Abstract
10-Hydroxy camptothecin (10-HCPT) is an antitumor agent effective in the treatment of several solid tumors but its use is hampered by poor water solubility, low lactone stability, short plasma half-life and dose-limiting toxicity. These limits of 10-HCPT had been overcome by our group through preparing super macromolecule prodrug: 10-HCPT-hydroxyethyl starch (HES) conjugate. In this study, we mainly evaluated in vitro and in vivo behavior of the prodrug, containing cytotoxicity assay, pharmacodynamics study, vascular irritation test, hemolysis experiment and tissue distribution test of rats. The irritation test results achieved much lower irritation than the commercial injection. The tissue distribution results showed that HES-10-HCPT conjugate increased significantly the 10-HCPT concentration in the tumor, liver and spleen site, whereas decreased the drug concentration in the heart and kidney. The hemolysis effect of the prepared conjugate was not obvious. The pharmacodynamics results indicated that HES-10-HCPT prodrug had a better antitumor efficiency against mice with H22 tumor than the commercial injection, and the inhibition ratio of tumor was 85.2% and 31.1%, respectively at the same dosage. These findings suggest that HES-10-HCPT prodrug is a promising drug delivery system providing improved good injection safety, greater tolerance and antitumor effect.
Introduction
10-Hydroxy camptothecin (10-HCPT) is one of the camptothecin (CPT) analogs with a powerful cytotoxic effect for cancer therapies and a broad range of anticancer activity in animal models (Song et al., Citation2006; Botella et al., Citation2011; Grillet et al., Citation2011; Ci et al., Citation2013). The molecular target of the CPTs family is the enzyme DNA topoisomerase I (topo I), a ubiquitous enzyme, which is overexpressed in a variety of tumor cell lines and is involved in the regularization of DNA topology during replication, recombination and transcription period (Wu et al., Citation2013). However, several major drawbacks of 10-HCPT have made them less attractive for clinical use, such as, low aqueous solubility and poor lactone stability in vitro and in vivo, short plasma half-life, severe clinical toxicity and side effects (Berna et al., Citation2014). Based on the properties of 10-HCPT, several approaches have been used to stabilize the lactone ring prior to entry into the site of action and increase the solubility in order to maximize therapeutic activity and minimize toxicity. For example, some 10-HCPT analogs possessing stabilized lactone structures and high water solubility have been developed to overcome this challenge, and topotecan and irinotecan are currently approved for the treatment of many types of cancer in humans (Babaei et al., Citation2015; Kim et al., Citation2015; Tseng et al., Citation2015). Among a variety of approaches, the use of biocompatible polymer conjugated anticancer drugs is a well-known and widely exploited technique to modify the properties of active agents and to improve the therapeutic response (Jiang et al., Citation2014; Li et al., Citation2014b; Lv et al., Citation2014; Wang et al., Citation2014). In terms of polymer–drug conjugate, the selection of polymer plays a crucial role in the preparation of conjugate. Hydroxyethyl starch (HES) is a semi-synthetic biodegradable polymer widely used as a plasma volume expander and it is degraded in vivo by serum α-amylase, and it offers the advantage of controllable biodegradation behavior. These excellent properties make HES an important biomedical material, and it has been tested as a substitute for PEG to extend the circulation time of drugs with a short half-life, and as a cryoprotectant for peptides, proteins and other nanoparticulate systems (Noga et al., Citation2014; Filippov et al., Citation2015; Fichter et al., Citation2015). In our previous experiment, 10-HCPT and HES conjugate (HES-10-HCPT conjugate) was prepared as a novel drug delivery system to transport and release 10-HCPT (Li et al., Citation2014b). The results showed that the aqueous solubility of 10-HCPT was increased by more than 100 times when it was conjugated to HES, and the conjugated 10-HCPT remains in the lactone form. HES-10-HCPT conjugate exhibited apparent sustained release effect and good stability in PBS solutions, plasma and liver homogenate. HES-10-HCPT conjugate achieved much lower IC50 and higher cytotoxicity effects than the free 10-HCPT on Hep-3B liver cancer cells. The pharmacokinetics results of HES-10-HCPT conjugate demonstrated that the biological half-life of 10-HCPT was increased from 10 min to 4.38 h and the bioavailability was 40 times higher than the commercial 10-HCPT injection.
In this study, we further evaluate the in vivo and in vitro behavior of HES-10-HCPT prodrug by a series of studies. In vitro cytotoxicity assay, hemolysis and vascular irritation tests were investigated. In vivo tissue distribution and pharmacodynamics in mice with H22 liver cancer cells were also studied. The rabbit irritation test results achieved much lower irritation than the commercial 10-HCPT injection. The tissue distribution test results showed that HES-10-HCPT prodrug increased significantly the 10-HCPT concentration in the tumor, liver and spleen site, whereas decreased the drug concentration in the heart and kidney. The hemolysis results demonstrated that the hemolysis effects of the prodrug was not obvious. The pharmacodynamics results indicated that HES-10-HCPT prodrug had a better antitumor efficiency against mice with H22 tumor than the commercial 10-HCPT injection, and the inhibition ratio of tumor was 85.2% and 31.1%, respectively at the same administration dose.
To sum up, super macromolecule prodrug HES-10-HCPT conjugate are often necessary to enhance passive targeting, antitumor effect, safety and tolerance that also decrease vascular irritation and side effects. In addition, we can further build new drug delivery system on the basis of HES-10-HCPT conjugate, such as polymeric micelles, nanoparticles.
Materials and methods
Material and reagents
10-Hydroxy camptothecin and 10-Hydroxy camptothecin injection (1.0 mg/mL) were purchased from Wuhan Lishizhen Pharmaceutical Co., Ltd. (Wuhan, China). Diphenhydramine hydrochloride was purchased from Hubei Xindali Pharmaceutical Co., Ltd. (Wuhan, China). Distilled water was purified in a Barn-stead EASY pure II RF/UV ultrapure water system (Dubuque, IA, USA) and passed through a 0.22 μm filter prior to use in all the studies. Methanol, acetonitrile and isopropanol of HPLC grade were purchased from Fisher Scientific (Fair Lawn, NJ). All other chemicals were commercially available and of reagent grade.
Animals
Thirty male KunMing mice weighing 20 ± 2 g were obtained from the Laboratory Animal Center of Shenyang Pharmaceutical University. Mice were housed in groups of two or three with 12 h light/12 h dark cycle in an air-conditioned room (25 ± 2 °C), at a relative humidity of 45–60% for 1 week. The mice were fasted for 12 h but had free access to water prior to the administration.
Twenty-four Guinea pigs weighting 220–270 g were obtained from the Laboratory Animal Center of Shenyang Pharmaceutical University, which were housed in an air-conditioned room (25 ± 2 °C), at a relative humidity of 45–60%.
Three rabbits weighing 2.5–3.0 kg were obtained from the Laboratory Animal Center of Shenyang Pharmaceutical University. Rabbits were housed in an air-conditioned room (25 ± 2 °C), at a relative humidity of 45–60 %. All animal use procedures were in accordance with the regulation for animal experimentation issued by the State Committee of Science and Technology of the People’s Republic of China.
Synthesis of HES-10-HCPT conjugate
The synthesis process of HES-10-HCPT conjugate has been published in International Journal of Pharmaceutics. The reaction between -OH groups from HES and -COOH groups from 10-HCPT occurs (Li et al., Citation2014b).
Cell viability assay
MTT assay was carried out to assess cytotoxicity of HES-10-HCPT conjugate. The liver cancer cell line H22 was seeded into 96-well plates at 6 × 103/well. Cells were incubated in an incubator under 5% CO2 at 37 °C for 24 h and, subsequently, the medium was removed, and the conjugate in different concentrations were added to the cell wells for 48 h and 72 h. Positive control treatments included varying the concentration of free 10-HCPT, and a negative control with medium alone. Then, 50 μL MTT (0.25 mg/mL) was added to each well at predetermined times and, after 4 h incubation period at 37 °C, the supernatant was removed, and 100 μL DMSO was added to dissolve the precipitated formazan crystals. The plates were shaken for 15 min and the OD values were measured with an ELIASA reader at a wavelength of 492 nm. The apparent cytotoxicity was calculated according to the following equation, and the cytotoxicity data were expressed as IC50, and the concentrations of the test agent producing a 50% reduction in cell numbers were compared with control cultures.
Tissue distribution test of HES-10-HCPT conjugate
Tissue distribution test
The mice were randomly divided into two groups. 10-HCPT injection and HES-10-HCPT conjugate were intravenously administrated via tail vein at a dose of 1.5 mg/kg to mice. After injection, the mice were killed at 0.5, 1, 2, 4, 12, 24 h after drug administration. Heart, liver, spleen, lung, kidney, marrow and tumor were collected. Tissue samples were blotted with paper towel, rinsed in saline, blotted to remove excess fluid and stored at −20 °C.
Tissue sample analysis
Frozen tissue samples were thawed at room temperature. Each tissue would be cut into pieces, weighed and homogenized with methanol. Totally 100 μL diphenhydramine hydrochloride (100 ng/ml) as internal standard was added to 500 μL tissue homogenate, then vortexed for 1 min. The mixture samples were then extracted with 4.0 mL ethylacetate–isopropanol (95:5, v/v) by shaking for 5 min in a test-tube shaker. After centrifugation at 6000 rpm for 10 min, the upper organic layer was transferred to a conical test tube (7.0 mL) and evaporated to dryness at 40 °C. The residue was reconstituted in 100.0 μL acetonitrile water (80:20, v/v). After centrifugation at 12 000 rpm for 10 min and a 5.0 μL aliquot of the solution was injected into the UPLC-ESI-MS/MS system for analysis.
UPLC-ESI-MS/MS method
Chromatography was performed on an ACQUITYTM UPLC system (Water Corp., Milford) with a conditioned autosampler at 4 °C (Li et al., Citation2013, Citation2014a). The mobile phase consisted of acetonitrile and 0.1% formic acid water. The separation was carried out on an ACQUITYTM BEH C18 column (50 mm × 2.1 mm i.d., 1.7 μm; Waters Corp., Milford). The column temperature was maintained at 40 °C. The injection volume was 5 μL and the partial loop mode was used for sample injection.
The Waters ACQUITY™ TQD triple-quadrupole tandem mass spectrometer (Waters Corp., Manchester, UK) was connected to the UPLC system via ESI interface. The ESI source was operated in positive ionization mode with the capillary voltage of 3.2 kV. The cone voltage was 16 V for 10-HCPT and 20 V for diphenhydramine hydrochloride. Nitrogen was used as the desolvationgas (600 L/h) and conegas (50 L/h). The temperature of the source and desolvation was set at 150 °C and 400 °C, respectively. Argon was used as the collision gas and the collision energy is 14 eV both for 10-HCPT and diphenhydramine hydrochloride. The MRM mode was used for quantification. Transition reactions of the 10-HCPT and IS are shown in . All data collected in centroid mode were acquired using Masslynx™ NT4.1 software (Waters Corp., Milford, MA). Post-acquisition quantitative analyses were performed using a Quanlynx™ program (Waters Corp., Milford).
The tissue samples were analyzed by UPLC/MS/MS, and the data were analyzed using drug and statistics (DAS) software version 2.0 (Mathematical Pharmacology Professional Committee of China, Shanghai, China). A value of p < 0.05 was considered statistically significant using the Statistical Package for Social Science (SPSS Inc., Chicago, IL).
Intravenous injection safety assessment
Hemolysis test
Rabbit blood was applied to test the hemolysis effect of HES-10-HCPT conjugate (Shi et al., Citation2010). Briefly, 10 mL of rabbit blood was obtained from arteria cruralis and the fibrinogen was removed by stirring with glass rod. Ten milliliter of 0.9% saline injection solution was added into defibrinogen blood sample, and supernatant was removed after centrifugation at 1500 rpm for 15 min. The erythrocytes at the bottom of centrifuge were washed three times (centrifugation followed by re-dispersion) with 0.9% saline injection solution. Finally, 2% erythrocyte standard dispersion was obtained by adding adequate amount of saline injection to the tube.
Different amounts of HES-10-HCPT conjugate solution (1.0 mg/mL) volumes of 0.1, 0.2, 0.3, 0.4 and 0.5 mL were added into five tubes with 2.5 mL of 2% erythrocyte dispersion in each. Then, appropriate amount of 0.9% saline injection was added in every tube to get a final volume of 5 mL. Negative control was a drug-free solution that contained 2.5 mL 2% erythrocyte dispersion and 2.5 mL 0.9% saline injection, whereas the positive control was prepared by addition of 2.5 mL water into 2.5 mL 2% erythrocyte. After vortex, the tubes were incubated at 37 °C and observed microscopically from 15 min to 4 h.
Rabbits vascular irritation test of HES-10-HCPT conjugate
Three rabbits weighting 2.5–3.0 kg were used for this study. Each rabbit was injected with a daily dose of 0.5 mg/kg of HES-10-HCPT conjugate into the right ear-border vein for 3 d. The same dose of 10-HCPT injection was given into the left ear-border vein. An equivalent volume of 0.9% saline injection was given into ear-border vein as control. The rabbits were sacrificed 48 h after the last administration, and the ears were cut and fixed in 10% liquor formaldehyde. At the localizations of 1 and 5 cm from the injection site to proximal part, histological sections were prepared for histopathological examination.
Injection anaphylaxis of HES-10-HCPT conjugate
Guinea pigs (200–250 g) were randomly assigned to four groups (n = 6): (1) negative control group (0.9% saline injection); (2) positive control group (0.3% egg albumen solution); (3) HES-10-HCPT conjugate (0.5 mg/kg) and (4) HES-10-HCPT conjugate (1.0 mg/kg). Animals were intraperitoneally injected every other day for three times, and the administration volumes of negative and positive groups were consistent with group (4). Ten days after the last administration, animals in each group were injected with a challenge dose of corresponding solution into the vein at the lateral of crus curvilineum (challenge dose was 2-fold of the administration dosage). The anaphylactic response was recorded in 3 h after the challenge injection.
The pharmacodynamic test of HES-10-HCPT conjugate
To assess antitumor activity in vivo of the conjugate, the pharmacodynamic test of HES-10-HCPT conjugate was performed in tumor bearing Kunming mouse. Kunming mouse weighing 20–25 g were randomly divided into four groups, consisting of six mice in each group, and subcutaneously transplanted with 1 × 105 H22 liver cancer cells on the right flank on Day 0. Mice developing palpable tumors reaching 10–12 mm in diameter within 5 d after the implantation began to receive injections via the femoral vein. HES-10-HCPT conjugate solutions at the dosage of 0.75 mg/kg and 1.5 mg/kg, normal saline as a blank control and commercial 10-HCPT injection at the dosage of 1.5 mg/kg as a reference were administered to each group separately. The injection was given twice a week for consecutive three weeks, on Monday and Thursday. The weight and tumor volume of the mice were measured every two days. After the tests were completed, the mice were killed and the tumors were totally excised. The pharmacodynamics data were analyzed by SPSS software. Based on the weight of tumors, the inhibition ratios of tumor were calculated according to the following equation:
In addition, in order to further study antitumor mechanism of different formulations, histological (H&E) analysis test of tumor samples from different treatment groups was carried out in pathology research center of China Medical University.
Results and discussion
Synthesis and characterization of HES-10-HCPT conjugate
The reaction between –OH groups from HES and –COOH groups from 10-HCPT occurs. The products are validated by NMR and TOF MS, and the results were reported in our previous paper.
In vitro cytotoxicity test
The effect of HES-10-HCPT conjugate concentrations on the proliferation of the cells and IC50 are shown in and , respectively. The results display that the HES molecule did not exhibit apparent inhibiting effects on cell proliferation at all concentrations, which demonstrated good cytocompatibility and safety of the HES. It can be seen that there was a positive correlation between the cytotoxicity effect and the dose. However, HES-10-HCPT conjugate exhibited lower IC50 than free 10-HCPT. The possible reason may be that the HES-10-HCPT conjugate had an excellent cell penetrating property and lactone stability, whereas the free 10-HCPT was rapidly hydrolyzed to the carboxylate form under physiological conditions and the carboxylate form was difficult to enter the cell because of high polarity. Additionally, HES-10-HCPT conjugate exhibited lower IC50 than free 10-HCPT group, which may result from the characteristics of 10-HCPT. On the one hand, 10-HCPT held a pH dependent equilibrium in aqueous medium between the lactone and the carboxylate forms. In general, the lactone form is essential for antitumor activity and the carboxylate form is almost inactive. Although the majority of free 10-HCPT would exist in carboxylate form in the cell culture solution (pH 7.4), it may result in the decrease of cytotoxicity. On the other hand, the polarity of 10-HCPT would increase when 10-HCPT existed in the form of carboxylate form, which may hinder the access of 10-HCPT into cell.
Figure 1. Cell viability of H22 liver call after 48 and 72 h cultured with the 10-HCPT-HES conjugate and free 10-HCPT at various 10-HCPT concentrations.
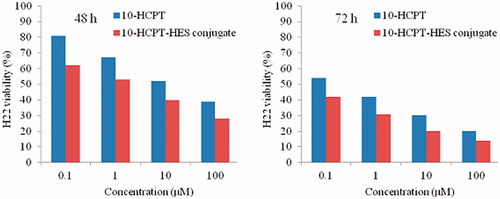
Table 1. IC50 values of H22 cell line cultured with 10-HCPT-HES conjugate and free 10-HCPT for 48 and 72 h.
Tissue distribution study
Tissue concentrations of 10-HCPT after intravenous administration of 10-HCPT injection and HES-10-HCPT conjugate were shown in , and AUCs in different tissues reported in .
Figure 2. Tissue distribution profiles of 10-HCPT after intravenous 10-HCPT injection and 10-HCPT-HES conjugate administration of 1.5 mg/kg.
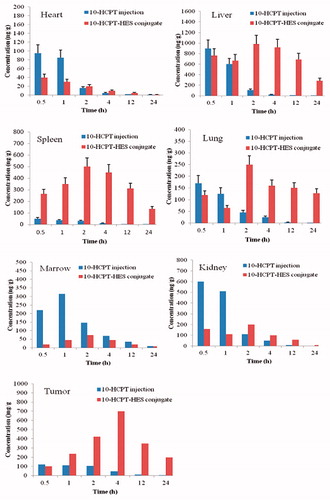
In the heart, the drug concentration of 10-HCPT injection group is higher than 10-HCPT conjugate group at the beginning of the administration, and the drug concentrations rapidly decreased in both groups with the extension of administration. In contrast, 10-HCPT conjugate group showed lower AUC value, which may avoid the generation of cardiac toxicity to some extent.
In terms of lung and liver, two formulations showed similar pharmacokinetics behavior. The drug concentration of 10-HCPT injection group is higher than 10-HCPT conjugate group 1 h after administration, and the 10-HCPT conjugate group showed higher plasma concentrations than injection group 2 h after the administration and the AUC value of conjugate group was 6 - and 18-fold higher than that of 10-HCPT injection group. The reason may be as following: when HES-10-HCPT conjugate was administrated by intravenous injection, they are usually taken up by the (reticuloendothelial system) RES in liver, spleen and lung. The uptake mechanism of the conjugate has been demonstrated to be either by phagocytosis or endocytosis because 10-HCPT conjugates possesses the properties of macromolecule solution. Additionally, HES-10-HCPT conjugate showed better long circulating effect and sustained effects during the transportation in vivo, which can promote the accumulation of 10-HCPT in tissues and organs. In comparison, the AUC values of 10-HCPT injection group were significantly lower than that of 10-HCPT conjugate group. This phenomenon can be attributed to the small molecule solution property and short half life time, because the small molecule solution could avoid the trapping by RES system and quickly distribute to each organization.
HES-10-HCPT conjugate mainly be uptake by spleen, and the AUC value was significantly higher than the 10-HCPT injection group, about 50 times higher. This may be due to that spleen contains a large number of macrophages with a phagocytosis, and HES-10-HCPT conjugate can be taken up by them. Meanwhile, spleen acts as a temporary reservoir of the drug, and some drugs can also return to the circulatory system again.
In the early administration, 10-HCPT injection showed higher drug concentration in kidney than 10-HCPT conjugate group, and the difference of Cmax about three times. It is well known that there is a close relationship between toxicity and Cmax values, so the severe side effects may be obtained from 10-HCPT injection group with further increase of the dose. In comparison, HES-10-HCPT conjugate may have a good tolerance for the patients.
It was found that the conjugate can significantly increase the drug accumulation in the tumor tissue, and the bioavailability was higher than that of 10-HCPT injection group, about 20 times. Such selective accumulation or passive targeting, which works especially good with tumors due to the lack of lymphatic drainage there, is currently known as enhanced permeability and retention (EPR) effect (Peer et al., Citation2007; Maeda Citation2010; Nichols & Bae, Citation2014). In addition, HES-10-HCPT conjugate showed better long circulating effect in vivo, which can promote the accumulation of 10-HCPT in tumor.
The drug concentration in the bone marrow significantly reduced with the preparation of HES-10-HCPT conjugate, and the AUC value of conjugate group was 4-fold lower than that of 10-HCPT injection group. As we known that the biggest flaw of anticancer drugs is the dose-limiting toxicity, and the dose-limiting toxicity is often bone marrow toxicity. 10-HCPT could induce the death of normal cell, especially for metabolically active cell, such as bone marrow stem cells, which resulted from in vivo distribution of 10-HCPT that lacked sufficient selectivity. However, HES-10-HCPT conjugate could not be taken up by marrow system due to the large particle size of the conjugate. Thus, the marrow toxicity of 10-HCPT significantly decreased with the preparation of conjugate, which had important implications for the treatment of clinical disease.
The pharmacodynamic test of HES-10-HCPT conjugate
In vivo antitumor activity of HES-10-HCPT conjugate was performed in Kunming mouse bearing H22 liver cancer cells, and the results are shown in and . It can be seen that the growth of tumor volume was rapid in negative control groups (treated with saline solution). In contrast, growth of tumor in mice treated with any 10-HCPT formulations significantly slowed down. HES-10-HCPT conjugate found to be more effective in inhibiting the growth of tumor in comparison with 10-HCPT injection group. For example, the average tumor weight of the negative control group was 4.216 g. For mice treated with 0.75 mg/kg and 1.5 mg/kg HES-10-HCPT conjugate, the mean tumor weight was 1.927 g and 0.625 g, respectively, which were significantly smaller than that of 10-HCPT injection group (2.906 g). The inhibition rate of HES-10-HCPT conjugate was 85.2%, which was 2.74-fold of 10-HCPT injection. The superior tumor inhibition effect of HES-10-HCPT conjugate might attribute to the efficient delivery to the tumor site via the EPR effect and the sufficient drug release after cellular uptake by tumor cells.
Figure 3. The excised tumor photo of 10-HCPT-HES conjugate on node mice (six animals in each group) xenografted with H22 tumors. Group A: saline group; group B: 10-HCPT injection group; group C: 0.75 mg/kg 10-HCPT-HES conjugate group; group D: 1.5 mg/kg 10-HCPT_HES conjugate group.
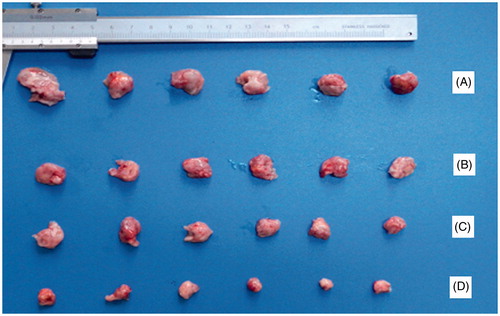
Table 2. Comparison of tissue of AUCs of 10-HCPT after i.v. administration of 10-HCPT injection and conjugate at a dose of 1.5 mg/kg.
Table 3. Antitumor effects of 10-HCPT-HES conjugate against H22 in Kunming mouse.
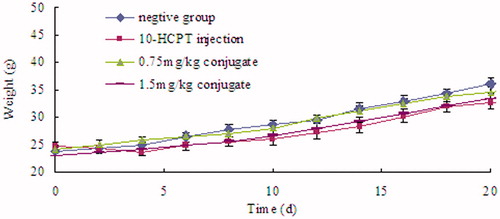
Body weight change is an indicator of systemic toxicity. As shown in , the body weight of PBS-treated mice showed a continuous and relatively fast increase, which might be due to the increase of tumor volume and low toxicity of PBS. In contrast, weight loss was observed after mice were injected with 10-HCPT injection in the first 2 d, revealing the toxicity of free 10-HCPT. HES-10-HCPT conjugate groups did not show significant weight loss in the early administration, and showed a continuous and slow increase during the trial, demonstrating the reduced systemic toxicity of the conjugated drug. With the high antitumor efficiency and excellent safety, this drug delivery system is promising in cancer therapy.
However, it is well known that one of the main problems is systemic side effects to normal tissues/organs. Thus, the in vivo tests of the conjugate against normal mice were carried out to systematically assess the potential toxicity and the biosafety of the conjugate. In this article, normal mice were randomly divided into three groups (n = 6), then intravenously injected with 10-HCPT injection, 10-HCPT conjugate and PBS (as control), respectively. The normal healthy mice were administrated every 3 d for 21 days with the dose of 10-HCPT, the same that was administrated in tumor bearing mice (1.5 mg/kg). The clinical signs and fluctuation in body weight, recognized as useful factors to assess in vivo toxicity of 10-HCPT injection and conjugate. Compared with the control group, the conjugate group was not found significant adverse effects among the administrated period. During the trial period, no apparent sighs of locomotor impairment, weight loss, anorexia and other symptoms associated with toxicity were observed in the groups of mice administrated with PBS and 10-HCPT conjugate. Meanwhile, there were no other abnormal physical signs and behaviors detected. In contrast, the mice treated with 10-HCPT injection showed significant pathological state, comprising body weight loss, lackluster hair and inactive mental mode. The group of conjugate displayed continuous body weight increase, which was close to that of the control group. However, the 10-HCPT injection group showed a significant body weight shift and the body weight of some of them even declined. All these results demonstrated that no significant in vivo toxicity might be obtained by conjugate compared with drug 10-HCPT.
Histological analysis of tumor samples
Histological (H&E) analysis test of tumor samples from different treatment groups was carried out in order to further study antitumor mechanism of different formulations. As shown in , the cross-section of tumor treated with PBS showed abundant and dense capillaries, and the tumor cells were bigger than other groups, whose tumor growth was in a good condition. In contrast, the mice treated with 10-HCPT injection showed less capillaries and smaller tumor cells than the control group. However, a large number of cells in mitosis and fusiform spindle can be seen. In addition, 10-HCPT injection could induce a small amount of tumor cells apoptosis. In terms of low dose 10-HCPT conjugate group, it showed similar pathological characteristics other than the number of capillaries and the volume of tumor cells further reduced. For mice treated with 1.5 mg/kg HES-10-HCPT conjugate, the number of tumor capillaries and the volume of tumor cells was much lower than other groups. In addition, there was a small amount of cells in mitosis seen occasionally. The most exciting thing is the proliferation of connective tissue of tumor, and the connective tissue can isolate and embed the tumor cells, which was causing a large number of tumor cell necrosis.
Figure 5. Histological (H&E) analysis of tumor samples from different treatment groups. Group A: saline group; group B: 10-HCPT injection group; group C: 0.75 mg/kg 10-HCPT- HES conjugate group; group D: 1.5 mg/kg 10-HCPT-HES conjugate group.
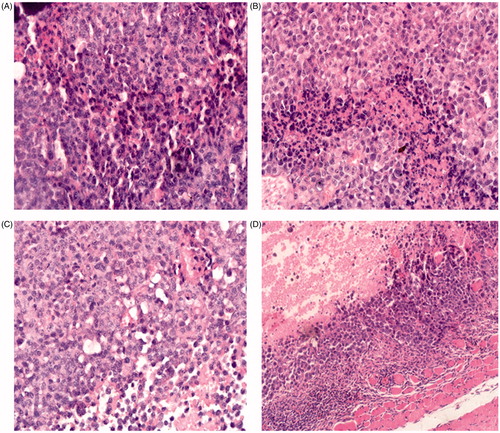
HES-10-HCPT conjugate showed high antitumor effect, the reason may be as following: the conjugate increased the solubility and lactone stability of 10-HCPT, and showed better long circulating effect in vivo, which can promote the accumulation of 10-HCPT in tumor; low pH environment of tumor tissue, EPR effect of tumor blood vessels and passive target of HES-10-HCPT conjugate can promote the drug to enter tumor tissues and release the drug rapidly. Additionally, it is well known that HES can be degraded by α-amylase; however, tumor tissues contain a small amount of α-amylase that may result in the accumulation of HES in the tumor tissue. The accumulation of HES would induce the proliferation of tumor connective tissue. The connective tissue can isolate and embed the tumor cells, which causes a large number of tumor cell necrosis because nutrients cannot be delivered to the tumor tissue.
Intravenous safety assessment
Hemolysis test
Complete hemolysis phenomenon was observed in the tube of positive control at 20 min, presenting as the red clear-diaphanous and no erythrocyte survived at the bottom of the tube. In contrast, the erythrocyte precipitated at the bottom of other six tubes (negative control and tested drug solution with different concentration of HES-10-HCPT conjugate) and dispersed after shaking in the 2 h observation. This phenomenon demonstrated that the prepared HES-10-HCPT conjugate at a concentration of 1.5 mg/mL would not induce hemolysis or erythrocyte agglutination at 37 °C.
Intravenous irritation assessment
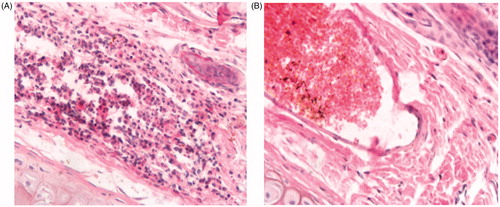
After a 3-day administration of 10-HCPT injection, HES-10-HCPT conjugate and 0.9% saline injection, no erythema, edema and tissue necrosis was observed at the injection site from HES-10-HCPT conjugate and 0.9% saline injection group. The histopathologic examination results are shown in . It can be seen that no thrombus, swelling or hyperplasia of endothelial cells appeared in the blood vessel of the rabbit ear-border vein. In addition, there were no pathological changes such as hemorrhage, edema, necrosis and inflammatory cell infiltrate in the vessel wall and surrounding tissues. The histopathologic examination results of the rabbit ears administrated with HES-10-HCPT conjugate were similar to those of the control group. All the results above indicated that no obvious intravenous irrigation was found in the ear vein of rabbit after intravenous administration of HES-10-HCPT at a dose of 0.5 mg/kg. In contrast, the histopathologic examination of the rabbit ear-border vein of 10-HCPT injection group showed severe thrombus, swelling or hyperplasia of endothelial cells.
Injection anaphylaxis
All the groups of the guinea pigs were in eucrasia during and after the administration. In contrast, significant anaphylaxis symptom such as dyspnea and convulsion was observed and all the guinea pigs died within 5 min. However, the HES-10-HCPT conjugate group and negative group did not show significant anaphylaxis phenomenon. Thus, it can be concluded that HES-10-HCPT conjugate used intravenously at the dose of 1.0 mg/kg did not cause hypersensitivity.
Conclusions
In conclusion, we have developed a simple and effective polymer-drug conjugate for antitumor drug delivery. We evaluated emphatically in vitro and in vivo behavior of the prepared HES-10-HCPT conjugate. It showed much lower IC50 and higher cytotoxicity against H22 cancer cells in comparison with the parent 10-HCPT. The in vivo study on H22 tumor bearing Kunming mice showed that HES-10-HCPT had superior tumor inhibition effect compared with 10-HCPT injection at the same drug dose, and the drug-related toxicity was negligible, demonstrating the great potential of super macromolecule conjugate for cancer therapy. After the conjugate is prepared, it can increase drug accumulation in the tumor site and decrease the drug concentration in heart and bone marrow significantly. Meanwhile, better intravenous safety than 10-HCPT injection has been obtained. Overall, with the simple synthetic route, well-defined structure, good biocompatibility, high antitumor effects, HES-10-HCPT conjugate also provided an excellent polymeric platform for the antitumor drug delivery.
Declaration of interest
The authors report no conflicts of interest. The authors are responsible for the content and writing of this article.
References
- Babaei M, Chadegani AR, Ghadam P. (2015). Binding of topotecan to chromatin: insights into cooperative binding and comparison with DNA. Int J Biol Macromol 80:57–63
- Berna GR, Sanjuan VM, Alvarez MG, et al. (2014). A promising camptothecin derivative: semisynthesis, antitumor activity and intestinal permeability. Eur J Med Chem 83:366–73
- Botella P, Abasolo I, Fernandez Y, et al. (2011). Surface-modified silica nanoparticles for tumor-targeted delivery of camptothecin and its biological evaluation. J Controlled Release 156:246–57
- Ci T, Li T, Chang G, et al. (2013). Simply mixing with poly (ethylene glycol) enhances the fraction of the active chemical form of antitumor drugs of camptothecin family. J Controlled Release 169:329–35
- Fichter M, Dedters M, Nguyen AP, et al. (2015). Monophosphoryl lipid a coating of hydroxyethyl starch nanocapsulesdrastically increases uptake and maturation by dendritic cells while minimizing the adjuvant dosage. Vaccine 33:838–46
- Filippov SK, Sergeeva OY, Vlasov PS, et al. (2015). Modified hydroxyethyl starch protects cells from oxidative damage. Carbohydr Polym 134:314–23
- Grillet F, Sabot C, Anderson R, et al. (2011). Intramolecular isomünchnone cycloaddition approach to the antitumor agent camptothecin. Tetrahedron 67:2579–84
- Jiang RJ, Zhao YL, Chen YJ, et al. (2014). Synthesis, characterization, and in vitro evaluation of artesunate-β-cyclodextrin conjugates as novel anti-cancer prodrugs. Carbohyd Res 400:19–25
- Kim JH, Jeong M, Lee SS, Song J. (2015). Camptothecin and topotecan inhibit adipocyte differentiation by inducing degradation of PPARγ. Biochem Bioph Res Co 463:1122–8
- Li GF, Cai CF, Ren TY, Tang X. (2014a). Development and application of a UPLC–MS/MS method for the pharmacokinetic study of 10-hydroxy camptothecin and hydroxyethyl starch conjugate in rats. J Pharm Biomed Anal 88:345–53
- Li GF, Li Y, Tang YL, et al. (2014b). Hydroxyethyl starch conjugates for improving the stability, pharmacokinetic behavior and antitumor activity of 10-hydroxy camptothecin. Int J Pharm 471:234–44
- Li N, Deng Y, Wang D, et al. (2013). Determination of glibenclamide and puerarin in rat plasma by UPLC-MS/MS: application to their pharmacokinetic interaction study. Talanta 104:109–15
- Li N, Li N, Yi QY, et al. (2014). Amphiphilic peptide dendritic copolymer-doxorubicin nanoscale conjugate self-assembled to enzyme-responsive anti-cancer agent. Biomaterials 35:9529–45
- Lv SX, Tang ZH, Zhang DW, et al. (2014). Well-defined polymer-drug conjugate engineered with redox and pH-sensitive release mechanism for efficient delivery of paclitaxel. J Controlled Release 194:220–7
- Maeda H. (2010). Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects. Bioconjugate chem 21:797–802
- Nichols JW, Bae YH. (2014). EPR: evidence and fallacy. J. Controlled Release 190:451–64
- Noga M, Edinger D, Wagner E, et al. (2014). Stability and activity of hydroxyethyl starch-coated polyplexes in frozen solutions or lyophilizates. Int J Pharm 469:50–8
- Peer D, Karp JM, Hong S, et al. (2007). Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol 2:751–60
- Shi S, Chen H, Lin X, Tang X. (2010). Pharmacokinetics, tissue distribution and safety of cinnarizine delivered in lipid emulsion. Int J Pharm 383:264–70
- Song L, Bevins R, Anderson BD. (2006). Kinetics and mechanisms of activation of α-amino acid ester prodrugs of camptothecins. J Med Chem 49:4344–55
- Tseng YY, Wang YC, Su CH, et al. (2015). Concurrent delivery of carmustine, irinotecan, and cisplatin to thecerebral cavity using biodegradable nanofibers: in vitro and in vivo studies. Colloids Surf B Biointerfaces 134:254–61
- Wang K, Zhang XF, Liu Y, et al. (2014). Tumor penetrability and anti-angiogenesis using iRGD-mediated delivery of doxorubicin-polymer conjugates. Biomaterials 35:8735–47
- Wu XW, Li HP, Song SE, et al. (2013). Facile synthesis of camptothecin intercalated layered double hydroxide nanohybrids via a coassembly route. Int J Pharm 454:453–61