Abstract
5-Fluorouracil (5-FU) is one among the anti-cancer agents in FOLFORINOX treatment along with oxaliplatin and irinotecan for the treatment of colorectal cancer. Despite its potential activity on the tumor cells, it lacks site specificity partly attributed by its biodistribution to healthy cells resulting in toxic effects to healthy cells. Therefore, we have formulated 5-fluorouracil enteric-coated nanoparticles (5-FUEC) to localize the drug in the colon area that enables its prolonged presence in target area in a sustained manner. The current work emphasizes on enhanced anti-cancer activity of 5-FUEC sequencing its apoptotic activity on HCT 116 colorectal cancer cell lines in vitro. MTT assay exhibited 5.5-fold decrease in IC50 value of nanoparticles comparable to 5-FU. Nuclear fragmentation with irregular edges in nucleus of cells justified its improved activity. Furthermore, flow cytometric analysis confirms the majority of cells gated in early apoptotic (39.75%) and late apoptotic phase (36.25%). Acridine orange/ethidium bromide staining (AO/EB) exhibited cells with red fluorescence (indicating apoptosis) comparable to the control and 5-FU. γ-Scintigraphic studies determined the applicability and feasibility of the enteric coating with mean gastric emptying time, mean intestinal transit time and mean colon arrival time of 1.89 ± 0.03, 2.15 ± 0.05 and 4.03 ± 0.27 h, respectively. Moreover, nanoparticulate approach was found significant in reducing tumor size and volume in xenograft tumor models in vivo along with sustained release. These superior anti-cancer activities exhibited by 5-FUEC indicated that it could be a potential alternative to chemotherapy for colorectal cancer.
Introduction
Cancer is the second leading cause of death in developing countries and comes along the most challenged one. Although, we witnessed the development of nanoscale technologies and its wide applicability, cancer still poses worldwide complications (Ozols et al., Citation2006). Various therapeutic treatment strategies for cancer are available at present includes surgery, chemotherapy, radiation and biological therapies, out of which chemotherapy is the widely followed in the present scenarios (Cirstoiu-Hapca et al., Citation2009; Tummala et al., Citation2014). However, associated benefits precede the toxic effects of chemotherapy thereby limiting its clinical applicability. Moreover, common features of tumor cells and healthy cells make healthy cells prone to anti-cancer agents suggesting their lack of site specificity. This limitation of chemotherapy makes the way for increase of dose, as therapeutic dose intended may not reach to the target resulting in sub-standard activity. In addition, this increase in the dose of chemotherapy further causes effects on healthy cells (Krishnaiah & Satyanarayana, Citation2001; Michor et al., Citation2005). Further summing up the limitations albeit with the rise of therapeutic tools comprises non-selective distribution of drug, enhanced toxicity causing side effects to healthy cells.
These effects can be minimized either by actively targeting the active pharmaceutical ingredient (API) to the target site or by localizing the API at the target region. We emphasized mainly on the localization of the drug in the target area, thereby minimizing the chances of drug distribution to the other organs in our previous studies (Tummala et al., Citation2015b). Essentially, coating of anti-neoplastics with polymers comprising of the classes like cellulose derivatives (Levine et al., Citation1987), acrylic polymers (Rasmussen et al., Citation1982) etc., that can deliver major amount of the drug directly to the large bowel thereby bypassing the systemic circulation can be explored. Enteric coating of the nanoparticles with polymer like eudragit S-100 helps the formulation in preventing release of the drug at gastric pH (as it is acidic) and releases once it arrives colon region (primarily distal ileum) (Rai et al., Citation2014).
5-Fluorouracil (5-FU) is one among the anti-neoplastic agent belonging to pyrimidine analogues, currently in use for the treatment of colorectal cancer (Tummala et al., Citation2014), breast cancer (Hutchins et al., Citation2005) and glioblastoma (Shapiro et al., Citation1992). It is basically a thymidylate inhibitor that majorly inhibits the synthesis of DNA thereby preventing excess DNA proliferation (Parker & Cheng, Citation1990; Longley et al., Citation2003). Coming apart from the beneficial terms, 5-FU poses limitations such as short half life, wide distribution to healthy tissues thereby depriving it of its clinical use (Fata et al., Citation1999; van Kuilenburg et al., Citation2000; Di Paolo et al., Citation2001; Zhang et al., Citation2014).
This study hypothesizes the localized activity of 5-FU enteric coated nanoparticles in comparison with pure drug. Polymeric nanoparticles that were enteric coated can be considered as an ideal platform that can prevent the drug release at gastric pH, control drug release and helps in attaining stability. Moreover, chitosan that was used as a polymer is biocompatible, and eudragit S 100 polymer was used for enteric coating (Ghorab et al., Citation2011). This nano formulation was hypothesized for better localization in the colon region, apoptotic activity with increased anti-cancer activity. We have previously reported the 5-FU enteric coated polymeric nanoparticles using chitosan and their characterization along with its in vitro drug release in various simulated fluids representing various areas of gastro intestinal tract (GIT). This study comes as a sequential to the former study to further investigate the cytotoxicity, apoptotic activity of the formulated 5-FU enteric coated nanoparticles with 5-FU:chitosan in ratio of 1:3 (5-FUEC). Flow cytometry studies were conducted for the formulation for its better analysis on HCT 116 cells during various phases of apoptosis. Cellular morphological changes in HCT 116 cells were emphasized by conducting fluorescence studies using DAPI. γ-Scintigraphic studies were conducted in male BABL/C nu/nu mice to assess the release pattern of the enteric-coated nanoparticles in various regions of GIT. Furthermore, nanoparticles activity in reducing tumor growth was observed in HCT 116 induced xenograft tumor model.
Materials and methods
Materials
5-Fluorouracil was used as active pharmaceutical ingredient and procured from Sigma Aldrich, Mumbai. 2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride solution (DAPI) was used for nuclear staining and purchased from Beyotime Institute of Biotechnology, Japan. Chitosan (purified viscosity grade 50 cps; molecular weight [MW] 150 kDa; deacetylation degree 85%) was used as the polymer and obtained from M/s Panacea Biotech, Chandigarh, India. Acridine orange, Ethidium bromide, JC-1 stain, MTT (3-(4,5-dimethylthiazolyl-2)-2,5-di-phenyltetrazolium bromide) and polyvinyl alcohol (PVA), which was used as surfactant and purchased from Sigma Aldrich, Mumbai. Annexin V-FITC plus propidium iodide (PI) double staining kit was purchased from Invitrogen (Carlsbad, CA). All cell culture medium and human colorectal cancer cell line (HCT 116) were procured from the National institute of health sciences (NIH), India. Acetonitrile (HPLC grade) was purchased from Himedia leading biosciences company, India. De-ionized Milli Q water was used for the preparation of all the samples.
Preparation of 5-FU enteric-coated nanoparticles
5-Fluorouracil (5-FU) polymeric enteric-coated nanoparticles were formulated and characterized in our previous studies. Briefly, 5-FU polymeric nanoparticles were formulated using chitosan as polymer by solvent emulsification method (Prabhaharan & Mano, Citation2004). Different ratios of drug:polymer (1:1, 1:2, 1:3 and 1:4) was used in order to optimize the best formulation. Acetic acid was used as an organic solvent for this method. 5-FU was dissolved in 40 ml water followed by addition polyvinyl alcohol (PVA) at a concentration of 0.5% w/v as surfactant using high-speed emulsifier. Stirring was later continued till 3 h and emulsion was centrifuged at 12 000 rpm for 30 min followed by thorough washing and lyophilizing (Christ Alpha 2-4 LD, Freeze Drying Solutions, UK) later on using mannitol as cryoprotectant. Extruder-spheronization technique (UFEE 60; Umang Pharmatech, Ahmedabad, India) was then used for formulating nanoparticles into pellets. Nanoparticles were mixed with deionized water and avicel to form wet mass followed by passing them through extruder having a sieve opening of 1 mm, screen thickness of 2.5 mm and extrude cut-off length of approximately 2–4 mm. The extrudes were spheronized in a spheronizer at 2200 rpm for 10 min. The pellets formed were dried properly at 35–40 °C prior to enteric coating using a conventional coating pan (rotating speed at 20 rpm, atomizing air pressure of 2 bar, inlet air temperature of 60–70 °C and outlet air temperature of 35–40 °C). Eudragit S100 with ammonia and triethyl citrate (coating solution) was sprayed through 1.1 mm spray nozzle to prepare enteric-coated pellets (Tummala et al., Citation2014, Citation2015a,b).
5-FU enteric-coated nanoparticles were characterized for particle size, entrapment efficiency, drug loading and in vitro drug release using dialysis bag method in various simulated fluids.
In vitro cytotoxicity assay
MTT assay was performed for 5-FU, NP (plain nanoparticles), 5-FUEC. HCT 116 cells were seeded onto 96 well plates at a seeding density of 1 × 104 cells/well. It was then left overnight with incubation for attachment of cells to the plate. In each well, the culture medium was replaced with 100 μl of medium with serial dilutions of all the samples for treatment. All the treatment samples were given with concentration of 0.5–10 μM and were left for 48 h. A 10 μl of MTT (5 mg/ml in phosphate buffer saline) was added at particular time intervals, which was followed by incubation for 4 h and removal of the culture solution. About 100 μl of DMSO was then added to each of the well separately in order to dissolve the formazan crystals while shaking the plates vigorously. Then the absorbance was determined for each well on a microplate reader (Thermo Scientific, Pittsburgh, PA) at a wavelength of 550 nm. The experiment was conducted in triplicate in parallel. IC50 is the concentration that can inhibit 50% of cell growth was calculated by SPSS software Version 19.0 (SPSS Inc., Chicago, IL). Along with that relative cell viability was calculated for different concentrations for all the samples, which was compared to the untreated control (Tummala et al., Citation2015a).
Annexin-PI assay
Assessment of apoptotic ratio induced by 5-FU enteric-coated nanoparticles was determined using an Annexin V-Fluorescein isothiocynate (FITC). Annexin-V which is a phospholipid protein that has higher affinity for phosphatidylsterine (PS). PS is generally exposed during early stages of apoptosis. Along with Annexin-V, propidium iodide (PI) is used that can enter the apoptotic cells and is a red fluorescent dye. Apoptosis is characterized generally by decreased cell size, condensation of chromatin and nuclear fragmentation. Briefly, HCT 116 cells were seeded in six well plates at a density of 1 × 105 cells/well that were left undisturbed for whole night to ensure proper attachment. Cells were treated with IC50 concentration of the pure drug (5-FU), enteric-coated nanoparticles (5-FUEC) and were harvested and washed thrice with PBS. Then the cells were resuspended in 500 μL of 1 × binding buffer followed by staining them with 5 μL of annexin-V FITC and 10 μL of PI and were later incubated at room temperature in dark area for 30 min. The stained cells were differentiated and analyzed by FAC scan flow cytometer (Beckton Dickinson, Franklin Lakes, NJ). Control cells were cultured with out any drug and were maintained separately till 48 h.
DAPI staining
Cell morphology can be visualized by DAPI staining. Briefly, HCT 116 cells were seeded in 6 well plates at a density of 1 × 10 5 cells/well. Cells were left undisturbed for one day followed by exposure to 5-FU and 5-FUEC containing media. IC50 concentration of 5–FU and 5-FUEC sample formulations was used. Cells, which were left untreated, were considered as negative control. Cells were left undisturbed for 24 h followed by the addition of acetic acid in methanol with ratio of 1:3 v/v and then stained with DAPI (Sigma-Aldrich, Mumbai). Fluorescence microscope (Olympus IX51, USA) was then used to visualize the stained cells morphology with a 430–490 nm filter (n = 6) (Chuah et al., Citation2014).
AO/EB assay
Apoptosis was further quantified by acridine orange (AO)/ethidium bromide (EB) studies for 72 h. Drug and 5-FUEC were used at IC50 concentrations for precise apoptotic quantification. Briefly, HCT 116 cells were seeded in 35 mm culture plate and were left placid for 24 h prior to addition of all the samples. Cells were then thoroughly washed with PBS followed by staining them in combination of 5 mg/ml of EB and 50 mg/ml of AO for 15 min at room temperature. Cells at different stages were determined by inverted fluorescence microscope (DMI 4000B; Leica Microsystems) at a magnification of 20×. Control cells were kept aside separately till 72 h that helps in further identifying the color changes. Cells with different colors observed were grouped as green (viable cells), bright green (preliminary phase of apoptosis), light orange (early apoptotic cells) and bright red (late apoptotic cells) (Nair et al., Citation2011).
Evaluation of mitochondrial membrane potential (ΔΨm) in HCT-116 cells
HCT-116 cells were seeded in 6-well plates and were subjected to trypsinization followed by suspending them in phosphate buffer consists of 10 μg/ml of JC-1 (Sigma Aldrich, St. Louis, MO). The cells were incubated for 15 min at 37 °C in the incubator before centrifugation in order to remove the supernatant. This was followed by suspending them in PBS for analyzing in flow cytometry. The cells, which were identified as green was considered to have exhibited change in the membrane potential (color attributed to the emission of green fluorescence from JC-1 monomers) (Vivek et al., Citation2014)
γ-Scintigraphic study of enteric coated nanoparticles
Nanoparticles were enteric coated to prevent its drug release at acidic pH and allow its release post the intestinal area to the colonic region. The release pattern of the nanoparticles with in the GIT of mice was assessed qualitatively by γ-Scintigraphic study. Mice (male) were selected (weight ranging from 35 to 45 g) and were fasted prior to administration of radiolabelled nanoparticles encapsulated in enteric-coated pellets to investigate the behavior of the dosage form. Radioactive 99mTc-DTPA (very low quantity; 1 μCi) was injected every 6 h through tail vein to obtain the outline of whole-body image. 99mTc-DTPA (1 mCi) at a high dose was encapsulated in enteric-coated pellets to assess the presence of pellets in various areas of GIT followed by measurement of the radioactivity by radioisotope dose calibrator (CRC-127 R; Capintech Inc., Pittsburgh, PA). Mice were kept under scintillation camera in a restraining cage after administration of enteric-coated pellets orally (with 2–3 ml of water). Gamma camera (E. Cam; Siemens, Munchen, Germany) for scanning (fitted with low-energy all-purpose collimeter) was used to monitor the transit of pellets at various time intervals through the GIT. Data were collected and stored in a linked online computer system with the camera (set for 100 K counts). Mean energy window of 99mTc was 140 ± 14 keV and the useful field was 256 × 256 mm. Anterior images of the rodents were recorded in supine position and archived on to a optical disk for subsequent analysis. Images were recorded immediately after drug administration followed by 10-min interval for 1 h, 30-min interval till 6 h and 60-min interval till the end of the experiment (Jain et al., Citation2010).
Xenograft tumor model in nude mice and in vivo treatment with chemotherapeutic agents
Male BABL/C nu/nu mice were selected and obtained upon animal ethical committee approval. They were well maintained under hygiene conditions free from pathogens. HCT 116 cell line, a human colonic adenocarcinoma cell lines were harvested followed by washing thrice in ice-cold serum free Dulbecco’s modified eagle medium (DMEM). Trypan blue exclusion was used for counting viable cells. Then, cells were again suspended in the DMEM media prior to injecting 0.25 ml of 2.5 × 106 cells subcutaneously (s.c) into the right flank of each mouse. It was allowed to grow for atleast three weeks followed by dissecting them and mechanical mincing. Then the tumor pieces with 3 mm3 size had been transplanted subcutaneously by trocar needle into 25 animals and waited till they reach 33–38 mm3. Animals were grouped into five groups each consiting of five animals. About 5 μM/kg of samples (saline, NP, 5-FU, 5-FUPNP and 5-FUEC) were administered orally every 2 days. Digital vernier callipers had been used for the determination of the tumor size every three days and tumor volume was also calculated very precisely using the formula (Radulovic et al., Citation1991; Guo et al., Citation2006; Li et al., Citation2015)
where a denotes long axis and b denotes short axis.
Further mice with tumor implants were euthanized after 2 weeks followed by excising them and weighing.
Statistical analysis
Statistical analysis was performed using minitab version 16 (Minitab Inc., Coventry, UK). ANOVA was mainly used to evaluate statistical differences between different mean values. All data were considered statistically significant at p < 0.05 and were represented as mean ± standard deviation (SD).
Results and discussion
The characterization of 5-fluorouracil enteric coated nanoparticles (5-FUEC) were carried out and reported in our previous paper. 5-FUEC with drug:chitosan ratio of 1:3 that was enteric coated with eudragit S 100 (optimized batch) have an average particle size of 138 ± 1.01 nm measured by dynamic light scattering (DLS), polydispersity value less than 0.2. 5-FUEC entrapment efficiency and drug loading was determined to be 69.18 ± 1.89 and 28.14 ± 0.19%, respectively. In vitro drug release for 5-FUEC in various simulated fluids representing GIT pH had conformed its stability in acidic pH and there after releasing the drug in the basic pH. Drug release was found to be only after 4 h once it enters the intestinal fluid and was localized along with its sustained release over a period of 24 h (Tummala et al., Citation2015b). In this study, extensive cellular and apoptotic studies were carried out to investigate the apoptotic activity of the 5-FUEC using Annexin-PI assay (using flow cytometry) and Acridine-orange/Ethidium bromide method. Anti-cancer activities of the formulations were evaluated for different time intervals till 72 h by MTT assay. DAPI studies were conducted to determine the apoptosis by morphological changes in the nucleus using fluorescence microscope. Mitochondrial membrane potential studies were performed to evaluate the apoptosis by change in the potential using JC-1 stain. γ-Scintigraphic study was also conducted in order to assess the release pattern of the drug at different times in various areas of gastrointestinal tract (GIT). 5-FUEC therapeutic activity on tumor growth was observed in BALB/c nude mice comparable with pure drug, plain nanoparticles, saline and 5-FU polymeric nanoparticles (5-FUPNP).
In vitro cytotoxicity activity
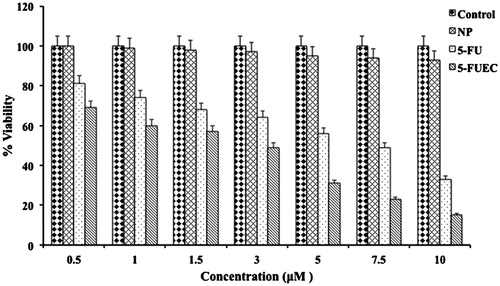
HCT-116 cell lines were selected in order to evaluate the cell toxicity activity of formulated 5-FUEC, NP and 5-FU. This can be observed clearly in . 5-FUEC had shown a considerable significant decrease in cell viability percentage comparable to pure drug and untreated control. 5-FU had shown decrease in cell viability, but not as significant as enteric coated nanoparticles in a dose and time dependent manner. The reason for the enhanced activity of 5-FUEC can be attributed to the release of the drug in the colon region thereby localizing it and allowing major amount of the drug incorporated to the colorectal tumors rather than distributing to other organs in the body. Furthermore, enhanced permeation and retention effect of the nanoparticles also helps in delivering the drug efficiently to the tumors there by enhancing its cytotoxicity activity. In addition, plain nanoparticles (NP) were tested for their activity and it was observed that cell viability was almost the same compared to untreated control. This also justifies that the cytotoxicity activity of the enteric-coated nanoparticles attributing to the anti-cancer property of 5-FU along with modified drug release pattern rather than nanoparticles alone thereby considering them as non-toxic. A 2-fold increase in cytotoxicity of 5-FUEC (at 2.12 ± 0.01 μM) nanoparticles equivalent to 0.89 ± 0.15 μM 5-FU) showed nearly 50% cell viability. The decrease in cell viability of 5-FUEC, 5-FU and NP at end of 48 h when given in the concentration range of 0.5–10 μM was found to be 69–15, 81–33 and 100–93%, respectively. Hence, we can conclude the predominant activity of enteric-coated nanoparticles over the others (p < 0.05) thereby justifying its release pattern (protecting the drug in the acidic pH and localizing the drug after entering the colon in the basic pH). IC50 value (2.12 ± 0.01 μM) of 5-FUEC had shown decline comparable to 5-FU (4.9 ± 0.23 μM). It should be noted that 2.12 ± 0.01 μM of enteric coated nanoparticles contain 5-FU equivalent of 0.89 ± 0.15 μM that showed its enhanced activity attributing to its 5.5 folds decrease in IC50 value comparable to the pure drug.
DAPI studies
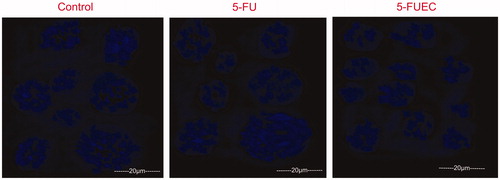
Microscopical observation of the cells can determine the evident morphological changes like irregular edges, nuclear fragmentation, etc., occurred during apoptosis. 5-FU was given as such at its IC50 concentration and minor changes in the nuclear morphology was observed after 24 h attributing to the fact that it has poor bioavailability contributing its minimal percentage of the drug at the colon area. By the fact that, as the pure drug is not capable of localizing the drug at the target site attributing to its release in the systemic circulation followed by its distribution to the healthy tissues along with the tumor tissues. Furthermore, 5-FUEC nanoparticles had shown significant changes in the nuclear morphology with most of them either fragment or in irregular shape that can be seen in . This activity can be justified by the absence of any signs in negative control group (untreated) and minimal signs in pure drug treated cells. Significant apoptotic activity can be attributed to the enteric coating of the nanoparticles that helps in preventing the release of the drug at stomach pH and thereby helps in bypassing the systemic circulation and localizing the drug at the colon site. The morphological changes by apoptosis in the nucleus for all the samples can be observed in .
Annexin-PI studies
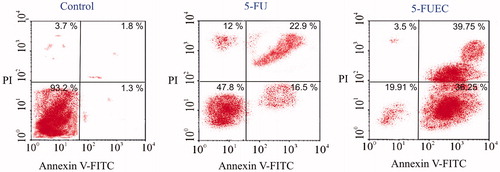
Assessment of the apoptotic ratio can evaluate the quantification of the cells at different stages using flow cytometry that can be seen in . Different stages comprise the viable cells, necrotic cells, early apoptotic cells and late apoptotic cells. These cells were quantified by visualizing in different quadrants by FACscan flow cytometer. PI stains the apoptotic cells, where as Annexin V-FITC binds to PS marking its affinity towards it thereby helping in determination of apoptotic ratio. Furthermore, cells were grouped into different categories: viable cells representing lower left quadrant (LL), necrotic cells representing upper left quadrant (UL), early apoptotic cells representing upper right quadrant (UR) and late apoptotic cells represented by lower right quadrant (LR). Control group of HT-29 cells were rather cultured separately and observed for its viable cells that showed 93%. 5-FU (pure drug) at its IC50 concentration had shown 47.8% of viable cells (LL), 22.9% of early apoptotic cells (UR) and 16.5% of late apoptotic cells (LR). 5-FUEC nanoparticles had shown 19.91, 39.75 and 36.25% of viable cells (LL), early apoptotic cells (UR) and late apoptotic cells (LR) respectively. Comparative evaluation determines the significant apoptotic activity of enteric-coated nanoparticles that can be justified by the increased percentage of the cells in early and late apoptotic quadrants. The enhancement of the apoptotic activity of the enteric-coated nanoparticles can be attributed to the eudragit S-100 coating that can prevent the drug release in the acidic pH and helps in localizing the drug at the colon area. This also suggests the increase in the therapeutic activity of 5-FU by localizing major amount of the drug in the colon and delivering it to the tumor by enhanced permeation and retention effect. Moreover, apoptosis ratio of the nanoparticles in the tumor cells was observed for a prolonged period of time justified by its sustained release achieved using chitosan polymer.
AO/EB studies
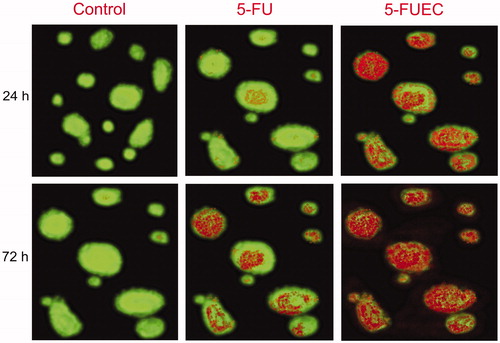
AO/EB studies were performed over a period of 72 h to visualize time dependent effect of apoptosis of the samples by fluorescence study. Acridium orange (AO) was selectively taken up viable cells and ethidium bromide (EB) stains apoptotic cells affirming characteristic chromatin condensation. Control cells (HT-29) were visualized by major amount (92%) of the cells with green fluorescence conforming to its viability. 5-FU (pure drug) had shown apoptotic activity with time, showing an apoptotic activity of 51% at 72 h visualized by minor number of cells with red and dull orange fluorescence. 5-FUEC nanoparticles had shown a significant and appreciable apoptotic activity compared to other samples. Visualization of HT-29 cells treated with 5-FUEC had shown major number of cells with red and orange fluorescence indicating apoptotic cells, minor number of cells with green fluorescence thereby indicating its decreased viability. Apoptotic cells for enteric-coated nanoparticles can be quantified as 45% at 24 h, 59% at 48 h and 78% at 72 h thereby justifying its localized and sustained release. Moreover, sustained property of the formulation helps in increasing the dosing interval and achieved maximal therapeutic effect with minimal dose. Cytochemical staining of all formulations along with control can be clearly observed in .
Figure 4. Fluorescent microscopic images of HCT 116 colon cancer lines with enteric coated nanoparticles, pure drug (5-FU) at 24 h and 72 h. Control was visualized by green fluorescence at all time intervals conforming its viability. 5-FU had shown minimal fluorescence of light orange and green representing minimal apoptosis. 5-FUEC had shown appreciable apoptosis attributing to the major part of the cells with red fluorescence.
Evaluation of mitochondrial membrane potential change (ΔΨm)
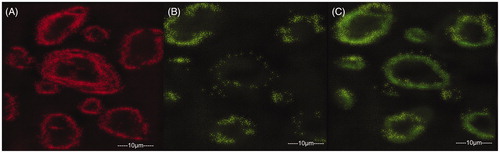
Release of cytochrome-C through the outer mitochondrial membrane is one of the major steps in the process of apoptosis that causes change in the mitochondria membrane potential. In general during cancer, pro-apoptotic caspase proteins were inhibited thereby resulting in more cell growth proliferation. Cytochrome C helps in activation of the caspases there by inducing apoptosis in the tumor cells inhibiting cell proliferation. 5-FU (pure drug) and 5-FUEC nanoparticles were evaluated for mitochondrial membrane potential change (ΔΨm) by JC-1 stain. Control cells that were untreated with 5-FU were observed with red fluorescence. 5-FU had shown minimal green fluorescence in mitochondria attributing to its apoptotic activity, whereas 5-FUEC had shown significant green fluorescence relating to its appreciable apoptotic activity by enhanced permeation and retention effect. The change in the fluorescence with mitochondrial potential change can be observed clearly in .
Figure 5. Mitochondria membrane potential (ΔΨm) change evaluation in HCT-116 cell lines induced by 5-FU and 5-FUEC by staining the cells with JC-1 followed by analyzing them in fluorescence microscope. (A) Control cells producing red fluorescence, (B) 5-FU (pure drug) producing partial green fluorescence representing minimal potential change and (C) 5-FUEC producing full green fluorescence representing complete mitochondrial potential change. Second and third boxes represent apoptotic activity of 5-FU and nanoparticles respectively.
γ-Scintigraphic study
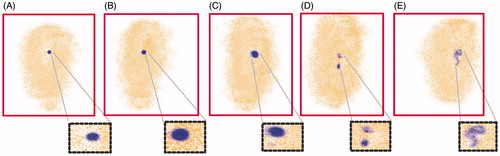
γ-Scintigraphic study was performed to assess the release pattern of prepared 5-FUEC nanoparticles in different areas of GIT by γ-scintigraphy. Mean gastric emptying time, mean intestinal transit time and mean colon arrival time of 5-FUEC was found to be 1.89 ± 0.03, 2.15 ± 0.05 and 4.03 ± 0.27 h, respectively. Tracer containing pellets of 5-FU had entered colon between 3.79 and 4.23 h after administration of the formulation. It can be observed from that the formulation remained intact and the tracer was not released in stomach conforming the successful enteric coating of the nanoparticles preventing its release in acidic pH. Moreover, slower transit times were observed at the initial time period for 5-FUEC followed by much faster transit times conforming to the reason that it takes time for the GIT fluid to penetrate the nanoparticles lessening the rigidity of formulation. Jain et al. (Citation2010) observed this kind of fast transit time after initial slow release with a colonic arrival time near to 5 h for oral administration in albino rats. Captured scintigraphs () clearly indicate no amount of tracer release in the stomach justifying its enteric coating, where as scintigraphs taken after 2 h (between 1.98 and 2.30) had shown minimal tracer release in the small intestine () due to its slight visibility around the pellet. Apart from the minimal tracer release, pellets remained intact passing through small intestine. However, tracer release was increased after 4 h due to the fact that it entered the ascending colon due to the change of pH to alkaline in the GIT solubilizing the enteric coat thereby releasing the tracer (). Pellets shape was distorted as the time progresses in the ascending colon leading to the dissolution of the coat at 9 h (). Captured scintigraphs suggested the entry of pellets into transverse colon at 12 h after releasing major amount of the tracer in the ascending colon. Scintigraphs observed after 12 h suggests the complete disintegration of the pellets and the radioactivity liberated was distributed across all the regions of colon. Liberated radioactivity suggests the presence of enteric-coated particles in the distal colon with increase in time till 24 h that can be observed in (). Therefore, these studies can conclude the feasibility and applicability of the prepared enteric-coated nanoparticles in preventing the release if the drug in the stomach and intestine followed by releasing major amount of the drug in the colon for successful localization of 5-FU.
Figure 6. Visualization of release of enteric coated nanoparticulate pellets in various regions of GIT at different time intervals using γ-scintigraphy. (A) Intactness of the pellet was found unaffected in stomach (<2 h). (B) Minimal tracer release in small intestine (<2 h). (C) Release of tracer in ascending colon (after 4 h). (D) Distortion of pellet by releasing the tracer with time as it progresses in ascending colon (9 h). (E) After 12 h, the major amount of tracer release in ascending colon, transverse colon followed by pellet entry into whole colon with complete disintegration (radioactivity in distal part of colon) with increase in time till 24 h. Red color boxes indicate the γ-scintigraphic image of the pellets, where as black dotted boxes highlights the pellets shape with enhanced magnification.
Inhibition of tumor growth
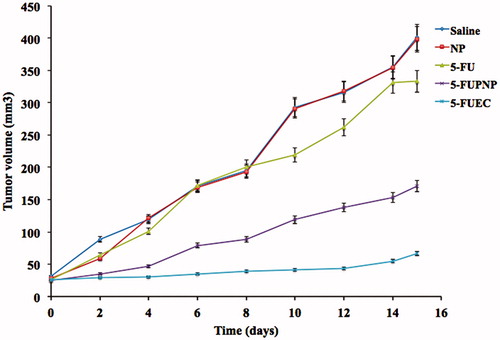
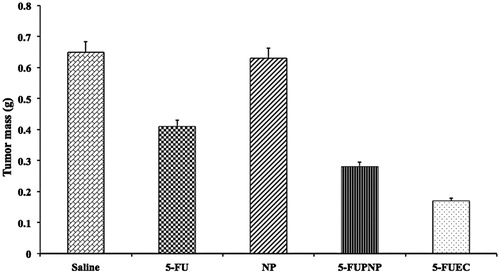
BALB/c nude mice were selected upon approval from animal ethical committee that were injected with HCT 116 xenografts subcutaneously into their right flank in order to determine their inhibition on the growth of tumor. Animals were divided randomly into five groups: saline, NP, 5-FU, 5-FUPNP and 5-FUEC. Tumor was allowed to attain a volume of 30 mm3 after treatment initiation and it was continued later on till 10 days followed by sacrificing the mice on 15th day. Untreated groups (saline and plain nanoparticles) had shown no effect on the tumor growth. 5-FUPNP that was not only enteric coated showed effect on the reduction in tumor growth, but also to a certain extent attributing to its release in the systemic circulation thereby minimizing its localization in the colon region. 5-FUEC had shown significant reduction in the tumor growth (slower growth) comparable to pure 5-FU and 5-FUPNP that justifies its enhanced anti-cancer activity along with its localization in the colon area. Eudragit nanoparticles once it enters the alkaline media, their integrity were disturbed leading to ionization thereby releasing the nanoparticles at the basic pH in the colon area. This helps in removal of the enteric coat leading to successful delivery of the nanoparticles inside the tumor where the pH is 4.5. The mean weight of the tumors was compared for all groups and it also showed lower tumor mass for 5-FUEC. The reduction in tumor mass and volume justified its increased anti-tumor activity there by enhancing its therapeutic effectiveness. Tumor mass and volumes for all the formulation samples can be observed in and . This enhanced anti-cancer activity also correlates with the cytotoxicity effect of the enteric-coated nanoparticles conform its clinical applicability.
Conclusion
In summary, biocompatible 5-FU chitosan nanoparticles that were enteric coated (5-FUEC) were formulated and evaluated for their enhanced apoptotic activity that may result in a consequently increased anticancer effect of 5-FU. 5-FUEC had shown 5.5 folds decrease in IC50 value comparable to the pure drug, whereas plain nanoparticles (NP) did not show any toxicity thereby suggesting the non-toxic nature of the nanoparticles. Morphological changes in the nucleus like nuclear condensation with irregular edges were observed for enteric-coated nanoparticles attributing to its ability in localizing the drug at the colon area thereby allowing major amount of drug deliverable to the nucleus by EPR. Furthermore, the number of gated cells in early apoptotic and late apoptotic phases with decreased viable cells can justify enhanced apoptotic activity of the enteric-coated nanoparticles. Moreover, AO/EB staining further proved the apoptotic activity by staining the apoptotic cells that can be differentiated by color fluorescence (primarily red in case of enteric coated nanoparticles). γ-Scintigraphic study was performed in order to determine the release pattern of prepared 5-FUEC conforming the intactness of the nanoparticulate pellets in stomach at 2 h and releasing major part of the drug once it arrives the ascending colon after 4 h. These enhancements endow improved anti-cancer activity of 5-FUEC making it capable to decrease tumor size and volume in HCT 116 induced tumor xenograft models in nude mice. Therefore, this study can conclude the current drug therapy to deliver the drug to the colon area in a localized manner by preventing drug degradation in stomach and by passing the systemic circulation resulting in enhanced anticancer activity.
Acknowledgements
Authors would like to dedicate their work to Dr M. N. Satish Kumar for his kind support in guiding them through out the research. Authors are very much thankful to Dr Ashish Wadhwani for supporting in vitro studies.
Declaration of interest
The authors report no declaration of interest
References
- Chuah LH, Roberts CJ, Billa N, et al. (2014). Cellular uptake and anticancer effects of mucoadhesive curcumin-containing chitosan nanoparticles. Colloids Surf B Biointerfaces 116:228–36
- Cirstoiu-Hapca A, Buchegger F, Bossy L, et al. (2009). Nanomedicines for active targeting: physico-chemical characterization of paclitaxel-loaded anti-HER2 immunonanoparticles and in vitro functional studies on target cells. Eur J Pharm Sci 38:230–7
- Di Paolo A, Danesi R, Falcone A, et al. (2001). Relationship between 5-fluorouracil disposition, toxicity and dihydropy rimidine dehydrogenase activity in cancer patients. Ann Oncol 12:1301–6
- Fata F, Ron IG, Kemeny N, et al. (1999). 5-Fluorouracil-induced small bowel toxicity in patients with colorectal carcinoma. Cancer 86:1129–34
- Ghorab DM, Amin MM, Khowessah OM, Tadros MI. (2011). Colon-targeted celecoxib-loaded Eudragit® S100-coated poly-ɛ-caprolactone microparticles: preparation, characterization and in vivo evaluation in rats. Drug Deliv 18:523–35
- Guo J, Verma UN, Tripathy D, et al. (2006). Efficacy of sequential treatment of HCT116 colon cancer monolayers and xenografts with docetaxel, flavopiridol, and 5-fluorouracil1. Acta Pharmacol Sin 27:1375–81
- Hutchins LF, Green SJ, Ravdin PM, et al. (2005). Randomized, controlled trial of cyclophosphamide, methotrexate, and fluorouracil versus cyclophosphamide, doxorubicin, and fluorouracil with and without tamoxifen for high-risk, node-negative breast cancer: treatment results of Intergroup Protocol INT-0102. J Clin Oncol 23:8313–21
- Jain A, Jain SK, Ganesh N, et al. (2010). Design and development of ligand-appended polysaccharidic nanoparticles for the delivery of oxaliplatin in colorectal cancer. Nanomedicine Nanotechnol Biol Med 6:179–90
- Krishnaiah Y, Satyanarayana S. (2001). Colon-specific drug delivery systems. Advances in controlled and novel drug delivery. New Delhi, India: CBS Publishers and Distributors, 89–119
- Levine DS, Raisys VA, Ainardi V. (1987). Coating of oral beclomethasone dipropionate capsules with cellulose acetate phthalate enhances delivery of topically active antiinflammatory drug to the terminal ileum. Gastroenterology 92:1037–44
- Li F, Zhao X, Wang H, et al. (2015). Multiple layer-by-layer lipid-polymer hybrid nanoparticles for improved FOLFIRINOX chemotherapy in pancreatic tumor models. Adv Funct Mater 25:788–98
- Longley DB, Harkin DP, Johnston PG. (2003). 5-Fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer 3:330–8
- Michor F, Iwasa Y, Lengauer C, Nowak MA. (2005). Dynamics of colorectal cancer. Semin cancer Biol 15:484–93
- Nair L, Jagadeeshan S, Nair SA, Kumar GV. (2011). Biological evaluation of 5-fluorouracil nanoparticles for cancer chemotherapy and its dependence on the carrier, PLGA. Int J Nanomedicine 6:1685–97
- Ozols RF, Herbst RS, Colson YL, et al. (2006). Clinical cancer advances 2006: major research advances in cancer treatment, prevention, and screening – a report from the American Society of Clinical Oncology. J Clin Oncol 25:146–62
- Parker WB, Cheng YC. (1990). Metabolism and mechanism of action of 5-fluorouracil. Pharmacol Ther 48:381–95
- Prabhaharan M, Mano JF. (2004). Chitosan-based particles as controlled drug delivery systems. Drug Deliv 12:41–57
- Radulovic S, Miller G, Schally AV. (1991). Inhibition of growth of HT-29 human colon cancer xenografts in nude mice by treatment with bombesin/gastrin releasing peptide antagonist (RC-3095). Cancer Res 51:6006–9
- Rai G, Yadav AK, Jain NK, Agrawal GP. (2014). Eudragit-coated dextran microspheres of 5-fluorouracil for site-specific delivery to colon. Drug Deliv. [Epub ahead of print]. DOI:10.3109/10717544.2014.913733
- Rasmussen S, Bondesen S, Hvidberg E, et al. (1982). 5-Aminosalicylic acid in a slow-release preparation: bioavailability, plasma level, and excretion in humans. Gastroenterology 83:1062–70
- Shapiro WR, Green SB, Burger PC, et al. (1992). A randomized comparison of intra-arterial versus intravenous with or without intravenous 5-fluorouracil, for newly diagnosed patients with malignant glioma. J Neurosurg 76:772–81
- Tummala S, Kumar MS, Gowthamarajan K, et al. (2014). Preparation, physicochemical characterization and in vitro evaluation of oxaliplatin solid lipid nanoparticles for the treatment of colorectal cancer. Indo Am J Pharm Res 4:3579–87
- Tummala S, Gowthamarajan K, Satish Kumar MN, Wadhwani A. (2015a). Oxaliplatin immuno hybrid nanoparticles for active targeting: an approach for enhanced apoptotic activity and drug delivery to colorectal tumors. Drug Delivery. [Epub ahead of print]. DOI:10.3109/10717544.2015.1084400
- Tummala S, Kumar MS, Prakash A. (2015b). Formulation and characterization of 5-Fluorouracil enteric coated nanoparticles for sustained and localized release in treating colorectal cancer. Saudi Pharm J 23:308–14
- van Kuilenburg AB, Haasjes J, Richel DJ, et al. (2000). Clinical implications of dihydropyrimidine dehydrogenase (DPD) deficiency in patients with severe 5-fluorouracil-associated toxicity: identification of new mutations in the DPD gene. Clin Cancer Res 6:4705–12
- Vivek R, Thangam R, NipunBabu V, et al. (2014). Multifunctional HER2-antibody conjugated polymeric nanocarrier-based drug delivery system for multi-drug-resistant breast cancer therapy. ACS Appl Mater Interfaces 6:6469–80
- Zhang J, Wang X, Liu T, et al. (2014). Antitumor activity of electrospun polylactide nanofibers loaded with 5-fluorouracil and oxaliplatin against colorectal cancer. Drug Deliv. [Epub ahead of print]. DOI:10.3109/10717544.2014.916768