Abstract
Context: Breast cancer is the most common cancer in female population. Breast cancer chemotherapy using doxorubicin (DOX) is well illustrated. However, a significant obstacle for successful chemotherapy with DOX is multidrug resistant (MDR) in breast cancer cells. Targeted nanocarriers have emerged as frontier research for the improvement of cancer chemotherapy.
Objective: Bombesin (Bn)-modified, DOX-loaded solid lipid nanoparticles (Bn-DOX/SLNs) were constructed. Doxorubicin-resistant MCF-7/MDR human breast cancer cells and the cancer animal models were applied for the evaluation of the in vitro and in vivo anti-tumor effect of Bn-DOX/SLNs.
Methods: Bn-conjugated lipids were synthesized. DOX was then loaded into Bn-modified SLNs. The physicochemical properties of the Bn-DOX/SLNs were investigated by particle size and zeta potential measurement, drug loading and drug-entrapment efficiency, and in vitro drug release behavior. In vitro cytotoxicity against MCF-7/MDR cells was investigated, and in vivo anti-tumor of SLNs was evaluated in human breast cancer mice models.
Results: Bn-DOX/SLNs showed an excellent in vitro cytotoxicity and in vivo anti-tumor effect both in MCF-7/MDR breast cancer cells and breast cancer animal model.
Conclusion: The results demonstrated that Bn-DOX/SLNs reversed the resistance of doxorubicin, suggesting that chemotherapy using this kind of targeted nanocarriers may benefit human breast MDR cancer therapy.
Introduction
Breast cancer is one of the most frequently diagnosed types of cancer in women worldwide (DeSantis et al., Citation2014). Despite a significant progress in early diagnosis and treatment, systemic chemotherapy is considered as a standard strategy against metastatic breast cancer. Doxorubicin, due to its excellent anti-tumor efficiency against various solid tumors, is one of the most interesting chemotherapeutic drugs in clinical regimens for breast cancer, especially for metastatic breast cancer (Cabeza et al., Citation2015). However, chemotherapeutic resistance, particularly to DOX, represents a major impediment to successfully treating breast cancer (Kim et al., Citation2015; Zhao et al., Citation2015). Therefore, new research efforts are necessary to overcome its poor tumor selectivity and severe side effects in healthy tissues, and to reduce the drug resistance associated with DOX (Carvalho et al., Citation2009; Toldo et al., Citation2013).
Multidrug resistance (MDR) is often the result of over-expression of drug efflux pumps (Liu et al., Citation2015). To prevent drug efflux, nanotechnology approaches such as magnetic nanoparticles and solid-core nanoparticles have been successfully designed (Kievit et al., Citation2011; Accardo et al., Citation2014). Solid lipid nanoparticles (SLNs) have come up as the latest development in the arena of lipid-based colloidal delivery systems after nanoemulsion and liposomes ever since their introduction in the early 1990s (Müller et al., Citation2011; Thukral et al., Citation2014). SLNs are composed of the lipid matrix, which is solid at body and room temperature (Doktorovova et al., Citation2014). Compared to lipid nanoemulsions, liposomes or polymeric nanoparticles, SLNs provide the following advantages: improved stability and drug-encapsulation ability; controlled release of an encapsulated drug; biodegradable excipients; and easily scaled up (Weber et al., Citation2014). In this text, DOX-loaded solid lipid nanoparticles (DOX/SLNs) were constructed. In order to improve the therapeutic index of DOX by reducing its toxicity or enhancing its efficacy, peptide targeting is being actively pursued in our study.
Peptides, based on their chemical and biological properties, could have a prevalent role to direct drug encapsulated nanoparticles. A considerable number of molecular targets for peptides are exclusively expressed on both cancer vasculature and cancer cells. Interesting results have been obtained by using the Bn peptide (Accardo et al., Citation2012). Bombesin (Bn) is a 14-amino-acid peptide present in amphibian tissues. Bn receptors, known as gastrin-releasing peptide receptors (GRPR), have been found to be overexpressed in breast, prostate, ovarian, etc (Chanda et al., Citation2010; Kulhari et al., Citation2014a). Therefore, Bn was chosen as the ligand for DOX/SLNs-targeted delivery.
In our present study, Bn-DOX/SLNs were engineered. The particle size, zeta potential, encapsulation efficiency and in vitro release were characterized. In vitro cytotoxicity against MCF-7/MDR cells was investigated, and in vivo anti-tumor of SLNs was evaluated on human breast cancer mice models.
Materials and methods
Chemicals and reagents
Bombesin, doxorubicin, stearic acid (SA), l-α-phosphatidylethanolamine (PE), Dulbecco’s Modified Eagle’s Medium (DMEM), and (3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl-2H-tetrazolium bromide] (MTT), were purchased from Sigma-Aldrich (St. Louis, MO). Injectable soya lecithin was purchased from Shanghai Taiwei Pharmaceutical Co, Ltd (Shanghai, China). 1,2-Dioleoyl-3-trimethylammonium-propane (DOTAP) were obtained from Avanti Polar Lipids (Alabaster, AL). COOH-PEG-COOH was purchased from Shanghai Yare Biotech Inc (Shanghai, China). All other chemicals were of analytical grade or of high-performance liquid chromatography (HPLC) grade.
Cell lines and animals
The MCF-7 human breast cancer cell lines were obtained from American Type Culture Collection (Manassas, VA). The MCF-7/MDR cell lines were generated through sequential exposure to increasing concentrations of doxorubicin (0.1–1 µM). The cells were maintained and cultured in DMEM, supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin at a temperature of 37 °C in a humidified atmosphere with 5% CO2. Doxorubicin (1 µM) was added to the culture medium to maintain the MDR characteristics of the MCF-7/MDR cells (Kim et al., Citation2015).
BALB/c mice (6–8 weeks old, 20–25 g weight) were purchased from the Medical Animal Test Center of Shandong University, maintained at 25 °C and 55% of humidity with free access to standard water and chow. All animal experiments were complied with the Animal Management Rules of the Ministry of Health of the People’s Republic of China.
Synthesis of Bn-PEG-l-α-phosphatidylethanolamine
Bn-PEG-l-α-phosphatidylethanolamine (Bn-PEG-PE) ligands were synthesized. Firstly, PEG-PE was synthesized via the reaction between the carboxyl group of COOH-PEG-COOH with the amino group of l-α-phosphatidylethanolamine (PE) (Wang et al., Citation2012; Jing et al., Citation2013). Then, Bn-NH2 and COOH-PEG-PE were reacted in the presence of 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide (EDC) and N-hydroxysuccinimide (NHS). The mixture was kept for stirring for 24 h (Kulhari et al., Citation2014b). Finally, Bn-PEG-PE solution was obtained and purified by dialyzing. The yield of Bn-PEG-PE was 75.8%.
Construction of Bn-DOX/SLNs
Bn-DOX/SLNs were prepared following the solvent evaporation method (Jiang et al., Citation2012). DOX (10 mg), stearic acid (50 mg) and injectable soya lecithin (20 mg) was accurately weighted and dissolved in 10 ml acetone. Bn-PEG-PE was dissolved in 0.1% DOTAP solution to form an aqueous phase. The organic phase was added dropwise into the aqueous phase being stirred at 400 rpm. When complete evaporation of the organic solvent had occurred, the redundant stabilizers were separated by ultracentrifugation at 10 000 rpm, 4 °C for 10 min. The pellet was resuspended in double distilled water.
DOX-loaded SLNs do not containing Bn (DOX/SLNs) were prepared using the same method with PEG-PE instead of Bn-PEG-PE. Blank SLNs do not containing DOX were prepared using the same method without adding the drug. The obtained SLNs suspensions were stored at 2–8 °C.
Physicochemical characterization of SLNs
The mean particle size, polydispersity index (PDI) and zeta potential of SLNs, were analyzed by photon correlation spectroscopy (PCS) with a Zetasizer 3000 (Malvern Instruments, Malvern, UK). The average particle size was expressed as volume mean diameter and the reported value was represented as mean ± SD (n = 3).
The drug-loading ability (DL) encapsulation efficiency (EE) of the Bn-DOX/SLNs and DOX/SLNs were determined by a subtraction method (Wu et al., Citation2014). Briefly, 0.2 ml of SLNs complexes solution was centrifuged through a filter (EMD Millipore, Billerica, MA) with molecular weight cutoff of 3 kDa. Free DOX could pass through the filter, but DOX-loaded SLNs could not pass through the filter. Unincorporated DOX in the solution was quantified by determining the absorbance at 485 nm using a spectrophotometer. DL and EE were calculated using the following equations:
In vitro release assay
To determine the in vitro release behavior of Bn-DOX/SLNs and DOX/SLNs, a dialysis technique was used (Liu et al., Citation2014). Preparations were added to a pretreated dialysis bag. Then, the dialysis bags were incubated with 30 ml of release medium (1 M sodium salicylate in PBS, pH 7.4) at 37 °C with stirring at 100 rpm. At predetermined time intervals, the NLCs suspensions were centrifuged and the amount of drug released in the supernatant was analyzed by the same method in the above section.
Cell viability analysis
The doxorubicin-resistant MCF-7/MDR cells were seeded at a density of 1 × 104 cells/well into a 48-well plate (Kim et al., Citation2015). The cells were treated with Bn-DOX/SLNs, DOX/SLNs, DOX solution, blank SLNs and 0.9% saline for 72 h. Subsequently, MTT solution (0.5 mg/ml) was added to the culture medium for 4 h incubation in a 37 °C and 5% CO2 atmosphere. The cells were then dissolved in dimethyl sulfoxide (DMSO). Relative viability was obtained from the absorbance at 570 nm using an ELISA reader (VERSA max microplate reader; Molecular Devices, Toronto, ON, Canada).
Anti-tumor effects in vivo
The anti-tumor effects of SLNs were investigated in gastric tumor-bearing BALB/c mice (Han et al., Citation2014). BALB/c mice were inoculating subcutaneously in the armpit with MCF-7/MDR cells suspended in PBS. When tumor volume reached about 100 mm3, transplanted mice were randomly divided into five groups separately. Bn-DOX/SLNs (10 mg DOX/kg), DOX/SLNs (10 mg DOX/kg), DOX solution (10 mg DOX/kg), blank SLNs, and 0.9% saline were injected through the tail vein of the mice of each group, once every 3 d. After drug administration, tumor growth was determined by caliper measurement every 3 days. Tumor volume was calculated as follows: Tumor volume (mm3) = (length × width2)/2.
The anti-tumor efficacy of each formulation was evaluated by tumor inhibition rate and was calculated using the following formula: Tumor inhibition rate (%) =(Wcontrol − Wsample)/Wcontrol × 100, where Wsample and Wcontrol represent the mean tumor weight of the samples and control group, respectively.
Statistical analysis
All studies were repeated three times and all measurements were carried out in triplicate. Results were reported as means ± SD (SD = standard deviation). The one-way analysis of variance (ANOVA) and Tukey’s multiple comparison post-tests were used. Differences less than 0.05 (p < 0.05) were considered statistically significant.
Results and discussion
Characterization of SLNs
Mean particle size, PDI and zeta potential of Bn-DOX/SLNs, DOX/SLNs and blank SLNs were characterized and displayed in . The size of Bn-DOX/SLNs, DOX/SLNs and blank SLNs was 187.4, 165.6 and 135.2 nm. These results show that the loading of DOX increased the size of SLNs, also the particles enlarged with the modification of Bn. Particle size can influence the in vitro and in vivo delivery behavior of the nanoparticles, including prolonged blood circulation time, and targeted ability to the tumor cells (Niknejad & Mahmoudzadeh, Citation2015). The zeta potential of SLNs was positive, positive surface charge would let the SLNs easily absorb onto the passive charged cell surface (Khurana et al., Citation2013). The EE of Bn-DOX/SLNs and DOX/SLNs were close (p > 0.05). This could be the evidence that the modification of Bn did not affect the encapsulation of DOX. The high EE achieved could offer advantages in the in vitro and in vivo anti-cancer efficiency.
Table 1. Characterization of SLNs.
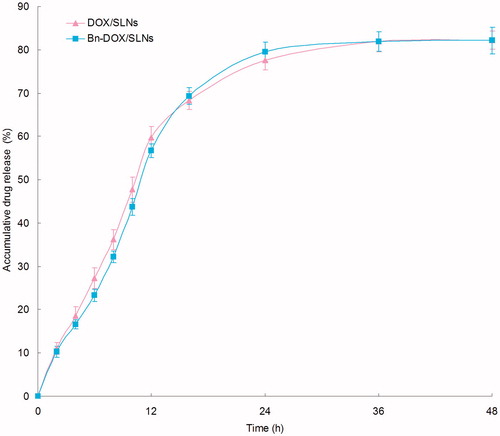
In vitro release profiles of Bn-DOX/SLNs and DOX/SLNs were illustrated in . Bn-DOX/SLNs and DOX/SLNs showed the similar sustained release behavior of DOX. Both SLNs formulations achieved nearly complete releases at 36 h. The sustained-release of SLNs may bring about long-term anti-tumor effect.
Cell viability analysis
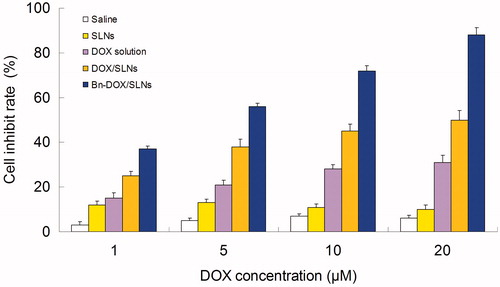
Using the MTT method, the cell inhibit rate of MCF-7/MDR cells with different samples were determined for cytotoxicity investigation. As shown in , Saline and blank SLNs groups showed low toxicity due to high cell viability (over 80%). Significantly breast tumor cell toxicity of Bn-DOX/SLNs, DOX/SLNs, DOX solution was observed at the concentration of 5–20 µM, complied with a dose-dependent cell inhibition behavior. The cytotoxicity of DOX/SLNs was significantly higher than DOX solution (p < 0.05), DOX solution showed no significant effect on the cells because of MDR effect of MCF-7/MDR cells. The significantly higher cell inhibition rate of SLNs groups shows the anti-MDR ability of the carriers in MDR breast tumor cells. Bn-DOX/SLNs exhibited better anti-tumor activity over a wide range of drug concentrations compared to their DOX/SLNs counterpart, shown as the targeted ability of Bn modification.
Anti-tumor effects in vivo
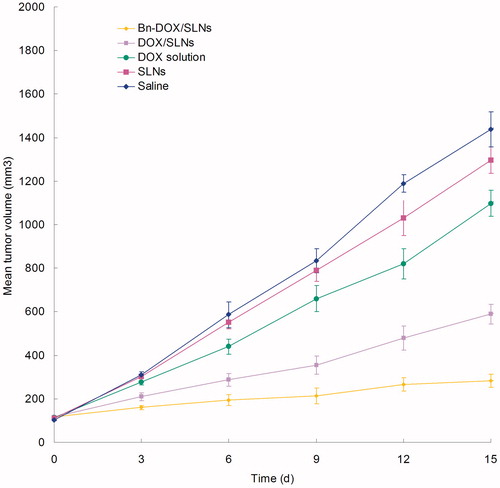
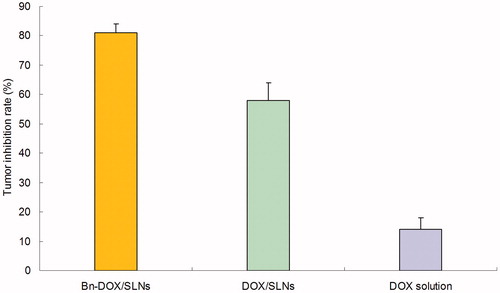
MCF-7/MDR cells bearing BALB/c mice models were used for the evaluation of the anti-tumor efficacy of Bn-DOX/SLNs, DOX/SLNs and DOX solution in vivo. illustrates the increase of tumor volume, which is significantly inhibited by SLNs groups than the solution groups (p < 0.05). The tumor volume of Bn-DOX/SLNs group was the smallest. The tumor inhibition rate of Bn-DOX/SLNs, DOX/SLNs and DOX solution were 81, 58 and 14%, respectively (). The Bn-DOX/SLNs showed far better anti-tumor activity than non-modified DOX/SLNs or free DOX on the inhibition of breast cancer in vivo.
Conclusions
Bn-DOX/SLNs could significantly enhance in vitro cytotoxicity and in vivo anti-tumor effect against MCF-7/MDR cells and gastric cancer animal model compared to the DOX/SLNs and free DOX solution. It could overcome the MDR of DOX and the vector could target the cancer cells effectively. The resulting Bn-modified SLNs can be used as a promising nano-sized drug delivery system for the treatment of breast carcinoma.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Accardo A, Aloj L, Aurilio M, et al. (2014). Receptor binding peptides for target-selective delivery of nanoparticles encapsulated drugs. Int J Nanomedicine 9:1537–57
- Accardo A, Salsano G, Morisco A, et al. (2012). Peptide-modified liposomes for selective targeting of bombesin receptors overexpressed by cancer cells: a potential theranostic agent. Int J Nanomedicine 7:2007–17
- Cabeza L, Ortiz R, Arias JL, et al. (2015). Enhanced anti-tumor activity of doxorubicin in breast cancer through the use of poly(butylcyanoacrylate) nanoparticles. Int J Nanomedicine 10:1291–306
- Carvalho C, Santos RX, Cardoso S, et al. (2009). Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem 16:3267–85
- Chanda N, Kattumuri V, Shukla R, et al. (2010). Bombesin functionalized gold nanoparticles show in vitro and in vivo cancer receptor specificity. Proc Natl Acad Sci U S A 107:8760–5
- DeSantis C, Ma J, Bryan L, et al. (2014). Breast cancer statistics, 2013. CA Cancer J Clin 64:52–62
- Doktorovova S, Souto EB, Silva AM. (2014). Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers – a systematic review of in vitro data. Eur J Pharm Biopharm 87:1–18
- Han Y, Zhang Y, Li D, et al. (2014). Transferrin-modified nanostructured lipid carriers as multifunctional nanomedicine for codelivery of DNA and doxorubicin. Int J Nanomedicine 9:4107–16
- Jiang Z, Sun C, Yin Z, et al. (2012). Comparison of two kinds of nanomedicine for targeted gene therapy: premodified or postmodified gene delivery systems. Int J Nanomedicine 7:2019–31
- Jing F, Li J, Liu D, et al. (2013). Dual ligands modified double targeted nano-system for liver targeted gene delivery. Pharm Biol 51:643–9
- Khurana B, Goyal AK, Budhiraja A, et al. (2013). Lipoplexes versus nanoparticles: pDNA/siRNA delivery. Drug Deliv 20:57–64
- Kievit FM, Wang FY, Fang C, et al. (2011). Doxorubicin loaded iron oxide nanoparticles overcome multidrug resistance in cancer in vitro. J Control Release 152:76–83
- Kim KY, Kim SH, Yu SN, et al. (2015). Salinomycin enhances doxorubicin-induced cytotoxicity in multidrug resistant MCF-7/MDR human breast cancer cells via decreased efflux of doxorubicin. Mol Med Rep 12:1898–904
- Kulhari H, Kulhari DP, Singh M, et al. (2014a). Colloidal stability and physicochemical characterization of bombesin conjugated biodegradable nanoparticles. Colloid Surfaces A 443:459–66
- Kulhari H, Pooja D, Shrivastava S, et al. (2014b). Peptide conjugated polymeric nanoparticles as a carrier for targeted delivery of docetaxel. Colloids Surf B Biointerfaces 117:166–73
- Liu M, Chen D, Wang C, et al. (2015). Intracellular target delivery of 10-hydroxycamptothecin with solid lipid nanoparticles against multidrug resistance. J Drug Target. [Epub ahead of print]. doi: 10.3109/1061186X.2015.1020427
- Liu Y, Wang L, Zhao Y, et al. (2014). Nanostructured lipid carriers versus microemulsions for delivery of the poorly water-soluble drug luteolin. Int J Pharm 476:169–77
- Müller RH, Shegokar R, Keck CM. (2011). 20 years of lipid nanoparticles (SLN and NLC): present state of development and industrial applications. Curr Drug Discov Technol 8:207–27
- Niknejad H, Mahmoudzadeh R. (2015). Comparison of different crosslinking methods for preparation of docetaxel-loaded albumin nanoparticles. Iran J Pharm Res 14:385–94
- Thukral DK, Dumoga S, Mishra AK. (2014). Solid lipid nanoparticles: promising therapeutic nanocarriers for drug delivery. Curr Drug Deliv 11:771–91
- Toldo S, Goehe RW, Lotrionte M, et al. (2013). Comparative cardiac toxicity of anthracyclines in vitro and in vivo in the mouse. PLoS One 8:e58421
- Wang W, Zhou F, Ge L, et al. (2012). Transferrin-PEG-PE modified dexamethasone conjugated cationic lipid carrier mediated gene delivery system for tumor-targeted transfection. Int J Nanomedicine 7:2513–22
- Weber S, Zimmer A, Pardeike J. (2014). Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for pulmonary application: a review of the state of the art. Eur J Pharm Biopharm 86:7–22
- Wu G, Wang Z, Bian X, et al. (2014). Folate-modified doxorubicin-loaded nanoparticles for tumor-targeted therapy. Pharm Biol 52:978–82
- Zhao Y, Zhou Y, Wang D, et al. (2015). pH-responsive polymeric micelles based on poly(2-ethyl-2-oxazoline)-poly(d,l-lactide) for tumor-targeting and controlled delivery of doxorubicin and P-glycoprotein inhibitor. Acta Biomater 17:182–92