Abstract
Traditional in situ gel forming systems are potential applications for parenteral administration but always accompanied with burst release. To overcome this limitation, the tinidazole (TNZ)-loaded in situ gel forming system using a diblock copolymer, monomethoxy poly(ethylene glycol)–block-poly(d,l-lactide) (mPEG–PDLLA), was designed. The formulation of the mPEG–PDLLA-based TNZ in situ gel forming system contained 5% (w/w) TNZ, 0.4% glycerol, 5 ml N-methyl pyrrolidone (NMP) and 35% (w/w) mPEG–PDLLA. The in situ gel forming system showed sustained TNZ release over 192 h with low burst effect (around 7% in the first 8 h) in the in vitro release study. Additionally, in vivo studies were performed on rabbits with ligature-induced periodontitis, and the concentration of TNZ in the gingival crevicular fluid (GCF) as well as the pharmacokinetic parameters was calculated and the pharmacological effect of TNZ-loaded in situ gel forming (mPEG–PDLLA)-based system was found effective. Finally, histological studies revealed that the gel was a safe formulation with low irritation. The desirable drug release kinetics combined with the excellent in vivo characteristics highlight the potential of the gel in the treatment of periodontitis. Therefore, these results confirmed that the TNZ-loaded in situ gel forming mPEG–PDLLA-based system could reduce burst release of TNZ and act as a sustained-release and injectable drug depot for periodontitis treatment.
Introduction
Periodontitis is a prevalent inflammatory disease characterized by rapid attachment loss, gingival bleeding and periodontal pocket formation, which occurs in the structures of the teeth containing gums, periodontal ligaments, alveolar bone and dental cementum (Seo et al., Citation2004). Dental plaque and pathogenic bacteria, such as Porphyromonas gingivalis, Fusobacterium nucleatum and Prevotella melaninogenica, are responsible for the inflammation associated with this disease (Socransky & Haffajee, Citation1994). It is reported that about 64.7 million of adults suffer from periodontitis in the United States (Eke et al., Citation2012).
Currently, systemic and local antibiotics therapies have been used in periodontitis treatment. However, the main adverse effects of systemic therapy are hypersensitivity, bacterial resistance as well as side effects of antibiotics (Schwach-Abdellaoui et al., Citation2000). To overcome these disadvantages, local drug delivery systems ensure the effective drug concentration at the site of inflammation in order to achieve controlled and sustained release of antimicrobial agents. Numerous local drug delivery systems for periodontitis treatment have been investigated, including implants (Do et al., Citation2014), fibers (Zamani et al., Citation2010; Reise et al., Citation2012), films (Labib et al., Citation2014), gels (Hosadurga et al., Citation2014) and in situ gel forming systems (Walker & Karpinia, Citation2002; Mownika & Srinivas, Citation2012; Mahadlek et al., Citation2013). The in situ gel forming systems are suitable candidates among these local drug delivery systems, and offer great advantages ranging from easy administration with relatively simple procedure, to time-controlled or sustained drug release rate and reduced side effects (Sandker et al., Citation2013).
Tinidazole (TNZ) is a 5-nitroimidazole effective antimicrobial with a half-life of 12–14 h (Wood et al., Citation1982), it is used against most prevalent anaerobic periodontal pathogens, including five species of the genus Prevotella, F. nucleatum and Veillonella spp. (Maestre et al., Citation2007). TNZ has longer half-life and higher bioavailability than metronidazole, which makes it a promising antimicrobial for periodontitis treatment. However, most TNZ were prepared as oral dosage forms, resulting in low concentration of TNZ in periodontal pocket and serious adverse reactions in gastrointestinal tract. Therefore, we developed monomethoxy poly(ethylene glycol)–block-poly(d,l-lactide) (mPEG–PDLLA)-based TNZ in situ gel forming system administrated locally in periodontal pocket in an aim to avoid these limitations.
On the one hand, the amphiphilic diblock copolymer mPEG–PDLLA synthesized by ring opening polymerization is widely applied as a promising carrier for its great biodegradability and noncytotoxicity (Zhang et al., Citation1996), and on the other hand the hydrophilicity and molecular weight of mPEG–PDLLA can be modified by different ratio of mPEG and PDLLA. On the basis of previous literature (Yang & Kao, Citation2006), mPEG–PDLLA has been used for in situ forming implants, which provides a valuable strategy for sustained drug release applications. However, the burst release is the main challenge for in situ formulation and an undesirable high initial release may exhaust the encapsulated drug from gels too rapidly and even cause toxicity problems or tissue irritation in the human body. There are two factors involved in the burst release. Firstly, before precipitation, quick diffusion of drug occurs as the diffusion of solvent into release medium. Secondly, after the formation of gel, drug diffuses through porous surface of polymeric matrix into release medium. In addition, mPEG–PDLLA can reduce burst effect of in situ formulation because the hydrophilicity improves the affinity to the drug; and then accelerates the diffusion of the drug as well as the swelling and erosion of the matrix structure, resulting in well-released drug with low initial release rate.
As for gel formulations, the increase of polymer hydrophobicity can contribute to the dynamics of water influx reduction during the depot forming, resulting in the formation of more dense network. Hence, we selected a ratio of 10:90 of mPEG–PDLLA. The objective of this study was to develop an mPEG–PDLLA-based (with mPEG to PDLLA ratio of 10:90) in situ gel forming system having a sustained delivery of TNZ over 192 h with low initial release in the treatment of periodontitis. Then we evaluated its in vitro properties as well as in vivo and histological properties in an animal model with ligature-induced periodontitis.
Materials and methods
Materials
Monomethoxy poly(ethylene glycol) (mPEG) with Mw of 2000 g/mol and stannous octoate were purchased from Sigma–Aldrich (San Francisco, CA). d,l-Lactic acid was purchased from Shanghai Ling Feng Chemical Reagent Co., Ltd. (Shanghai, China). TNZ was purchased from Zhejiang Supor Pharmaceuticals Co., Ltd. (99% purity, Shaoxing, China). N-methyl pyrrolidone was purchased from International Specialty Products and glycerol was purchased from Hunan Er-Kang Pharmaceutical Co., Ltd. (Hunan, China). Other reagents were of analytical grade. Rats and rabbits were purchased from the Experimental Animal Center of Nanjing Qinglongshan (Nanjing, China). All animal experiments were carried out according to the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by the Animal Ethics Committee of China Pharmaceutical University.
Synthesis and characterization of polymer
Synthesis of d,l-lactide (PDLLA)
d,l-Lactide was synthesized by condensation polymerization and depolymerization of d,l-lactic acid in the presence of stannic oxide as catalyst. A 300 ml d,l-lactic acid was placed in a 500 ml three-neck flask and heated to 80 °C in nitrogen atmosphere and stirred for 1 h. The heating temperature was raised to 150 °C and stannic oxide (1.57% of the volume of d,l-lactic acid) was then added under nitrogen atmosphere and the melted mixture was stirred for 3 h. Then the heating temperature was rinsed up to 280 °C with nitrogen under vacuum (≥0.095 MPa), the crude d,l-lactide was obtained by distillation under reduced pressure followed by recrystallization from ethyl acetate. The recrystallization was repeated three times. After drying at 45 °C under vacuum for 8 h, d,l-lactide was obtained as a white powder with a melting point of 129–130 °C (the yield was 18.8%).
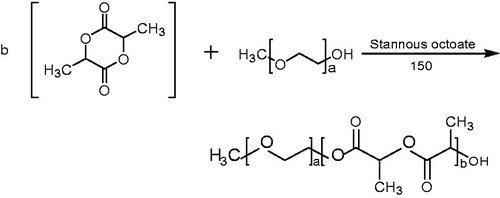
Synthesis of mPEG–PDLLA diblock copolymer (10/90)
The mPEG–PLA diblock copolymer was synthesized according to the ring-opening polymerization, with mPEG to PDLLA ratio of 2:3 w/w (Shi et al., Citation2015). Briefly, 40.0 g mPEG was heated under reduced pressure to 100 °C in a 250 ml three-necked flask, 60 g d,l-lactide and stannous octoate (0.5% of the total weight) were added after mPEG was completely melted. After stirring for 6 h at 150 °C under nitrogen, the mixture was dissolved in methylene chloride followed by precipitation in ice diethyl ether, filtered and concentrated under vacuum at room temperature for 24 h to yield a white solid (the yield was 93.6%).
Characterization of PDLLA and mPEG–PDLLA polymer
The chemical structure of PDLLA and mPEG–PDLLA was characterized by Fourier transform infrared spectroscopy (FT-IR) with potassium bromide (KBr) method using a TENSOR 27 instrument (Bruker, Germany). The chemical structure, molecular weight and the ratio of mPEG–PDLLA diblock copolymer were characterized by 1H NMR in CDCl3 using a 300 MHZ NMR spectrometer (Bruker, Switzerland) at 25 °C. The molecular weight of mPEG–PDLLA was determined by gel permeation chromatography (GPC) system equipped with Shodex column (KF-803, 4.6 × 300 mm, Waters) and Shimadau RID-10A differential refractometric detector. The samples were dissolved in tetrahydrofuran (THF) flowing at a rate of 1.0 ml/min and the column was maintained at 40 °C.
Preparation of TNZ-loaded in situ gel forming system
Five percent (w/w) TNZ was dissolved in 5 ml NMP to obtain a clear TNZ solution at 37 °C. Thirty-five percent (w/w) mPEG–PDLLA was added, then the solution was vortex-mixed thoroughly at room temperature by WH-3 Vortex mixing apparatus (Shanghai, China) until dissolution, then additional 0.4% glycerol was added and completely vortex-mixed at room temperature. Then the solution of TNZ-loaded in situ gel forming system was obtained and the samples were stored in the refrigerator at 4 °C. There were many factors influencing the release rate of TNZ, including TNZ content, the ratio of NMP and polymer and the kind of additives (see Supplementary Figures S1–S4). Then we selected the formulation, containing 5% (w/w) TNZ, 0.4% glycerol, 5 ml N-methyl pyrrolidone (NMP) and 35% (w/w) mPEG–PDLLA, for further studies. Since its great potential of sustained released to 192 h with low burst effect.
Characterization of TNZ-loaded in situ gel forming system
Morphology
The structural morphology of the gel was analyzed by scanning electron microscope (SEM, Quanta 200, Field Emission Inc., Hillsboro, OR). In brief, after gelation and lyophilization for 4 h, the sample was coated with gold before measurement.
Gelation time determination
A 30 mg TNZ-loaded in situ gel forming system was placed into a glass vial, 5 ml PBS 7.4 was added to the sample in a temperature-controlled bath at 37 °C. Furthermore, gelation time was defined as the amount of time needed for the solution to pass to non-flowability condition.
Rheological characterization
Rheological analysis of TNZ-loaded in situ gel forming system was measured using a Brookfield DV-IIIUltra Viscometer equipped with a programmable rheometer (Brookfield Engineering Labs., Inc., Middleboro, MA). The viscosity of the sample was investigated at 0, 25 and 37 °C, respectively using a suitable spindle (no. 25 spindle). All measurements were carried out in triplicates.
Drug content
The TNZ concentration was determined by reverse phase HPLC system (Shimadzu, Kyoto, Japan) equipped with a LC-20AT pump, a SIL-20A autosampler and an SPD-20A UV detector set at a wavelength of 310 nm. The mobile phase consisted of 0.05 mol/l monopotassium phosphate (adjusted to pH 3.5 with phosphoric acid) and methanol (80:20) flowing at a rate of 1.0 ml/min. The chromatographic separations were performed on a reversed phase C18 column (4.6 × 250 mm, 5 μm, Waters XTerra) and the column was maintained at 30 °C. The experiments were repeated three times. Briefly, before each analysis, the gel was dispersed by deionized water and diluted to the appropriate concentration with 1 mg TNZ, and centrifuged at 16 000 rpm for 5 min. Then the solution was diluted by deionized water to give TNZ concentrations of 100 μg/ml. Twenty microliters of samples were then analyzed by HPLC. The drug content was calculated from the standard calibration curve obtained previously with known concentrations of TNZ.
In vitro release study
TNZ release was measured by three loading conditions: TNZ-loaded in situ forming system, TNZ freely mixed with NMP and TNZ–PLA formulation which was prepared by the same method as the test group except that we replaced the polymer PLA with mPEG–PDLLA. Phosphate buffer saline (PBS pH 7.4, 0.01 mol/l) was selected as the release medium that could provide sink condition for TNZ. A 30 mg TNZ-loaded formulation was transferred into the crucible at 37 °C. After adding 5 ml PBS, the surface of formulation began to precipitate, forming gel approximately 5 mm in diameter and 1 mm in thickness. Therefore, we could assure the same surface area among different batches. Then shook in a shaking water bath at a speed of 100 rpm for 24 h. At predetermined time points (2, 4, 8, 12, 24 h until completely released), 3 ml release medium was removed and replaced by an equal volume of fresh PBS. All experiments were done in triplicate.
The concentration of TNZ was determined by HPLC method as described before. The release kinetic parameters of TNZ from the in situ forming system were calculated by the following formula (Yao et al., Citation2009)
where Ve and V0 stand for the volume of removed medium and PBS, respectively (ml). n is the number of samples. Ci and Cn are the concentration of TNZ at i and n times, respectively (mg/ml), and mdrug is the weight of TNZ in the gel.
To investigate the mechanism of drug release, the release data were simulated with zero order (1), first order (2), Higuchi (3) and Peppas kinetic models (4) (Costa & Sousa Lobo, Citation2001; Zhang et al., Citation2010)
(1)
(2)
(3)
(4)
where t is the release time, Q is the percentage of drug released at t, k0, k1 and k2 are the release rate constants. And
/
is the fractional release of the drug, K is a constant incorporating structure and geometric characteristics of the dosage form, n is the release exponent, which indicates the drug release mechanism.
In vivo pharmacokinetic studies
Rabbit periodontitis model
To induce periodontitis, rabbits were anesthetized with 20% urethane into the lateral ear vein. The ligatures were placed at the application sites – the buccal and lingual sides of the first molar on left mandible regions, and 10 μl P. gingivalis was injected directly to the ligated teeth (Hasturk et al., Citation2009). The formation of periodontal pocket was monitored, and all rabbits were provided with 10% sucrose water for two weeks.
Ten rabbits were randomly divided into two groups to investigate the pharmacokinetics of TNZ-loaded in situ gel forming system. Group one (test group, n = 5) was injected subcutaneously with 50 μl TNZ-loaded in situ gel forming system (including 2.95 mg TNZ), while group two (control group, n = 5) was given 50 μl NMP solution of TNZ (including 2.95 mg TNZ). Prior to administration, the two groups were fasted for 12 h and provided with only water. Both formulations were administered by microinjector with flat needles into the periodontal pocket of rabbits, and then added a drop of water in them for gelation. The gingival crevicular fluid (GCF) samples were removed from the periodontal pocket (Ito et al., Citation2014) and collected into eppendorf tubes by test strip (3 mm × 20 mm) at predetermined time points (3, 6, 12, 24, 48, 72, 96, l20, 144, 168, 192 h) (Egelberg & Attström, Citation1973). The GCF samples were dried in nitrogen atmosphere and stored at −20 °C until analysis. In the in vivo study, the concentration of TNZ in GCF was analyzed by HPLC. Briefly, 300 μl mobile phase was added into dry GCF samples, after centrifugation at 10 000 r/min for 10 min, the supernatant of the samples were injected into HPLC, as described in section “drug content”.
Pharmacokinetic parameters were calculated by WinNonlin software (V6.3) using SPSS 12.0 (SPSS, Inc., Tokyo, Japan). Differences between the groups were analyzed by two-tailed t test, p values < 0.05 were accepted as statistically significant. All data were presented as the mean ± standard deviation.
Pharmacological effect evaluation
On the 7th day after administration, rabbits (n = 5 each group) were sacrificed, the gingiva (5 × 5 mm) of the first molar on labial sides of left mandible regions was dissected for pharmacological effect evaluation, while the gingival tissues on the same place of right side was used as control. The specimens were fixed in 10% formalin for 24 h then embedded in demineralized EDTA and paraffin. The embedded specimens were sliced into thin slices of 5 μm in thickness and stained with hematoxylin and eosin (H&E). Evaluations were made by morphometric analysis of staining sections.
Histological safe evaluation
Rats were randomly divided into two groups to investigate the histology of TNZ. After being anesthetized using 20% urethane via an intraperitoneal injection, rats of test groups (n = 5) were injected by 0.1 ml TNZ-loaded in situ gel forming system in the sub-margin of the rat's left mandibular second molar, meanwhile a drop of water was added to the injection site to allow gelation. Whereas the rats in the control group (n = 5) received the physiological saline with the same dose of anesthesia for standardization purposes. Rats were sacrificed 7 days after drug injection and the oral mucosa was removed from the surrounding tissues, fixed with 10% formalin solution and embedded in paraffin. The embedded specimens were sliced into 4–5 μm thin slices and stained with H&E. The stained sections were processed for morphometric analysis of mucosa and histological evaluation.
Results and discussion
Characterization of PDLLA and mPEG–PDLLA polymer
The structure of mPEG–PDLLA is shown in . It is a copolymer with hydrophilic mPEG and hydrophobic PDLLA segments.
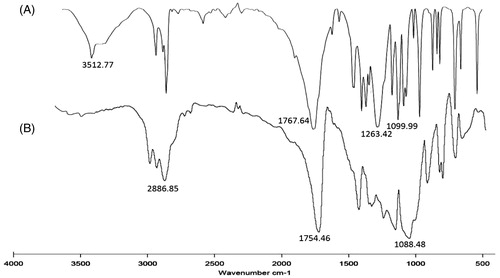
shows the FT-IR spectra of PDLLA and mPEG–PDLLA. The absorption peaks at 1767.64 cm−1 represented C = O group of d,l-lactide. The peaks at 1263.42 and 1099.90 cm−1 were attributed to the bending vibration of C–O group, which could also confirm the formation of d,l-lactide. The weak signals at 3512.77 cm−1 corresponded to the hydroxyl group of lactic acid, which was degraded from d,l-lactide. The diblock copolymer mPEG–PDLLA () showed the characteristic peaks of mPEG at 2886.85 cm−1, which belonged to the stretching vibration of C–H group. While the peaks at 1754.46 and 1088.48 cm−1 were similar to the site of C=O group and C–O group of d,l-lactide, respectively.
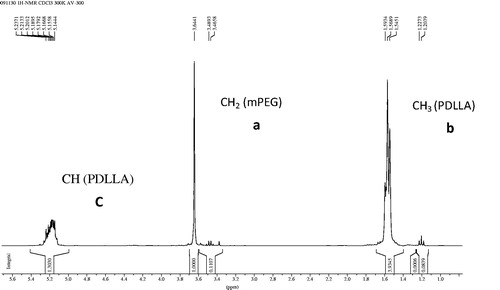
The 1H NMR spectra of mPEG–PDLLA is displayed in . The peaks shown at 3.6 ppm were attributed to methylene protons of mPEG, while the signals at 1.5 and 5.1 ppm were assigned to methyl and methylene protons of PDLLA, respectively. These results confirmed that the diblock copolymer of mPEG–PDLLA was successfully synthesized.
As shown in , the ratio and the number average molecular weights of mPEG–PDLLA were calculated from the areas of peak a and c in the 1H NMR spectra following Equations (5)–(7), while the molecular weight distribution was obtained from GPC. Furthermore, the degree of substitution was very close to 90:10, the molecular weight distribution of mPEG–PDLLA was relatively narrow, which could confirm the formation of the diblock copolymer of mPEG–PDLLA.
(5)
(6)
(7)
Table 1. Molecular weight and molecular weight distribution of mPEG–PDLLA.
Characterization of TNZ-loaded in situ gel forming system
Morphology
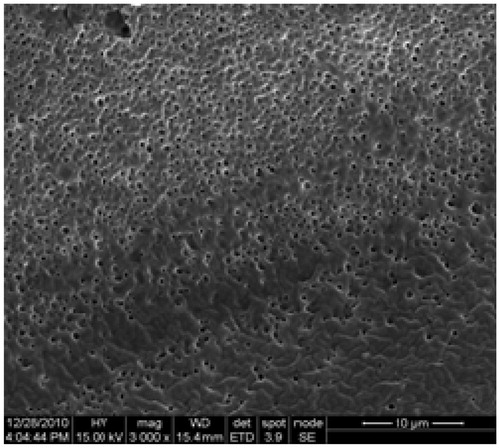
The TNZ-loaded in situ gel forming system had a clear yellow color with stable physical characteristics. The morphology of the gel was determined by SEM. As it can be observed, TNZ-loaded in situ gel forming system had a smooth surface with a high porosity (). The surface of TNZ-loaded in situ gel forming system displayed homogenous surface, indicating that water could penetrate inside and allow TNZ, which was located inside the formulation, to be sustainably released through the pores.
Gelation time and drug content
The sol-to-gel phase transition time was 6 s after the drop of PBS 7.4 was added in the TNZ-loaded in situ gel forming system. The formulation then turned into white and lost its flowability, and the rapid gelation could avoid drug burst from the liquid formulation.
In terms of drug content, the formulation exhibited fairly uniform drug content ensuring adequacy of the method of preparation of the in situ gel forming system.
Rheological characterization
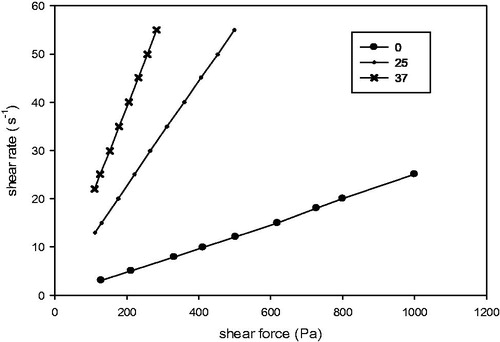
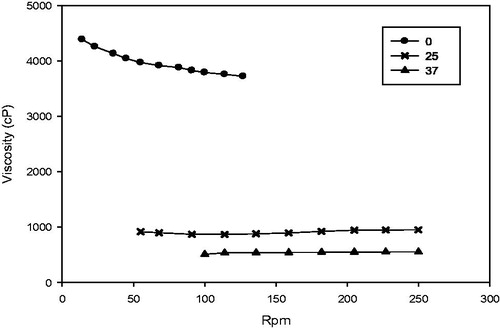
As the gel was intended to inject into the periodontal pocket, it was necessary to determine the rheological properties of the formulation. Rheological properties of TNZ-loaded in situ gel forming system were investigated by the relationship between shear stress and stress rate according to Newton's law of viscosity Equation (8). Flow curves of TNZ-loaded in situ gel forming system at 0, 25 and 37 °C are shown in . It clearly indicated that the shear stress had a linear relation with the stress rate at these three temperatures. Thus, TNZ-loaded in situ gel forming system was Newtonian fluid and followed Equation (8) (Zhu et al., Citation2010). As shown in and , it was noted that the viscosity of the system increased with the increase of the shear rate at 0 °C. However, the viscosity was only in relationship with temperature at 25 and 37 °C. At 25 °C, the viscosity was about 900 cP, which demonstrated that the gel could avoid needle clogging during injection at room temperature. Furthermore, TNZ-loaded in situ gel forming system was found to be a promising drug system since it exhibited suitable viscosity (around 550 cP), namely, mucoadhesive properties, which could ensure retention of the gel at the periodontal pocket, moreover, it could be easily injected at 37 °C.
(8)
where S is shear stress while D is stress rate and η is viscosity.
In vitro TNZ release study
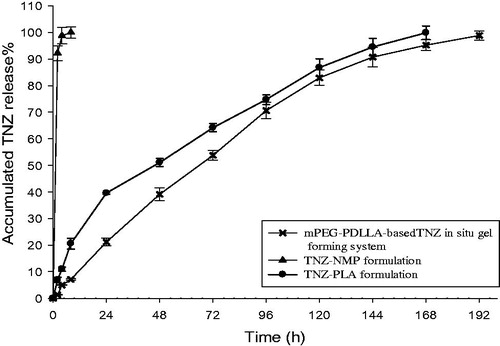
Based on the solubility studies of TNZ in PBS, the saturated solubility of TNZ was 5.88 mg/ml. Therefore, 5 ml PBS was used to maintain sink condition and the concentration of TNZ would never reach more than 10% of its saturated solubility. The TNZ in situ gel forming system with 5% TNZ, 0.4% glycerol, 5 ml NMP and 35% (w/w) mPEG–PDLLA, TNZ–NMP formulation with 5% TNZ and 5 ml NMP, and TNZ–PLA formulation with 5% TNZ, 0.4% glycerol, 5 ml NMP and 35% (w/w) PLA were prepared. The in vitro release study was carried out. The cumulative percentage of TNZ released from in situ gel forming system, TNZ–NMP formulation and TNZ-PLA formulation over 7 days is shown in . Clearly, TNZ in situ gel forming system was sustainedly released with a relative low initial release 7.11% in the first 8 h, followed by a smooth release up to 192 h. And the cumulative percentage of TNZ was 50%, 70%, 90% and 99% at 48, 96, 168 and 192 h, respectively. In comparison, TNZ–PLA formulation exhibited a significant 20.55% burst within 8 h of release, and almost all TNZ released within first 8 h by TNZ–NMP formulation. Therefore, TNZ in situ gel forming system with mPEG–PDLLA can greatly reduce burst effect and achieve sustained release of TNZ over 192 h. The initial burst effect was observed by Gad et al. (Citation2008), whose in situ implants (containing 25% PLA, 35% PLA, 25% PLGA and 35% PLGA, respectively) showed burst effect which ranged from 10% to 15% after 15 min. The in situ forming gel (containing 15% (w/v) PLA) prepared by Kang & Singh (Citation2005) showed high burst release (35–50%), which could decrease with the increase of PLA concentration to 30% (w/v). As for microparticles, microparticles containing oligonucleotide had high initial burst, which was around 50% in first 8 h with 10%, 17% and 20% oligonucleotide loadings (Ahmed et al., Citation2008). In comparison, TNZ in situ gel forming system based on mPEG–PDLLA was a promising formulation capable of reducing initial burst effect around 7% in the first 8 h.
Figure 7. Cumulative percent release of mPEG–PDLLA-based TNZ in situ gel forming system, TNZ–NMP formulation and TNZ–PLA formulation in PBS. Data are shown as mean ± standard deviations, n = 3.
To understand TNZ release mechanism, the results above were simulated with zero order, first order, Higuchi and Peppas kinetic models (Costa & Sousa Lobo, Citation2001; Zhang et al., Citation2010). The results showed that Peppas model best fitted the TNZ release data with a correlation coefficients of 0.9959, the release exponent (n value) was observed between 0.5 and 1.0 and was found to be 0.536, indicating that TNZ release mechanism was assumed to be non-Fickian diffusion, i.e. anomalous transport. Hence, the release was controlled by both erosion of the gel and diffusion of the drug. This was likely due to the swelling and erosion of the matrix structure formed by mPEG–PDLLA (Zhou et al., Citation2010). The release process of the gel could be described as follows: when exposure to the release medium made the polymer form a viscous gel layer, which gradually started to erode and swell. Then TNZ diffuses into the surrounding aqueous liquid through the viscous gel layer.
In vivo pharmacokinetic studies
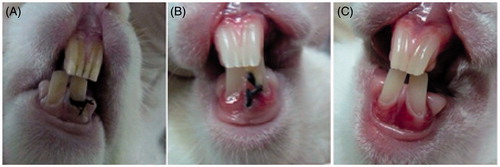
For rabbit periodontitis models, the initial symptoms were observed on the 7th day and severe hyperaemia and oedema appeared at the application site. On the 14th day, large ulcerations were found with enlarged infiltrates and massive abscesses at the gingival margin of the ligated teeth. Periodontal pockets were formed as well ().
Figure 8. Pictures of rabbits with ligated tooth and control tooth at different days after operation, A: 0 day; B: 7 days; C: 14 days.
and show the GCF concentrations of TNZ in two groups over 168 h after administration. The pharmacokinetic parameters were calculated by WIN-NONLIN using non-compartmental analysis as listed in . It was obvious that the test group showed a sustained TNZ release compared with the control group. Moreover, the mean Cmax was higher in the test group in comparison to the control group (1174.856 ± 213.657 and 1008.573 ± 194.661 μg/ml, respectively). And the mean concentration of TNZ in GCF of in situ gel forming system at each time point after 12 h was significantly higher than that of NMP solution, resulting in higher AUC0–168 and AUC0–∞ (95987.44 ± 5979.843 and 4559.758 ± 661.033 versus 10 0611 ± 7521.075 and 4622.01 ± 677.116 h μg/ml, respectively, ). The concentration of TNZ in GCF in the test group rose slowly and reached a maximum at 24 h after administration, then declined gradually within the next 144 h and reached 111.680 μg/ml after 168 h, which was largely higher than the effective concentration (8 μg/ml) (Wüst, Citation1977). After the calculations, the parameters of TNZ-loaded in situ gel forming system are listed in , the comparison of t1/2 and tmax for both the groups showed statistically significant differences (p < 0.05), t1/2 and tmax were dramatically higher in the test group (27.84 ± 6.937 and 1.665 ± 0.167 versus 24 and 3 h). Additionally, the mean residence time (MRT) of the test group was 72.14 h, which was much longer than that of the control group and enabled the promising TNZ-loaded in situ gel forming system to be applied in periodontitis clinical therapy.
Figure 9. TNZ concentration–time profiles in GCF after administration of TNZ-loaded in situ gel forming system (test group), and NMP solution of TNZ (control group). Data are expressed as the mean ± standard, n = 5.
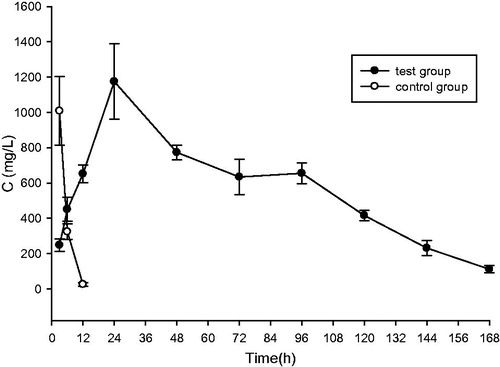
Table 2. The average concentration of tinidazole in GCF of rabbits (n = 5).
Table 3. Pharmacokinetic parameters of test and the control groups by WinNonlin using none-compartmental model.
These results clearly indicated that TNZ-loaded in situ gel forming system (test group) was a sustained release gel and a promising formulation for periodontitis, and the TNZ depot formulation allowed the oral administration of the gel into the periodontal pocket of the rabbits. TNZ was released sustainably due to the saliva erosion and chewing effect, followed by absorption and distribution.
Pharmacological evaluation
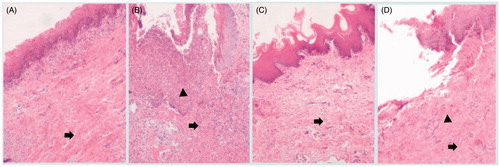
For the purpose of evaluating the efficiency of the gel in vivo, pharmacological studies were carried out based on rabbit periodontitis models. shows H&E-stained sections of control groups and test groups on 1st day and 7th day after injection. After comparison with normal rabbits without any operations which is shown in , inflammatory cells, i.e. neutrophils and eosinophils, were present in the gingival tissues of the test group after the 1st day induction of periodontitis (). As shown in , less number of fibroblasts and collagen fibers appeared due to degeneration or loss. In the TNZ-loaded in situ forming gel group on the 7th day, no inflammatory cell infiltration appeared in the gingival tissues, and a large number of fibroblasts were observed within a network of collagen fibers (). Nevertheless, the epithelial tissue of the control group was irregular and appeared to be damaged, and the presence of inflammatory cell infiltration is expected in . Thus, TNZ-loaded in situ gel forming system could obviously decrease the gingival inflammatory response, and the morphological characteristics were basically similar to healthy rabbits.
Figure 10. Pictures of H&E-stained gingival tissues, (A) healthy rabbits without any operation, (B) rabbits with periodontitis after 1 day of operation without treatment, (C) periodontitis rabbits receiving TNZ-loaded in situ gel forming system after 7 days of administration, (D) periodontitis rabbits receiving NMP solution of TNZ after 7 days of administration. → the collagen fibrils, and ▴ the inflammatory cell.
Histological safety evaluation
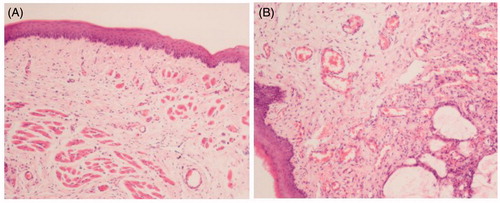
Histological safety evaluation was evaluated for changes in mucosal tissues of rats. The test rats were injected by TNZ-loaded in situ forming gel while the control group received the physiologic saline. We could see from , blood vessels and mucosal tissues were intact without any red swelling or inflammatory infiltration in the control group. In comparison, there was no significant difference between the two groups. Briefly, in the test group, blood vessels dilated slightly with minor bleeding (), suggesting that the inflammatory responses in mucosal tissues for the group that received TNZ-loaded in situ gel forming system were generally mild, and slightly irritating to mucosal tissues.
Conclusion
Based on the results of this study, it was concluded that mPEG–PDLLA based in situ gel forming system was effective on the one hand in delivering TNZ sustainably with low burst release, and on the other hand, in treating periodontal inflammation induced by P. gingivalis in rabbits. The in vitro release profiles of TNZ from the formulation containing 5% (w/w) TNZ, 0.4% glycerol, 5 ml NMP and 35% (w/w) mPEG–PDLLA was examined over 192 h and found that it exhibited a sustained release with low burst release (around 7% in the first 8 h). Our findings suggested that drug content, polymer concentration and the kind of additives had a huge effect on the release of TNZ in vitro (see Supplementary Figures S1–S4). Moreover, pharmacological evaluation of the formulation imposed it as a potential candidate for further applications, which was performed on H&E-stained sections and showed the reduction of inflammatory cell infiltration. In addition, the histological safety evaluation showed that the inflammatory changes in rats received TNZ in situ gel forming system were negligible compared to healthy rats, indicating that the safety of mPEG–PDLLA-based in situ gel forming system was considerably high. The desirable drug release kinetics combined with the excellent in vivo characteristics made the formulation suitable for mPEG–PDLLA-based TNZ in situ gel forming system. Therefore, the present findings revealed that TNZ in situ gel forming system could act as an injectable drug depot that can sustainably release TNZ with low burst effect and irritation for periodontitis treatment. Further experiments about periodontal repair processes on large animals over longer experimental periods using TNZ in situ forming gel are currently underway.
Supplementary material available online
Supplementary figures S1-S4
Supplementary Figures S1-S4
Download PDF (117.3 KB)Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Ahmed AR, Dashevsky A, Bodmeier R. (2008). Reduction in burst release of PLGA microparticles by incorporation into cubic phase-forming systems. Eur J Pharm Biopharm 70:765–9
- Costa P, Sousa Lobo JM. (2001). Modeling and comparison of dissolution profiles. Eur J Pharm Sci 13:123–33
- Do MP, Neut C, Delcourt E, et al. (2014). In situ forming implants for periodontitis treatment with improved adhesive properties. Eur J Pharm Biopharm 88:342–50
- Egelberg J, Attström R. (1973). Comparison between orifice and intracrevicular methods of sampling gingival fluid. J Periodontal Res 8:384–8
- Eke PI, Dye BA, Wei L, et al. (2012). Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res 91:914–20
- Gad HA, El-Nabarawi MA, Abd El-Hady SS. (2008). Formulation and evaluation of PLA and PLGA in situ implants containing secnidazole and/or doxycycline for treatment of periodontitis. AAPS PharmSciTech 9:878–84
- Hasturk H, Goguet-Surmenian E, Blackwood A, et al. (2009). 1-Tetradecanol complex: therapeutic actions in experimental periodontitis. J Periodontol 80:1103–13
- Hosadurga RR, Rao SN, Jose J, et al. (2014). Evaluation of the efficacy of 2% curcumin gel in the treatment of experimental periodontitis. Pharmacogn Res 6:326–33
- Ito H, Numabe Y, Sekino S, et al. (2014). Evaluation of bleeding on probing and gingival crevicular fluid enzyme activity for detection of periodontally active sites during supportive periodontal therapy. Odontology 102:50–6
- Kang F, Singh J. (2005). In vitro release of insulin and biocompatibility of in situ forming gel systems. Int J Pharm 304:83–90
- Labib GS, Aldawsari HM, Badr-Eldin SM. (2014). Metronidazole and Pentoxifylline films for the local treatment of chronic periodontal pockets: preparation, in vitro evaluation and clinical assessment. Expert Opin Drug Deliv 11:855–65
- Maestre JR, Bascones A, Sánchez P, et al. (2007). Odontogenic bacteria in periodontal disease and resistance patterns to common antibiotics used as treatment and prophylaxis in odontology in Spain. Rev Esp Quimioter 20:61–7
- Mahadlek J, Charoenteeraboon J, Phaechamud T. (2013). Benzoyl peroxide in situ forming antimicrobial gel for periodontitis treatment. Key Eng Mater 545:63–8
- Mownika G, Srinivas P. (2012). Formulation and evaluation of simvastatin injectable in situ implants. Am J Drug Discov Dev 2:87–100
- Reise M, Wyrwa R, Müller U, et al. (2012). Release of metronidazole from electrospun poly (l-lactide-co-d/l-lactide) fibers for local periodontitis treatment. Dent Mater 28:179–88
- Sandker MJ, Petit A, Redout EM, et al. (2013). In situ forming acyl-capped PCLA–PEG–PCLA triblock copolymer based hydrogels. Biomaterials 34:8002–11
- Schwach-Abdellaoui K, Vivien-Castioni N, Gurny R. (2000). Local delivery of antimicrobial agents for the treatment of periodontal diseases. Eur J Pharm Biopharm 50:83–99
- Seo BM, Miura M, Gronthos S, et al. (2004). Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364:149–55
- Shi J, Zhang J, Shen Y, et al. (2015). Arginine-stabilized mPEG–PDLLA (50/50) polymeric micelles of docetaxel by electrostatic mechanism for tumor-targeted delivery. Drug Deliv 22:168–81
- Socransky SS, Haffajee AD. (1994). Evidence of bacterial etiology: a historical perspective. Periodontology 2000 5:7–25
- Walker C, Karpinia K. (2002). Rationale for use of antibiotics in periodontics. J Periodontol 73:1188–96
- Wood BA, Faulkner JK, Monro AM. (1982). The pharmacokinetics, metabolism and tissue distribution of tinidazole. J Antimicrob Chemother 10:43–57
- Wüst J. (1977). Susceptibility of anaerobic bacteria to metronidazole, ornidazole, and tinidazole and routine susceptibility testing by standardized methods. Antimicrob Agents Chemother 11:631–7
- Yang H, Kao WJ. (2006). Thermoresponsive gelatin/monomethoxy poly(ethylene glycol)–poly(d,l-lactide) hydrogels: formulation, characterization, and antibacterial drug delivery. Pharm Res 23:205–14
- Yao DG, Sun KX, Mu HJ, et al. (2009). Preparation of cyclosporine A loaded mPEG–PLGA copolymer micelles and study its pharmacokinetics in rats. Yao Xue Xue Bao = Acta Pharm Sin 44:1410–15
- Zamani M, Morshed M, Varshosaz J, et al. (2010). Controlled release of metronidazole benzoate from poly epsilon-caprolactone electrospun nanofibers for periodontal diseases. Eur J Pharm Biopharm 75:179–85
- Zhang X, Jackson JK, Burt HM. (1996). Development of amphiphilic diblock copolymers as micellar carriers of taxol. Int J Pharm 132:195–206
- Zhang Y, Huo M, Zhou J, et al. (2010). DDSolver: an add-in program for modeling and comparison of drug dissolution profiles. AAPS J 12:263–71
- Zhou X, Fu CM, He Y, et al. (2010). In vitro balanced sustained-release of Panax notoginseng saponins controlled with various matrix materials. Acta Pharm Sin 45:505–9
- Zhu J, Jiang P, Yan H, et al. (2010). Preparation and evaluation of pilocarpine nitrate in situ gel for ophthalmic use. Chin Tradit Herbal Drugs 41:720–4