Abstract
Purpose: Topical administration is the preferred route of drug delivery for ophthalmic ailments. However, poor permeation through ocular surface and significant systemic absorption, makes the topical drug delivery challenging. Furthermore, distribution of topically delivered drugs varies with their physicochemical properties and the type of formulation used. Hence, this study was done to understand the pattern of ocular drug distribution of topically applied hydrophilic and lipophilic substances in two different formulations.
Methods: 5-Carboxyfluorescein and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate were used as representative candidates for hydrophilic and lipophilic substances, respectively. They were formulated in solution and liposomes. Single drop of either formulation containing hydrophilic or lipophilic substance was instilled topically, unilaterally to rat eyes. Subsequently, rats were sacrificed at 10, 30 and 120 min post-instillation. Eyes were cryosectioned and examined under confocal microscope to determine the fluorescence intensity in ocular tissues.
Results: Corneal permeation of hydrophilic and lipophilic substances in both formulations peaked at 30 min post-instillation. Liposomal–lipophilic dye and non-liposomal–hydrophilic dye showed better corneal distribution. Fluorescence was absent in contralateral eyes of non-liposomal–hydrophilic dye-treated animals but was present in contralateral eyes of liposomal–hydrophilic dye-treated animals. Fluorescence in contralateral eyes of liposomal–lipophilic dye-treated animals was significantly higher compared to non-liposomal–lipophilic dye-treated animals.
Conclusions: Topically applied liposomal formulation of lipophilic substance provides higher corneal concentration of drug with lesser systemic absorption compared to its solution. For hydrophilic substance, topical use of solution provides greater corneal concentration compared to liposomes which is more likely to be absorbed systemically.
Introduction
Topical administration of drugs is the preferred choice for the treatment of ophthalmic ailments. This route of administration provides targeted drug delivery and aims to cause less systemic adverse effects due to avoidance of systemic delivery of drugs. However, intraocular delivery of drugs from topical formulations faces challenges due to several layers of ocular barrier. Three layered tear film and the tight junctions of corneal epithelium form a significant barrier for transcorneal permeation of drugs (Gaudana et al., Citation2010). Moreover, rich network of capillaries in the conjunctiva and sclera provides an alternative route for systemic absorption of topically administered drugs (Raviola, Citation1983; Steuhl & Rohen, Citation1983; Thombre & Himmelstein, Citation1984; Urtti et al., Citation1985). Corneal epithelium forms a significant barrier for the absorption of hydrophilic substances, and hence such substances are more likely to be absorbed through conjunctiva (Maurice & Mishima, Citation1984; Prausnitz & Noonan, Citation1998). Poor absorption of topically administered hydrophilic substances perhaps leads to longer exposure of ocular surface and hence greater possibility of its systemic absorption. Lipophilic substance are relatively better absorbed from corneal surface, however, these substances also permeate well through capillary walls and hence are likely to be systemically absorbed to significant extent.
In order to circumvent the challenges in topical drug delivery and achieve higher ocular bioavailability, colloidal drug delivery systems have been investigated. One of these drug delivery systems is liposomes. Liposomes are vesicular structures that consist of an outer covering of lipids and an aqueous core. Liposomes can entrap hydrophilic drugs in the aqueous core and lipophilic drugs in lipid covering (Nii & Ishii, Citation2005; Agarwal et al., Citation2014; Alyautdin et al., Citation2014). Hence, they are capable of entrapping both the hydrophilic and lipophilic drugs. For the first time, we have carried out this study in order to understand the pattern of ocular drug delivery of single drop application of hydrophilic and lipophilic substances. We used 5-carboxyfluorescein and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate as a representative candidate for hydrophilic and lipophilic drugs, respectively. Moreover, we used these substances both in solution and liposomal formulations to understand the differences in the pattern of drug distribution with change in formulation.
Methods
Chemicals
Chloroform, methanol, cholesterol, l-α-phosphatidylcholine (PC), 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), 5-carboxyfluorescein (CF), paraformaldehyde, dimethyl sulfoxide (DMSO) and phosphate buffered saline PBS; (1×; 0.01 M Na2HPO4, 0.002 M KH2PO4, 0.0027 M KCl, 0.137 M NaCl, pH 7.4) were purchased from Sigma Aldrich, St. Louis, MO. Sucrose was purchased from Fisher Scientific, Loughborough, UK. All other chemicals were of analytical grade.
Animals
Sprague–Dawley rats weighing 190–250 g were used in this study. Animals were obtained from Laboratory Animal Care Unit of Universiti Teknologi MARA. Animals were caged under standard laboratory conditions (12L:12D) and were given food and water ad libitum. All animals were examined prior to the study and those found normal were included. All experiments and animal handling were performed in accordance with the ARVO statement for the use of animals in ophthalmic and vision research as well as the local institutional ethical guidelines.
Formulation of dyes for topical application
Both DiI and CF were prepared in liposomal and non-liposomal forms for topical application. Liposomes containing DiI were prepared using hydration method. Cholesterol (5 µmol), PC (20 µmol) and DiI (110 µl of 10 mg/ml solution) were dissolved in a 5:1 solution of chloroform and methanol. The lipid solution was subjected to evaporation for 2 h to obtain a thin lipid film. The lipid film was rehydrated with 2 ml of PBS, and after 30 min of shaking, another 2 ml of PBS was added. The multilamellar vesicle solution was obtained, which was then sonicated for 10 min (Sonic Dismembrator, Fisher Scientific, Waltham, MA). The solution then underwent further reduction in particle size using hand-held extruder for 10 times through each of the 400, 200 and 100-nm polycarbonate membranes (Avanti Polar Lipids, Inc., Alabaster, AL). Liposome containing CF were also prepared as for DiI, however, CF was incorporated into liposomes during the rehydration process with PBS.
Non-liposomal preparation of DiI and CF were prepared in DMSO and double distilled water. DiI, 110 µl of 10 mg/ml, was dissolved in 4 ml of DMSO solution, while the same preparation for CF consisted of 600 µg per ml of double distilled water.
Characterization of liposomes
Liposome formulation (n = 3) was characterized for particle size and zeta potential using dynamic light scattering (Malvern Zetasizer, Nano ZS, Worcestershire, UK).
Study design
Four groups of animals (n = 12) received either 10 µl of topical treatment with freshly prepared liposomal DiI (LD), liposomal CF (LC), non-liposomal DiI (D) or non-liposomal CF (C), unilaterally in one of the randomly chosen eyes. The contralateral eye received no treatment and served as control. Animals were euthanized at 10, 30 and 120 min post-instillation. Eyes were washed with ice-cold PBS and enucleated. Enucleated eyeballs were immediately transferred to 4% paraformaldehyde solution at 4 °C for overnight fixation. Subsequently, eyeballs were immersed in 20% sucrose solution at 4 °C for 48 h for cryoprotection and then were embedded in Optimal Cutting Temperature (O.C.T.) Compound (Sakura Finetechnical Co., Ltd., Tokyo, Japan) at −20 °C.
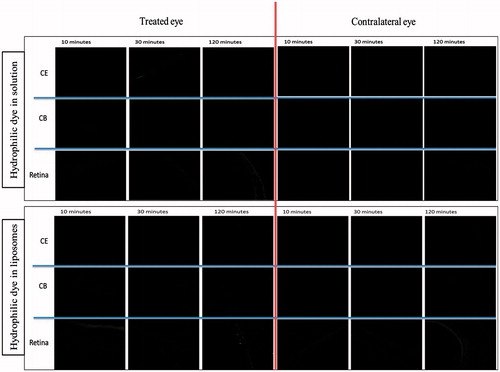
Cryostat sectioning was done using cryostat machine (Cryostat UV 1850, Leica Instrument GmbH, Nussloch, DE) at −20 °C. Eyeballs were sectioned at 10 µm thickness vertically using brush technique. Selected sections at the level of optic nerve insertion were then viewed under confocal microscope (TCS SPE, Leica Microsystems, Wetzlar, DE). The ocular structures visualized included cornea (CE), ciliary body (CB) and retina. In retina, fluorescence intensity (FI) was measured separately in retinal ganglion cell (RGC) layer, inner nuclear and inner plexiform layer (INL/IPL), outer nuclear and outer plexiform layer (ONL/OPL) and outer segment (OS) consisting of photoreceptors and retinal pigment epithelium. Cornea represented the ocular surface, ciliary body the anterior segment of eye and retina represented the posterior segment of eye. Images were analyzed for intensity measurement using Leica LAS AF Lite 2.0 software (Leica Microsystems, Buffalo Grove, IL).
Statistical analysis
All values were expressed as mean ± standard deviation (SD). Statistical comparison was done using two-way ANOVA with post hoc multiple comparison and Bonferroni correction. p < 0.05 was considered significant.
Results
Characterization of liposomal formulation
The mean particle size for liposomal formulation was 116.20 ± 1.98 nm and 341.83 ± 76.77 nm for hydrophilic and lipophilic dyes, respectively. The zeta potential recorded for liposomal formulation was 2.99 ± 0.12 and 2.83 ± 0.39 mV for hydrophilic and lipophilic dyes, respectively.
Distribution of hydrophilic dye in treated and contralateral eyes
The extent of distribution of a non-liposomal hydrophilic dye in cornea, ciliary body and retina at each time point is shown in . At 30 min post-instillation, significantly high level was observed in cornea compared to any other part in C-treated eyes. The corneal distribution of FI was significantly lower at 10 and 120 min compared to 30 min post-instillation (p < 0.001). At 10 and 120 min post-instillation, we observed a comparable level of FI in all parts of eye. Contralateral eyes showed complete absence of FI in all parts of at all time-points.
Figure 1. Distribution of non-liposomal hydrophilic dye in treated eye at three time points post-instillation. CE – corneal epithelium; CB – ciliary body; RGC – retinal ganglionic cell layer; INL/IPL – inner nuclear and inner plexiform layer; ONL/OPL – outer nuclear and outer plexiform layer; OS – outer segment.
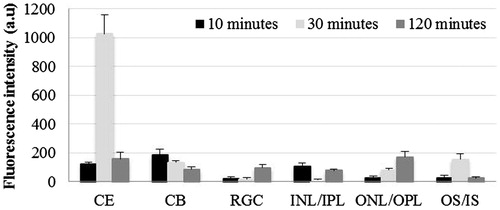
shows comparative distribution of LC at different time points in cornea, ciliary body and retina. At 10 min post-instillation, higher quantity of dye was observed in outer retina compared to all other parts of eye (p < 0.05). At 120 min post-instillation, significantly high FI was observed in cornea and all retinal layers compared to that seen at 30 min post-instillation (p < 0.05). In the contralateral eyes, cornea as well as retinal layers showed increasing FI from 30 to 120 min post-instillation (p < 0.05; and ).
Figure 2. Distribution of hydrophilic dye used in liposomal formulation in treated eye at three time points post-instillation. CE – corneal epithelium; CB – ciliary body; RGC – retinal ganglionic cell layer; INL/IPL – inner nuclear and inner plexiform layer; ONL/OPL – outer nuclear and outer plexiform layer; OS – outer segment.
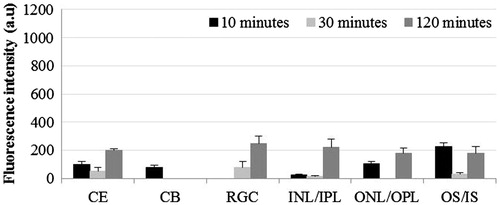
Figure 3. Distribution of liposomal hydrophilic dye in contralateral eye at three time points post-instillation. CE – corneal epithelium; CB – ciliary body; RGC – Retinal ganglionic cell layer; INL/IPL – inner nuclear and inner plexiform layer; ONL/OPL – outer nuclear and outer plexiform layer; OS – outer segment.
Comparative distribution of hydrophilic dye in non-liposomal and liposomal formulation
In the cornea, comparison of FI in C- and LC-treated groups showed that corneal distribution of C was 21-fold higher at 30 min compared to that at 10 and 120 min, post-instillation (p < 0.001). However, at 10 min and later at 120 min post-instillation, the FI was comparable (p > 0.05). The contralateral eyes in C-treated groups did not show the presence of dye at any time point in any parts of eye. On the other hand, in LC-treated group, there was a progressive increase in FI from 30 to 120 min with significantly higher values at 120 min in cornea and retina (p < 0.001).
In ciliary body, significantly higher FI level was seen in C-treated eyes compared to LC-treated eyes at all-time points (p < 0.05). The presence of dye was not detectable in the contralateral eyes of C-treated group at any time point. However, comparable level of FI was detected in the contralateral eyes of LC-treated group at 30 and 120 min post-instillation.
The RGC layer in LC-treated eyes showed significantly higher FI compared to corresponding eyes in C-treated group at 30 and 120 min post-instillation (4.8- and 2.6-fold, respectively; p < 0.05). In INL/IPL layer of LC-treated eyes, a gradual increase in FI was observed from 30 min onwards reaching a 2.8-fold higher intensity compared to C-treated eyes at 120 min post-instillation (p < 0.01). In ONL/OPL, the FI was significantly higher in LC-treated group at 10 min (4.1-fold; p < 0.01) and it was higher both at 10 and 120 minutes in OS (9.8- and 7.4-fold, respectively; p < 0.05).
Distribution of lipophilic dye in treated and contralateral eyes
Comparison of FI in different parts of eye at each time point after instillation of DiI in non-liposomal formulation is shown in . Although we did not observe the significant presence of FI in cornea and retina at 10 min post-instillation, significantly higher FI was detectable in ciliary body at this time point (p < 0.001 compared to all other parts of eye). In cornea, significant FI appeared only at 30 min post-instillation (p < 0.001). Subsequently, at 120 min post-instillation, gradually increasing levels of FI were observed from RGC layer to ONL/OPL.
Figure 5. Distribution of non-liposomal lipophilic dye in treated eye three time points post-instillation. CE – corneal epithelium; CB – ciliary body; RGC – Retinal ganglionic cell layer; INL/IPL – inner nuclear and inner plexiform layer; ONL/OPL – outer nuclear and outer plexiform layer; OS – outer segment.
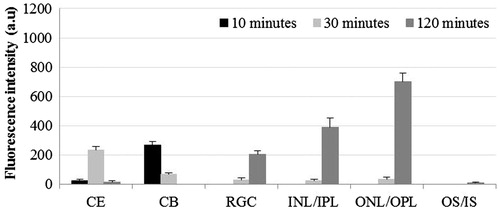
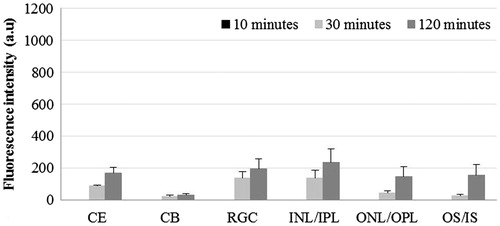
When DiI was used in liposomal formulation, cornea showed the presence of maximum FI at 30 min and minimum at 120 min post-instillation (p < 0.01: 30 min versus 10 min; p < 0.001: 30 min versus 120 min; p < 0.01: 10 min versus 120 min). ONL/OPL showed significantly higher FI at 120 min compared to 10 and 30 min post-instillation (p < 0.001). In OS, the FI was comparable at all three time points (). At both the 30 and 120 min post-instillation, the FI in ONL/OPL and OS was significantly higher compared to inner retina (p < 0.01).
Figure 6. Distribution of liposomal lipophilic dye in treated eye at three time points post-instillation. CE – corneal epithelium; CB – ciliary body; RGC – Retinal ganglionic cell layer; INL/IPL – inner nuclear and inner plexiform layer; ONL/OPL – outer nuclear and outer plexiform layer; OS – outer segment.
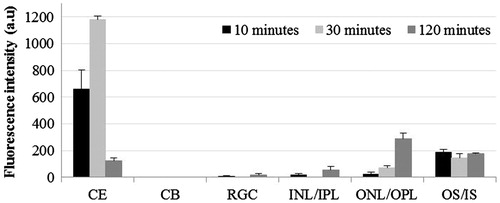
Contralateral eyes receiving LD showed the presence of significant FI in outer layers of retina at 30 and 120 min post-instillation (). The contralateral eyes treated with D showed the presence of FI only in ONL/OPL at 120 min post-instillation, however, this was significantly lower than that in treated eyes (p < 0.001; and ).
Figure 7. Distribution of liposomal lipophilic dye in contralateral eye at three time points post-instillation. CE – corneal epithelium; CB – ciliary body; RGC – Retinal ganglionic cell layer; INL/IPL – inner nuclear and inner plexiform layer; ONL/OPL – outer nuclear and outer plexiform layer; OS – outer segment.
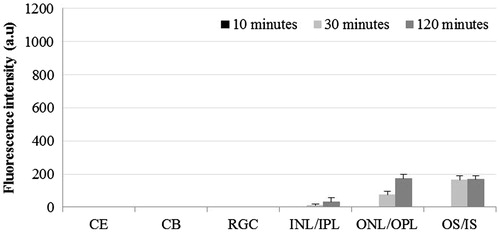
Figure 8. Distribution of non-liposomal lipophilic dye in contralateral eye at three time points post-instillation. CE – corneal epithelium; CB – ciliary body; RGC – Retinal ganglionic cell layer; INL/IPL – inner nuclear and inner plexiform layer; ONL/OPL – outer nuclear and outer plexiform layer; OS – outer segment.
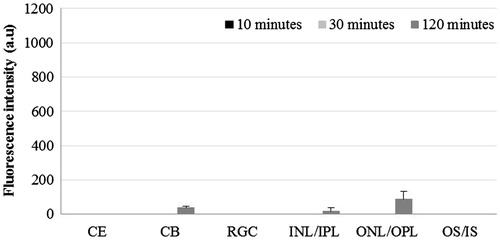
Comparative distribution of lipophilic dye in non-liposomal and liposomal formulation
The distribution of DiI in cornea was significantly higher in LD-treated group at all three time points compared to D-treated group (p < 0.01). In LD-treated eyes, negligible FI was observed in the ciliary body and inner part of retina compared to D-treated eyes that showed significantly high FI in ciliary body at 10 min (p < 0.001) and in inner parts of retina at 120 min post-instillation (p < 0.01). In ONL/OPL, we did observe, FI in LD-treated eyes at 120 min, however, it was significantly lower than D-treated eyes (p < 0.001). We also observed the presence of dye in ONL/OPL of contralateral eyes of both the LD and D-treated eyes. In the OS, significantly high level of FI was present at all time points in LD-treated eyes compared to D-treated eyes (p < 0.001) and at 30 and 120 min in the contralateral eyes of LD-treated group compared to D-treated group (p < 0.01).
Discussion
In this study, we demonstrated the distribution of hydrophilic and lipophilic substance in different parts of eye after topical application as solution or in liposomal formulation. We also studied the ocular distribution of these substances in the contralateral eye which represents the systemic absorption of the drugs following topical application.
The corneal permeation of both the hydrophilic and lipophilic substances in either formulation peaked at 30 min post-instillation (,,). This finding is in accordance with previous studies that also showed that peak concentrations of dye in the anterior chamber are normally attained after approximately 30 min (Davis et al., Citation2004; Wilson, Citation2004). We also observed that lipophilic substance in liposomes and hydrophilic substance in solution achieve better corneal distribution at this time point. Corneal distribution of hydrophilic substances in liposomes, although, poor compared to solution, was present both in the treated and contralateral eyes. Importantly, we observed complete absence of fluorescence in contralateral eyes of animals treated with hydrophilic dye in solution but the significant presence of FI in contralateral eyes of LC-treated animals. This indicates the possibility of greater systemic presence of hydrophilic substance if used in liposomes for topical application.
We detected the presence of fluorescence in ciliary body in eyes treated with the solution of hydrophilic dye (), however, the pattern of distribution over time was not in accordance with the corneal distribution over time. Similar trend was observed for retinal distribution. Hence, it is likely that ciliary and retinal distribution of the dye is primarily due to its systemic absorption rather than the intraocular passage after transcorneal permeation. Previous studies have shown that for topically applied drugs, the route of drug delivery to ciliary body and retina is through conjunctiva and sclera. Since these tissues have rich network of capillaries, it is likely that in our study, the presence of dye in these tissues is subsequent to systemic absorption (Chien et al., Citation1990; Acheampong et al., Citation2002).
Interestingly, despite the presence of significant FI in cornea with liposomal hydrophilic dye, its presence in ciliary body was minimal particularly in the treated eye (). In line with this observation, we also found that in the inner retina, FI increased from 30 to 120 min with liposomal formulation of hydrophilic dye minimally in treated eye but more so in contralateral eye. A similar trend was observed in outer layers of retina. This pattern of distribution indicates that when a hydrophilic substance is used in liposomal formulation topically, the route for its distribution into the ciliary body and retina is more through systemic circulation rather than the intraocular passage from transcorneal penetration after topical application. Previous studies have shown that hydrophilic substances are preferably absorbed through non-corneal routes hence allowing greater systemic absorption (Ahmed & Patton, Citation1985, Citation1987; Chien et al., Citation1990). Ahmed & Patton (Citation1985, Citation1987) demonstrated that inulin, a compound with poor transcorneal permeability, was able to gain intraocular access via non-corneal route with mechanical blockage of corneal access. Chien et al. (Citation1990) compared compounds with different lipophilicity and showed that the least lipophilic compounds used non-corneal routes to gain intraocular access. Non-corneal routes include absorption through bulbar conjunctiva and sclera to reach the uveal tract and vitreous humor (Järvinen et al., Citation1995). In addition, liposome distribution through endothelial cells has been shown to be slow, which may be the reason on later peak of fluorescence detected in the retina (Keuter et al., Citation2003).
When liposomes encapsulating lipophilic dye were applied topically, we did not observe the presence of fluorescence in ciliary body as well as inner parts of retina (). However, we did observe the presence of FI in outer parts of retina. This pattern of retinal distribution of lipophilic substance in liposomes could be attributed to highly significant absorption into the cornea leaving only smaller amounts of dye to enter the circulation, which fails to reach the inner parts of retina in significant quantities. At the same time, as was the case with hydrophilic dye, the intraocular passage of drug through transcorneal permeation fails to provide significant concentration in the posterior segment of eye. The difference is the corneal distribution of hydrophilic and lipophilic dyes in liposomes could also be attributed to the difference in the size of liposomes ( and ). Previous studies have shown that large and multilamellar liposomes have longer corneal retention time compared to smaller and unilamellar liposomes (Schaeffer & Krohn, Citation1982). Fitzgerald et al. (Citation1987) showed that restricted drainage of larger liposomal particle through the inner canthal region leads to higher corneal residence time.
In contrast to hydrophilic dye, use of lipophilic dye in solution resulted in relatively higher FI in ciliary body at earlier time points and in retina at the later time points (), compared to liposomal formulation. This pattern of distribution of lipophilic dye in solution is explained by its relatively lesser corneal absorption compared to liposomal formulation allowing greater systemic availability and hence appearance in ciliary body and inner parts of retina.
Conclusions
In conclusion, topical administration of lipophilic substance in liposomal formulation provides high corneal concentration of entrapped drug with relatively lesser systemic absorption compared to topical use of lipophilic substance in solution. For hydrophilic substance, topical use of solution provides greater corneal concentration compared to its liposomal formulation, which is more likely to be absorbed systemically. However, hydrophilic substances containing liposomes are more unstable and cause leakage of entrapped drug (Sasaki et al., Citation1987). Hence it is likely that the use of liposomes containing hydrophilic drugs will provide lesser intraocular and greater systemic delivery compared to lipophilic substances containing liposomes.
Acknowledgement
We acknowledge the administrative and facility support by Research Management Institute, Institute of Medical Molecular Biotechnology (IMMB) and Laboratory Animal Care Unit (LACU), Universiti Teknologi MARA, Malaysia.
Declaration of interest
The authors acknowledge the financial support by Ministry of Higher education, Government of Malaysia, under the grant no. 600-RMI/RAGS 5/3 (39/2014) and 600-RMI/RAGS 5/3 (40/2014).
References
- Acheampong AA, Shackleton M, John B, et al. (2002). Distribution of brimonidine into anterior and posterior tissues of monkey, rabbit, and rat eyes. Drug Metab Dispos 30:421–9
- Ahmed I, Patton TF. (1985). Importance of the noncorneal absorption route in topical ophthalmic drug delivery. Invest Ophthalmol Vis Sci 26:584–7
- Ahmed I, Patton TF. (1987). Disposition of timolol and inulin in the rabbit eye following corneal versus non-corneal absorption. Int J Pharm 38:9–21
- Agarwal R, Iezhitsa I, Agarwal P, et al. (2014). Liposomes in topical ophthalmic drug delivery: an update. Drug Deliv. [Epub ahead of print]. doi:10.3109/10717544.2014.943336
- Alyautdin RN, Iezhitsa IN, Agarwal R. (2014). Transcorneal drug delivery: prospects for the use of liposomes. Vestn Oftalmol 130:117–22
- Chien DS, Homsy JJ, Gluchowski C, Tang-Liu DDS. (1990). Corneal and conjunctival/scleral penetration of p-aminoclonidine, AGN 190342, and clonidine in rabbit eyes. Curr Eye Res 9:1051–9
- Davis JL, Gilger BC, Robinson MR. (2004). Novel approaches to ocular drug delivery. Curr Opin Mol Ther 6:195–205
- Fitzgerald P, Hadgraft J, Wilson CG. (1987). A gamma scintigraphic evaluation of the precorneal residence of liposomal formulations in the rabbit. J Pharm Pharmacol 39:487–90
- Gaudana R, Ananthula HK, Parenky A, Mitra AK. (2010). Ocular drug delivery. AAPS J 12:348–60
- Järvinen K, Järvinen T, Urtti A. (1995). Ocular absorption following topical delivery. Adv Drug Deliv Rev 16:3–19
- Keuter J, Ramge P, Petrov V, et al. (2003). Direct evidence that polysorbate-80-coated poly(butylcyanoacrylate) nanoparticles deliver drugs to the CNS via specific mechanisms requiring prior binding of drugs to the nanoparticles. Pharm Res 20:409–16
- Maurice DM, Mishima S. (1984). Ocular pharmacokinetics. In: Sears ML, ed. Pharmacology of the eye. Vol. 69. Berlin: Springer, 19–116
- Nii T, Ishii F. (2005). Encapsulation efficiency of water-soluble and insoluble drugs in liposomes prepared by the microencapsulation vesicle method. Int J Pharm 298:198–205
- Prausnitz MR, Noonan JS. (1998). Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J Pharm Sci 87:1479–88
- Raviola G. (1983). Conjunctival and episcleral blood vessels are permeable to blood-borne horseradish peroxidase. Invest Ophthalmol Vis Sci 24:725–36
- Sasaki H, Matsukawa Y, Hashida M, Sezaki H. (1987). Characterization of alkylcarbamoyl derivatives of 5-fluorouracil and their application to liposome. Int J Pharm 36:147–56
- Schaeffer HE, Krohn DL. (1982). Liposomes in topical drug delivery. Invest Ophthalmol Vis Sci 22:220–7
- Steuhl P, Rohen JW. (1983). Absorption of horse-radish peroxidase by the conjunctival epithelium of monkeys and rabbits. Graefes Arch Clin Exp Ophthalmol 220:13–18
- Thombre AG, Himmelstein KJ. (1984). Quantitative evaluation of topically applied pilocarpine in the precorneal area. J Pharm Sci 73:219–22
- Urtti A, Salminen L, Miinalainen O. (1985). Systemic absorption of ocular pilocarpine is modified by polymer matrices. Int J Pharm 23:147–61
- Wilson CG. (2004). Topical drug delivery in the eye. Exp Eye Res 78:737–43