Abstract
Context: Silicone oils, such as dimethicone, are commonly administered against gastrointestinal gas accumulation and are attributed with mucoprotective features.
Objective: Evaluation of thiolated silicone oil as advanced antiflatulence with a prolonged retention on small intestinal mucosa as an intended site of action.
Materials and methods: 3-Mercaptopropionic acid (MPA) as a thiol ligand was covalently attached to silicone oil. This thiomer was assessed with regard to foam inhibiting action, droplet size of a suitable self-emulsifying system, mucoadhesion and cytotoxicity.
Results: Antifoaming activity of silicone–MPA was complying with United States Pharmacopeia (USP) requirements for simethicone as standard antiflatulence. Another antifoaming test performed on porcine mucosa supported silicone–MPA's outstanding foam destruction, as this thiomer was superior in comparison to non-thiolated silicone oil and dimethicone with 14.7 ± 2.1 versus 73.3 ± 9.1 and 66.3 ± 7.5 s, respectively. A significantly enhanced mucoadhesiveness (p < 0.02) with 26.2 ± 7.1% silicone formulation remaining on small intestinal mucosa after 8 h was evident for the thiomer without any toxic effect.
Conclusion: Thiolated silicone oil was found to exhibit excellent antifoaming and superior mucoadhesive features. The prolonged residence time of thiolated silicone oil promises to be beneficial in the treatment of flatulence, aerophagy and inflammation throughout the whole gastrointestinal tract.
Introduction
Silicones with their immense diversity are an essential part in many fields of our daily life. In view of pharmaceutical applications, polydimethylsiloxanes, commonly referred to as silicone oil, are regarded to be of advantage for versatile gastrointestinal indications. Among them, dimethicone and simethicone are well-known representatives, whereby the difference between them is that simethicone additionally contains small amounts of silicone dioxide. Those oils are commonly administered as defoaming agents and were evaluated beneficial in the treatment of gastrointestinal gas accumulation (Rider, Citation1968) or before anorectal endoluminal ultrasonography (de la Portilla et al., Citation2003). Furthermore, mucosa-protective features with respect to reflux oesophagitis (Ogilvie & Atkinson, Citation1986; Smart & Atkinson, Citation1990) as well as prevention of gastric lesion formation induced by bile salts have been attributed to dimethicone (Bergmann et al., Citation1989). Commercial solid dosage form products containing dimethicone, such as ILIO Funkton®, Pankreoflat® or Drofaron® are indicated for flatulence, excessive gas accumulation or relief of heartburn and acid indigestion. However, these formulations need to be taken several times per day, which is unfavorable for the patient. Hence, the drawback of current formulations is the limited time of gas inhibiting action due to digestion. Consequently, a more sustained delivery system for antifoaming agents would be beneficial.
Thiomers as innovative bioadhesives show high affinities for mucosal membranes (Bernkop-Schnürch & Steininger, Citation2000). After the covalent attachment of free thiol groups on the polymeric backbone, the thiolated polymer can interact with the mucus layer and attach due to the formation of disulfide bonds (Leitner et al., Citation2003). An enhanced mucoadhesiveness has already been shown for thiolated silicone oil: MPA as well as cysteine was coupled as thiol ligands to a silicone oil side chain resulting in a prolonged residence time on small intestinal mucosa for up to 8 h (Partenhauser et al., Citation2015).
The aim of this study was the in vitro evaluation of thiolated silicone oil as advanced mucoadhesive antiflatulence with a prolonged residence time on small intestinal mucosa as the site of action. Two benefits, a superior mucoadhesion as well as excellent antifoaming activity, should be combined within silicone oil thiomers. Taking the mucoprotective aspect into account, thiolated silicone oils might be of advantage not only for the therapy of gas accumulation, but also for other inflammatory gastrointestinal issues, such as reflux oesophagitis or Crohn's disease. For this study, dimethicone as well as an amino-modified polydimethylsiloxane polymer with a functional group equivalent weight of 4400, both with a viscosity of 100 cSt, and simethicone as standard antiflatulent were chosen as non-thiolated controls. For thiomer synthesis, the silicone oil with free primary amino groups on the side chain was coupled to the carboxylic acid group of MPA as a thiol ligand via carbodiimide reaction as described previously (Partenhauser et al., Citation2015). Due to the high viscosity of the thiomer, a self-emulsifying system for a homogenous silicone–MPA delivery was designed. The influence of three parameters, namely concentration, temperature and pH, on the behavior of the self-emulsifying drug delivery system (SEDDS) was investigated. The silicone oils were evaluated with regard to their antifoaming activity as emulsion or purely applied. Adhesive properties on freshly excised porcine small intestinal mucosa as well as cytotoxic effects on Caco-2 monolayers were additionally assessed.
Materials and methods
Materials
Poly[dimethylsiloxane-co-(3-aminopropyl)methylsiloxane] with a functional group equivalent weight (FGEW) of 4400 Da (silicone oil), N,N′-diisopropylcarbodiimide (DIC), 1-hydroxybenzotriazole hydrate (HOBt), 3-mercaptopropionic acid (MPA), dichloromethane (DCM), l-cysteine hydrochloride monohydrate (cysteine), 4,4′-dithiodipyridine (DTDP), triethylamine, Span 80, Brij O 10, stearic acid, n-butanol and 1-(2-methoxyphenylazo)-2-naphthol (sudan red G), Triton X 100 and minimum essential medium (MEM) were purchased from Sigma Aldrich (Steinheim, Germany). Simethicone (C100LV; Q7-2243) and dimethicone (Xiameter PMX-200) silicone oil were kindly donated from Biesterfeld Spezialchemie (Hamburg, Germany) and Chemische Produkte Dieter Wilde (Bochum, Germany). All other chemicals, reagents and solvents were received from commercial sources and were of analytical grade.
Synthesis and purification of thiolated silicone oil
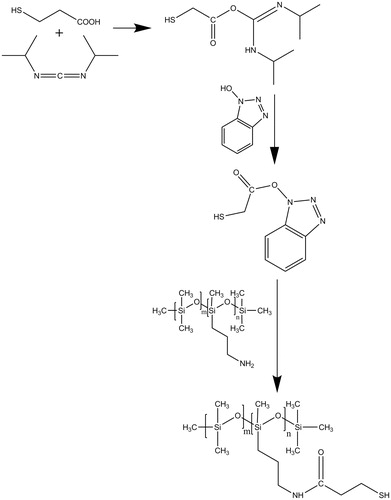
Based on previously described procedures (König & Geiger, Citation1970; Windridge & Jorgensen, Citation1971; Carpino & El-Faham, Citation1999), the active ester HOBt was applied in the combination with carbodiimides. This approach was modified for silicone oils as has recently been published by our research group (Partenhauser et al., Citation2015). In brief, 1 mmol (1 equiv.) of silicone oil and 2 equiv. of triethylamine were dissolved in 40 mL DCM. Then, 2 equiv. of MPA was dissolved in 2 mL of DMSO and 2 equiv. of HOBt was added. The mixture was cooled to 0 °C and 2 equiv. of DIC was dropwisely added. The solution was stirred for 1 h at 0 °C and for 24 h at room temperature. The modified silicone oil solution was purified via filtration and five washing steps with demineralized water until the pH of the aqueous phase was neutral. The residual solvent was removed under vacuum. The thiolated silicone oil was finally centrifuged two times for 10 min at 13 400 rpm and stored at 4 °C until further use.
Determination of thiol groups with DTDP
The total amount of thiol groups attached to the polymeric backbone was determined with DTDP (Egwim & Gruber, Citation2001) as has been described previously for thiolated silicone oils (Partenhauser et al., Citation2015). In brief, the reaction medium was prepared by addition of triethylamine (0.1%, v/v) to DCM and stirring for 10 min in ambient air. One milligram of the polymer conjugate was dissolved in 500 µL of the reaction medium. Subsequently, 500 µL of DTDP reagent (0.9 mg in 10 mL of reaction medium) was added. After 5 min the reaction was terminated with 30 µL of neat acetic acid. Aliquots of 200 µL were transferred to a microtitration plate and the absorbance at 360 nm was read against DCM with a microtitration-plate reader (Tecan infinite M200 spectrophotometer, Grödig, Austria). The quantity of bound thiol ligand was calculated using a standard curve obtained by the thiol group determination of a series of solutions containing increasing concentrations of cysteine.
Composition and preparation of SEDDS
All SEDDS batches contained the respective silicone oil, two surfactants, a co-solvent and, if mentioned, stearic acid as a thickening agent. Several mixtures of excipients were tested with respect to their self-emulsification behavior. During pre-tests, different surfactant combinations and various amounts of all ingredients were applied to optimize the lipid phase and to investigate the maximum silicone load. For a first selection, the stability of the lipid phase overnight and the emulsification behavior of a 1% (w/v) dilution in phosphate buffer pH 6.8 was taken into account. The resulting SEDDS formulations contained 34.5% of both Span 80 and Brij O10 as well as 5% n-butanol, 1% stearic acid and 25% silicone oil. For the lipid core, all components except the silicone were admixed and heated to dissolve the thickening agent in order to achieve a clear phase. After cooling down, modified or unmodified silicone oil as the last component was added. The polydimethylsiloxane was emulsified within the surfactant core via drawing-up with a syringe for 30 times. For the homogenization of an oil-in-oil emulsion with polydimethylsiloxane, a similar approach is described (Lunter et al., Citation2014).
Characterization of self-emulsifying systems
To characterize the SEDDS, particle size measurements were performed under varying conditions. The mean diameter size of the respective emulsion was determined by means of dynamic light scattering using a PSS NICOMP TM 380 DLS (Santa Barbara, USA). First, the effect of dilution was evaluated with increasing concentrations of the SEDDS (0.1%, 0.25%, 0.5%, 1%, 2%) in phosphate buffer, pH 6.8. Second, the droplet size was analyzed in a physiological pH range (pH 1 with 0.1 M HCl, pH 4.5 with 0.1 M acetate buffer and pH 6.8 with 0.1 M phosphate buffer). In addition to that the emulsification behavior at different temperatures (0, 22 and 37 °C) with respect to the droplet size of the SEDDS was investigated.
Antifoaming activity
Testing based on USP method
The applied method was based on the USP Defoaming Activity Test for simethicone as well as a slightly modified version of the test (Torrado et al., Citation1999). The assay was only slightly varied in so far, as no oscillating shaker was applied and the solutions were shaken for the required 10 s without that device. First, a 1% (w/v) Triton X 100 solution in water was prepared as foaming solution. Then, for the respective sample solution, 300 mg of silicone or 1200 mg of SEDDS (only 25% m/m silicone loading; equivalent to 300 mg pure silicone) was diluted with water to 30 g and shaken thoroughly. As non-thiolated controls, the amino-modified silicone oil as well as dimethicone and simethicone were chosen. For each test, a 250-mL glass jar with cap was used. One hundred milliliteres of the foaming solution was filled in and 500 µL of sample solution were dropwisely added. The mixture was shaken for 10 s to allow excessive foam formation. Then, the time was recorded for foam collapse, which is the instant the first portion of foam-free liquid surface appears. The USP acceptance criteria limit this time to 15 s.
“In vivo mimicking” antifoaming activity assay
To the best of our knowledge, no assay to evaluate the defoaming properties under more physiological conditions has been published yet. For the assay, freshly excised intestinal mucosa of approximately 16 cm2, simulated intestinal fluid (0.1 M phosphate buffer pH 6.8; Stippler et al., Citation2004) and a temperature of 37 °C was applied. As non-thiolated controls, the amino-modified silicone oil as well as dimethicone and simethicone was chosen. The mucosa was glued to the bottom of a 1-L vessel. Two hundred and fifty milliliters of a 1% (w/v) Triton X solution in 0.1 M phosphate buffer (pH 6.8) was added and excessive foam to reach a volume of 500 mL was produced. 12.5 mg of pure simethicone, dimethicone or unmodified control and accordingly 50 µL of the respective SEDDS formulation containing either dimethicone, unmodified control or silicone–MPA, was immediately added. The amount of silicone was chosen equivalent to the USP method, which is 0.005% m/v for the silicones and accordingly 0.02% m/v for the SEDDS formulations as they have a silicone payload of 25%. To compare the different antifoaming activities, the time for foam collapse, which is the instant the first foam-free liquid surface appears, was determined. In contrast to the above-mentioned experiment, no sample solution was prepared as the silicone or SEDDS formulation was directly applied to the foam.
In vitro evaluation of mucoadhesive properties
The residence time for up to 8 h of thiolated and non-thiolated silicone oil SEDDS as well as dimethicone and simethicone was determined in vitro by a previously described method (Rahmat et al., Citation2013). For the test, formulations were stained with sudan red G as lipophilic dye. One gram of each sample was admixed with 12 mg of dye and incubated on a thermomixer (Thermomixer Comfort, Eppendorf, Hamburg, Germany) at 30 °C for 3 h. Afterwards the mixture was centrifuged (5 min, 3400 rpm) and the supernatant was withdrawn. The stained formulation was cooled down, admixed five times via a syringe and further used for the study. Freshly excised porcine mucosa was cut into pieces of the same size (approximately 6 cm2) and mounted on a half-pipe using a cyanoacrylate adhesive. The pipes were put in an incubator and positioned at an angle of 45 ° with 100% humidity and a temperature of 37 °C. The mucosa was humidified for an equilibration period of 2 min. Thereafter, 50 mg of the respective SEDDS formulation with unmodified or thiolated silicone oil, dimethicone or simethicone was administered to the mucosa. 0.1 M phosphate buffer pH 6.8 was thoroughly rinsing the mucosa with a constant flow rate of 1 mL/min. After 1, 2, 4 and 8 h mucosa samples in triplicate were taken and incubated for 30 min in DCM. The absorbance was measured at 500 nm with a microtitration-plate reader (Tecan infinite M200 spectrophotometer, Grödig, Austria). Humidified mucosa samples with 50 mg of modified or unmodified stained silicone oil SEDDS, dimethicone or simethicone, were prepared for each incubation time without buffer rinsing and put in the incubator just like the other samples. They were also extracted with DCM and served as 100% control.
Resazurin assay
The potential cytotoxic effect of 0.5% (m/v) SEDDS formulation with silicone–MPA or unmodified control was determined by resazurin assay (O'Brien et al., Citation2000). Approximately 2.5 × 104 cells per well were seeded to a 24-well plate. The cells were incubated for 14 days in MEM supplemented with 10% (v/v) heat inactivated fetal calf serum (FCS) and penicillin/streptomycin solution (100 units/0.1 mg/L) at 95% humidity and 37 °C in an atmosphere of 5% CO2. Experiments were performed during cell passages 14–22. Pure MEM and Triton X 100 served as negative and positive controls, respectively. After 1, 3, 6 and 12 h of incubation, samples were removed from the cells and washed with isotonic phosphate buffered saline. Subsequently, 250 µL of resazurin solution (44 µM) in FCS- and penicillin/streptomycin-free MEM was added to each well and incubated for 3 h. The fluorescence of the supernatant was measured after background subtraction using an excitation wavelength of 540 nm and an emission wave length of 590 nm. Cell viability rates were calculated according to Equation (1):
(1)
where As is the fluorescence of samples and Ac is the fluorescence measured after treatment of cells with MEM.
Statistical data analysis
Statistical data analysis was performed using the student's t-test with p < 0.05 as the minimal level of significance. Results are expressed as means of at least three experiments ± SD.
Results and discussion
Characterization of thiolated silicone oil
Thiolated silicone oil was synthesized by a one-pot procedure via amide bond formation as has recently been described by our research group (Partenhauser et al., Citation2015). MPA as a thiol ligand was covalently attached to the silicone oil side chain. The respective amide bonds were formed between the carboxylic acid group of MPA and the primary amino group of the silicone oil. Regarding the postulated mechanism in , the first step is likely to be the activation of the carboxylic acid moiety of the thiol ligand with DIC and HOBt as an active ester. This combination of coupling reagents has been evaluated more efficient than lipophilic carbodiimides alone (Partenhauser et al., Citation2015). During the second step, this reactive derivative is able to form an amide bond with the amino-modified silicone oil. As the amino-modified silicone oil has a FGEW of 4400, a maximum thiol coupling of 227 µmol/g seems feasible in theory. Previously, for silicone–MPA an almost quantitative coupling efficiency of around 97% was determined (Partenhauser et al., Citation2015). The results in this study were correlating with a coupling rate of approximately 96% and a total thiol group amount of 217 ± 18 µmol/g. The silicone oil–MPA conjugate was used for all further studies.
Design of thiolated silicone oil formulation
Due to the high viscosity of the silicone–MPA, a self-emulsifying system for a homogenous silicone–MPA delivery was designed. To the best of our knowledge, such a delivery system for silicone oil has not been developed yet. Various surfactant combinations were evaluated based on the literature about silicone oil emulsions, which were prepared with blended lauryl ethoxylates (Binks & Dong, Citation1998; Purohit et al., Citation2012) and Span/Tween mixtures (Hu et al., Citation2012). Unmodified silicone oil with a payload of 20% within the formulation and a HLB of 8–9 (Zhang et al., Citation2007) was chosen for a first evaluation (). Higher HLB values, as were obviously feasible for silicone oil emulsions (Nazir et al., Citation2011; Purohit et al., Citation2012), were not applicable for the desired self-emulsifying systems. Concerning convenient surfactants, oleic acid side chains were superior with regard to the emulsification behavior. One reason for that might be that this rather long unsaturated fatty acid is able to stabilize lipophilic silicone molecules within the micelle due to hydrophobic interactions. A blend of Span 80 and Brij O 10 was identified as appropriate regarding handling and emulsification, whereby an HLB of 8.2 seemed to be a feasible compromise. It was hydrophilic enough to ensure an appropriate self-emulsification in an aqueous medium on the one hand and lipophilic enough to enable a thorough homogenization of the silicone phase within the surfactants. In a second step, butanol (Hu et al., Citation2012) was identified as suitable co-solvent due to its superior solubility for the silicone oil in comparison to propylene glycol or ethanol. Subsequently, the maximum silicone oil payload as well as co-solvent content was determined. Although the applied silicone oil is amino-modified, it does not show valuable surfactant properties (Mehta et al., Citation2009), which limits the maximum silicone content for a homogenous emulsification. The selection criterion was, apart from appropriate self-emulsification properties, no phase separation overnight both for the undiluted SEDDS as well as a 1% w/v emulsion. For the unmodified silicone oil this goal was achieved at a co-solvent and silicone content of 5% and 25%, respectively. In contrast to that, the equally prepared SEDDS formulation with silicone–MPA conjugate showed a phase separation on the next day. Alginate, carboxymethyl cellulose and methylcellulose have already been applied as thickening agents in silicone emulsions (Nazir et al., Citation2011; Zhang et al., Citation2007) as well as in commercial products (30% simethicone emulsion USP from Dow Corning®). So, a lipophilic thickening agent was added to enhance the stability of the silicone oil system. Concentrations up to 3% (w/v) were analyzed, but the addition of 1% seemed to be the best choice concerning an increase in stability without decreasing the emulsification properties. As an overall result of the preliminary testing, the final composition of the formulation was 34.5% Span 80 and Brij O10, 5% n-butanol, 1% stearic acid and 25% unmodified or thiolated silicone oil as listed in . Self-emulsifying systems containing mucoadhesive thiolated silicone oil might be used for versatile medicinal applications. Mucoprotective features on the one hand open the door for the therapy of mucosal inflammations throughout the gastrointestinal tract. On the other hand, antifoaming properties ensure the inhibition of excessive gas accumulation in case of flatulence, aerophagy or before endoscopical surgeries.
Table 1. Formulation compositions during optimization of a self-emulsifying system for thiolated or unmodified silicone oil.
Characterization of thiolated silicone oil system via dynamic light scattering (DLS)
Robustness to dilution
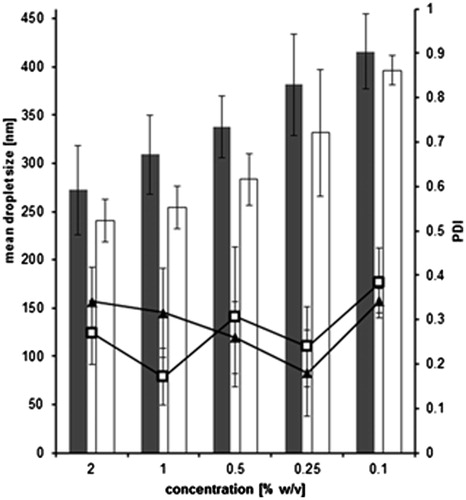
In , the droplet sizes of various SEDDS dilutions are depicted. Droplets from diluted formulations containing unmodified silicone are not significantly different from systems containing thiomer (p > 0.05), although silicone–MPA is a gel unlike unmodified control (Partenhauser et al., Citation2015). This is in contrast to data gained for silicone oil emulsions, where the droplet size was increased with raising silicone oil viscosities (Nazir et al., Citation2014). Concerning the polydispersity index (PDI), no remarkable differences between lower and higher concentrated emulsions are evident. Nevertheless, a 20-fold increase in the concentration of the diluted formulation from 0.1% to 2% only results in a size increase of about 1.5-fold. Therefore, the droplet size can be regarded rather robust upon dilution ensuring a constant surface area after self-emulsification.
Figure 2. Effect of dilution on droplet size of optimized SEDDS formulation containing unmodified silicone (gray bars) and silicone–MPA (white bars) as well as on PDI values for unmodified silicone (▴) and silicone–MPA (□) emulsified in 0.1 M phosphate buffer pH 6.8. (means ± SD; n = 3). No significant difference between unmodified silicone oil and silicone–MPA at p = 0.05.
Influence of temperature
To further investigate the thermodynamic aspect of the self-emulsification behavior, three different temperatures were investigated. In , the results for both formulations are listed. For the formulations with incorporated thiolated silicone, a slight droplet decrease toward higher temperatures was evident. Apart from that, unmodified silicone oil systems did not show a pronounced droplet size tendency for the investigated temperatures. However, the biggest droplets were determined for 0 °C as well. In connection to that, the PDI for both emulsions was decreased at 37 °C, which is a good indicator for a rather monodisperse emulsification at the physiological temperature of the small intestine. All emulsions were kept at the respective temperatures for up to 3 h without any change in droplet size (data not shown), whereby the samples were allowed to equilibrate to room temperature for 10 min before the measurement. Both the probes at 0 °C as well as 37 °C were not measurable without temperature adjustment, most likely due to a decreased or increased Brownian motion in comparison to the samples at room temperature. As DLS distinguishes droplets according to their Brownian motion, it seemed reasonable to measure all emulsions at the same temperature (21 °C) after their previous emulsification at different temperatures.
Figure 3. Influence of temperature on droplet size of optimized SEDDS formulation containing unmodified silicone (gray bars) and silicone–MPA (white bars) as well as on PDI values for unmodified silicone (▴) and silicone–MPA (□) emulsified in 0.1 M phosphate buffer pH 6.8 (means ± SD; n = 3). No significant difference between unmodified silicone oil and silicone–MPA at p = 0.05.
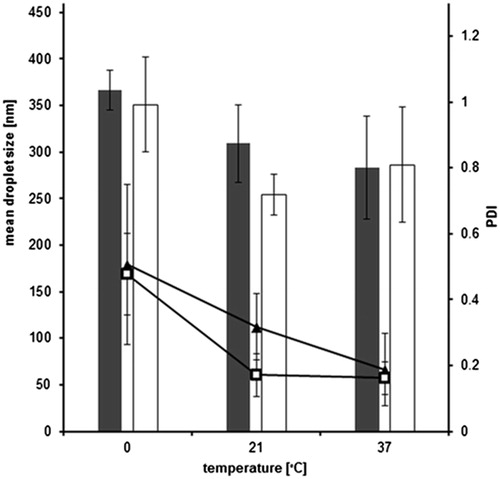
The reason for the higher droplet size as well as PDI value at 0 °C might be that the thermodynamic process of the emulsification is decelerated. As a consequence the micelles are not formed as fast and homogenous as at physiological temperatures. Another hint for this assumption is the fact that the emulsification of the SEDDS droplets in buffer solution was visibly smoother and faster at a temperature of 37 °C than at 0 °C.
Influence of pH
Three different pH values with respect to certain in vivo circumstances were chosen. For emulsions containing unmodified silicone oil, a slight droplet size decrease with increasing pH values was detected (). However, non-thiolated silicone oil droplets are significantly bigger (p < 0.02) than silicone–MPA droplets. This might be due to a protonation of the amino-modified silicone oil as the biggest droplets were determined at pH 1. The droplet size might increase because of an interaction between the cationic amino group and the surfactant micelle. Concerning the size of thiolated silicone oil droplets, no influence of pH was remarkable. PDI values were comparable to the data gained within the dilution studies.
Figure 4. Influence of pH on droplet size of optimized SEDDS formulation containing unmodified silicone (gray bars) and silicone–MPA (white bars) as well as on PDI values for unmodified silicone (▴) and silicone–MPA (□) emulsified to 1 % (w/v) solutions (means ± SD; n = 3). Significant difference between unmodified silicone oil and silicone–MPA at p < 0.02.
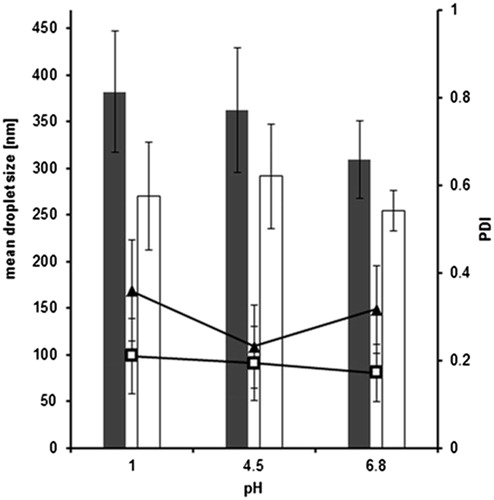
Overall, the thiolation of silicone oil does not seem to interfere with droplet properties as the two systems are quite similar concerning size and PDI values. Neither dilution nor different temperatures or pH conditions seem to remarkably affect the emulsification behavior of both thiomer formulations with a droplet size of mainly around 300 nm.
Antifoaming activity
Testing based on USP method
Antifoaming activity, which is therapeutically used against gastrointestinal gas accumulation, is supposed to be due to two mechanisms: On the one hand the drainage of liquid from foam films and on the other hand the rupture of relatively thick liquid films (Brecević et al., Citation1994). For simethicone, an assay to determine the antifoaming activity of an emulsion is listed in the USP. In , the time for foam destruction of pure silicones or accordingly diluted self-emulsifying formulations are listed. Simethicone was applied as control and fulfilled the USP requirement with a foam collapse within 15 s. During the test, striking differences in antifoaming activity were remarkable at first sight. For the diluted SEDDS formulations containing unmodified silicone oil or dimethicone it took around 7 min for a first foam collapse. This is still twice as fast as without any silicone sample solution, where around 15 min was necessary for foam collapse (data not shown), but far away from the USP requirements. For unmodified silicone oil and dimethicone, the antifoaming activity was increased when they were applied as pure oils. Unfortunately, it was not possible to homogenously distribute silicone–MPA conjugate in pure form within a sample solution as only one big droplet was achievable. As a consequence, the thiomer was evaluated as self-emulsifying system (composition see “Design of thiolated silicone oil formulation” section and ) with respect to its antifoaming activity. Silicone–MPA showed pronounced foam destruction and the USP limit was kept. As this result was so diverse from the other investigated formulations (except simethicone), also lower silicone–MPA amounts were evaluated. For 200 mg and 100 mg added thiomer, the time for foam destruction was higher, but the defoaming activity was still outstanding in comparison to the sample solutions with unmodified silicone oil or dimethicone. The results are in correlation with previously published data, where a low in vitro defoaming activity has already been outlined for commercial products containing dimethicone, such as Pankreoflat (Torrado et al., Citation1999). However, the thiolation of silicone oils, which increases the viscosity of the thiol-bearing conjugate in comparison to the unmodified silicone oil due to disulphide crosslinking (Partenhauser et al., Citation2015), boosts its defoaming properties. The correlation between viscosity and defoaming activity has not been elucidated into detail so far, but one suggestion is that low viscosities are associated with more effective foam-inhibiting action (Ross & Young, Citation1951). So, the thiolated silicone oil might have another mechanism of action to enhance defoaming properties apart from its viscosity change.
Table 2. Antifoaming activity assay based on USP method.
“In vivo mimicking” antifoaming activity assay
In order to support the assumption that the thiolated silicone has advanced foam destructive features, another test was performed. Freshly excised porcine mucosa was involved to elucidate whether any substances, e.g. in the mucus, were interfering with the defoaming activity. So, the direct foam inhibiting action of pure silicone oil or self-emulsifying systems was investigated in comparison to diluted emulsions of the respective formulations within the USP method. Results are illustrated in , whereby simethicone was again used as control. The same antifoaming rank order was evident as for the USP method outlined earlier. The lowest antifoaming activity was determined for SEDDS formulation with dimethicone, however, this assay showed a 3.4-fold faster foam destruction in comparison to the USP method. Both applied silicone oils as well as SEDDS formulation with unmodified control needed slightly more than 1-min for foam inhibition, which is also faster than determined within the USP method. Nevertheless, for silicone–MPA and simethicone, the time stayed almost unaltered below 15 s.
Figure 5. Time for foam destruction determined with freshly excised porcine mucosa in 0.1 M phosphate buffer pH 6.8 at 37 °C. Results are listed for simethicone, dimethicone, non-thiolated silicone oil and silicone–MPA alone or incorporated in SEDDS, respectively (means ± SD; n = 3).
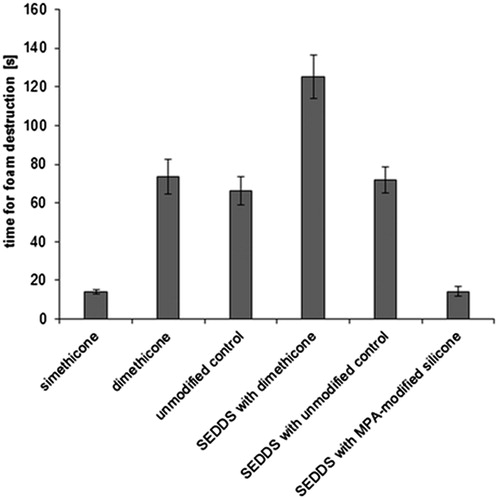
Taking all antifoaming data into account, neither pre-dilution nor pure application of the thiolated silicone oil system affected its foam destructive properties. Especially for surgical applications in the gastrointestinal tract, such as endoscopy, these features might be of advantage for a premedication as well as during the intervention. In case the antifoaming premedication is not sufficient or the visibility is disturbed due to excess gas accumulation, the surgeon would be able to directly apply the undiluted thiolated silicone formulation. This would save time during the surgery and be more comfortable for the patient.
Mucoadhesive properties
To evaluate the mucoadhesive properties, the residence time of silicone formulations on small intestinal porcine mucosa was investigated. Due to the low antifoaming activity, the self-emulsifying system containing dimethicone was excluded from this experiment. As a low mucoadhesive capacity for small intestinal mucosa has already been shown recently for the purely applied amino-modified silicone oil (Partenhauser et al., Citation2015), it was also excluded from the test. highlights that the formulation containing unmodified silicone and dimethicone was washed away very fast. After 8 h of incubation, both formulations could no longer be detected on the mucosa samples. The thiolated silicone oil system, on the other hand, remains on the small intestine pieces until the end of the experiment with a remaining final amount of almost 30% after 8 h. This residue is significantly higher (p < 0.02) than for simethicone, which was determined only marginally at the end of the experiment. As mucus glycoproteins have cysteine-rich subdomains (Gum et al., Citation1992) featuring free thiol groups, those regions dimerise due to disulfide bond formation in the human body. It has already been shown that also thiolated polymers can interact with free mucus thiol groups and form disulfide bonds (Leitner et al., Citation2003). This mechanism is supposed to be the reason for the enhanced mucoadhesiveness of thiomers. As a result, silicone–MPA conjugate shows enhanced mucoadhesion on small intestinal mucosa in comparison to all other silicones. For this thiomer, a prolonged retention time on porcine mucosa has already been shown, when it was applied as pure oil (Partenhauser et al., Citation2015). Thus, the incorporation into self-emulsifying systems does not seem to affect the enhanced mucoadhesiveness of thiolated silicone oil.
Figure 6. Comparison of mucoadhesive properties of simethicone (black bars), dimethicone (shaded bars) and formulations with unmodified silicone (gray bars) and silicone–MPA (white bars) after 1, 2, 4 and 8 h incubation with 0.1 M phosphate buffer pH 6.8 at 37 °C on freshly excised porcine mucosa (means ± SD; n = 3). Significant difference between simethicone and silicone–MPA at p < 0.02.
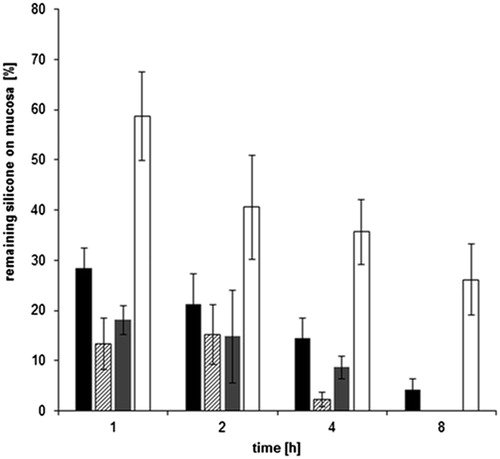
Regarding the performed antifoaming activity tests, silicone–MPA seems to have an as pronounced defoaming action as the gold standard simethicone. However, with free thiol groups on its side chains, silicone–MPA has a prolonged residence time on small intestinal mucosa as the site of action. This can be regarded as a major advantage in comparison to currently available products and an accordingly reduced dosing regimen will foster patient's compliance. The presented silicone thiomer system seems to be a suitable novel formulation for superior small intestine antiflatulence delivery. Apart from that, as silicone oils can be attributed with mucoprotective features, the therapy of relux oesophagitis and inflammatory bowel diseases, such as Crohn's disease, might also be a pharmaceutical targets.
Cytotoxicity
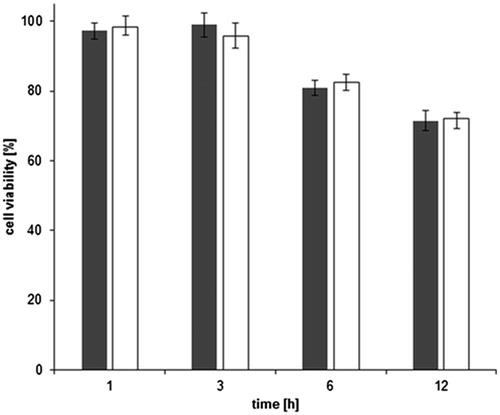
As far as the cytotoxic effects of amino-modified as well as thiolated silicone oil formulation are concerned, the experiment performed on a Caco-2 monolayer showed a cell viability of over 80% for both silicone oils for up to 6 h (). Thus, as the normal human small intestine transit time does only sporadically exceed 5 h (Kim, Citation1968; Fynne et al., Citation2011), neither amino-modified silicone oil nor silicone–MPA seems to have a toxic effect. Even though silicone–MPA is mucoadhesive, the above-outlined study showed that only around one quarter of the formulation remained on the mucosa after 8 h. So, cell viability after 12 h can be considered as a not very likely time frame in vivo and was only chosen to extendedly assess the safety profile of the formulations. Nevertheless, there was only a slight decrease of cell viability between 6 and 12 h. In addition to that, under physiological conditions, the silicone oil system will most-likely be diluted to a greater extent after several hours than the tested 0.5% (w/v). However, even a lower concentration might be enough for the treatment of excessive gas accumulation in vivo as both antifoaming activity tests showed an enhanced effect on foam collapse for a silicone concentration of only 0.02% m/v. This lower amount of thiomer still has the desired properties for the intended antiflatulence. No significant difference between the unmodified and the thiolated silicone SEDDS was evident (p > 0.05), which proofed that the thiolation of the polymer had no toxic effect on the cells. This has already been shown for purely applied thiolated silicone oil (Partenhauser et al., Citation2015) as well as other thiolated polymers such as chitosan (Müller et al., Citation2013).
Conclusion
Within this study, silicone–MPA as thiolated silicone oil was evaluated as enhanced antifoaming and mucoadhesive biomaterial. According to droplet size measurements, which were evaluating the influence of dilution, temperature and pH as three decisive parameters, the thiolated silicone oil system seems to be robust against such fluctuations. A USP conforming high antifoaming activity was evident for the accordingly diluted silicone–MPA conjugate. The striking defoaming action remained, when the thiomer formulation was purely applied on porcine small intestinal mucosa in vitro. For thiolated silicone oil, an advanced mucoadhesion and thus increased residence time on small intestinal mucosa in comparison to non-thiolated control, dimethicone and simethicone was evident. Apart from that, conjugation of amino-modified silicone oil with MPA as the thiol ligand does not increase the cytotoxicity for up to 12 h. Silicone–MPA thus seems to be suitable for the future therapy of excessive gas accumulation with a dosing reduction due to a prolonged residence time on mucosal membranes. In addition, inflammatory diseases throughout the gastrointestinal tract might as well benefit from adhesive and mucoprotective features of the thiolated silicone oil. Reflux oesophagitis, colitis ulcerosa or Crohn's disease, for example, might be accessory pharmaceutical targets.
Acknowledgements
The authors thank J. Mayr and co-workers from the slaughterhouse Natters for supply of porcine intestinal mucosa.
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
References
- Bergmann JF, Simoneau G, Chantelair G, et al. (1989). Use of dimethicone to reduce the fall in gastric potential difference induced by bile salts. Eur J Clin Pharmacol 36:379–81
- Bernkop-Schnürch A, Steininger S. (2000), Synthesis and characterisation of mucoadhesive thiolated polymers. Int J Pharm 194:239–47
- Binks BP, Dong J. (1998). Emulsions and equilibrium phase behaviour in silicone oil + water + nonionic surfactant mixtures. Coll Surfaces A Physicochem Eng Aspects 132:289–301
- Brecević L, Bosan-Kilibarda I, Strajnar F. (1994), Mechanism of antifoaming action of simethicone. J Appl Toxicol 14:207–11
- Carpino LA, El-Faham A. (1999), The diisopropylcarbodiimide/1-hydroxy-7-azabenzotriazole system: segment coupling and stepwise peptide assembly. Tetrahedron 55: 6813–30
- de la Portilla F, Ynfante I, Fernández A, et al. (2003). Improved quality of anorectal endoluminal ultrasonography using emulsion of dimethicone. Dis Colon Rectum 46:1436–7
- Egwim IO, Gruber HJ. (2001). Spectrophotometric measurement of mercaptans with 4,4′-dithiodipyridine. Anal Biochem 288:188–94
- Fynne L, Worsøe J, Gregersen T, et al. (2011). Gastrointestinal transit in patients with systemic sclerosis. Scand J Gastroenterol 46:1187–93
- Gum JR, Hicks JW, Toribara NW, et al. (1992). The human MUC2 intestinal mucin has cysteine-rich subdomains located both upstream and downstream of its central repetitive region. J Biol Chem 267:21375–83
- Hu Z, Liao M, Chen Y, et al. (2012). A novel preparation method for silicone oil nanoemulsions and its application for coating hair with silicone. Int J Nanomed 7:5719
- Kim SKY. (1968). Small intestine transit time in the normal small bowel study. Am J Roentgenol 104:522–4
- König W, Geiger R. (1970), Eine neue Methode zur Synthese von Peptiden: Aktivierung der Carboxylgruppe mit Dicyclohexylcarbodiimid unter Zusatz von 1-Hydroxy-benzotriazolen. Chem Berich 103:788–98
- Leitner VM, Walker GF, Bernkop-Schnürch A. (2003). Thiolated polymers: evidence for the formation of disulphide bonds with mucus glycoproteins. Eur J Pharm Biopharm 56:207–14
- Lunter DJ, Rottke M, Daniels R. (2014). Oil-in-oil-emulsions with enhanced substantivity for the treatment of chronic skin diseases. J Pharm Sci 103:1515–19
- Mehta SC, Somasundaran P, Kulkarni R. (2009). Variation in emulsion stabilization behavior of hybrid silicone polymers with change in molecular structure: phase diagram study. J Coll Interface Sci 333: 635–40
- Müller C, Ma BN, Gust R, Bernkop-Schnürch A. (2013). Thiopyrazole preactivated chitosan: combining mucoadhesion and drug delivery. Acta Biomater 9: 6585–93
- Nazir H, Lv P, Wang L, et al. (2011). Uniform-sized silicone oil microemulsions: preparation, investigation of stability and deposition on hair surface. J Coll Interface Sci 364:56–64
- Nazir H, Zhang W, Liu Y, et al. (2014). Silicone oil emulsions: strategies to improve their stability and applications in hair care products. Int J Cosmet Sci 36:124–33
- O'Brien J, Wilson I, Orton T, Pognan F. (2000). Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem 267:5421–6
- Ogilvie AL, Atkinson M. (1986). Does dimethicone increase the efficacy of antacids in the treatment of reflux oesophagitis? J Roy Soc Med 79:584–7
- Partenhauser A, Laffleur F, Rohrer J, Bernkop-Schnürch A. (2015). Thiolated silicone oil: synthesis, gelling and mucoadhesive properties. Acta Biomater 16:169–77
- Purohit PS, Kulkarni R, Somasundaran P. (2012). Investigation of colloidal properties of modified silicone polymers emulsified by non-ionic surfactants. J Coll Interface Sci 383:49–54
- Rahmat D, Müller C, Barthelmes J, et al. (2013). Thiolated hydroxyethyl cellulose: design and in vitro evaluation of mucoadhesive and permeation enhancing nanoparticles. Eur J Pharm Biopharm 83:149–55
- Rider JA. (1968). Experience with the use of a defoaming agent in the treatment of gastrointestinal gas. Ann NY Acad Sci 150:170–7
- Ross S, Young GJ. (1951). Action of antifoaming agents at optimum concentrations. Indus Eng Chem 43:2520–5
- Smart HL, Atkinson M. (1990). Comparison of a dimethicone/antacid (Asilone gel) with an alginate/antacid (Gaviscon liquid) in the management of reflux oesophagitis. J Roy Soc Med 83:554–6
- Stippler E, Kopp S, Dressman JB. (2004). Comparison of US pharmacopeia simulated intestinal fluid TS (without pancreatin) and phosphate standard buffer pH 6.8, TS of the international pharmacopoeia with respect to their use in in vitro dissolution testing. Dissolut Technol 11:6–11
- Torrado G, García-Arieta A, de los Ríos F, et al. (1999). Quantitative determination of dimethicone in commercial tablets and capsules by Fourier transform infrared spectroscopy and antifoaming activity test. J Pharm Biomed Anal 19:285–92
- Windridge GC, Jorgensen EC. (1971). 1-Hydroxybenzotriazole as a racemization-suppressing reagent for the incorporation of im-benzyl-l-histidine into peptides. J Am Chem Soc 93:6318–19
- Zhang G, Liu Y, Wei C. (2007). Preparation of TS emulsion type organic silicon defoamer. China Surf Detergent Cosmet 37:128