Abstract
To develop a novel neomycin sulfate-loaded hydrogel dressing (HD), numerous neomycin sulfate-loaded HDs were prepared with various amounts of polyvinyl alcohol (PVA), polyvinyl pyrrolidone (PVP) and sodium alginate (SA) using freeze-thawing technique, and their physical dressing properties, drug release, in vivo wound curing and histopathology in diabetic-induced rats were assessed. SA had a positive effect on a swelling capacity, but a negative effect on the physical dressing properties and drug release of HD. However, PVP did the opposite. In particular, the neomycin sulfate-loaded HD composed of drug, PVA, PVP and SA at the weight ratio of 1/10/0.8/0.8 had excellent swelling and bioadhesive capacity, good elasticity and fast drug release. Moreover, this HD gave more improved wound curing effect compared to the commercial product, ensured the disappearance of granulation tissue and recovered the wound tissue to normal. Therefore, this novel neomycin sulfate-loaded HD could be an effective pharmaceutical product for the treatment of wounds.
Introduction
Wound curing is a complex and dynamic process of replacing devitalized cellular structures and tissue layers. Generally, an effective wound dressing should maintain a moist environment at the wound, allow gas exchange, act as a barrier to microorganisms and remove excess exudates (Zhang et al., Citation2015). Nowadays, advanced dressings, such as hydrocolloid, foam and hydrogel, offer wounds a moist environment (van Leen et al., Citation2014). Hydrogel is a three-dimensional (3D) network composed of hydrophilic polymer chains that is able to retain a large amount of water within their structures, leading to a drug carrier system (Chang et al., Citation2015) and wound dressing (Lee et al., Citation2010; Choi et al., Citation2014).
Recently, various types of wound dressing have been studied but some flaws, such as poor mechanical properties and low fluid absorption capability, have been reported (Kim et al., Citation2008a; Chakavala et al., Citation2012; Jaiswal et al., Citation2013). Polyvinyl alcohol (PVA) has been widely used as a base of wound-dressing system owing to its biocompatibility, nontoxicity, noncarcinogenicity and excellent gel-forming capacity (Lim et al., Citation2010; Choi et al., Citation2014; Coşkun et al., Citation2014). However, the single use of PVA provided insufficient swelling and mechanical properties to the wound-dressing system. To solve these problems, hydrophilic polymers, such as polyvinylpyrrolidone (PVP), sodium alginate (SA), sodium carboxymethylcellulose, chitosan and poloxamer, were used together with PVA as the base of wound dressing systems; however, these mixed polymer systems barely gave satisfactory swelling and mechanical properties, and they were a little ameliorated. Thus, a wound-dressing system with better swelling and mechanical properties must be developed for practical pharmaceutical product.
In this study, instead of one type of hydrophilic polymer, two different kinds of hydrophilic polymers, PVP and SA were used together with PVA as the base of a wound-dressing system. Namely, to develop a novel neomycin sulfate-loaded hydrogel dressing (HD), numerous neomycin sulfate-loaded HDs were prepared with various amounts of PVA, PVP and SA using the freeze-thawing technique, and their physical dressing properties, drug release, in vivo wound curing and histopathology in diabetic-induced rats were assessed.
PVP has been used as the main component of temporary skin covers and dressings due to its high hydrophilicity, complexation ability and biocompatibility (Singh & Pal, Citation2011; Taheri et al., Citation2011; Tan et al., Citation2013). SA, the salt of a natural polysaccharide, is biocompatible and biodegradable and possesses excellent swelling capacity (Balakrishnan et al., Citation2005; Shalumon et al., Citation2011; Fu et al., Citation2015). Furthermore, because the process of wound curing could be accelerated by antibiotics (Daeschlein, Citation2013; Kevadiya et al., Citation2014; Mosti et al., Citation2015), neomycin sulfate was added as a drug in this hydrogel dressing. Neomycin sulfate, a widely used broad spectrum aminoglycoside antibiotic, has been topically used on the skin and mucous membranes (Spann et al., Citation2003; Nitanan et al., Citation2013).
Materials and methods
Materials
Neomycin sulfate was purchased from Shanghai Fine Chem. Co. (Shanghai, China). Polyvinyl alcohol (PVA, typical average Mw = 146 000–186 000; +99% hydrolyzed), polyvinyl pyrrolidone (PVP) and sodium alginate (SA) were purchased from Sigma-Aldrich Co. (St. Louis, MO), BASF (Ludwigshafen, Germany) and FMCBioPolymer Co., (Philadelphia, PA), respectively. Commercial product (Carejel™; hydrogel dressing type) was purchased from Daeil Chem. Co. (Sungnam, South Korea). Streptozotocin was purchased from Sigma Chemicals Co. (St. Louis, MO). All other chemicals were used without any further purification.
Animals
Male CrlOri:SKH1-hr strain hairless mice weighing 25–30 g and male SD rats weighing 250–280 g were used for the evaluation of bioadhesive strength and in vivo wound curing effect HDs, respectively. On the other hand, to make a diabetes-induced rat, streptozotocin (60 mg/kg) in sodium citrate buffer (pH 4.5)-saline solution was intravenously administered to each rat and the blood was then sampled from the tail vein of rats after 5 days. Employing a blood glucose meter (Accu-chek®, Roche Diagnotics Korea Co., Seoul, South Korea), its glucose amounts was determined. After anesthetizing the diabetes-induced rats by i.p. injection of Zoletil 50® (tiletamine/zolazepam), their dorsal hair was shaved with an electric trimmer. All animal care and procedures were implemented consistent with the NIH Policy and Animal Welfare Act under approval by the Institutional Animal Care and Use Committee (IACUC) at Hanyang University.
Preparation of HDs
Various neomycin sulfate-loaded HDs were obtained by the freezing-thawing technique. Solutions containing 10% w/w PVA and 1% drug were dissolved into distilled water at room temperature using a mechanical stirrer (MS304S; Mettler Toledo, Toledo, OH) at the speed of 400 rpm for 6 h. Different proportions of aqueous solutions containing PVP or SA were added in the above solution, blended entirely by vortexing at room temperature for 1 h, and poured into petri dishes. For three consecutive cycles, these hydrogels were then frozen at −20 °C for 18 h and then thawed at room temperature for 6 h. The final neomycin sulfate-loaded HD formulations are given in .
Table 1. Compositions of neomycin sulfate-loaded HDs.
In vitro physical dressing property
Determination of swelling ratio
The HD pieces (2 cm × 2 cm) were dried at 60 °C under vacuum for 12 h (Winitial). Subsequently, they were soaked in phosphate buffer (pH 7.4) at 37 °C (Wsoaked) for 24 h. The swelling ratio (SR) was calculated using the following equation (Kim et al., Citation2008b; Sung et al., Citation2010). SR % = (Wsoaked/Winitial) × 100; where Winitial and Wsoaked are the weights of HD pieces dried for 12 h at 60 °C and soaked in phosphate buffer at 37 °C, respectively.
Determination of tensile strength and elongation at break
Referred to ASTM D882, the tensile strength of the HD pieces (2 cm × 2 cm) was determined by the maximum weight at the time of rupture employing texture analyzer (TA.XT2, stable microsystems, Haslemere, Surrey, UK) (Kim et al., Citation2008a; Jin et al., Citation2015a). In addition, the elongation at break (%) was assessed by comparing the length of HD pieces at breakage and its length before being drawn by the appliance.
Determination of bioadhesive strength
The measurement of bioadhesive strength of HDs was conducted employing the texture analyzer (TA.XT2; Haslemere, Surrey, UK) with a 5-kg-loaded cell (Jin et al., Citation2015a). After the elimination of all hair, fats and debris, the skin of hairless mouse was secured on the upper movable probe while the HD piece was placed onto the lower fixed probe. Then, the upper probe was lowered at a speed of 1 mm/sec until making contact with the tissue at a force of 1 N for 30 s. At a rate of 1 mm/s, the upper probe was lifted to a distance of 5 mm. An acquiring rate of 200 points/s was chosen for the analysis. The bioadhesive strength was determined as a minimum force required to detach the skin tissue from the HDs.
In vitro drug release test
The release of neomycin sulfate-loaded HDs was evaluated using the USP dissolution apparatus V (paddle over disk; Varian, Walnut Creek, CA) in distilled water. The paddles were rotated at 50 rpm at temperature 37 ± 0.5 °C for 4 h. The concentration of neomycin sulfate was determined at excitation and emission wavelengths of 260 and 315 nm, respectively, using a Water 2795 HPLC system consisted of a Waters 2795 Separation module and a Waters 470 fluorescence detector. The column (4.6 mm I.D × 250 mm, 5 um) was an Inertsil ODS-2 C18 column (GL Sciences Inc., Tokyo, Japan). The mobile phase consisted of acetonitrile/water (90:10, v/v) was filtered (0.45 μm) and eluted at a flow rate of 1.0 ml/min. The interday and intraday precision and accuracy were within the acceptable limits (r2 = 0.999).
In vivo wound-curing evaluation and histopathology
The dorsal hairs of diabetes-induced rats anaesthetised by i.p. injection of Zoletil 50® (tiletamine/zolazepam) were shaved with an electric razor, and cleaned with 70% ethanol (Jin et al., Citation2015a). The abrasion wounds (15 mm × 15 mm) were induced on the back of each animal employing coarse sandpaper and acetone until skin bleeding and oozing of fluid were shown, indicating that only the superficial portion of the skin was damaged. Each wound was covered with sterile gauze (control), the drug-free HD, the neomycin sulfate-loaded HD and the commercial product, respectively. The drug-free HD and neomycin sulfate-loaded HD were composed of PVA/PVP/SA (10/0.8/0.8, weight ratio) and drug/PVA/PVP/SA (1/10/0.8/0.8, weight ratio), respectively. In particular, the wound was covered using an elastic adhesive bandage (Soft cloth tape®, 3 M, Maplewood, MN). All rats were separately kept in individual cages. Moreover, each dressing sample was changed every day for the experimental periods (Nacer Khodja et al., Citation2012). The images of the wounds were preserved at 0, 2, 3, 4 and 6 days using a digital camera followed by surveying with the Adobe® Acrobat® 7 Program. The relative wound size reduction was calculated using the following equation (Kim et al., Citation2008a; Jin et al., Citation2015b). Relative wound size reduction (%) = [(WSo − WSt)/WSo] × 100, where WSo and WSt are the wound size at initial time and time “t”, respectively.
Additionally, the rats were sacrificed after 6 days postoperation, the healed wound area of skin containing dermis and hypodermis was then sampled and carefully trimmed with cutter. All trimmed skins were fixed in 10% neutral buffered formalin followed by paraffin embedding and preparing 3–4 μm sections. Representative sections were stained with haematoxylin and eosin dyes for microscopic examination (Jang et al., Citation2012; Al-Hoqail et al., Citation2014).
Results and discussion
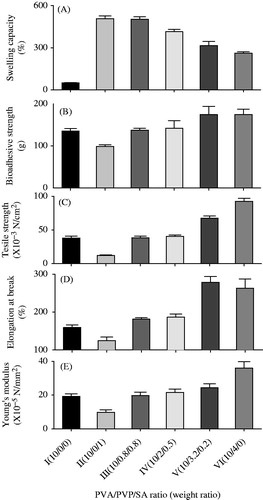
To investigate the influence of PVP/SA ratio on the physical dressing properties of neomycin sulphate-loaded HDs, various HD formulations were prepared with various ratios of PVP and SA, while keeping the amount of 1% drug and 10% PVA (). In the preliminary study on swelling ratio, their swelling ratios per hour were determined for 30 h, leading to reaching the equilibrium in swelling degree from 24 h; thus, the swelling ratio for 24 h was decided to be assessed in this study (Kumar et al., Citation2012; Zhang et al., Citation2015). As shown in , the HDs prepared with PVA, PVP and SA (formulation II–VI) improved the swelling capacity of HD compared with the HD prepared with only PVA (formulation I). As the PVP/SA ratios were increased, the swelling capacities were decreased. Our results indicated that SA had a higher swelling capacity than PVP, suggesting that the addition of SA had a positive effect on the swelling capacity of HD. In particular, the HDs prepared with the PVA/PVP/SA ratios of 10/0/1 (formulation II) and 10/0.8/0.8 (formulation III), which had no significant difference, gave an approximately 10-fold swelling capacity compared with that prepared with only PVA (507.3 ± 20.1%; 502.3 ± 18.1% versus 50.1 ± 1.0%). Compared to PVA/PVP/SA ratios of 10/0/0 (formulation I), 10/0/1 (formulation II) and 10/1/0 (formulation VI), the bioadhesive strength (), tensile strength (), elongation at break () and Young’s modulus () of HD increased in the order of SA < PVA < PVP. Thus, the more increased the PVP/SA ratios were, the more increased these physical dressing properties were. Our results suggested that the addition of SA had a negative effect on the physical dressing properties of HD, unlike the swelling capacity. Moreover, the HDs prepared with the PVA/PVP/SA ratios of 10/0.8/0.8 (formulation III) and 10/2/0.5 (formulation IV) gave better bioadhesive strength, tensile strength, elongation at break and Young’s modulus than those prepared with only PVA (formulation I). However, there were no significant differences in the physical dressing properties of HD between these HDs. Among the HDs prepared with PVA, PVP and SA, the HD prepared with the 10/0.8/0.8 ratio (formulation III) gave relatively good values in all physical dressing properties, including swelling capacity. In particular, this neomycin sulphate-loaded HD had the swelling capacity of 502.3 ± 18.1%, bioadhesive strength of 117.2 ± 4.5 g, maximum tensile strength of 38.2 ± 2.5 × 10−3 N/mm2, elongation at break of 181.5 ± 4.3% and Young’s modulus of 19.7 ± 2.0 × 10−5 N/mm2. Thus, the HD prepared with the PVP/SA ratio of 10/0.8/0.8 (formulation III) was selected as an optimal formulation of neomycin sulphate-loaded HD, because it had excellent swelling and bioadhesive capacity and good elasticity.
Figure 1. Effect of PVP/SA ratio on the physical dressing property of neomycin sulfate-loaded HDs: (A) swelling capacity; (B) bioadhesive strength; (C) tensile strength; (D) elongation at break; and (E) Young’s modulus. The neomycin sulfate-loaded HDs were composed of PVA, PVP and SA at the weight ratio. Each value represents the mean ± SD (n = 3).
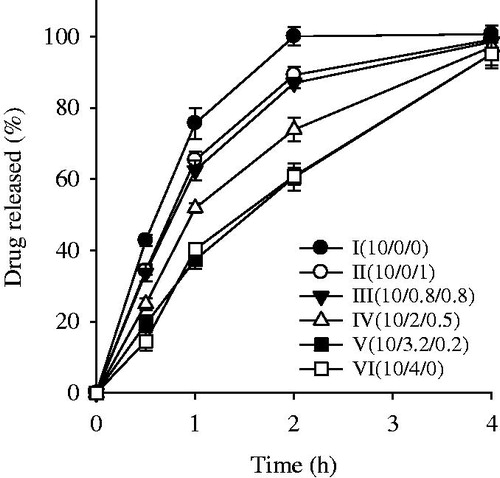
The neomycin sulfate-loaded HD formulations were tested for drug release behaviour over a period of 4 h (). The formulation I prepared only with PVA gave higher release rate until 2 h compared to the other formulations prepared with the addition of PVP and/or SA to the PVA base, even if the former had lower swelling ratio than did the latters. The neomycin sulfate might weakly interact with relatively less hydrophilic PVA in the wound dressing; thus, PVA might not prevent the penetration of drug from the crosslinking matrix (HD), resulting in relatively fast release. However, the drug might strongly interact with relatively more hydrophilic PVP and SA and be slowly released from the HD due to no prevention of these hydrophilic polymers (Wen et al., Citation2008; Kulkarni et al., Citation2010; Lee et al., Citation2010; Singh & Pal, Citation2011). Thus, in this study, the interaction of drug with the polymer more affected the release rate of drug from the HD than did the swelling ratio. As the PVP/SA ratios were increased in the HD, these HD formulations showed a high release rate. PVP delayed the drug release for 2 h compared with SA, since the crosslinking bond between PVA and SA might be weak compared to that between PVA and PVP (Kulkarni et al., Citation2010; Singh & Pal, Citation2011). However, all these formulations gave the release rates of about 100% for 4 h irrespective of dressing polymers, because neomycin sulphate was very soluble in water (Lim et al., Citation2010; Nitanan et al., Citation2013). The HDs prepared with the PVA/PVP/SA ratios of 10/0.8/0.8 (formulation III) and 10/2/0.5 (formulation IV) gave a higher initial release rate than those prepared with PVP/SA. However, there were no significant differences in the initial release rate between formulation III and IV. Particularly, the PVA/PVP/SA ratio of 10/0.8/0.8 (formulation III) showed about 85% of drug released within 2 h, indicating relatively fast initial drug release.
Figure 2. Effect of PVP/SA ratio on the drug release from neomycin sulfate-loaded HDs. The neomycin sulfate-loaded HDs were composed of PVA, PVP and SA at the weight ratio. Each value represents the mean ± SD (n = 6).
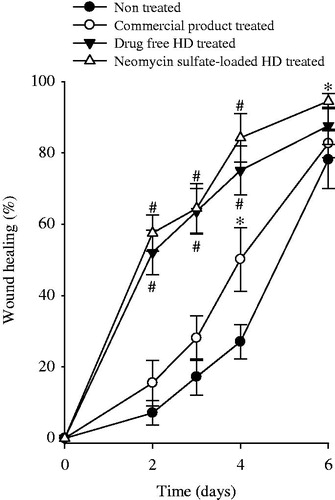
The diabetic wound model is useful in assessing the wound curing potential, because wound curing is known to be delayed in the diabetic wound model compared to the non-diabetic wound models (Romero-Cerecero et al., Citation2014; Wu et al., Citation2015). The representative images of wounds are presented in . Furthermore, wound-curing profiles are revealed in . Compared to nontreated wounds (), the commercial HD product-treated wounds (), drug-free HD-treated wounds () and neomycin sulfate-loaded HD-treated wounds () looked healthier and showed accelerated curing. Compared to nontreated wounds, the commercial HD product-treated wounds gave significantly higher wound curing only on day 4 but did not on the other days (). However, from the initial stage, wound curing in the drug-free HD-treated wounds and neomycin sulfate-loaded HD-treated wounds was significantly better than nontreated wounds and the commercial product-treated wounds (p < 0.05). Compared to drug-free HD, the neomycin sulfate-loaded HD-treated wounds gave higher wound curing; however, there were not significantly different (p > 0.05). At day 6, wound curing in nontreated wounds, the commercial product treated wounds, drug-free HD-treated wounds and neomycin sulfate-loaded HD-treated wounds were 78.1 ± 8.1, 82.7 ± 4.0, 87.6 ± 5.2 and 94.5 ± 2.1%, respectively (). Our results suggested that the neomycin sulfate-loaded HD gave a more enhanced wound curing effect compared to the commercial product. Such an excellent wound curing effect of the HD was mainly attributed to the moist environment with better swelling capacity. A moist environment was very useful for the migration of cell growth factors, cytokine fibroblasts and keratinocytes (Kim et al., Citation2008a; Kim et al., Citation2008b; Jin et al., Citation2015b). Moreover, neomycin sulfate, a loaded drug had a little positive influence on the wound-curing effect (Lee et al., Citation2010; Lim et al., Citation2010).
Figure 3. The representative images of wound model: (A) nontreated; (B) commercial product treated; (C) drug-free HD treated; (D) neomycin sulfate-loaded HD treated. The neomycin sulfate-loaded HDs were composed of drug, PVA, PVP and SA at the weight ratio of 1/10/0.8/0.8.
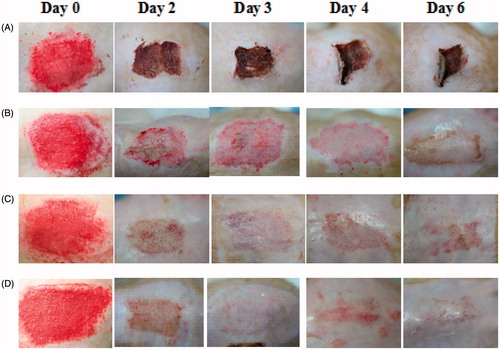
Figure 4. Wound-curing profile of wound model in nontreated, commercial product treated, drug-free HD treated and neomycin sulfate-loaded HD treated. The neomycin sulfate-loaded HDs were composed of drug, PVA, PVP and SA at the weight ratio of 1/10/0.8/0.8. Each value represents the mean ± SD (n = 4). *p < 0.05 and #p < 0.05 compared with nontreated and the commercial product, respectively.
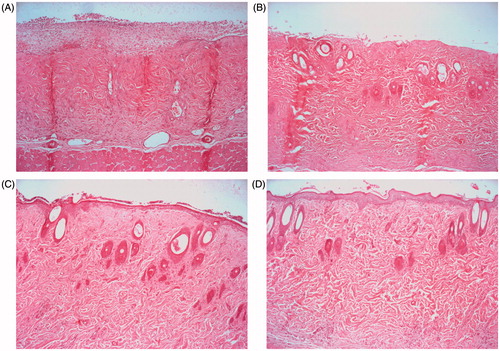
Histological investigations were performed with three wound tissues on day 6 after the evaluation of wound curing. The figures of representative tissue sections in nontreated wounds, the commercial product, drug-free HD-treated wounds and the neomycin sulfate-loaded HD-treated wounds are given in . The nontreated wound showed no epithelialization but bleeding (). In wounds treated with commercial product (), the wound region was not entirely covered by a crust. They gave the incomplete keratin and recovery in the epidermis matrix. However, the wounds treated with the neomycin sulfate-loaded HD and drug-free HD gave a crust covered in the wound region (). However, compared to the drug-free HD, the neomycin sulfate-loaded HD gave no noticeable changes in the histological image. As the wound curing evaluation, the moist environment with better swelling capacity improved the histological healing. Thus, this neomycin sulfate-loaded HD could ensure the disappearance of granulation tissue and recover the wound tissue to the normal.
Conclusion
The neomycin sulfate-loaded HD composed of drug, PVA, PVP and SA at the weight ratio of 1/10/0.8/0.8 could improve the physical dressing property and drug release. Moreover, this HD gave a more improved wound curing effect compared to the commercial product, and ensure the disappearance of granulation tissue and recover the wound tissue to the normal. Therefore, this novel neomycin sulphate-loaded HD could be an effective pharmaceutical product for the treatment of wounds.
Declaration of interest
This work was supported by the National Research Foundation of South Korea (NRF) grant funded by the South Korea government (MEST) (No. 2015R1A2A2A05027872). The authors report that there are no conflicts of interest.
References
- Al-Hoqail RA, Sadat-Ali M, Al-Habdan IM. (2014). The role of growth factor Sadat-Habdan mesenchymal stimulating peptide in healing of burn wounds. J Craniofac Surg 25:639–44
- Balakrishnan B, Mohanty M, Umashankar PR, Jayakrishnan A. (2005). Evaluation of an in situ forming hydrogel wound dressing based on oxidized alginate and gelatine. Biomaterials 26:6335–42
- Chakavala SR, Patel NG, Pate NV, et al. (2012). Development and in vivo evaluation of silver sulfadiazine loaded hydrogel consisting polyvinyl alcohol and chitosan for severe burns. J Pharm Bioallied Sci 4:S54–6
- Chang G, Chen Y, Li Y, et al. (2015). Self-healable hydrogel on tumour cell as drug delivery system for localised and effective therapy. Carbohydr Polym 122:336–42
- Choi YK, Din FU, Kim DW, et al. (2014). Amniotic membrane extract-loaded double-layered wound dressing: evaluation of gel properties and wound healing. Drug Dev Ind Pharm 40:852–59
- Coşkun G, Karaca E, Ozyurtlu M, et al. (2014). Histological evaluation of wound healing performance of electrospun poly(vinyl alcohol)/sodium alginate as wound dressing in vivo. Biomed Mater Eng 24:1527–36
- Daeschlein G. (2013). Antimicrobial and antiseptic strategies in wound management. Int Wound J 10:9–14
- Fu R, Li C, Yu C, et al. (2015). A novel electrospun membrane based on moxifloxacin hydrochloride/poly(vinyl alcohol)/sodium alginate for antibacterial wound dressings in practical application. Drug Deliv. [Epub ahead of print]
- Jaiswal M, Gupta A, Agrawal AK, et al. (2013). Bi-layer composite dressing of gelatine nanofibrous mat and poly vinyl alcohol hydrogel for drug delivery and wound healing application: in-vitro and in-vivo studies. J Biomed Nanotechnol 9:1495–508
- Jang SI, Mok JY, Jeon IH, et al. (2012). Effect of electrospun non-woven mats of dibutyryl chitin/poly(lactic acid) blends on wound healing in hairless mice. Molecules 17:2992–3007
- Jin SG., Kim KS, Yousaf AM, et al. (2015a). Mechanical properties and in vivo healing evaluation of a novel Centella asiatica-loaded hydrocolloid wound dressing. Int J Pharm 490:240–7
- Jin SG, Yousaf AM, Jang SW, et al. (2015b). In vivo wound-healing effects of novel benzalkonium chloride-loaded hydrocolloid wound dressing. Drug Dev Res 76:157–65
- Kevadiya BD, Rajkumar S, Bajaj HC, et al. (2014). Biodegradable gelatine-ciprofloxacin-montmorillonite composite hydrogels for controlled drug release and wound dressing application. Colloids Surf B Biointerfaces 122:175–83
- Kim JO, Choi JY, Park JK, et al. (2008a). Development of clindamycin-loaded wound dressing with polyvinyl alcohol and sodium alginate. Biol Pharm Bull 31:2277–82
- Kim JO, Park JK, Kim JH, et al. (2008b). Development of polyvinyl alcohol–sodium alginate gel-matrix-based wound dressing system containing nitrofurazone. Int J Pharm 359:79–86
- Kulkarni RV, Sreedhar V, Mutalik S, et al. (2010). Interpenetrating network hydrogel membranes of sodium alginate and poly(vinyl alcohol) for controlled release of prazosin hydrochloride through skin. Int J Biol Macromol 47:520–27
- Kumar PT, Lakshmanan VK, Anilkumar TV, et al. (2012). Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wounddressing: in vitro and in vivo evaluation. ACS Appl Mater Interfaces 4:2618–29
- Lee JH, Lim SJ, Oh DH, et al. (2010). Wound healing evaluation of sodium fucidate-loaded polyvinylalcohol/sodium carboxymethylcellulose-based wound dressing. Arch Pharm Res 33:1083–89
- Lim SJ, Lee JH, Piao MG, et al. (2010). Effect of sodium carboxymethylcellulose and fucidic acid on the gel characterization of polyvinylalcohol-based wound dressing. Arch Pharm Res 33:1073–81
- Mosti G, Magliaro A, Mattaliano V, et al. (2015). Comparative study of two antimicrobial dressings in infected leg ulcers: a pilot study. J Wound Care 24:121;124–7
- Nacer Khodja A, Mahlous M, Tahtat D, et al. (2012). Valuation of healing activity of PVA/chitosan hydrogels on deep second degreeburn: pharmacological and toxicological tests. Burns 39:98–104
- Nitanan T, Akkaramongkolporn P, Rojanarata T, et al. (2013). Neomycin-loaded poly(styrene sulfonic acid-co-maleic acid) (PSSA-MA)/polyvinyl alcohol (PVA) ion exchange nanofibers for wound dressing materials. Int J Pharm 448:71–8
- Romero-Cerecero O, Zamilpa A, Díaz-García ER, Tortoriello J. (2014). Pharmacological effect of Ageratina pichinchensis on wound healing in diabetic rats and genotoxicity evaluation. J Ethnopharmacol 156:222–7
- Shalumon KT, Anulekha KH, Nair SV, et al. (2011). Sodium alginate/poly(vinyl alcohol)/nano ZnO composite nanofibers for antibacterial wound dressings. Int J Biol Macromol 49:247–54
- Singh B, Pal L. (2011). Radiation crosslinking polymerisation of sterculia polysaccharide-PVA-PVP for making hydrogel wound dressings. Int J Biol Macromol 48:501–10
- Spann CT, Tutrone WD, Weinberg JM, et al. (2003). Topical antibacterial agents for wound care: a primer. Dermatol Surg 29:620–6
- Sung JH, Hwang MR, Kim JO, et al. (2010). Gel characterisation and in vivo evaluation of minocycline-loaded wound dressing with enhanced wound healing using polyvinyl alcohol and chitosan. Int J Pharm 392:232–40
- Taheri A, Atyabi F, Dinarvnd R. (2011). Temperature-responsive and biodegradable PVA:PVP k30:poloxamer 407 hydrogel for controlled delivery of human growth hormone (hGH). J Pediatr Endocrinol Metab 24:175–9
- Tan SP, McLoughlin P, O'Sullivan L, et al. (2013). Development of a novel antimicrobial seaweed extract-based hydrogel wound dressing. Int J Pharm 456:10–20
- van Leen M, Rondas A, Neyens J, et al. (2014). Influence of superabsorbent dressings on non-healing ulcers: a multicentre case series from the Netherlands and the UK. J Wound Care 23:543–44, 546, 548–50
- Wen H, Morris KR, Park K. (2008). Synergic effects of polymeric additives on dissolution and crystallization of acetaminophen. Pharm Res 25:349–58
- Wu X, Alberico S, Saidu E, et al. (2015). Organic light emitting diode improves diabetic cutaneous wound healing in rats. Wound Repair Regen 23:104–14
- Zhang D, Zhou W, Wei B, et al. (2015). Carboxyl-modified poly(vinyl alcohol)-cross-linked chitosan hydrogel films for potential wound dressing. Carbohydr Polym 125:189–99