Abstract
To improve the targeting delivery efficiency of anticancer drug to tumor sites, a new strategy combining cell-permeable peptide (CPP) and ultrasound was reported in this article. In this study, we devised and tested a strategy for functional payload delivery to cells by loading CPP–camptothecin conjugate (CPP–CPT) into nanobubble (CPP–CPT NB). Here, CPP existing in the conjugation form of CPP and CPT was hidden in nanobubble to cloak the penetration activity of CPP. Meanwhile, local tumor ultrasound was utilized to achieve specific targeting of CPP–CPT to the tumor cells. The mean particle size of the prepared CPP–CPT NB was ∼200 nm, and the drug entrapment efficiency was >80%. Stimulated by ultrasound, over 90% of the entrapped CPP–CPTs would release from the nanobubbles. Subsequent research demonstrated that the CPP–CPT NB showed effective cellular uptake and significant cytotoxic activity in HeLa cells in vitro. Additionally, after systemic administration in mice, CPP–CPT NB with ultrasound showed a higher tumor inhibition effect in nude mice xenografted HeLa cells tumors and excellent body safety when compared with normal CPT injection group. In conclusion, the carrier constructed in this study would be a safe and efficiently drug delivery system for specific cancer treatment.
Introduction
To reach the action site, therapy agents need to cross many biological barriers, such as organs, cells and intracellular compartments (Torchilin, Citation2000). To address this issue, a new approach employing “cell-penetrating peptides (CPPs)” for payload delivery seems promising. CPPs are positively charged short peptide sequences, rich in lysine or arginine, which are also known as protein transduction domains (PTDs), protein translocation domains, membrane translocating sequences and Trojan peptides (Derossi, Citation1998). These cationic peptides can facilitate cellular internalization of therapeutic agents, which is attributed to interaction between the negatively charged plasma membrane and the positively charged carrier (Zorko, Citation2005; Lindgren & Langel, Citation2011). A CPP/cargo combination can block the endocytosis pathway and translocate directly into the cells without consuming energy. However, CPP is non-specific functional molecule that can penetrate any cell upon encountering it. This drawback limited the utilization of CPPs in drug delivery systems (Vivès, Citation2005).
With the focus of solving this dilemma, stimulus-responsive nanostructures have been introduced to build “off–on” switches to control CPPs’ activity (Taghizadeh et al., Citation2015), which were based on sensitivity to endogenous triggers (e.g. enzymatic activity and pH) and external triggers (e.g. light, ultrasound and temperature). For example, Xiang et al. (Citation2013) developed a system, named “activatable cell-penetrating peptides” (ACPPs), for targeted delivery of siRNA to prostate-specific antigen (PSA)-positive prostate tumors (Xiang, Citation2013). The cellular uptake of CPP was blocked by masking the Arg positive charges with polyanionic segment (polyglutamate) that was linked via a PSA cleavable sequence. The over-expressed MMPs in the vicinity of the tumor would initiate cell penetration of CPP. Because reliance on the above-mentioned endogenous stimulus, this triggered release mechanisms may suffer from some disadvantages, mainly due to the large inter-individual variability in the expression levels of enzymes or intratumoral pHs. To address such drawback, some researchers investigated the external triggered release methodology, which was independent of the extracellular features of the tumor microenvironment. In one example, we reported a liposome modified with photo-sensitive peptide (PSP) (Xie et al., Citation2015). The cationic residues of CPP were temporarily masked by photolabile protecting group (PG) to minimize non-specific cellular uptake. Upon near-infrared (NIR) light illumination, the group would be cleaved, the CPPs regained its activity and facilitated rapid intracellular delivery of the liposomes into cells. However, as the CPPs were immobilized on the surface of the nanocarriers in this strategy, the CPPs may not well be protected from enzymatic degradation in vivo before approaching the targeting sites.
It was proposed that CPP could be incorporated into “smart” carrier delivery platform to enhance its performance (Yang et al., Citation2014). In this strategy, during the first phase of drug delivery, the non-specific function of CPP is sterically shielded. After reaching the target site and exposed under external triggers, the cell penetration of CPP would be activated and thus facilitated the carrier through the cell membrane followed by intracellular delivery of its cargo. Among the various external triggers, ultrasound is especially preferable, as it can penetrate deeply into tissues and be focused on regions of tumor growth to effectively activate sonosensitizers while the peripheral healthy tissue is preserved. Ultrasound-mediated drug delivery can be amplified by acoustic disruption of microbubbles (MB) that undergo cavitation (Escoffre et al., Citation2013). In recent years, MBs have been used successfully in preclinical research for drug delivery (Tsu-Yin et al., Citation2013; He et al., Citation2015). On the other hand, the nanoparticles can accumulate in the tumor tissue via a passive targeting mechanism known as the enhanced permeability and retention (EPR) effect (de Saint Victor et al., Citation2014). Therefore, by utilizing the ascendancies of nanobubbles, a new strategy by encapsulating CPP–drug conjugates into the nanobubbles was hypothesized to mask CPPs’ function as well as improve their in vivo stability in the circulation in this work.
Camptothecin (CPT) is a naturally occurring alkaloid isolated from the Chinese tree Camptotheca acuminata, with promising anti-tumor activity against various leukemia cell lines (Zhang et al., Citation2011). However, due to the lack of reaction selectivity, the use of CPT can cause severe side effects. Recently, various CPP–drug conjugates have been developed to improve anti-tumor efficacy (Lindgren et al., Citation2006; Aroui et al., Citation2010). Inspired by these results, in this study, CPPs would be encapsulated into NBs in the form of CPP–CPT conjugates to realize intracellular delivery of CPT at the tumor sites, and enhance its delivery efficiency.
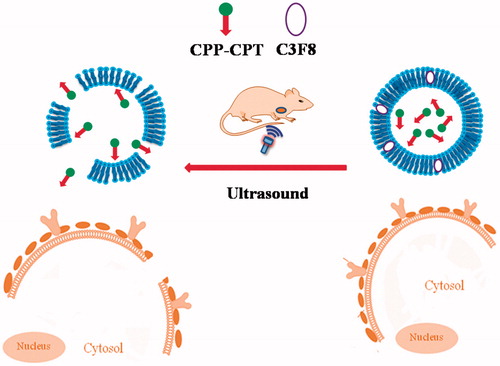
Here, we hypothesized to construct a drug delivery system (designed as CPP–CPT NB). The working scheme of CPP–CPT NB is shown in . CPP (CKRRMKWKK), derived from Penetratin, being reported to have increased membrane translocation efficiency (Fischer et al., Citation2000) would be first conjugated with CPT to form a conjugation of CPP–CPT via chemical reactions; then the CPP–CPTs would be encapsulated into NBs. After systemic administration, CPP–CPT NB would pass or accumulate in tumor sites by the EPR effect. As the target site exposed to ultrasound, CPP–CPTs would release from NBs destructed by ultrasound irradiation. CPP delivered CPT directly and actively through cellular membranes into cells. In this article, we investigated the physicochemical and biological characters of the CPP–CPT-loaded NBs at cellular level, and the in vivo tumor therapy efficiency of the CPP–CPT NB was also explored.
Experimental materials
Materials
CPT was provided by Yuxin Pharmaceutical Co., Ltd. (Sichuan, China). CPP (CGRRMKWKK) was custom-synthesized by Shanghai GL Biochem Co., Ltd. (Shanghai, China). 1,2-Dipalmitoyl-snglycero-3-phosphatidylcholine (DPPC), 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-methoxy (polyethylene glycol) (ammonium salt) (DSPE-mPEG2000) were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL). All chemicals were of reagent grade and were obtained from Sigma-Aldrich (St. Louis, MO), unless otherwise stated.
Methods
Synthesis of CPP–CPT
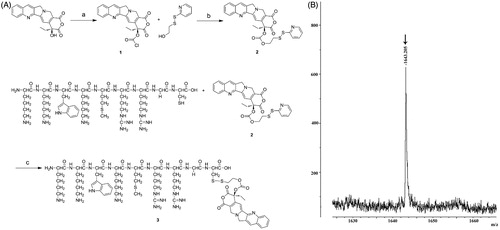
CPP–CPT conjugate was synthesized by coupling CPP with CPT via a disulfide linker as literatures described (Pessah et al., Citation2004; Henne et al., Citation2006; Zhang et al., Citation2011). Synthesis of the disulfide CPP–CPT is showed in . Briefly, CPT (1.5 g, 4.3 mmol) and triphosgene (0.35 equiv) were dissolved in dichloromethane (DCM) (45 mL) at 0 °C, followed by the addition of 4-dimethylaminopyridine (DMAP, 6 equiv). The resulting solution was stirred at room temperature for 0.5 h to obtain compound 1. When the reaction was completed by monitored with (thin-layer chromatography) TLC, 2-(2-pyridinyldithio)-ethanol (2 equiv) was added. The resulting mixture was stirred for 10 h at room temperature to form compound 2. The DCM was removed under reduced pressure. The remaining residue was purified by column chromatography on silica gel (ethyl acetate, 100%), yielding compound 2.
Compound 2 (500 mg, 0.89 mmol), CPP (1 equiv) and 18 mL of dimethyl sulfoxide (DMSO) were mixed and stirred at room temperature for 6 h. The progress of the reaction was monitored by TLC. The DMSO was removed under reduced pressure (0.09 Mpa) in a rotating flask (100 rpm, 45 °C) for 4 h and the remaining residue was purified by column chromatography on silica gel (ethyl acetate, 100%), giving a 67.3% yield of compound 3 in the form of near yellow powders. The final product was obtained through vacuum drying (0.005 Mpa, 30 °C) and verified by a MALDI-TOF/MS (Ultraflex III, Bruker Daltonics, Billerica, MA).
Preparation of nanobubbles
All lipid compositions consisted of DSPE-PEG2000, DSPC and DPPC at a molar ratio of 10:10:90. NBs were prepared as previously described with some modifications (Zhou et al., Citation2013; Zhu et al., Citation2015). In brief, the lipids (50 mg) and CPT (4 mg) or CPP–CPT (equals to 4 mg CPT) were mixed and dissolved in 20 mL of chloroform–methanol mixture (3:1, v/v), then dried by a rotary evaporator for 15 min at 40 °C. The obtained lipid film was hydrated using 15 mL of PBS (25 mM, pH = 7.0) in a rotating flask at the speed of 150 rpm at 50 °C for 30 min to obtain a homogeneous dispersion. The liposome dispersion was homogenized three circulations under 10 000 psi pressure in a high-pressure homogenizer (EmulsiFlex-C3; Avestin, Ottawa, Canada), and was then subjected to extrusion through polycarbonate membrane (200 nm, Whatman, Shanghai China) using an extruder (EmulsiFlex-C3; Avestin). Then the precursor solution was transferred to 3-mL vials and sealed, the headspaces were exchanged with perfluorobutane (C3F8; Flura, Newport, TN). At last, nanobubbles were formed by mechanically shaking the sealed vials with a VialMix (ImaRx Therapeutics, Tucson, AZ) at 3200 rpm for 45 s.
Characterization of CPP–CPT NB
Photo correlation spectroscopy (Nanophox, Sympatec GmbH, Germany) was used to measure the diameter of the nanobubble samples. The nanobubble suspension was diluted with distilled water and then analyzed at the laser intensity of 50–60% under 25 °C. The measuring mode was cross correlation. The morphology of the samples was observed by a transmission electron microscopy (TEM). The samples were diluted with distilled water and deposited onto carbon-coated copper grid, air-dried for 10 min at room temperature and removed excessive water in the sample by a filter paper. Phosphotungstic acid solution (2%, w/v) was added onto the grid as a staining solution. The excess staining solution was removed as described above. The resulting negatively stained samples were air-dried at room temperature and observed by a TEM (H-7650, HITACHI Ltd., Tokyo, Japan) quickly.
Determination of drug content
The amount of CPT or CPP–CPT loaded into the NB was determined by high-pressure liquid chromatography (HPLC) (Agilent 1211, Agilent Technologies, Santa Clara, CA), with Akasil C18 column (4.6 × 250 mm, 5 μm, Agilent Technologies, Santa Clara, CA) and an isocratic mobile phase consisting of 25% (v/v) acetonitrile and 2% (w/v) triethylamine acetate. The detection wavelength was 254 nm.
Entrapment efficiency (EE) of CPT or CPP–CPT in NBs was determined by a minicolumn method as Zhang et al. (Citation2005) described. Briefly, dextran gel (Sephadex G-25) was firstly put into distilled water for 24 h; then, the swelled gel was added into a column (plastic injector); after that, the column was centrifuged for 2 min at 350 g to remove resident water. 0.2 mL of NBs sample was introduced into the dry column and centrifuged at 350 g for 2 min to separate free CPT from the NBs entrapped drug; 0.2 mL of distilled water was introduce into column and centrifuged at 350 g for 2 min to elute residual NBs, the above operation was repeated twice. The eluted NBs were collected and analyzed by an HPLC. The EE was calculated by the following equation:
Fifty microliters of nanobubble sample was mixed with 20 μL of acetonitrile, followed by vortexing to disrupt the membrane and release CPT or CPP–CPT. The resulting samples were then centrifuged at 5000 g for 5 min. The supernatant was injected into the HPLC to determinate the CPT or CPP–CPT content.
In vitro stability of CPP–CPT NB in serum
The stability of CPP–CPT NB in serum (50% FBS in PBS) was determined by evaluating the variations of particle size and size distribution with Turbiscan Lab® Expert (Formulaction, L’Union, France). Briefly, a total of 200 μL of CPP–CPT NB was added to 4 mL of culture medium containing 50% FBS in PBS and incubated at 37 °C, 5% CO2. At predetermined time points, 4 mL of the sample was placed into cylindrical glass tubes and subjected to Turbiscan Lab® Expert stability analysis.
Ultrasound triggered release of payload
The payload CPP–CPT or CPT was released from nanobubbles by insonation using an ultrasound instrument. Briefly, the samples were diluted 20-fold with serum (50% FBS in PBS) and incubated at 37 °C for 5 min and immediately transferred into the bath ultrasound (KQ116, Ultrasonic Instrument Co. Ltd., Kunshan, China). The ultrasound treatment condition was 1 MHz, output power density at 1 W/cm2 for 10 s and with a 10 s pause for a total of 60 s. In this study, these samples treated with ultrasound were named as CPP–CPT NB (+US) and CPT NB (+US). Controls were treated without insonation and named as CPP–CPT NB and CPT NB. The choice of acoustic parameters was based on the reference description with some modification (Du et al., Citation2011). At predetermined time intervals, 100 μL of samples was withdrawn, and the CPP–CPT concentration was determined as described above.
Cell culture
HeLa cells purchased from the Cell Resource Centre of IBMS (Beijing, China) were maintained in culture medium consisted of RPMI1640 medium supplemented with 10% FBS, 100 IU/mL penicillin, and 100 mg/mL streptomycin. The cells were maintained in a 37 °C humidified incubator under a 5% CO2 atmosphere.
Analysis of cellular uptake
The enhancement in cellular uptake offered by CPP-mediated drug delivery system was quantitatively confirmed using flow cytometry with HeLa cells. The cells were seeded in six-well plates at a density of 2 × 105 cells per well overnight. After the attachment period (24 h), the cells were rinsed with PBS and incubated with free CPP–CPT, free CPT–CPT NB or CPP–CPT NB, respectively. The autofluorescence of the cells was used as the control. In nanobubble groups CPT NB or CPP–CPT NB, the cell media contained with NB samples were pre-treated with or without ultrasound (1 MHz, output power density 1 W/cm2 for 10 s with a 10 s pause for a total of 60 s) and then incubated for 4 h at 37 °C. After incubation, the cells were trypsinized, washed twice with cold PBS, and then immediately analyzed using a flow cytometer (BD FACSCalibur, San Jose, CA). In each analysis, the used cells were >105, and the collected cells were 104.
In vitro cytotoxicity assay
The cytotoxicity of free CPP–CPT, free CPT–CPT NB and CPP–CPT NB against HeLa cells was measured using the MTT assay. The cells were seeded into a 96-well plate at a density of ∼4000 cells/well. After incubation for 24 h (37 °C, 5% CO2), the cells were treated with various samples at a range of concentrations. In nanobubble groups (CPT NB or CPP–CPT NB), the cell media contained with NB samples were pre-treated with or without ultrasound, as mentioned above. After the cells were further incubated for 72 h, 20 μL of MTT solution (5 mg/mL in cultural medium) was added to each well and the plates were incubated for another 4 h. Next, the MTT medium was replaced by 200 μL of DMSO in each well, and the mixture was shaken at room temperature to dissolve the reacted dye. The absorbance of each well was measured using a Microplate Reader (Model 680, BIO-RAD Laboratories, Hercules, CA) at a wavelength of 570 nm. All samples were evaluated in sextuplicate.
Animal model
Female BALB/c nude mice (weighing 18–22 g, 5-week old) were purchased from Vital River Laboratories (Beijing, China). All procedures involving animal housing and treatment were approved by the Animal Care and Use Ethics Committee of the Academy of Military Medical Sciences. The anti-tumor efficacy in vivo was evaluated in HeLa cells tumor-bearing female nude mice. Briefly, the mice were subcutaneously injected in right axilla with 0.2 mL of cell suspension containing 5 × 106 HeLa cells. Tumor sizes were measured with calipers across two perpendicular diameters. The estimated tumor volume was calculated using the formula: volume (mm3) = (length × width2)/2.
In vivo anti-tumor efficacy
When the tumor volume reached ∼200 mm3, the HeLa cells tumor-xenografted mice were intravenously injected via the tail vein with 5% glucose (control), 10 mg/kg of CPT, CPP–CPT, CPT NB and CPP–CPT NB on the 6th, 9th, 12th and 15th day. Thirty minutes after administration, the tumor xenograft mice were fixed and the surface of the tumor sites were covered with 1.0 cm thick gel interfaces (EcoGel 100 Imaging Ultrasound Gel, Eco-Med Pharmaceutical Inc., Mississauga, Ontario, Canada), then each tumor site was treated with a HUT-105 ultrasound sonication system (Institute of Biomedical Engineering, Huazhong University of Technology, Wuhan, China) with a sonication area of 0.8 cm2 (Zhu et al., Citation2015), given ultrasound (1 MHz, output power density at 1 W/cm2 for 10 s with a 50 s pause for a total of 30 min). Meanwhile, the mice in groups treated with CPT NB or CPP–CPT NB not subjecting to the ultrasound were also designed as the control. The tumor volumes and mouse body weight were measured. The treatment over control ratio T/C% was calculated as T/C% = (mean RTV of treated group)/(mean RTV of control group) × 100%. The relative tumor volume (RTV) was calculated as RTV = (tumor volume at day n)/(tumor volume at day 0).
Statistical analysis
The data are presented as the mean ± standard deviation (SD). The difference between any two groups was determined via analysis of variance (ANOVA). The comparison of survive cures was by Log-rank (Mantel–Cox) Test. p < 0.05 was considered to be statistically significant. The statistical software SPSS 10.0 (SPSS Inc., Somers, NY) was employed to process the experiment data.
Results and discussion
Synthesis of functional conjugates
The synthetic procedure for CPP–CPT is depicted in . The whole procedure could be divided into the following three steps: Firstly, CPT was handled with triphosgene with the existence of DMAP to generate the chloroformate camptothecin (compound 1). DMAP was used in the reaction to maintain basic conditions to keep the E-lactone ring form of CPT; secondly, 2-(2-pyridinyldithio)-ethanol was added in the reactor to form the pyridyldithioethyl carbonate camptothecin (compound 2); finally, the free thiol of CPP was conjugated to the pyridyldithiol carbonate arm of camptothecin (compound 2) via disulfide exchange and formed the target product of CPP-CPT (compound 3). The molecular weight of the final modified peptide (compound 3) was confirmed by MALDI-TOF/MS (Autoflex III; Bruker Daltonics Inc., Billerica, MA). CPP–CPT expected mass was 1642.7 Da and observed mass was 1643.205 Da (), which implied that the desired CPP–CPT conjugates were successfully synthesized. It is a common reception that the nuclear magnetic resonance spectroscopy (NMR) graph of peptide was hard to analyze and it was usually replaced by the spectrum of MALDI-TOF/MS. Thus, the NMR analysis was not conducted here. The solubility of free CPT and the CPP–CPT conjugate (Moon et al., Citation2008) were estimated to be 28.2 and 122.4 μg/mL, respectively. This 4-fold increase in CPT solubility by the CPP–CPT conjugate might be attributed to the hydrophilic nature of CPP. Besides the enhanced water solubility of CPP–CPT, the conjugate was presumed to stabilize the lactone ring of CPT (Henne et al., Citation2006) and may release the unmodified camptothecin in cells via endosomal disulfide reduction (Zhang et al., Citation2011).
Nanobubble characteristics
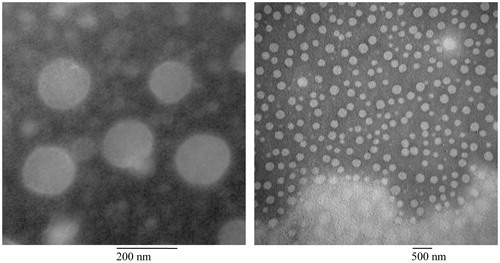
The physicochemical properties of the two distinct nanobubble formulations (CPT NB and CPP–CPT NB are summarized in . As shown in , the CPT NB or CPP–CPT NB encapsulation efficiency of each nanobubbles was >80%. The sizes of CPT NB and CPP–CPT NB were ∼189 ± 10 and 198 ± 9 nm as evidenced from photon correlation spectroscopy, respectively. As there was no bubble like structure found in the visual field of TEM, the bubbles were supposed to be destroyed in the sample preparation and observing process. According to a reference reported, the nanobubbles could not be observed by TEM because perfluorobutane would escape from the liposome and destruct its structure under high vacuum (Du et al., Citation2011). As an alternative, liposomes (CPP–CPT LP) without perfluorobutane were used to represent the nanobubbles and observed under a TEM. As shown in , transmission electron micrograph of CPP–CPT LP demonstrated that the particle sizes were close to the values measured using the laser particle analyzer. In addition, the prepared nanobubbles could be stable at room temperature for 6 d, after that the ultrasound could not trigger the drug release.
Table 1. Characteristics of the nanobubbles.
MB as ultrasound contrast agents have been used in clinical for decades (Stride, Citation2015), and people have developed many new methods to prepare MB during this time (Parhizkar et al., Citation2014; Mahalingam et al., Citation2015). It is a common reception that nanoparticles made ∼200 nm can take the advantage of EPR effect, and thus, we prepared the nanobubbles in this work. The nanobubble reported here was prepared based on methods reported by references with some modification (Gao et al., Citation2013; Zhou et al., Citation2013), and its structure was liposome with perfluorobutane molecules in its bilayer membrane (). In fact, the prepared nanobubble was thermosensitive liposome contained with ultrasound sensitive agent perfluorobutane. However, the detail structure of the prepared nanobubbles remains further studies and we will report it in the future.
In vitro stability in serum and release study
As exhibited in , the transmission or backscattering profile hardly changed for CPP–CPT NB, indicating that there was no aggregation in the presence of 50% FBS in PBS, which was potentially owed to the PEGylation that stabilized the NBs.
Figure 4. Stability of CPP–CPT NB in the presence of 50% FBS. The transmission and backscattering profiles of each formulation was measured at each time point by using Turbiscan Lab® Expert. Data are reported as a function of time (0–24 h) and sample height (from 33 to 48 mm) (A). Release behaviors of CPT or CPP–CPT from nanobubbles with or without ultrasound (B). The data are presented as the mean ± SD (n = 3).
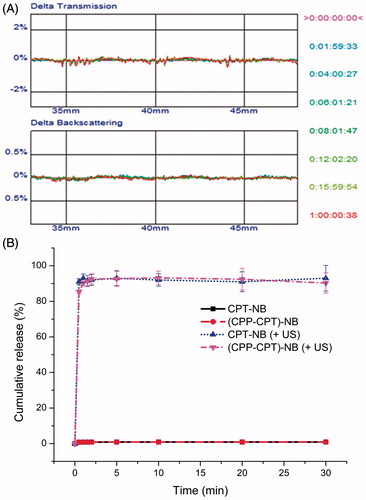
The release of CPP–CPT from various nanobubbles by insonation is depicted in . The data revealed the release of payload from various nanobubbles exhibited an ultrasound-dependent characteristic. About 90% of the entrapped payload was released from nanobubbles after insonation (1 MHz, output power density at 1 W/cm2 for 10 s with a 10 s pause for a total of 60 s). On the contrary, without ultrasound stimulus, the payload encapsulated nanobubbles exhibited minimal payload leakage in serum medium with cumulative release <1.5% after the incubation at 37 °C for 30 min. Therefore, it was anticipated that no drug would release from nanobubbles into circulation (at least for 30 min) before the application of ultrasound.
In vitro intracellular uptake of CPP–CPT
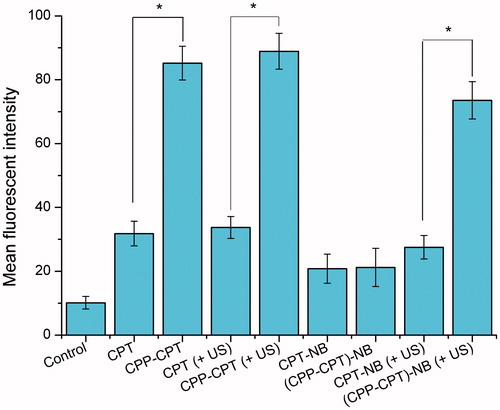
After confirming that nanobubble could work properly via ultrasound stimulus, its ability to deliver drugs into the tumor cells was further assessed. Whether the “functional molecule” CPP could work after ultrasound stimulus is crucial for the success of this specific targeting nanobubble. According to the design strategy, when nanobubble is triggered by ultrasound, the cellular uptake of the CPT is expected to enhance due to the CPP penetration effect. To verify this hypothesis, HeLa cells were used to evaluate the in vitro cellular uptake of CPP–CPT toward tumor cells by flow cytometry. As shown in , there were no statistical cell uptake between the free CPT and the CPT NB ultrasonic group (p > 0.05), so did the CPT–CPP correlated ones; Compared with CPT, CPP–CPT exhibited significantly enhanced cellular uptake in the cell lines (p < 0.05). Furthermore, CPP–CPT NB (+US) exhibited stronger intracellular fluorescence than CPP–CPT NB (p < 0.05), which revealed the contribution of ultrasound-triggered uncaging of CPP–CPTs in NBs to cellular uptake in the cell lines. Taken together, these results indicated that when the nanobubbles were triggered by ultrasound, the cellular uptake of CPT would be enhanced due to the CPP induced cell penetration.
Cytotoxicity
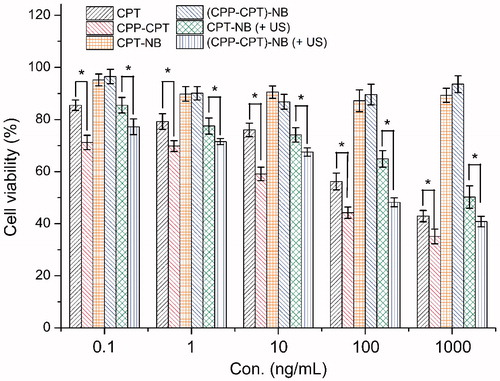
The cytotoxicities of CPT, CPP–CPT and various nanobubbles were evaluated after incubation with the aforementioned cells for 72 h. The potential anti-proliferative activities of CPT, CPP–CPT and various nanobubbles were studied by incubating the cells with these samples at series concentrations from 0.1 to 1000 ng/mL. As shown in , the anti-proliferation effect was in the order of CPP–CPT > CPP–CPT NB (+US) > CPT ≈ CPT NB (+US) > CPP–CPT NB ≈ CPT NB. The results depicted that CPP-DOX showed stronger anti-proliferative activity than DOX in HeLa cell lines (p < 0.05). It might be explained by this, CPP–CPT conjugates can increase the intracellular concentrations of CPT (Fonseca et al., Citation2009). The improved cellular uptake led to an anticipated enhanced anti-proliferation effect. For the similar reason, the molecular CPT with relatively good cell penetration ability displayed a higher cellular uptake than CPT NB. With the aid of ultrasound, CPT NB (+US) showed a comparable cellular uptake to CPT. In addition, the results also demonstrated that the ultrasound stimulus could trigger the CPP–CPTs release from NBs, and thus the CPP promoted the anti-proliferative activities of nanobubbles in the HeLa cells. The data verified that ultrasound stimulus could improve the antiproliferative activity of nanobubbles to cells. In summary, these results () were consistent with previous studies () that demonstrated the ultrasound responsive feature and effective cellular uptake of CPP–CPT NBs by the effect combining CPP and ultrasound stimulus.
In vivo anti-tumor efficiency of the targeted nanobubbles
To determine whether CPP–CPT NB (+US) displays anti-tumor activity in vivo, the effects of CPT NB (+US), CPT NB, CPP–CPT NB (+US) and CPP–CPT NB on tumor growth inhibition in animals were investigated. As shown in , the treatment over control ratio T/C% of CPT, CPP–CPT, CPT NB (+US), CPT NB, CPP–CPT NB (+US) and CPP–CPT NB was 71.0, 57.9, 44.5, 68.3, 22.6 and 49.7%, respectively. The maximal reduction in tumor growth was noted in the group treated with CPP–CPT NB (+US). This correlated with the above-mentioned data revealing the advantage of CPP–CPT NB (+US) over the other carriers tested in intracellular uptake in vitro () and apoptosis induction in vitro (), indicating the combined processes of CPP and ultrasound stimulus. CPP–CPT possessed a lower anti-tumor efficacy in vivo than CPP–CPT NB (+US) and CPT NB (+US), which could be attributed to CPP’s lack of selectivity. However, this in vivo trend was different from the in vitro results, in which the higher uptake and anti-proliferation effect of CPP–CPT-treated cells ( and ) derives from the promoted entry of CPT into cells via CPP. This is because in the in vitro cell experiment the drugs were centered in the cell wells with a relative high concentration, while in the in vivo animal models the drugs were distributed in the circulation with a relative low blood drug concentration.
Figure 7. Anticancer efficacy in the HeLa xenografts in nude mice after the treatment with varying formulations (A). Body weight changes of the HeLa xenografts in nude mice after the treatment with varying formulations (B). The data are presented as the mean ± SD (n = 10). Asterisk indicates p < 0.05.
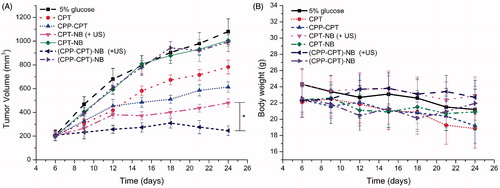
The changes in the body weights of the animals were recorded as an indication of safety. As shown in , there was no significant change in the body weight of the CPT NB (+US) or CPP–CPT NB (+US) group of mice during the experimental period (p > 0.05). These results suggest that there is negligible acute or severe toxicity related to the indicated treatment at the test dose. However, >11% weight loss was detected in the CPT and CPP–CPT-treated group at the end of the experimental period. The weight loss of the CPT or CPP–CPT group was likely due to non-targeted effects of the drugs and tumor cachexia.
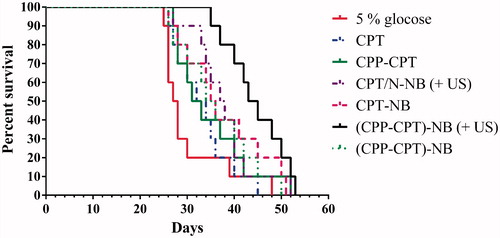
The median survival time of the group treated with 5% glucose, CPT, CPP–CPT, CPT NB (+US), CPT NB, CPP–CPT NB (+US) and CPP–CPT NB were 27.5, 33.0, 32.0, 37.5, 35.5, 44.0 and 35.0 d, respectively (). By statistical analysis, the CPP–CPT NB (+US) group has the longest median survival time in the HeLa models (p < 0.05).
Conclusion
CPP has a good ability to aid drug carrier penetrating into cells, but its non-specific affinity limits its application in drug delivery systems. In this study, a dual function of CPP–CPT NB, combining efficiently cell-penetrating ability of CPPs and the ultrasound-triggered drug release characteristics of nanobubble, was successfully developed. The constructed vesicles showed a suitable particle size, high drug EE and good stability in the presence of FBS. The entrapped CPP–CPT would release under the stimulation of ultrasound. Furthermore, the prepared carriers demonstrated strong tumor inhibitory activities both in vitro and in vivo. Compared with the group without ultrasound treatment, the CPP–CPT NB (+US) showed an increased tumor inhibitory efficacy. The salient advantage of this delivery system is that it overcomes the drawback of the CPPs by “cloaking” them in the carriers and triggers their penetrating function by external ultrasound. Although preliminary, the results of this study demonstrate the tremendous potential of the nanobubble system for the efficient delivery of therapeutic agents for oncotherapy.
Declaration of interest
We are grateful for the financial support from the National Natural Science Foundation of China (Grant No. 81202466).
References
- Aroui S, Brahim S, De Waard M, Kenani A. (2010). Cytotoxicity intracellular distribution and uptake of doxorubicin and doxorubicin coupled to cell-penetrating peptides in different cell lines: a comparative study. Biochem Biophys Res Commun 391:419–25
- de Saint Victor M, Crake C, Coussios CC, Stride E. (2014). Properties, characteristics and applications of microbubbles for sonothrombolysis. Expert Opin Drug Deliv 11:187–209
- Derossi D. (1998). Trojan peptides: the penetratin system for intracellular delivery. Trends Cell Biol 8:84–7
- Du L, Jin Y, Zhou W, Zhao J. (2011). Ultrasound-triggered drug release and enhanced anticancer effect of doxorubicin-loaded poly(D,L-lactide-co-glycolide)-methoxy-poly(ethylene glycol) nanodroplets. Ultrasound Med Biol 37:1252–8
- Escoffre JM, Mannaris C, Geers B, et al. (2013). Doxorubicin liposome-loaded microbubbles for contrast imaging and ultrasound-triggered drug delivery. IEEE Trans. Ultrason Ferroelectr Freq Control 60:78–87
- Fischer PM, Zhelev NZ, Wang S, et al. (2000). Structure-activity relationship of truncated and substituted analogues of the intracellular delivery vector Penetratin. J Pept Res 55:163–72
- Fonseca SB, Pereira MP, Kelley SO. (2009). Recent advances in the use of cellpenetrating peptides for medical and biological applications. Adv Drug Deliv Rev 61:953–64
- Gao W, Xiang B, Meng TT, et al. (2013). Chemotherapeutic drug delivery to cancer cells using a combination of folate targeting and tumor microenvironment-sensitive polypeptides. Biomaterials 34:4137–49
- He X, Wu DF, Ji J, et al. (2015). Ultrasound microbubble-carried PNA targeting to c-myc mRNA inhibits the proliferation of rabbit iliac arterious smooth muscle cells and intimal hyperplasia. Drug Deliv. [Epub ahead of print]. doi: 10.3109/10717544.2015.1014947
- Henne WA, Doorneweerd DD, Hilgenbrink AR, et al. (2006). Synthesis and activity of a folate peptide camptothecin prodrug. Bioorg Med Chem Lett 16:5350–5
- Lindgren M, Langel U. (2011). Classes and prediction of cell-penetrating peptides. Methods Mol Biol 683:3–19
- Lindgren M, Rosenthal-Aizman K, Saar K, et al. (2006). Overcoming methotrexate resistance in breast cancer tumour cells by the use of a new cell-penetrating peptide. Biochem Pharmacol 71:416–25
- Mahalingam S, Raimi-Abraham BT, Craig DQ, Edirisinghe M. (2015). Formation of protein and protein-gold nanoparticle stabilized microbubbles by pressurized gyration. Langmuir 31:659–66
- Moon C, Kwon YM, Lee WK, et al. (2008). A novel polyrotaxane-based intracellular delivery system for camptothecin: in vitro feasibility evaluation. J Biomed Mate Res A 84:238–46
- Parhizkar M, Stride E, Edirisinghe M. (2014). Preparation of monodisperse microbubbles using an integrated embedded capillary T-junction with electrohydrodynamic focusing. Lab Chip 14:2437–46
- Pessah N, Reznik M, Shamis M, et al. (2004). Bioactivation of carbamate-based 20(S)-camptothecin prodrugs. Bioorg Med Chem 12:1859–66
- Stride E. (2015). Physical principles of microbubbles for ultrasound imaging and therapy. Front Neurol Neurosci 36:11–22
- Taghizadeh B, Taranejoo S, Monemian SA, et al. (2015). Classification of stimuli-responsive polymers as anticancer drug delivery systems. Drug Deliv 22:145–55
- Torchilin VP. (2000). Drug targeting. Eur J Pharm Sci 11:S81–91
- Tsu-Yin W, Wilson KE, Machtaler S, Willmann JK. (2013). Ultrasound and microbubble guided drug delivery: mechanistic understanding and clinical application. Curr Pharm Biotechnol 14:743–52
- Vivès E. (2005). Present and future of cell-penetrating peptide mediated delivery systems: “is the Trojan horse too wild to go only to Troy?” J Control Release 109:77–85
- Xiang B, Dong DW, Shi NQ, et al. (2013). PSA-responsive and PSMA-mediated multifunctional liposomes for targeted therapy of prostate cancer. Biomaterials 34:6976–91
- Xie X, Yang Y, Yang Y, et al. (2015). A photo-responsive peptide- and asparagine–glycine–arginine (NGR) peptide-mediated liposomal delivery system. Drug Deliv. [Epub ahead of print]. doi: 10.3109/10717544.2015.1008707
- Yang Y, Yang YF, Xie XY, et al. (2014). Preparation and characterization of photo-responsive cell-penetrating peptide-mediated nanostructured lipid carrier. J Drug Target 22:891–900
- Zhang JA, Anyarambhatla G, Ma L, et al. (2005). Development and characterization of a novel Cremophorw EL free liposome-based paclitaxel (LEP-ETU) formulation. Eur J Pharm Biopharm 59:177–87
- Zhang W, Song J, Zhang B, et al. (2011). Design of acid-activated cell penetrating peptide for delivery of active molecules into cancer cells. Bioconjugate Chem 22:1410–15
- Zhou ZY, Zhang P, Ren JL, et al. (2013). Synergistic effects of ultrasound-targeted microbubble destruction and TAT peptide on gene transfection: an experimental study in vitro and in vivo. J Control Rel 170:437–44
- Zhu F, Jiang Y, Luo F, Li P. (2015). Effectiveness of localized ultrasound-targeted microbubble destruction with doxorubicin liposomes in H22 mouse hepatocellular carcinoma model. J Drug Target 23:323–34
- Zorko M ÜL. (2005). Cell-penetrating peptides: mechanism and kinetics of cargo delivery. Adv Drug Deliv Rev 57:529–45