solution group and DXSP-DRM group. The control group was administered with PS, and both the dosages of DXSP solution group and DXSP-DRM group were 10 mg/kg. These mice were administered once a day and continuously for 10 days. Meanwhile, they were weighed before administration everyday and the variation of the body weight was recorded during the 10 days. All animals were sacrificed on the 10th day and their spleen and thymus were collected. After cleaned by filter paper, the spleen and thymus were weighed and the viscera indexes was calculated according the following equation:
Therapy of DXSP-DRM on the experimental colitis mouse induced by TNBS
Establishment of colitis models induced by TNBS
The induction of colitis was performed according to the Wang et al. (Citation2001) method. Specifically, after fasting for 12 h, mice were anesthetized with ether before induction of colitis. About 0.1 ml 50% (v/v) ethanol which contained 2.5% (v/v) TNBS was instilled into the colon 3.5–4.0 cm from the anus by a gavage needle. Mice were kept in a head-down position for 30 s to prevent the leakage of the intracolonic instillation. In this study, mice who received PS instead of TNBS were set up as the blank control (p.o., aqueous solution of CMC-Na, n = 15). The dosage of TNBS groups treated with DXSP solution or DXSP-DRM was 2.5 mg/kg (p.o., n = 15), respectively. The TNBS group (p.o., aqueous solution of CMC-Na, n = 15) was also set up. The administration started after 12 h.
Measurement of survival rate and body weight changes
At the end of the experiment, namely, 5 days later after administration, the number of the ultimately surviving mouse was recorded and the final survival rate was calculated. As the colitis was induced by TNBS, the appetite and weight of mouse decreased significantly after modeling. Therefore, a trend graph of mouse body weight changes was drawn according to the weight variation through the daily records of mouse weight.
Measurement of disease activity index (DAI)
Disease activity index (DAI) reflects the therapeutic effect of different drugs on the colitis induced by TNBS (Patel et al., Citation2012). shows the DAI scoring criteria of mouse. The percentage of weight loss which was calculated according to the daily records of the weight variation combined with the characteristics of the mouse excrement was recorded and scored according to . Moreover, the test of fecal occult blood was also added to calculate the composite score. And, the DAI was the sum of the three scores. The test of mouse fecal occult blood was performed using o-tolidine. Specifically, a small amount of mouse feces was smeared on a white plate. About 2–3 drops of 10 g/l of O-benzidine in glacial acetic acid solution was dripped into the feces and blended evenly. Then 2–3 drops of 3% H2O2 solution was added in the mixture, which was followed timing as well as observing the results immediately. The evaluation criteria of fecal occult blood level were shown in . In this study, the daily DAI of each group was recorded and the figure of DAI varying trend was drawn. And, the differences between groups were evaluated simultaneously.
Table 1. The scoring system of disease activity index (DAI) of mouse.
Table 2. The assay criteria of O-benzidine to determine the fecal occult blood of mouse.
Measurement of MPO Activity
The measurement of Myeloperoxidase (MPO) is to determine the activity of myeloperoxidase which is used to measure the accumulation of neutrophils (McCabe et al., Citation2001). Specifically, the weighted colonic tissue of mouse was homogenized in 10 volumes of pH 7.4 phosphate buffer. After homogenizing for 30 s at 4 °C, the homogenate was centrifuged at 12 000 rpm for 30 min, then the supernatant was discarded. Fifty milligrams precipitated tissue was taken to mix with 0.5% HTAB solution followed the centrifugation at 12 000 rpm for 15 min. Then 0.1 ml supernatant was collected and added into 2.9 ml ODD solution. After rapidly mixing, the sample was measured continuously in 3 min at 460 nm using an UV spectrophotometer followed recording the absorbance of the system and calculating the value of absorbance change per minute. In this study, one enzyme unit was defined as the amount of 1 μmol peroxide decomposed per minute at 25 °C.
Statistical analysis
Statistical analyses were performed with SPSS version 13.0 (SPSS Inc., Chicago, IL) for Windows. All results were expressed as mean ± SD. Data between groups were compared using Student’s t-test. And data among multiple groups were compared using one-way ANOVA test. p < 0.05 were considered to be statistically significant.
Results
Preparation of DXSP-IER and DXSP-DRM
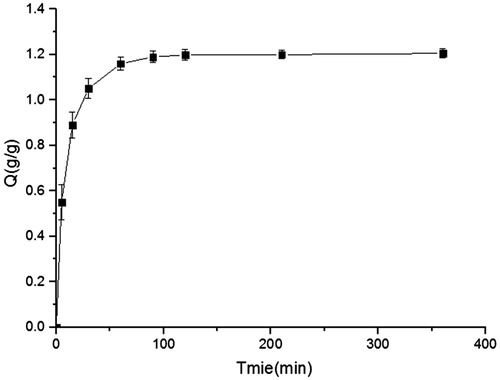
In this work, DXSP was encapsulated in IER through the batch process (Ichikawa et al., Citation2001). During the drug-loading process, samples collected at each time internal were determined by HPLC and the drug-loading capacity (Q) was the difference between the total dosage and the residual DXSP in solution. presents the drug-loading capacity of resins changes versus time curve. As shown in , the ion-exchange reaction achieved balance at 100 min, and the drug-loading capacity was 1.20 g/g. In the coating process, in-liquid drying method was used to prepare DXSP-DRM (Li et al., Citation2008). Liquid paraffin which was stable and not easy to volatilize was selected as the continuous phase. The dispersed phase was acetone which could dissolve the coating material (Eudragit S100) and be volatilized easily. Span 80 and PEG 400 were used as emulsifier and plasticizer, respectively. Moreover, after immersed in the PEG 4000 solution, the swelling degree of DXSP-IER significantly reduced, thus preventing the occurrence of burst release after the film-coated layer (composed by Eudragit S100) of DXSP-DRM was degraded. In this work, the DXSP-DRM was obtained through removing the volatile dispersed phase by heating and stirring, thus guarantying the shape and dispersion of DXSP-DRM.
In vitro release study
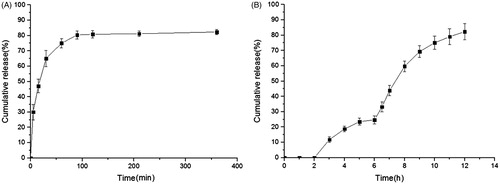
shows the in vitro release behavior of the DXSP-IER. As shown in , the release rate increased rapidly within the first 100 min, then reduced significantly. And, the cumulative release rate (%) reached the maximum at the 2 h (82.3%). presents the in vitro release curve of DXSP-DRM at different pH values. This experiment imitated the pH values and the transit time of stomach, small intestine and colon. Since an excellent colon-targeted preparation should not release or release less drug in the gastric environment (pH 1.2); releasing parts of drug in the intestinal environment (pH 6.8); while releasing lots of drug in the colonic environment (pH 7.4). The results of the in vitro release experiment confirmed the good colon-targeting of DXSP-DRM. As shown in , DXSP-DRM did not release any DXSP in the acidic stomach environment. However, the release rates changed significantly when the pH values varied. In the intestinal environment (pH 6.8), the accumulated release was <30%. While in the colonic environment (pH 7.4), massive DXSP released from the DXSP-DRM (>80%). This release behavior benefited drugs be concentrated at the targeted-site and paly a therapeutic role.
Colon-targeting evaluation of DXSP-DRM
Pharmacokinetic study
A linear relationship within the range of 0.15–40 μg/ml was found between the peak areas of DXSP in blood and theoretical concentrations. And the regression equation of calibration curves was A = 6121C − 2484, R = 0.9997. The lowest detectable concentration of DXSP in blood was 0.15 μg/ml. For recovery detection, the absolute recoveries of DXSP in blood ranged from 95.7% to 103.6% () and no obvious difference occurred among the 3 concentrations (0.15, 5 and 40 μg/ml). For precision test, the intra- and inter-day RSD of blood assay were less than 5% in three different concentrations (). These results indicated that the reproducibility and recovery were acceptable over the studied concentration range.
Table 3. Recovery and precision for DXSP determination in rat plasma by HPLC.
The plasma concentration–time profiles of DXSP and DXSP-DRM after oral administration are shown in . The corresponding pharmacokinetic parameters were summarized in . The single oral dose was both 5 mg/kg DXSP per kg. After oral administration of DXSP, the peak blood concentrations (Cmax = 6.98 μg/ml) of DXSP appeared at 0.69 h post-dose. Nevertheless, when orally taking DXSP-DRM, the variation of blood concentrations of rats was stable and no sharp changes occurred. The peak blood concentrations (Cmax = 4.08 μg/ml) of DXSP appeared at 2.25 h post-dose. The Cmax of the oral DXSP-DRM group was far lower than that of the oral DXSP group. The AUC0–12 of the experimental group was far lower than that of the control group and the former was 0.41 times of the latter, indicating that the DRM could control the release of DXSP through the changes of pH value and ionic strength in the digestive tract, thereby reducing and delaying the absorption of DXSP.
Figure 3. The plasma concentration–time curves in rats of DXSP solution (p.o. administration of 5 mg/kg) and DXSP-DRM solution (p.o. administration of hydrogel containing 5 mg/kg dexamethasone; data are mean ± SD, n = 6).
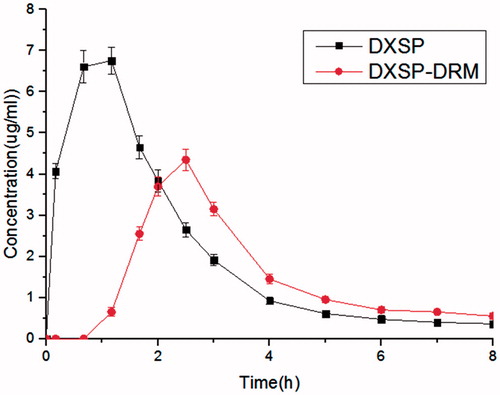
Table 4. Pharmacokinetic parameters of DXSP and DXSP-DRM in rat plasma after p.o. administration of DXSP solution (5 mg/kg) and DXSP-DRM containing 5 mg/kg DXSP (data are mean ± SD, n = 6).
Determination of the intestinal drug residues
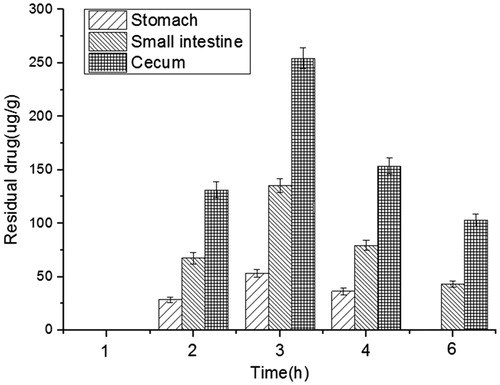
The distribution trend of DXSP in the different parts of the rat’s digestive tract is shown in . From , DXSP could not be detected in the whole digestive tract after administration for 1 h. However, the free DXSP could be detected in the parts of the stomach, small intestine and cecum after 2 h and the concentration of DXSP in cecum was much higher than in the small intestine. After administration for 3 h, the concentration of the free DXSP in the cecum reached the maximum. Then, due to the peristalsis and metabolism of the intestinal tract, the residual of DXSP decreased gradually. However, the concentration of DXSP in the cecum was always greater than in the small intestine.
Improvement of DXSP-DRM on the adverse reaction induced by DXSP
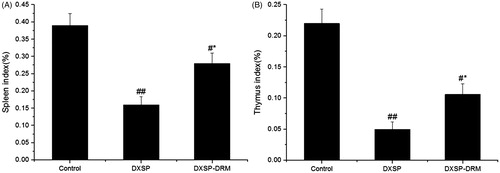
The variation of the mouse body weight influenced by DXSP-DRM is shown in , and the presents the influence of DXSP-DRM on the ultimate mouse body weight. From , the body weight of the mouse administered with PS showed the fastest growth rate, then followed the mouse administered with DXSP-DRM. The body of the mouse administered with DXSP showed the slowest growth rate. From , the results of the t-test indicated that the body weights of DXSP and DXSP-DRM group all showed significant differences compared with the control group. Moreover, the difference of DXSP group (p < 0.01) was more significant than the DXSP-DRM group (p < 0.05). The spleen index is shown in and the thymus index was shown in . Both the results of showed that each group administered with DXSP or DXSP-DRM appeared different degree of toxic reaction. The spleen and the thymus index of the DXSP declined significantly compared with the control group (p < 0.01). However, the DXSP-DRM group decreased less (p < 0.05).
Figure 5. The influence of DXSP and DXSP-DRM on the body weight changes (A) and the ultimate body weight after 10 days (B) of mouse in the improvement of DXSP-DRM on the adverse reaction induced by DXSP (data are mean ± SD, n = 12). #,*Mean values with different superscript symbols were significantly different. ##p < 0.01, #p < 0.05 compared to the control group; *p < 0.05 compared to the TNBS group. Control, control group; DXSP, DXSP p.o. group; DXSP-DRM, DXSP-DRM p.o. group.
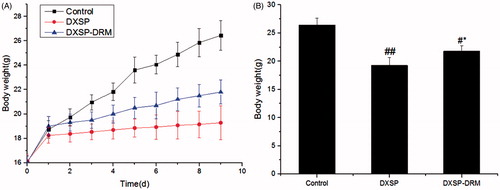
Figure 6. The influence of DXSP and DXSP-DRM on the spleen index (A) and the thymus index (B) of mouse (data are mean ± SD, n = 12). #,*Mean values with different superscript symbols were significantly different. ##p < 0.01, #p < 0.05 compared to control group; *p < 0.05 compared to TNBS group. Control, control group; DXSP, DXSP p.o. group; DXSP-DRM, DXSP-DRM p.o. group.
Effect of DXSP-DRM on the survival rate and body weight changes
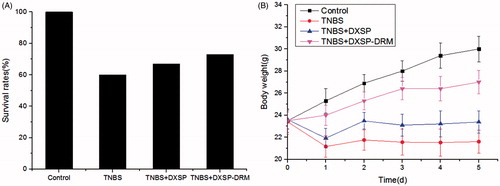
is the influence of DXSP-DRM on the survival rate of mouse. From , the mouse survival rates showed little difference between other two groups apart from the control group, which might be due to the relatively large individual differences of the resistance of mouse to TNBS. presents the influence of DXSP-DRM on body weight changes. From , the weight of the control group always maintained stable growth. However, the TNBS group showed sharp decline after modeling, then the decreasing trend slowed down. Furthermore, both weights of the DXSP-DRM group and the DXSP solution group began to rise gradually from the second day after administration and showed stable growth trend. However, compared with the DXSP solution group, the DXSP-DRM group showed a more obvious growth trend, indicating the better treatment effect of DXSP-DRM.
Effect of DXSP-DRM on the results of DAI and MPO activity
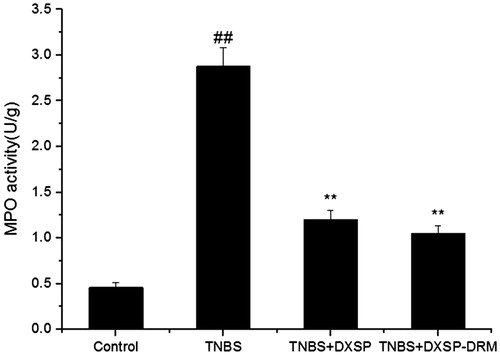
After modeled by TNBS, some conditions, such as bloody stools, diarrhea, reduced activity and weight loss, could be observed on the modeled mouse after 24 h. reflects the variation tendency of the DAI of each group and presents the influence of DXSP-DRM on the ultimate DAI. As shown in , the DAI of control group was 0. However, due to the influence of inflammation, the DAI of TNBS group was significantly higher than the DXSP-DRM group and DXSP group. After treatment, both DAI indices of DXSP-DRM group and DXSP group showed obvious decline. The results of indicate that both DAI indices of DXSP-DRM group and DXSP group showed significant difference compared with the TNBS group (p < 0.01). And they showed similar decreased trend. As a peroxidase, MPO is generated by the azurophil granules of neutrophils and is mainly distributed in the neutrophils and monocytes. Since the neutrophils and monocytes play a major role in the inflammatory response, the increase of MPO in colon tissue reflects the increase of neutrophils at colon lesions, thereby reflecting the existence of inflammatory at the colon site. is the influence of DXSP-DRM on the activity of MPO. As shown in , compared with the TNBS group, both MPO indices of DXSP-DRM group and DXSP group decreased significantly (p < 0.01). And, the downward trend of them was also similar. Both results of the variation of DAI and MPO indices suggested the good therapeutic effect of DXSP-DRM on the TNBS induced colitis of mouse.
Figure 8. The influence of DXSP and DXSP-DRM on the variation of DAI index (A) and the ultimate DAI index after 5 days (B) of mouse (data are mean ± SD, n = 15). ##,**Mean values with different superscript symbols were significantly different. ##p < 0.01, compared to control group; **p < 0.01 compared to TNBS group. Control, control group; TNBS, TNBS group; DXSP, DXSP p.o. group; DXSP-DRM, DXSP-DRM p.o. group.
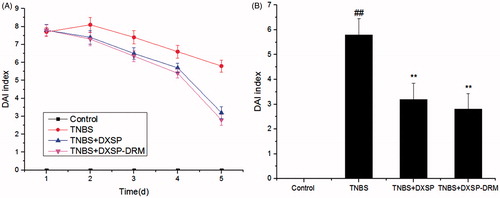
Figure 9. The influence of DXSP and DXSP-DRM on the activity of MPO in the colonic tissue of mouse. ##,**Mean values with different superscript symbols were significantly different. ##p < 0.01 compared to control group; **p < 0.01 compared to TNBS group. Control, control group; TNBS, TNBS group; DXSP, DXSP p.o. group; DXSP-DRM, DXSP-DRM p.o. group.
Discussion
Since Hench successfully used cortisone to therapy the rheumatoid arthritis in 1949, corticosteroids as a good anti-inflammatory and immunosuppressive agent have been widely used in clinic and have become an important means for the clinical treatment of various diseases (Lundberg et al., Citation2004). For the treatment of the UC, corticosteroids have good inhibitory effects on the increased vascular permeability, vascular dilatation and leukocyte infiltration caused in the early stage of the inflammation. And, they also inhibit the vascular proliferation, fibroblast and collagen deposition formed in the later stage of the inflammation. However, the large number or long-term of oral administration will cause serious side-effects (Friend, Citation2005). Therefore, it is necessary to develop colon-targeted preparation to prevent corticosteroids from being absorbed in the upper gastrointestinal in advance, thus not only concentrating drug at the colon site to play a treatment role, but also reducing its toxic side-effects.
In this study, the 717 anion exchange resin which was mainly composed of styrene–divinylbenzene copolymer was used to encapsulate DXSP. Its functional group was the quaternary ammonium groups which contained positive charge, and the active ion was chloride ion. After the resin was immersed in the drug solution, the DXSP anions replaced the chloride ions which were connected with quaternary ammonium groups through ionic bonds. In the in vitro release study of the drug-loaded resin, the release rate increased significantly in the first 100 min with the increase of time. However, it obviously slowed down after 100 min. Moreover, the cumulative release rate reached the maximum in 2 h. The results indicated that in the medium with a certain concentration, the release of the drug-loaded resin depends on the exchanging speed between the drug ions in the resin and the medium ions. Therefore, due to the limitation of the resin volume and the number of active groups, with the increasing of the medium concentration or the prolonging of the immersing time, the resin could finish releasing in a short time. In addition, in the different parts of the human digestive tract, the ionic strength is different, thus making the resin release drug in advance before reaching the colon site. Therefore, only depending on the IER could not achieve colon-targeting. In this study, Eudragit S100 was employed to encapsulate the DXSP loaded-resin through the in-liquid dying method to prepare the drug-resin microcapsule (DXSP-DRM). From the in vitro release study, the release rate of the DXSP-DRM was much lower in the solution environment pH < 7.0 (the cumulative release rate was <30% and almost nothing was obtained at pH 1.2). However, DXSP gradually released at pH 7.4 and the cumulative release rate reached the maximum at the 12th h (80%), indicating that due to the rapid degradation of Eudragit S100 at pH 7.4, the DXSP-loaded resin could exchange the DXSP anions with other ions in the dissolution medium and thus releasing drugs at higher pH value.
In the in vivo study, the result of pharmacokinetic study suggested that DXSP was quickly absorbed in the acidic stomach environment, so that it could reach the peak concentration within a short time (Tmax = 0.69 μg/ml, Cmax = 6.98 μg/ml). However, before reaching the peak concentration, the plasma concentration of rats orally administered with DXSP-DRM rose slowly, indicating that in the acidic environment, because the Eudragit S100 did not degrade, DXSP could not be released through the ion exchange. When the DXSP-DRM reached the colon environment, the Eudragit S100 was degraded in the alkaline environment. Therefore, the drug-loaded resin was exposed to the colon site and gradually released DXSP (Tmax = 2.25 μg/ml, Cmax = 4.08 μg/ml). Moreover, after orally administered, most of the released drug was distributed in the colon and cecum, suggesting that the DXSP-DRM could effectively targeted to the colon and release DXSP, thereby improving the drug concentration in the colonic lesions and contributing to the therapy of colitis. (The preparation, administration and in vivo transportation of DXSP-DRM were depicted in Scheme 1.)
The use of corticosteroids makes the immune organs (e.g. spleen and thymus) be shriveled and the body weight reduced. In this study, through determining the spleen and thymus indices, we investigate the influence of corticosteroids on the immune function of mouse. As discussed above, DXSP was first loaded in the resin and then packaged by the Eudragit S100. This method prevented the DXSP from being released in the upper gastrointestinal (e.g. in stomach), thus avoiding the toxic side-effects which was caused by DXSP been absorbed in advance. In this study, the body weight growth of mouse administered with the DXSP-DRM was obviously faster than mouse administered with DXSP. Moreover, the spleen and the thymus indices of the former were all higher than the latter. All data suggested that the DXSP-DRM could effectively reduce the toxic side-effects caused by DXSP.
The results of mouse survival rate and body weight changes showed that after the treatment of DXSP-DRM, both the survival rate and weight of mouse increased, indicating that the DXSP-DRM group had good therapeutic effect on the experimental colitis mouse induced by TNBS. Moreover, the measurement of DAI and MPO activity also confirmed that there was no significant difference occurred between the DXSP-DRM group and DXSP solution group about the therapeutic effect on the TNBS induced colitis mouse. As mentioned above, the DXSP-loaded resin encapsulated in the coating film composed of Eudragit S100 did not release drug at lower pH values (e.g. at stomach). However, in the colonic environment, due to the degradation of Eudragit S100, the DXSP-loaded resin was exposed to the colon site and began to release DXSP. Moreover, due to the weak electrolyte concentration at the colon site, the exchanging process of DXSP anions with external ions was relatively slower and DXSP could be gradually released, thus greatly reducing the absorption of DXSP in the upper gastrointestinal tract and effectively avoiding the occurrence of side-effects. These results indicate that at the condition of the same dosage, compared with DXSP, the DXSP-DRM not only has a good therapeutic effect on UC, but also play a better role on reducing the toxic side-effects caused by DXSP.
Conclusion
In this study, the dexamethasone sodium phosphate (DXSP) was encapsulated in the 717 anion exchange resin through a batch process. Furthermore, to improve the colon-targeting and the therapeutic effects of DXSP-DRM, the coating polymer material, Eudragit S100, was employed to package the DXSP-DRM to prepare the colon-targeted drug resin microcapsule (DXSP-DRM) through the in-liquid drying process. The results of in vitro release study showed that the TBSS-DRM displayed a pH-sensitive release behavior. In the in vivo study, the pharmacokinetic study and the determination of the intestinal drug residues in rat confirmed the good colon-targeting of the DXSP-DRM. And the DRM effectively reduced the toxic side-effects induced by DXSP on spleen and thymus of mouse. Moreover, The DXSP-DRM showed good therapeutic effect on the experimental colitis mouse induced by TNBS. All data indicated that the DXSP-DRM not only has good colon-targeted characteristics, but also significantly reduces the toxic side-effects induced by DXSP.
Declaration of interest
This work was financially supported by Fundamental Research Funds for the Central Universities (xj08142016 and xjj2013054), China Postdoctoral Science Foundation Funded Project (2014M562429) and Natural Science Foundation of China (No. 30973578 and No. 81473177). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Ethical approval
The study protocol was approved by the Xi’an Jiaotong University Medical and Biological Research Ethics Committee.
References
- Amsel LP, Hinsvark ON. (1984). Recent advances in sustained-release technology using ion-exchange polymer. Pharm Technol 4:28–48
- Anand V, Kandarapu R, Garg S. (2001). Ion-exchange resins: carrying drug delivery forward. Drug Discov Today 6:905–14
- Barnes PJ. (2006). How corticosteroids control inflammation: quintiles prize lecture 2005. Br J Pharmacol 148:245–54
- Blonski W, Buchner AM, Lichtenstein GR. (2014). Treatment of ulcerative colitis. Curr Opin Gastroenterol 30:84–96
- Buchman AL. (2001). Side effects of corticosteroid therapy. J Clin Gastroenterol 33:289–94
- Burton S, Washington N, Steele RJC, et al. (1995). Intragastric distribution of ion-exchange resins: a drug delivery system for the topical treatment of the gastric mucosa. J Pharm Phamacol 47:901–6
- Chen Y, Burton MA, Codde JP, et al. (1992). Evaluation of ion-exchange microspheres as carriers for the anticancer drug Doxorubicin: in-vitro studies. J Pharm Pharmacol 44:211–15
- Dahl R. (2006). Systemic side effects of inhaled corticosteroids in patients with asthma. Respir Med 100:1307–17
- Das S, Subuddhi U. (2014). Controlled delivery of dexamethasone to the intestine from poly(vinyl alcohol)-poly(acrylic acid) microspheres containing drug-cyclodextrin complexes: influence of method of preparation of inclusion complex. RSC Adv 4:24222–31
- De Cassan C, Fiorino G, Danese S. (2012). Second-generation corticosteroids for the treatment of Crohn's disease and ulcerative colitis: more effective and less side effects? Dig Dis 30:368–75
- Deeb G, Becker BA. (1960). Absorption of sustained release oral amphetamine in rats. Toxicol Appl Farmacol 2:410–17
- Dial EJ, Lichtenberger LM. (1986). Development of gastric-mucosal protection against acid in the rat-role of corticosteroids and prostaglandins. Gastroenterology 91:318–25
- Dong K, Dong Y, You C, et al. (2014). Assessment of the safety, targeting, and distribution characteristics of a novel pH-sensitive hydrogel. Colloids Surf B Biointerfaces 123:965–73
- Eroglu H, Kas HS, Oner L, et al. (2001). The in-vitro and in-vivo characterization of PLGA:L-PLA microspheres containing dexamethasone sodium phosphate. J Microencapsul 18:603–12
- Freeman HJ. (2013). Developments in the treatment of moderate to severe ulcerative colitis: focus on adalimumab. Ther Clin Risk Manag 9:451–6
- Friend DR. (2005). New oral delivery systems for treatment of inflammatory bowel disease. Adv Drug Deliv Rev 57:247–65
- Guo X, Chang RK, Hussain MA. (2009). Ion-exchange resins as drug delivery carriers. J Pharm Sci 98:3886–902
- Ichikawa H, Fujioka K, Adeyeye MC, Fukumori Y. (2001). Use of ion-exchange resins to prepare 100 mm-sized microcapsules with prolonged drug-release by the Wurster process. Int J Pharm 216:67–76
- Jaskari T, Vuorio M, Kontturi K, et al. (2000). Controlled transdermal iontophoresis by ion-exchange fiber. J Control Release 67:179–90
- Jeong SH, Park K. (2008). Development of sustained release fast-disintegrating tablets using various polymer-coated ion-exchange resin complexes. Int J Pharm 353:195–204
- Jewell DP. (1989). Corticosteroids for the management of Ulcerative-colitis and Crohns-disease. Gastroenterol Clin North Am 18:21–34
- Kopacz DJ, Bernards CM, Allen HW, et al. (2003). A model to evaluate the pharmacokinetic and pharmacodynamic variables of extended-release products using in vivo tissue microdialysis in humans: bupivacaine-loaded microcapsules. Anesth Analg 97:124–31
- Lennardjones JE. (1983). Toward optimal use of corticosteroids in Ulcerative-colitis and Crohns-disease. Gut 24:177–81
- Li HQ, Xie WR, Strong JA, Zhang JM. (2007). Systemic antiinflammatory corticosteroid reduces mechanical pain behavior, sympathetic sprouting and elevation of proinflammatory cytokines in a rat model of neuropathic pain. Anesthesiology 107:469–77
- Li M, Rouaud O, Poncelet D. (2008). Microencapsulation by solvent evaporation: state of the art for process engineering approaches. Int J Pharm 363:26–39
- Lilienfield LS, Zapolski EJ. (1983). Controlled release dextromethorphan using advanced ionexchange technology. Curr Ther Res 33:692–702
- Lundberg IE, Grundtman C, Larsson E, Klareskog L. (2004). Corticosteroids – from an idea to clinical use. Best Pract Res Clin Rheumatol 18:7–19
- McCabe AJ, Dowhy M, Holm BA, Glick PL. (2001). Myeloperoxidase activity as a lung injury marker in the lamb model of congenital diaphragmatic hernia. J Pediatr Surg 36:334–7
- Meier J, Sturm A. (2011). Current treatment of ulcerative colitis. World J Gastroenterol 17:3204–12
- Mondino C, Ciabattoni G, Koch P, et al. (2004). Effects of inhaled corticosteroids on exhaled leukotrienes and prostanoids in asthmatic children. J Allergy Clin Immunol 114:761–7
- Monteleone G, Pallone F, Macdonald TT. (2011). Emerging immunological targets in inflammatory bowel disease. Curr Opin Pharmacol 11:640–5
- Myou S, Fujimura M, Leff AR. (2004). Additive effect of cysteinyl leukotriene or thromboxane modifiers to inhaled corticosteroids in asthmatic patients. Allergol Int 53:211–17
- Narisawa S, Nagata M, Danyoshi C, et al. (1994). An organic acid induced sigmoidal release system for oral controlled release preparations. Pharm Res 11:111–16
- Patel SH, Rachchh MA, Jadav PD. (2012). Evaluation of anti-inflammatory effect of anti-platelet agent-clopidogrel in experimentally induced inflammatory bowel disease. Indian J Pharmacol 44:744–8
- Samprasit W, Akkaramongkolporn P, Ngawhirunpat T, et al. (2013). Meloxicam taste-masked oral disintegrating tablet with dissolution enhanced by ion exchange resins and cyclodextrin. AAPS PharmSciTech 14:1118–28
- Sohi H, Sultana Y, Khar RK. (2004). Taste masking technologies in oral pharmaceuticals: recent developments and approaches. Drug Dev Ind Pharm 30:429–48
- Thakral S, Thakral NK, Majumdar DK. (2013). Eudragit (R): a technology evaluation. Expert Opin Drug Deliv 10:131–49
- Truelove SC, Witts LJ. (1954). Cortisone in Ulcerative colitis-preliminary report on a therapeutic trial. Br Med J 2:375–8
- Turner D, Walsh CM, Steinhart AH, Griffiths AM. (2007). Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol 5:103–10
- Wang WP, Guo X, Koo MW, et al. (2001). Protective role of heme oxygenase-1 on trinitrobenzene sulfonic acid-induced colitis in rats. Am J Physiol Gastrointest Liver Physiol 281:G586–94
- Yewale CP, Rathi MN, Kore GG, et al. (2013). Formulation and development of taste masked fast-disintegrating tablets (FDTs) of Chlorpheniramine maleate using ion-exchange resins. Pharm Dev Technol 18:367–76
- Yu L, Li S, Yuan Y, et al. (2006). The delivery of ketoprofen from a system containing ion-exchange fibers. Int J Pharm 319:107–13
- Zhao J, Cui Y, Wang AH, et al. (2011). Side effect reduction of encapsulated hydrocortisone crystals by insulin/alginate shells. Langmuir 27:1499–504