Abstract
The aim of this study was to develop docetaxel (DTX)-loaded poly-d,l-lactide (PDLLA) nanofibers and evaluate their therapeutic effect in preventing local breast cancer recurrence. DTX was incorporated into biodegradable PDLLA nanofibers by electrospinning. The surface morphology of the DTX/PDLLA nanofibers was characterized using scanning electron microscopy and wide angle X-ray diffraction. The in vitro release behavior of DTX from the fiber mats was also studied in detail. The cytotoxicity of DTX/PDLLA nanofibers was evaluated by MTT assay in 4T1 breast cancer cells. Flow cytometry revealed that DTX/PDLLA nanofibers exhibited apoptotic activity in 4T1 cells. In vivo antitumor efficacy of DTX/PDLLA nanofibers was evaluated in BALB/c mice bearing local breast tumors. Locoregional recurrence after primary tumor resection decreased obviously in mice treated with subcutaneously (16.7%) administered DTX-loaded PDLLA nanofibers, compared with the blank PDLLA nanofibers (88.9%), systemic (75.0%) or locally (77.8%) administered DTX and the control group (100%) (p < 0.05). Finally, after subcutaneous transplantation in mice, the DTX/PDLLA scaffolds presented excellent biocompatibility, as exhibited by the minimal presence of inflammatory cells in the region surrounding the scaffolds. Our results suggest that DTX/PDLLA nanofibers could have great potential for clinical application requiring local chemotherapy.
Introduction
Nowadays, breast cancer is the most frequent tumor in young women. The risk of local regional recurrence (LRR) is an important factor that determines the postoperative survival rate for breast conservation therapy (BCT). According to reported clinical trials, in the second decade following BCT, local recurrences continue to occur at ∼1% per year and rates of local recurrence vary between 2% and 10% after 5 years and between 5% and 15% at 10 years, with or without adjuvant radiotherapy (Arriagada et al., Citation2003; Voogd et al., Citation2005). Despite the evolution of advanced tumor resection surgery and postoperative chemotherapy and radiotherapy treatment modalities, LRR after surgery remains a challenge, causing significant relapse of breast cancer mortality. Moreover, a diagnosis of locally recurrent disease, confirmed by histopathology, is associated with a higher risk of breast cancer-specific mortality than the initial tumor (Chung & Harris, Citation2007). Therefore, there is a great need for the development of agents that can effectively control locally recurrent disease, to minimize serious recurrence rates and improve the quality of life of patients who have previously received treatment.
Docetaxel (DTX), which is a semi-synthetic product obtained from the European yew Taxus baccata, is currently one of the most important chemotherapeutic agents, since it can bind tubulin to stabilize microtubules and reduce their dynamicity, promoting mitotic arrest, thereby leading to apoptosis at the molecular level in a variety of tumor cells (Ceruti et al., Citation1999; Xiao et al., Citation2006). DTX has a similar structure to paclitaxel, but it has a higher affinity for binding to β-tubulin, and thus resides inside cells for a longer period than paclitaxel. In addition, DTX has a more favorable pharmacological profile and is more potent than paclitaxel (Gligorov & Lotz, Citation2004). Previous studies have shown that DTX has a broad spectrum of activity against a variety of tumors and has been effectively used to treat breast tumors, ovarian tumors, non-small cell lung cancer and gastric cancer (Kaye et al., Citation1997; Gradishar, Citation2001; Petty et al., Citation2002; Ajani, Citation2006; Bergh et al., Citation2012; Ganta et al., Citation2014). Despite its high performance at the molecular and cellular levels, its clinical application is limited by its intrinsic or acquired multidrug resistance (MDR), poor water solubility and severe toxic side effects, such as hematological, gastrointestinal and neuromotor toxicities, which are attributed to formulating vehicles (Vaishampayan & Hussain, Citation2008).
To eliminate toxicities associated with the formulation, polymeric nanoparticles, emulsions and liposomes have been applied as drug delivery systems (DDSs) (Hwang et al., Citation2008; Qiu et al., Citation2008; Yanasarn et al., Citation2009; Wang et al., Citation2011; Ernsting et al., Citation2012; Mazzaferro et al., Citation2012; Tang et al., Citation2014). Studies investigating toxicities associated with first generation DTX products have received a lot of attention, especially those looking for better alternative formulations that are less toxic and provide better penetration and targeting to the tumor tissues (Hennenfent & Govindan, Citation2006).
One method to reduce the limitations of DTX, so that it can be used to treat local breast cancer recurrence, is the use of a delivery vehicle, such as electrospun fibers (Muthu et al., Citation2012; Cai et al., Citation2013). The electrospun DDSs can be implanted into the tumor site, allowing for local release and delivery of the drug. Local delivery is an important method for cancer chemotherapy. When anticancer agents are delivered systemically, they must cross several barriers to reach their site of action within tumor cells, including the capillary walls, the extracellular space and tumor cell membranes (Fung & Saltzman, Citation1997). Implanted nanofibers localize to specific anatomic sites, providing a continuous sustained release of anticancer drugs while minimizing systemic exposure. In addition, local delivery can also reduce the severe side effects of DTX. The local delivery and controllable release profiles of anticancer drug-loaded electrospun ultrafine fibers have good potential for wide application in the prevention of LRR of breast cancer.
During the past few years, the fabrication of nanofibers using electrospinning has received much attention for their wide range of applications in the biomedical field, such as tissue engineering, wound healing and DDSs (Li et al., Citation2006; Choi et al., Citation2008; Kenawy et al., Citation2009; Yoo et al., Citation2009; Orlova et al., Citation2011; Khadka & Haynie, Citation2012; Zhang et al., Citation2014). The use of electrospun fibers as drug carriers have some advantages for preparing DDSs, as follows: the nanofibers possess high surface-to-volume ratio, which can facilitate drug release; they are highly porous, which can be manipulated to control the release rate and amount of the drug released; more than one drug can be encapsulated directly into the fibers. In particular, the fibrous carriers can offer site-specific delivery of drugs to the body. Nanofibers made from biodegradable polymers are good candidates for applications in chemotherapy owing to their biocompatibility and degradation in vivo. In this study, DTX-loaded poly-d,l-lactide (PDLLA) fibers were fabricated to examine the advantages of the biodegradable properties of the polymer in regulating the drug release rate. The drug release in buffer solutions of pH 7.4 and antitumor activity of the DTX-containing fibers were investigated in vitro. Furthermore, we investigated this DDS in breast cancer for the prevention of recurrent disease after surgical resection in vivo. The results revealed that DTX/PDLLA nanofibers provides a system for sustained drug release in a model of recurrent breast cancer, promotes cell apoptosis in vitro and in vivo, reduces locoregional recurrence rates and improves the postoperative treatment efficiency.
Materials and methods
Materials
Poly-d,l-lactide (Mw = 135 000) was purchased from Nature Works LLC (Minneapolis, MN). Docetaxel, Gibco® RPMI 1640 medium and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma (St. Louis, MO). Acetonitrile (HPLC grade) was purchased from Fisher Scientific (UK). Dimethyl sulfoxide (DMSO), ethanol, methanol were purchased from KeLong Chemicals (Chengdu, China).
Female BALB/c mice, weighing 20 ± 2 g, were purchased from the Beijing HFK Bioscience (Beijing, China). The animals were sex-separately housed in a controlled temperature of 20–22 °C, relative humidity of 50–60% and 12-h light–dark cycles. Free access to food and water was allowed. All the studies involving mice were approved by the Animal Care and Use Committee of Sichuan University.
Preparation of DTX/PLLA nanofibers
The electrospun solution was prepared by incorporation of DTX into a solution of 6% (w/v) PDLLA/CH2Cl2. The mixed emulsion of DTX and PDLLA was added via a 20-ml syringe attached to a circular-shaped metal capillary. The flow rate of the solution was controlled within 7 ml/h using a precision pump (model WZ-50C66T, Smiths Medical Instrument, Zhejiang Co., Ltd., Hangzhou, China). Then DTX/PDLLA suspension was electrospun at a high voltage of 18 kV (electrospinning apparatus, High Voltage Technology Institute, Beijing, China). The distance between the tip of the needle and the 10 cm × 10 cm grounded aluminum collector was adjusted to 12 cm. We obtained 5%, 10% and 20% DTX-loaded PDLLA nanofibers, with the 10% DTX nanofibers being used for the in vivo studies. The obtained DTX/PDLLA fibers were collected and dried in a vacuum oven at 37 °C for at least 48 h to eliminate the solvent, and then stored at −20 °C for further characterization and treatment.
Surface morphology
Surface morphologies of the DTX/PDLLA nanofibers were observed by an electron scanning microscopy (SEM, JEM-100CX, Japan). All the samples were analyzed at 20 kV. An energy-dispersive spectrometer (EDS) was used in conjunction with SEM for elemental analysis of the deposited mineral phase.
X-ray diffraction
The crystalline states of DTX, PDLLA and DTX/PDLLA nanofibers were analyzed by wide-angle X-ray diffraction (WAXD, X' Pert Pro MPD DY1291, PHILIPS, The Netherlands). The samples were measured from 5 °C to 50 °C at a scanning rate of 5 °C/min, using Cu–K alpha radiation.
The drug release study
The release profile of DTX from nanofibers was evaluated. DTX-loaded nanofibers were placed into a centrifugal tube containing 30 ml of phosphate buffer saline (PBS, pH 7.4) as the release media. The system was maintained at 37 °C, with stirring rate of 100 rpm. The release media (0.2 ml) was sampled at predetermined time intervals and replaced with an equal volume of PBS. The concentration of DTX was determined using high-performance liquid chromatography (Alliance 2695 HPLC System, Waters, Irvine, CA). Chromatographic separations were performed on a reversed phase C18 column (4.6 mm 150–5 mm, SunFire Chromatography Column, Waters, Irvine, CA). Acetonitrile/deionized water (48/52, v/v) was used as eluent, at a flow rate of 1 ml/min. The amount of paclitaxel remaining in each film was extracted and measured by HPLC by dissolving films in 1 ml dichloromethane.
Analysis of cytotoxicity
The cytotoxicity of DTX/PDLLA nanofibers to 4T1 cell line was evaluated by MTT method. 4T1 cells were plated at a density of 2 × 104 cells per well in 100 ml of RPMI 1640 medium, containing 10% FBS, in 24-well plates and grown for 24 h. Cells were then exposed to a series of DTX/PDLLA nanofibers at different concentrations, for different time points. The viability of cells was measured using the MTT method. Results were the mean of six test runs.
Apoptosis
Cell apoptosis was detected by assessment of nuclear morphology by staining with 4′,6-diamidino-2-phenylindole (DAPI, Sigma, St. Louis, MO). 4T1 cells were treated with different ratio DTX/PDLLA nanofibers for 48 h. Then, the cells were washed and fixed with 4% paraformaldehyde. After staining with DAPI (10 mg/ml) at room temperature for 10 min, the cells were observed on a fluorescence microscope. Apoptotic cells were determined by dual staining with an Annexin V and propidium iodide kit (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. In brief, cells were incubated with DTX/PDLLA nanofibers (5 mg) in 6-well plates, for the indicated time periods, and collected by trypsinization using 0.125% of Trypsin solution, without EDTA. Then, the cells were washed twice with PBS (pH 7.2) and resuspended in 500-μl binding buffer, at a density of 1 × 106 cells/ml. Five microliters of Annexin V-FITC and 5 μl of propidium iodide (PI) were added and incubated at room temperature for 15 min in the dark. The cells were analyzed by flow cytometry (Becton Dickinson, Sunnyvale, CA) within 1 h.
Docetaxel concentrations in local tissue and plasma
To assess local and systemic drug levels in vivo, tumor-naive mice that did not receive sham surgery were subcutaneously injected with 250-ml DTX/PDLLA nanofibers or intravenous free DTX (20 mg/kg). The tail vein plasma and local thoracic tissue were collected at postoperative days 1, 4 and 7 (n = 3 for each time point). The surgical surrounding tissue was harvested (50–150 mg) and the drug concentrations were assessed by HPLC as described earlier.
In vivo breast cancer locoregional recurrence
Female BALB/c mice (6–8 weeks old) were subcutaneously inoculated with 4T1 cells (5 × 105 cells in 100 µl RPMI 1640 medium) on day 0. Tumor resection was performed ∼10 days after inoculation, when the primary tumors had reached an estimated volume of 150 mm. Prior to surgery, all mice were anesthetized by intraperitoneal injection of 10% chloral hydrate (60–100 µl). Generally, tumor resection involved a 1- to 2-cm incision lateral to the tumor, followed by complete dissection of the tumor without macroscopic residuals. To ensure a high recurrence rate in untreated mice, the surrounding skin was preserved among all the tumor-bearing mice. All the eligible animals survived the surgery and were then randomized into five groups and numbered. Immediately after the surgery (day 0), DTX nanofibers (100 mg/kg) containing DTX (20 mg/kg) were subcutaneously injected into the original tumor site in Group 1. The other four groups were injected intravenously with free DTX (20 mg/kg), subcutaneously with free DTX (20 mg/kg), subcutaneously with blank PDLLA (100 mg/kg) or subcutaneously with saline (250 µl) alone. Locoregional recurrence in the experimental animals was defined as any palpable subcutaneous nodule anywhere on the chest wall. All mice were monitored every other day for body weight, wound healing, LRR and survival for up to 70 days.
Histological analysis in vivo
To further evaluate the tissue responses to the implanted scaffold in vivo, the DTX/PLLA fibrous scaffold was subcutaneous transplanted into the mice. The mice were randomly sacrificed at predetermined intervals of 2, 4, 6 and 8 weeks, and the excised implant and surrounding tissue were fixed in 10% formalin for histological analysis. The fixed specimens were serially dehydrated in a graded series of ethanol washes and embedded in paraffin. Then, 8-μm thick sections were cut and stained according to standard protocols for hematoxylin and eosin (H&E) staining. All H&E results were analyzed with a digital image analysis system (Nikon E 600 Microscope with a Nikon Digital Camera DXM 1200, Nikon Co., Tokyo, Japan).
Results
Characterization of nanofibers
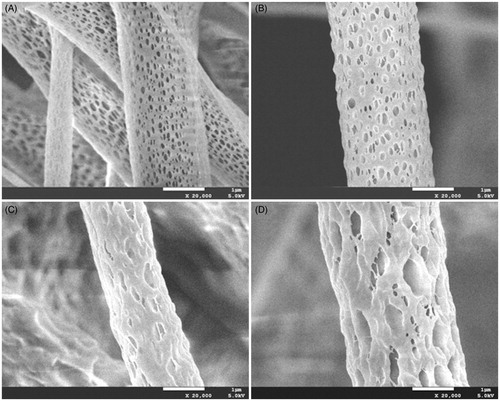
Docetaxel was easily dissolved in PDLLA/dichloromethane solution, and the mixture solution was stable and homogeneous. The electrospun scaffolds of PDLLA with or without DTX were fabricated in this study. The content of DTX in the fibers was 5, 10 and 20 wt%, with respect to PDLLA content. SEM micrographs of the fibers obtained are shown in . The surfaces of the fibers were polyporate and no drug crystals were found. The loading of DTX changed the porosity and diameter distribution of fibers. Nanopore areas were greater in fibers with a higher proportion of the DTX. A broad diameter distribution of fibers was observed upon incorporation of DTX. The diameter range of the fiber mats was 0.4–12.6 µm. Increasing DTX concentration from 5 to 20 wt% did not appear to significantly change fiber morphology.
Figure 1. SEM photographs of docetaxel (DTX)/PDLLA nanofibers containing (A) 0 wt%, (B) 5 wt% DTX/PDLLA, (C) 10 wt% DTX/PDLLA and (D) 20 wt% DTX/PDLLA (100 × and 5000×).
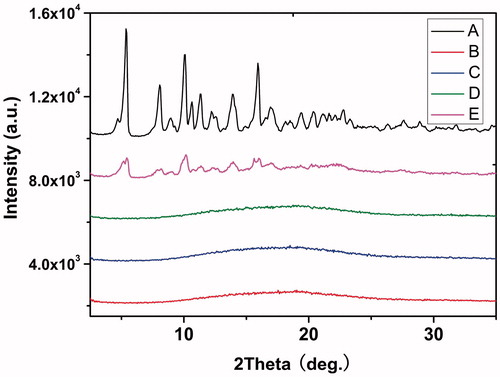
Blank- and DTX-loaded nanofibers were characterized by WAXD. shows the wide-angle XRD analysis, with the relevant patterns of DTX crystals (A), DTX/PDLLA composite (B), 5% DTX-loaded PDLLA nanofibers (C), 20 wt% DTX-loaded PDLLA nanofibers (D) and pure PDLLA nanofibers (E). In , the crystalline peaks of DTX at 2θ = 5.36°, 8.06°, 10.1° and 15.98°, which are the characteristic peaks of DTX. The diffraction intensities of the DTX-loaded fibers at the peaks in pure DTX had disappeared and look very like pure PDLLA, indicating that DTX is finely incorporated into the electrospun fibers.
In vitro release profile and toxicity studies of nanofibers
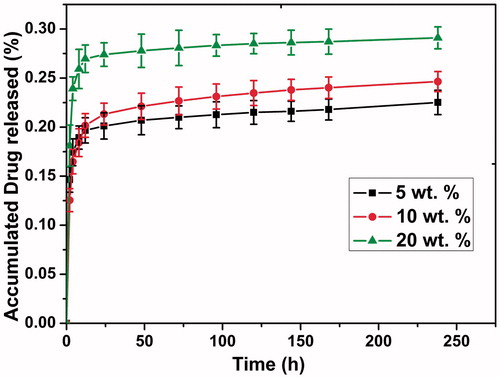
shows the in vitro release profiles of DTX from DTX/PDLLA nanofibers in PBS. A sustained release profile could be visibly observed when DTX was released from PDLLA nanofibers (). When DTX was released from the nanofibers, a visible initial burst release (within 12 h) occurred, which was followed by a slow release ratio. The release rate of DTX increased with increasing DTX and PDLLA mass ratio content during the entire release period. Approximately 23.3%, 25.3% and 29.6% of DTX was released in 24 days from the 5, 10 and 20 wt% DTX/PDLLA scaffolds, respectively.
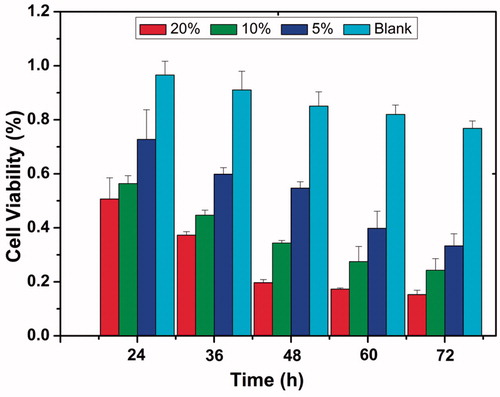
The cell growth inhibition ratios of various fiber mats against 4T1 cells are shown in . For the negative control and blank fibers (without DTX), the samples did not display any cytotoxicity to the 4T1 cells until 72 h display slightly cytotoxicity to the cell. For the DTX/PDLLA fibers, all the samples showed decreasing cell viability with increased culture time. For example, in the cases of 5, 10 and 20 wt% DTX/PDLLA nanofibers achieved cell growth inhibition rates of 20%, 34% and 54%, respectively, were achieved at 48 h after administration. The data showed that DTX/PDLLA exhibited significant anti-tumor cytotoxicity in vitro.
Apoptosis analysis by flow cytometry
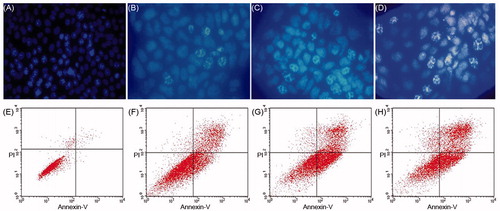
As shown in , chromatin condensation and apoptotic-body formation can be observed for some of the cells treated with DTX (). This result indicates that the cytotoxicity of DTX could induce 4T1 cell apoptosis in vitro. The apoptosis of 4T1 cells induced by DTX nanofibers was also shown by flow cytometry. Annexin V-PI staining could distinguish early apoptosis from late apoptosis cells. In 4T1 cells, the control treatment group shows negligible presence of apoptosis and necrotic cells (<5%) (). DTX-treated cells were found in the lower right (Annexin V-positive and PI-negative) and upper right quadrants (Annexin V-positive and PI-positive), with all of the DTX/PDLLA nanofiber-treated cells having died by apoptosis. The percentage of apoptotic cells was higher in the case of cells treated with a higher percentage DTX ().
Figure 5. Docetaxel (DTX) induced cell apoptosis on 4T1 breast cancer cells. Cells were treated with (A) blank control, (B) 5 wt% DTX/PDLLA, (C) 10 wt% DTX/PDLLA and (D) 20 wt% DTX/PDLLA, and stained with DAPI (blue). Apoptosis efficiency was evaluated. (E) Blank control without treatment, (F) 5 wt% DTX/PDLLA, (G) 10 wt% DTX/PDLLA and (H) 20 wt% DTX/PDLLA. Cells were stained with Annexin-V-FITC and propidium iodide (PI). Flow cytometry profile represented by Annexin-V-FITC staining on the X axis and PI on the Y axis.
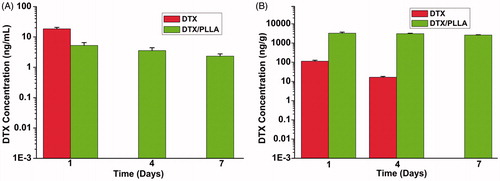
Docetaxel concentrations in local tissue and plasma
In the current study, we detected the systemic concentrations and local tissue concentrations of DTX to compare drug content. As shown in , DTX plasma concentrations were measured in animals at days 1 and 7 following implantation of DTX-loaded nanofibers or intraperitoneal injection of the equivalent 300-μg DTX dose (). At day 1, the concentration of DTX at the local site was over 28.8-fold higher than with free DTX group at day 1 (3238.2 ± 426.5 ng/g versus 122.4 ± 14.2 ng/g, p < 0.01). Plasma concentrations remained low at days 1 and 7 compared with the free DTX, confirming that drug delivery is concentrated at the local site. These initial results suggest that high local tissue levels of DTX are associated with a reduction of LRR, whereas high plasma concentrations may be related to increased risk of systemic toxicity and do not prevent recurrence.
Figure 6. The concentration measured via HPLC within plasma or tissues near the surgical site following implantation of DTX/PLA nanofibers or intravenous injection of an equivalent docetaxel dose. (A) DTX/PLA nanofibers result in higher docetaxel concentration in local tissue and (B) small quantities of DTX were detected in plasma.
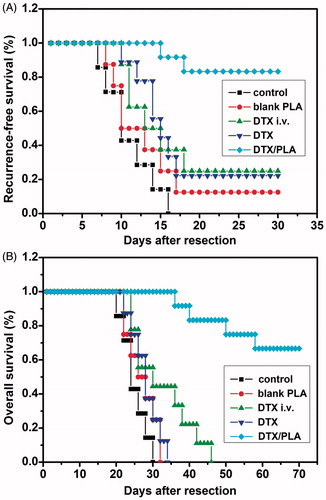
Docetaxel-loaded nanofibers prevent local recurrence in vivo
Based on the toxicity studies and the apoptosis analysis of DTX-loaded PDLLA nanofibers in vitro, we assessed whether DTX nanofibers prevented LRR of breast cancer in vivo after primary tumor resection. Mice were randomized into five treatment groups after resection of the primary tumor. There were no differences in the number of days of primary tumor growth before resection and characteristics of primary tumors (tumor volume and weight) among the five experimental treatment groups. No mice died of treatment-related toxicity.
Two of the 12 mice treated with DTX nanofibers developed locoregional tumor recurrence. LRRs were observed within 30 days in six of the eight mice treated with local administration of DTX (77.8%), eight of the nine mice that received blank PDLLA nanofibers (88.9%), seven of the nine mice that were intravenously injected with DTX (75%), and all eight mice in the control group (100%), with a median time to recurrence of 13, 11.7, 11.9 and 11 days, respectively. The recurrence time of the only mouse in the DTX nanofibers group was significantly delayed to 15 days after tumor resection. After complete resection of the primary tumors, mice were randomized into five groups: DTX-loaded film, unloaded film, local subcutaneous injection of DTX 15 mg kg−1, or intraperitoneal injection of DTX and control group. With virous treatment, the survival curves of mice in each treatment group are shown in . These results suggest that DTX nanofibers can efficiently inhibit local recurrence of 4T1 breast cancer and prolong the survival of mice.
Figure 7. Implantation of docetaxel (DTX)-loaded nanofibers prevents local recurrence. (A) Locally administrated DTX/PLA obviously reduced locoregional recurrence after resection of primary tumor (p < 0.05). (B) Overall survival was improved in the DTX/PDLLA group compared with all the other groups (p < 0.05).
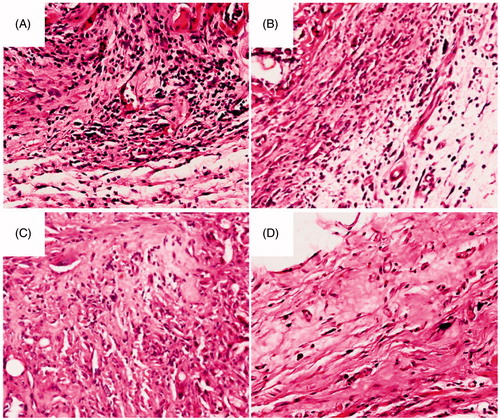
In vivo compatibility study
The DTX/PDLLA fibrous scaffold was transplanted into the subcutaneous region of mice and later examined by histology to evaluate the tissue responses in vivo. The host response was determined by measuring inflammatory cell infiltration.
shows the histopathology of the representative tissue sections taken from subcutaneous tissue adjacent to the implantation site. The in vivo biocompatibility study revealed that DTX/PDLLA implants induced mild inflammatory reactions. With increasing time, an expressive decrease of leukocytes, neutrophils and macrophages was observed, and the number of polymorphonuclear and other cell types decreased significantly.
Figure 8. Histological analysis of tissue response to the DTX/PDLLA nanofibers after (A) 2 weeks, (B) 4 weeks, (C) 6 weeks and (D) 8 weeks. There was a dense accumulation of inflammatory cells presented around the DTX/PDLLA fibrous scaffolds at week 2, which degraded over time and disappeared at week 8.
Discussion
Breast cancer is the most common cancer and the most frequent leading cause of cancer mortality among women (Farquhar et al., Citation2007). Breast conservative surgery and mastectomy are the main treatments for breast cancer. These are followed by conventional adjuvant treatment strategies, including systemic therapies (hormonal therapy and chemotherapy), which are now more effective and recommended to a higher proportion of patients than ever before. Postoperative radiotherapy is also adopted for local therapy. Despite a variety of treatment modalities, the present situation of LRRs remains poor. In addition, the combined treatment of chemotherapy followed by radiotherapy can cause substantial toxicity in patients. Treatment of LRR in breast cancer patients remains a therapeutic challenge. There is a need for further clinical studies to achieve the primary goal of management: maximize the quality of life, by palliation of symptoms without unnecessary side effects.
Several novel DDSs, such as nanoparticles, and chitosan hydrogels, have been applied to treat and inhibit tumor recurrence in previously published studies (Ruel-Gariépy et al., Citation2004; Liu et al., Citation2011; Lei et al., Citation2012). Drug-loaded nanoparticles that have an initial burst release fail to achieve a sustainable drug-release profile, and are thus more likely to delay LRR rather than completely inhibit tumor regrowth. Over the last decade, emerging thermosensitive hydrogels have been reported as sustainable DDSs; however, hydrogels have a relatively fast degradation speed. Polymeric films have effectively prevented cancer relapse in the resection site of primary cancers (Liu et al., Citation2010, Citation2012). In agreement with the polymer films, the PDLLA nanofibers used in our study exhibited outstanding biocompatibility and biodegradability, as well as a consistent drug-release rate. Owing to their large surface area and that they can offer site-specific delivery of drugs, electrospun fibers have been identified as local DDSs. Electrospun fibers also afford flexibility in shape to the contacting tissue edge. The use of electrospun nanofibers as drug carriers could be promising for use in future biomedical applications, especially postoperative local chemotherapy.
In the current study, we first successfully acquired the DTX-loaded PDLLA nanofibers, which we then subsequently characterized. The in vitro release study indicated that DTX/PDLLA nanofibers could slowly release DTX over time. The nanofibers had a long and stable low-dose drug-release profile. The cytotoxicity analysis indicated that DTX nanofibers showed clear cytotoxicity to 4T1 breast cancer cells. The apoptosis of 4T1 cells induced by DTX nanofibers was confirmed by flow cytometry. Thus, as the drug-loaded nanofibers exhibited cytotoxicity to cancer cells in vitro, we hypothesized that they could directly kill cancer cells in vivo and inhibit the LRR of breast cancer with the use of logical chemotherapy.
In this study, the results of the release profile demonstrated that DTX exhibited a sustained drug-releasing profile, without an initial burst phase. The previous theory suggested that the prolonged delivery of low-dose anticancer drugs at the site of tumor resection for several weeks would be significantly more effective at preventing tumor recurrence in vivo than a polymer “burst” delivery system that rapidly exhausts (Liu et al., Citation2010). In support of this hypothesis, LRR could be prevented when DTX was delivered via PDLLA nanofibers for low-dose and sustained drug delivery. Low concentrations of DTX (1–50 nM) are sufficient to reach the IC50 of cancer cells and as DTX exhibits a great affinity to β-tubulin, targeting centrosome organization, it affects cells in all the three phases of the cell cycle (S/G2/M), thus resulting in cell apoptosis (Sampath et al., Citation2003). Increased susceptibility of non-mitotic cells to high-dose DTX may also result in the killing of normal bystander cells and likely contribute to impaired healing, inflammation and increased infection, neurotoxicity and cardiotoxicity, which are seen clinically as dose-limiting toxicities. As shown in and , the current study provides a significant advantage with the use of DTX-loaded PDLLA nanofibers, as these maintained a systemically low, but locally effective drug concentration long term, thereby maximizing tumor cytotoxicity, while minimizing systemic toxicity. Thus, DTX/PDLLA nanofibers could be sufficient for preventing LRR.
Furthermore, the release of DTX from the composite nanofibers becomes slower as time progresses. We believe that if we can enhance the drug release speed, the effect in preventing breast cancer recurrence and reducing mortality may be more significant and will focus our future research on this mechanism.
Early subacute inflammation with the use of nanofibers occurred, and a creation of acidic microenvironment might be induced by PDLLA degrading near the implanting site. We briefly performed an experiment on tissue responses to the PDLLA nanofibers in vivo. Acute inflammatory reaction presented at week 2, but attenuated at week 4 without unfavorable influence on the ambient muscle, indicating that the DTX/PDLLA fibrous scaffolds are biocompatible with the surrounding tissues in vivo. Overall, we can conclude that our DTX/PDLLA nanofibers could serve as a potential candidate for the inhibition of LRR of breast cancer, with good biocompatibility.
Conclusions
In summary, the current study demonstrates that electrospun DTX-loaded PDLLA nanofibers provide prolonged delivery, and sufficient cytotoxic drug to the local site, which can prevent local tumor recurrence following surgical resection. Simultaneously, the DTX/PDLLA nanofibers can avoid high systemic concentrations that lead to systemic toxicities. The beneficial potential of PDLLA nanofibers in the clinic depends on enhanced anti-tumor efficacy in the local region, decreased systemic toxicity and good biocompatibility. Considering its impressive efficiency in local control with DTX, further assessments of the efficacy of nanofibers as DDCs will be performed by incorporating them with other antineoplastic agents.
Declaration of interest
This work was financially supported by National Natural Science Foundation (NSFC31222023, NSFC51303203), Luzhou Science and Technology Plan (2013CDLZ-S21) and Distinguished young scholars of Sichuan University (2011SCU04B18).
References
- Ajani JA. (2006). The role of docetaxel in gastric cancer. Eur J Cancer Suppl 4:4–9
- Arriagada R, Le M, Guinebreti RE, et al. (2003). Late local recurrences in a randomised trial comparing conservative treatment with total mastectomy in early breast cancer patients. Ann Oncol 14:1617–22
- Bergh J, Mariani G, Cardoso F, et al. (2012). Clinical and pharmacokinetic study of sunitinib and docetaxel in women with advanced breast cancer. Breast 21:507–13
- Cai S, Xu H, Jiang Q, Yang Y. (2013). Novel 3D electrospun scaffolds with fibers oriented randomly and evenly in three dimensions to closely mimic the unique architectures of extracellular matrices in soft tissues: fabrication and mechanism study. Langmuir 29:2311–18
- Ceruti M, Tagini V, Recalenda V, et al. (1999). Docetaxel in combination with epirubicin in metastatic breast cancer: pharmacokinetic interactions. Il Farmaco 54:733–9
- Choi JS, Leong KW, Yoo HS. (2008). In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF). Biomaterials 29:587–96
- Chung CS, Harris JR. (2007). Post-mastectomy radiation therapy: translating local benefits into improved survival. Breast 16:78–83
- Ernsting MJ, Foltz WD, Undzys E, et al. (2012). Tumor-targeted drug delivery using MR-contrasted docetaxel–carboxymethylcellulose nanoparticles. Biomaterials 33:3931–41
- Farquhar CM, Marjoribanks J, Lethaby A, Basser R. (2007). High dose chemotherapy for poor prognosis breast cancer: systematic review and meta-analysis. Cancer Treat Rev 33:325–37
- Fung LK, Saltzman WM. (1997). Polymeric implants for cancer chemotherapy. Adv Drug Deliv Rev 26:209–30
- Ganta S, Singh A, Rawal Y, et al. (2014). Formulation development of a novel targeted theranostic nanoemulsion of docetaxel to overcome multidrug resistance in ovarian cancer. Drug Deliv. [Epub ahead of print]. doi: 10.3109/10717544.2014.923068.
- Gligorov J, Lotz JP. (2004). Preclinical pharmacology of the taxanes: implications of the differences. Oncologist 9:3–8
- Gradishar WJ. (2001). Primary (neoadjuvant) chemotherapy with docetaxel in breast cancer. Clin Breast Cancer 2:S31–5
- Hennenfent K, Govindan R. (2006). Novel formulations of taxanes: a review. Old wine in a new bottle? Ann Oncol 17:735–49
- Hwang H-Y, Kim I-S, Kwon IC, Kim Y-H. (2008). Tumor targetability and antitumor effect of docetaxel-loaded hydrophobically modified glycol chitosan nanoparticles. J Control Release 128:23–31
- Kaye S, Piccart M, Aapro M, et al. (1997). Phase II trials of docetaxel (taxotere®) in advanced ovarian cancer – an updated overview. Eur J Cancer 33:2167–70
- Kenawy E-R, Abdel-Hay FI, El-Newehy MH, Wnek GE. (2009). Processing of polymer nanofibers through electrospinning as drug delivery systems. Mater Chem Phys 113:296–302
- Khadka DB, Haynie DT. (2012). Protein-and peptide-based electrospun nanofibers in medical biomaterials. Nanomed Nanotechnol Biol Med 8:1242–62
- Lei N, Gong C, Qian Z, et al. (2012). Therapeutic application of injectable thermosensitive hydrogel in preventing local breast cancer recurrence and improving incision wound healing in a mouse model. Nanoscale 4:5686–93
- Li M, Guo Y, Wei Y, et al. (2006). Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications. Biomaterials 27:2705–15
- Liu R, Khullar OV, Griset AP, et al. (2011). Paclitaxel-loaded expansile nanoparticles delay local recurrence in a heterotopic murine non-small cell lung cancer model. Ann Thorac Surg 91:1077–84
- Liu R, Wolinsky JB, Catalano PJ, et al. (2012). Paclitaxel-eluting polymer film reduces locoregional recurrence and improves survival in a recurrent sarcoma model: a novel investigational therapy. Ann Surg Oncol 19:199–206
- Liu R, Wolinsky JB, Walpole J, et al. (2010). Prevention of local tumor recurrence following surgery using low-dose chemotherapeutic polymer films. Ann Surg Oncol 17:1203–13
- Mazzaferro S, Bouchemal K, Skanji R, et al. (2012). Intestinal permeation enhancement of docetaxel encapsulated into methyl-β-cyclodextrin/poly (isobutylcyanoacrylate) nanoparticles coated with thiolated chitosan. J Control Release 162:568–74
- Muthu MS, Kulkarni SA, Raju A, Feng S-S. (2012). Theranostic liposomes of TPGS coating for targeted co-delivery of docetaxel and quantum dots. Biomaterials 33:3494–501
- Orlova Y, Magome N, Liu L, et al. (2011). Electrospun nanofibers as a tool for architecture control in engineered cardiac tissue. Biomaterials 32:5615–24
- Petty WJ, Rathmann J, Dragnev KH, Rigas JR. (2002). The role of docetaxel in nonplatinum-based combination chemotherapy for non-small-cell lung cancer. Clin Lung Cancer 3:S12–16
- Qiu Y, Gao Y, Hu K, Li F. (2008). Enhancement of skin permeation of docetaxel: a novel approach combining microneedle and elastic liposomes. J Control Release 129:144–50
- Ruel-Gari PY, Shive E, Bichara M, et al. (2004). A thermosensitive chitosan-based hydrogel for the local delivery of paclitaxel. Eur J Pharm Biopharm 57:53–63
- Sampath D, Discafani CM, Loganzo F, et al. (2003). MAC-321, a novel taxane with greater efficacy than paclitaxel and docetaxel in vitro and in vivo. Mol Cancer Ther 2:873–84
- Tang J, Zhang L, Gao H, et al. (2014). Co-delivery of doxorubicin and P-gp inhibitor by a reduction-sensitive liposome to overcome multidrug resistance, enhance anti-tumor efficiency and reduce toxicity. Drug Deliv. [Epub ahead of print]. doi: 10.3109/10717544.2014.990651
- Vaishampayan U, Hussain M. (2008). Update in systemic therapy of prostate cancer: improvement in quality and duration of life. Expert Rev Anticancer Ther 8:269–81
- Voogd A, van Oost F, Rutgers E, et al. (2005). Long-term prognosis of patients with local recurrence after conservative surgery and radiotherapy for early breast cancer. Eur J Cancer 41:2637–44
- Wang L, Liu Z, Liu D, et al. (2011). Docetaxel-loaded-lipid-based-nanosuspensions (DTX-LNS): preparation, pharmacokinetics, tissue distribution and antitumor activity. Int J Pharm 413:194–201
- Xiao H, Verdier-Pinard P, Fernandez-Fuentes N, et al. (2006). Insights into the mechanism of microtubule stabilization by Taxol. Proc Natl Acad Sci 103:10166–73
- Yanasarn N, Sloat BR, Cui Z. (2009). Nanoparticles engineered from lecithin-in-water emulsions as a potential delivery system for docetaxel. Int J Pharm 379:174–80
- Yoo HS, Kim TG, Park TG. (2009). Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv Drug Deliv Rev 61:1033–42
- Zhang J, Wang X, Liu T, et al. (2014). Antitumor activity of electrospun polylactide nanofibers loaded with 5-fluorouracil and oxaliplatin against colorectal cancer. Drug Deliv. [Epub ahead of print]. doi: 10.3109/10717544.2014.916768