Abstract
Baohuoside I, extracted from the Herba epimedii, is an effective but a poorly soluble antitumor drug. To improve its solubility, formulation of baohuoside I-loaded mixed micelles with lecithin and Solutol HS 15 (BLSM) has been performed in this study. We performed a systematic comparative evaluation of the antiproliferative effect, cellular uptake, antitumor efficacy, and in vivo tumor targeting of these micelles using non-small cell lung cancer (NSCLC) A549 cells. Results showed that the obtained micelles have a mean particle size of around 62.54 nm, and the size of micelles was narrowly distributed. With the improved cellular uptake, BLSM displayed a more potent antiproliferative action on A549 cell lines than baohuoside I; half-maximal inhibitory concentration (IC50) was 6.31 versus 18.28 µg/mL, respectively. The antitumor efficacy test in nude mice showed that BLSM exhibited significantly higher antitumor activity against NSCLC with lesser toxic effects on normal tissues. The imaging study for in vivo targeting demonstrated that the mixed micelles formulation achieved effective and targeted drug delivery. Therefore, BLSM might be a potential antitumor formulation.
Introduction
Herba epimedii has been traditionally used in China as a tonic, an aphrodisiac, and an antirheumatic drug for many years. Baohuoside I (also known as icariside II) is the main active flavonoid component of H. epimedii (Qian et al., Citation2012; Zhai et al., Citation2013; Yu et al., Citation2013; Xiao et al., Citation2014). Baohuoside I can induce apoptosis in human non-small cell lung cancer cells (NSCLC) via reactive oxygen species-mediated mitochondrial pathway and also inhibits the growths of U266 multiple myeloma and pre-osteoclastic RAW264.7 cells (Choi et al., Citation2008; Kim et al., Citation2011; Jin et al., Citation2012a; Song et al., Citation2012; Liu et al., Citation2014).
Baohuoside I has very poor solubility in water. Moreover, baohuoside I possesses a low absorptive permeability and a high rate of efflux via apical efflux transporters such as multidrug resistance-associated proteins 1 and 2 (MRP 1 and MRP 2) and P-glycoprotein (P-gp). However, the membrane permeability of baohuoside I is slightly better than that of other flavonoids in H. epimedii, and the rate of efflux (PBA/PAB) of baohuoside I reached 9.84 in a previous study using Caco-2 cells (Chen et al., Citation2008). These peculiarities restrict the application of baohuoside I in the management of cancer therapy. Thus, poor aqueous solubility, low membrane permeability, and a severe efflux phenomenon all limit the therapeutic use of baohuoside I in humans (Jeong et al., Citation2005; Jin et al., Citation2012b).
Therefore, improvement in the aqueous solubility and membrane permeability and reduction of the efflux phenomenon of baohuoside I are essential for determining the future applications of baohuoside I. In previous studies, various drug delivery systems, including lecithin complexes and nanoparticles, have been developed to overcome the aforementioned limitations of baohuoside I (Jin et al., Citation2012a,Citationb). Among the several micellar formulations evaluated as carriers of anticancer drugs, mixed micelles are the appropriate choice for the carriers of baohuoside I. Micellar systems have many advantages such as increasing the drug solubility, circumventing the uptake by the reticuloendothelial system (RES), improving circulation time, and passive tumor targeting by the enhanced permeability and retention (EPR) effect (Fang et al., Citation2011; Duan et al., Citation2015). In addition, mixed micelles have synergistic properties such as increased drug stability and drug loading efficiency compared with that of the individual components (Potluri & Betageri, Citation2006; Chen et al., Citation2013). To the best of our knowledge, no studies have focused on the influence of mixed micelles for the delivery of baohuoside I. Therefore, we prepared mixed micelles of baohuoside I consisting of lecithin and a novel drug carrier materials Solutol HS 15, in order to increase the aqueous solubility and membrane permeability and to improve the efficacy of baohuoside I.
Lecithin is a kind of amphoteric surfactant which plays an important role in the biocompatibility of biological membranes. It has strong solubility and emulsifying ability without any toxicity but excellent biocompatibility (Kumar et al., Citation2014; Mouri et al., Citation2014). The mixed micellar system composed of lecithin has become a hotspot in current research, in which the lecithin/bile salt mixed micelles has been systematically and extensively studied to enhance the solubility of fat soluble vitamins, paclitaxel, diazepam, etc. (Ballinger et al., Citation1996; Magee et al., Citation2003; Cheng et al., Citation2014), Compared with the ion surfactant, the non-ionic surfactant has high stability and will not be easily disassembled by strong electrolyte or acid–base. In addition, it has the compatibility with most drugs but less toxicity and hemolysis (Wachter et al., Citation2008; Abidin et al., Citation2015).
A non-ionic surfactant called Solutol HS 15 (polyoxyethylene esters of 12-hydroxystearic acid) is structurally made by fusing fatty acids and end-capped methoxy polyethylene glycol (mPEG), which are connected via chemically and biologically labile linkers. Solutol HS 15 is a new type of amphiphilic surfactant with high performance, low toxicity, and excellent biocompatibility (Rajebahadur et al., Citation2006; Kim et al., Citation2008). The solubility capacity against hydrophobic drug showed a linear growth as the solvent concentration increases. Moreover, no matter what the maximum dose of the drug's dissolution or drug chemical structure is, the particle of micelles, finally formed, will remain unchanged. Therefore, high-solubility capacity of Solutol HS 15 enables the injection, characteristic of low volume and high dose, become possible. In addition, there is no need to take an antihistamine and corticosteroids before using it. The low hemolytic property of Solutol HS 15 suggests that it has a low toxicity and irritation. Solutol HS 15 has been reported to modulate the cytotoxicity and the accumulation of anticancer drugs by P-gp inhibition (Coon et al., Citation1991; Singh & Lamprecht, Citation2015). It is suspected that this inhibition is mediated by changing the plasma membrane fluidity of multi-drug resistance (MDR) cells.
To increase the solubility and membrane permeability and enhance the anticancer effect of baohuoside I, we prepared baohuoside I-loaded mixed micelles consisting of lecithin and Solutol HS 15 (BLSM) by using a solvent evaporation method and optimized the preparation process. We selected the NSCLC A549 cells to investigate the in vitro and in vivo anticancer effects of baohuoside I-loaded mixed micelles when compared with baohuoside I. In addition, we examined in vivo targeting behavior of the mixed micelles in this study.
Materials and methods
Materials
Baohuoside I was obtained from the Nanjing Zelang Medical Technology Co. Ltd (Nanjing, China) and the purity was 98.22%. Injection grade high-purity egg yolk lecithin (PC-98T) was purchased from Advanced Vehicle Technology L.T.D. Co. Ltd. (Shanghai, China). Solutol HS 15 (BASF) was purchased from Beijing Fengli Jingqiu Commerce and Trade Co. Ltd. (Beijing, China). Plastic cell culture dishes and plates were purchased from Corning Incorporated (Corning, NY). Coumarin-6 and DAPI were purchased from Sigma (Shanghai, China). DiR was purchased from Ganhua Trade Co. Ltd. (Shanghai, China). All reagents were of analytical grade except methanol, which was of chromatographic grade.
HPLC analysis
The concentration of Baohuoside I in the dissolution medium was determined by high-pressure liquid chromatography (HPLC, Agilent 1260, Agilent Technologies, Santa Clara, CA) equipped with a Diamonsil™ RP-C18 column (250 mm × 4.6 mm, 5 μm). The mobile phase of methanol and water (75:25, v:v) was used at a flow rate of 1.0 mL·min−1. The UV detector was set at 270 nm to analyze the column effluent and the column temperature was 30 °C. The entire solution was filtered through a 0.45 μm membrane filter (Millipore Corp., San Diego, CA) and degassed prior to use. The injection volume was 10 μL. The recovery rates for baohuoside I were in the range of 99–101%, and the RSD were less than 2%. Intra-day and inter-day precisions for baohuoside I were below 2%.
Preparation of baohuoside I-loaded mixed micelles
Baohuoside I-loaded mixed micelles were prepared by a thin-film hydration method (Tong et al., Citation2012). In brief, 50 mg of baohuoside I and 500 mg of a mixture of lecithin and Solutol HS 15 at different ratios (3:1, 1:1, 1:3; w/w) were dissolved in absolute ethyl alcohol by the ultrasonic method in a round-bottom flask. The solvent was subsequently evaporated by rotary evaporation to obtain a thin film. The film was then kept in a vacuum overnight at room temperature to remove the residual ethanol. The dried film was hydrated with 20 mL 0.9% NaCl solution and a clear micelle solution was formed. The solution was then filtrated through 0.22 µm membrane filter to remove the non-incorporated baohuoside I, followed by lyophilization. The mixed micelles prepared by this method can be well dispersed in water.
Baohuoside I-loading efficiency and encapsulation ratio
Briefly, 20 μL of baohuoside I-loaded micelles were mixed with 80 μL of methanol for micelles disruption and dissolved in mobile phase consisting of acetonitrile and deionized water (50:50, v/v) to 1 mL. The solution was filtered through 0.45 μm syringe filter before transferred into HPLC vial. All samples were analyzed in triplicate. The encapsulation efficiency (EE) and the drug loading efficiency (DE) were calculated using the following equations:
Characterization of baohuoside I-loaded mixed micelle
The particle size of all micelles was measured by dynamic light scattering (DLS) using a Zetasizer Nano ZS (Malvern Zetasizer 3000; Malvern Instruments Ltd, Malvern, UK). The particle size was carried out in triplicate. The morphological evaluation was performed using transmission electron microscopy (TEM; H7650; HITACHI, Tokyo, Japan). The micellar solution was diluted in the concentration of 100 µg/ml and stirred for 24 h. A few drops of sample was taken to film the copper net formation of droplets and dried in the natural air. It was negative stained with phosphomolybdic acid and observed under the transmission electron microscope.
In vitro baohuoside I release
The in vitro release behavior of baohuoside I from baohuoside I-loaded mixed micelles was monitored in a phosphate-buffered saline (PBS) (PH7.4) medium containing 0.5% Tween 80 to obtain pseudo sink conditions. Briefly, aliquots of baohuoside I-loaded mixed micelles were introduced into a dialysis bag (MWCO = 3500 Da, Green Bird Inc, Shanghai, China), the sealed end of which was immersed fully into 50 mL of a release medium at 37 °C; this medium was stirred at 100 rpm for 24 h. At fixed time intervals, 0.5 mL aliquots were withdrawn and replaced with an equal volume of fresh medium. Baohuoside I releases from the stock solution were conducted under the same conditions as the controls. The concentration of baohuoside I in the samples was determined by HPLC as described above, with correction for the volume replacement.
Cell lines and cell culture
The human lung adenocarcinoma cell line A549 was obtained from Nanjing KeyGEN Biotech. Co. Ltd, Nanjing, China, which was cultured with RPMI 1640 contained 10% FBS, 100 IU/mL penicillin, and 100 µg/mL streptomycin sulfates at 37 °C in 5% CO2.
Animals and tumor implantation
Male BALB/c nude mice (22 ± 2 g) were obtained from Changzhou Cavens Lab Animal Co. Ltd, Changzhou, China. All animal experiments were performed in accordance with protocols evaluated and approved by Jiangsu Provincial Academy of Chinese Medicine’s Experimental Animal Center. Pathogen free BALB/c mice were housed in separate cages with normal access to food and water and kept on a 12-h light–dark cycle. To generate tumors, the flanks of 6-week-old male BALB/c mice were shaved and 200 μL of single cell suspension containing 1 × 106 A549 cells in serum-free DMEM was injected subcutaneously under anesthesia.
In vitro cytotoxicity assay
The cytotoxicity of baohuoside I and BLSM against A549 cells was measured using the MTT assay. The cells were seeded into a 96-well plate 100 μL of single cell suspension containing 1 × 105 A549 cells. After incubation for 24 h, the cells were treated with baohuoside I at a range of concentrations for 24 h, 48 h, and 72 h. Then 10 µL MTT (5 mg/mL) was added to each well. After incubation for 4 h, the medium was removed and replaced with 100 µL DMSO solution to dissolve. The absorbance of each well was measured by a SpectraMax 190 microplate reader (Molecular Devices, Sunnyvale, CA) at the wavelengths of 570 nm and 630 nm. Each experiment was repeated three times.
In vitro cellular uptake: quantitative study and qualitative study
Quantitative study
The A549 cells were seeded into a 6-well culture plate. After incubation for 24 h, the original culture medium was removed and the cells were treated with baohuoside I and BLSM at a concentration of 25 µM for 1, 2, and 4 h incubation to evaluate the effects of the relationship between uptake and uptake time. After the incubation, the medium was fast discarded, and the cold PBS was ingested and the cells were washed three times. One milliliter of distilled water was added and cells were scraped. The suspended fluid of cells were collected and placed in an ice bath under ultrasonic cell disruption, centrifuged, and the supernatant was in another clean centrifuge tube, dried with nitrogen, dissolved in methanol, with 0.45 μm millipore filtration, constant volume to 1 ml, and used HPLC determination to measure the content of baohuoside I.
Qualitative study
In vitro qualitative study of cellular uptake of the micellar formulations was assessed by the fluorescence microscope. After reaching confluence, cells were detached, counted, and seeded in a 24-well plate overnight. Then, the medium was replaced by coumarin-6-loaded micelles (CLSM) suspension at a concentration of 0.2 mg/ml and incubated for 2 h. The cells were washed twice with pre-warmed PBS and fixed with 95% ethanol for 20 min. After that, the cells were washed twice with PBS and then the nuclei were counterstained by DAPI for 30 min. The cells were washed again twice by PBS and immersed in PBS for microscopic imaging.
In vivo antitumor activity evaluation
For in vivo implantation, nude mice were subcutaneously injected in the right flank with 0.2 mL of cell suspension containing 1.7 × 106 A549 cells. The in vivo antitumor studies were started when the tumor volumes reached about 60 mm3 (designated as day 0). Mice were randomly divided into three groups (n = 6): group 1 for saline solution, group 2 for baohuoside I (10 mg/kg), and group 3 for BLSM (10 mg/kg). Mice were administered according to their group protocol, through the tail vein for five times every 2 d. Tumor volume and mouse weight were monitored daily. Tumor volume was calculated by the following equation: V = (a × b2)/2, where a represents the longest diameter and b represents the shortest diameter perpendicular to length. At the end of the experiment, the animals were sacrificed and the tumor, thymus, and spleen masses harvested and weighed.
In vivo imaging
DiR-M (DiR-loaded mixed micelles composed of lecithin and Solutol HS 15) was prepared by a solvent evaporation method according the prescription of baohuoside I-loaded mixed micelles. When tumors reached an acceptable size, DiR-M was injected into the tail vein of the tumor-bearing mice at a dose of 5.0 mg/kg to investigate the in vivo distribution. The mice were anesthetized and imaged at the predetermined time (1, 3, 6, 12, and 24 h) after intravenous injection using the Maestro in vivo imaging system (NightOWL II LB983, Berthold, Germany).
Results
Formulation and characterization of mixed micelles
We prepared baohuoside I-loaded mixed micelles by using a thin-film hydration method. Result showed that the EE% and the DE% of BLSM were increased by the increase of lecithin proportion while the particle size could be increased. Thus, in consideration of the particle size EE% and DE%, the ratio of lecithin:Solutol HS 15:baohuoside I was 1:5:5(w/w) (). BLSM prepared had smaller particle size around 62.54 and higher encapsulation efficiencies above 80% under such condition. The mean diameter of BLSM was determined using DLS and TEM ().
Figure 1. Characteristics of the BLSM. Size distribution of BLSM as determined by dynamic light scattering (A). Transmission electron microscope (TEM) image of BLSM in 100 nm scale (B). Baohuoside I release profiles from the micelles in vitro in a PBS (PH7.4) (C). Data are presented as the mean ± SD (n = 3). (BLSM, baohuoside I-loaded mixed micelles composed of lecithin and Solutol HS 15).
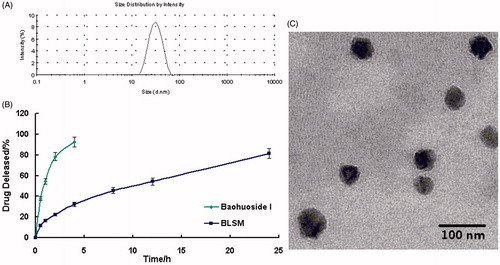
Table 1. Physicochemical characterizations of baohuoside I-loaded micelles (n = 3).
The accumulated percentage release of baohuoside I from the active pharmaceutical ingredient baohuoside I and BLSM in PBS (pH 7.4) containing 0.1% (w/v) Tween 80 is shown in . More than 90% of baohuoside I in a stock solution was released within the first 4 h, suggesting that baohuoside I could freely diffuse through the dialysis membrane. BLSM showed no burst release followed by controlled release. After 24 h of dialysis in PBS (pH 7.4), the percentage of baohuoside I released from the mixed micelles was 81.43%. Compared with baohuoside I, BLSM showed a significant (p < 0.05) increase in the in vitro drug release.
In vitro evaluation of baohuoside I micelle formulations
We examined the in vitro cytotoxicity of baohuoside I formulated in baohuoside I and BLSM and compared it with that of paclitaxel at an equivalent concentration on A549 cells after incubation at 37 °C for 24, 48, and 72 h (). The BLSM had lower IC50 values than baohuoside I, which indicated that compared with the free drug, the mixed micelle formulation could significantly increase the cytotoxicity of baohuoside I against A549 cells in vitro. In addition, mixed micelles without the drug did not show any antiproliferative effects when the concentration of micellar materials was higher than 500 μg/mL. It indicated that lecithin and Solutol HS 15 had very low toxicity and irritation.
Table 2. IC50 values (μg/mL) of free baohuoside I or BLSM against A549 cells (n = 6).
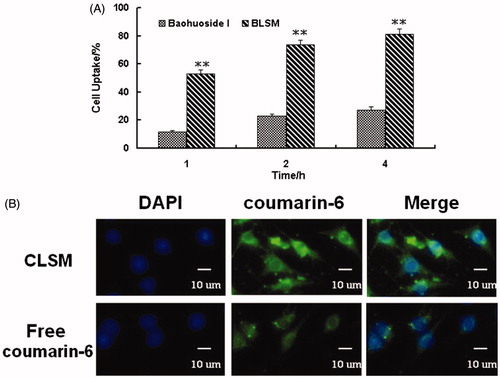
The quantitative uptake by A549 cells after incubation with baohuoside I loaded mixed micelles for 1, 2, and 4 h is shown in . We observed a trend of increased uptake of mixed micelles by the cells with an increase in the incubation time. The cellular uptake of the BLSM was significantly higher than that of the free drug. The qualitative uptake by A549 cells after incubation with the coumarin-6-loaded mixed micelles for 2 h is shown in . Our results showed that the green fluorescence in the A549 cells, which corresponds to the coumarin-6-mixed micelles loaded by lecithin and Solutol HS 15 (CLSM), was stronger than that of the free coumarin-6.
Figure 2. Cellular uptake efficiency of the baohuoside I and BLSM by A549 cells after 1, 2, and 4 h incubation (A). The results were presented as the mean ± SD (n = 6). **p < 0.01, compared with baohuoside I group. Fluorescence microscope of A549 cells after 2 h incubation with the free fluorescent coumarin-6 and the coumarin-6-loaded mixed micelles (B). (BLSM, baohuoside I-loaded mixed micelles composed of lecithin and Solutol HS 15).
In vivo antitumor efficacy
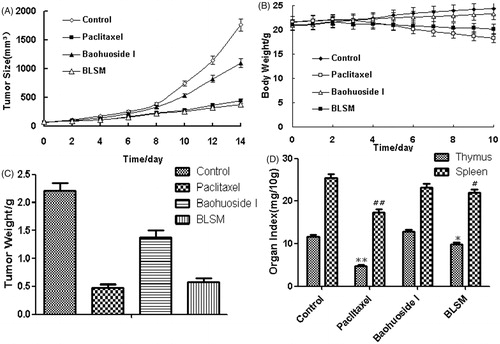
The micelles showed remarkable antitumor effects in vitro; therefore, we examined the antitumor effects of the micelles after systemic application. The tumor volume–time curve and weight analysis are shown in . Our results showed that tumor growth in the control group was greater than that in the baohuoside I groups, which indicated that baohuoside I was efficient at inhibiting tumor growth to varying degrees. Because of the effect of Solutol HS 15, BLSM produced stronger effects than baohuoside I, which could be concluded from the volume of tumors and weights. The variations in the body weights of the mice were plotted against time as shown in . The body weights of mice in the control group and baohuoside I slightly increased, which could be related to the increasing tumor weight. But the increasing degree baohuoside I-treated group was less than the control group increased, which might be related to the impact of baohuoside I. The body weights of the mice treated with BLSM decreased, which might be because of the efficient at inhibiting tumor growth of BLSM that is much better than baohuoside I. In addition, the body weights of the mice in the paclitaxel group decreased to a greater extent than those in the BLSM-treated groups which indicated that BLSM had lesser toxicity to normal organism than paclitaxel. The results of organ index (percentage of organ weight to body weight of each mouse) in different groups are shown in . Compared with the control group, the baohuoside I group showed a decrease in the organ index of the thymus and spleen to varying degrees, which may be attributed to the inherent toxicity of baohuoside I, but the values remained within the tolerance range. BLSM-treated groups showed an increase in the organ index of the thymus and spleen when compared with baohuoside I-treated group, which might be that micellar systems could circumvent the uptake by RES and have passive tumor targeting by EPR effect. However, compared with the control group, the paclitaxel group showed a significant decrease in the organ index. Thus, our results showed that compared with paclitaxel, BLSM had lesser toxic effects on normal tissues.
Figure 3. In vivo antitumor study of BLSM in Balb/c nude mice implanted with A549 cells. Tumor volumes (A) and body weight (B) were monitored daily. Tumor weight (C) and organ index (D) were monitored at the end of the experiment. The results were presented as the mean ± SD (n = 6). *p < 0.05, **p < 0.01, compared with the control group.
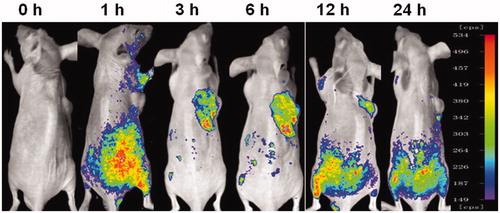
Imaging to determine in vivo targeting of baohuoside I mixed micelles
To investigate whether mixed micelles composed with lecithin and Solutol HS 15 can specifically target A549 cells in vivo, we established the tumor model by intravenously injecting mice with A549 cells in the right sides. Mice treated with DiR-M showed a time-dependent increase in the fluorescence signals, and they were mainly distributed on the tumor (). Fluorescence signals were clearly observed at the tumor site and maintained for 24 h. Therefore, with the modification of Solutol HS 15, the lecithin/Solutol HS 15 mixed micelles were efficiently delivered and retained by the lung tumors under in vivo conditions.
Discussion
The anti-tumor effect of flavonoids has been extensively examined. Owing to their strong anticancer effects and low toxicity, flavonoids have a great potential of being developed as anticancer drugs (Ravishankar et al., Citation2013; Martinez-Perez et al., Citation2014; Orlikova et al., Citation2014). Baohuoside I is the main active flavonoid component of Herba epimedii and has impressive pharmacological effects. Although baohuoside I has anti-tumor characteristics, it has poor solubility and permeability and it may be pumped out by the intestinal efflux transporters, and thus, its use for the treatment of human diseases is limited (Chen et al., Citation2009). To improve the solubility and the cellular uptake of baohuoside I, mixed micelles were prepared. In this study, the purity of baohuoside I was 98.22%, and the solubility that we determined was 0.0124 g/L. The solubility of baohuoside I in the micelles was 1.894 g/L, which was 152.7 times that of the baohuoside I.
The IC50 value reflected the MDR reversing ability of certain formulations. Free baohuoside I was easily effluxed from cells via the P-gp. The IC50 value of BLSM was lower than that of free baohuoside I, indicating that the mixed micelles had MDR reversing ability. Solutol HS 15 has been used as an excipient for overcoming MDR and as an inhibitor of P-glycoprotein (P-gp). Based on the chemical structure of the Solutol HS 15, Buckingham et al. showed that the ethoxylated stearic and oleic acid ester in the Solutol HS 15 induced the P-pg inhibition (Buckingham et al., Citation1995, Citation1996). Our results indicate that the superior cytotoxicity of BLSM might be attributed to the drastic increase in drug accumulation in A549 cells by reduced efflux and increased uptake. Lecithin is an important component of biological cell membrane and it has good biocompatibility, increasing the permeability of baohuoside I inside, and thus enhancing the cell uptake. The cellular uptake of baohuoside I in BLSM was 3.2 times that of baohuoside I.
Lecithin is a kind of ampho-surfactants with hydrophilic and hydrophobic aliphatic hydrocarbon chains. It has a strong emulsifying capacity, and it is an important component of biological cell membrane. It has good biocompatibility and exhibited none toxicity, which make it suitable for injection formulations. The use of lecithin and surfactant in the formation of micelles can greatly improve the solubilization and stabilization of insoluble drugs, thus improving its clinical use. The study of lecithin/ionic surfactant micelle has been reported on solubilization of drugs and improving drug bioavailability. However, in the current study of lecithin mixed micelles, the non-ionic surfactant is still less and the non-ionic surfactant species are previously mainly around the polyoxyethylene surfactant (Özcan et al., Citation2013). Solutol HS 15, a new type of PEG series, is used as a non-ionic surfactant with low toxicity and high safety. It can meet the requirements of strict technical requirements for injection drug delivery (Ji et al., Citation2012).
Coumarin-6 is a kind of lipid soluble laser dyes with high conversion efficiency and stable performance. It is reported that the nanoparticles which contain 0.05% of coumarin-6 can be observed under the fluorescence microscope with high sensitivity (Panyama et al., Citation2003). Therefore, in recent years, coumarin-6 is often used as a fluorescent substance, which is contained in the drug delivery system to study the cellular uptake and transport mechanism of the drug delivery system (Makhlof et al., Citation2009; Hu et al., Citation2009). However, the emission wavelength of coumarin-6 is too short to penetrate tissue. Because of this, it is not suitable for in vivo imaging to study the tissue distribution and target. It requires a fluorescent substance with long wavelength (general >700 nm). 1,1-Dioctadecyl-3,3,3,3 tetramethylindotricarbocyanine iodide (DiR iodide) is a fluorescent substance whose emission wavelength is 780 nm, and the combination between DiR and the cell membrane is stable (Wen et al., Citation2011).
Conclusion
In this study, we designed mixed micelles consisting of lecithin and Solutol HS 15 to entrap the poorly soluble anticancer drug baohuoside I. The baohuoside I-loaded mixed micelles (BLSM) had 85% higher encapsulation efficiency and particle size around 62.54 nm. Compared with free baohuoside I, baohuoside I from BLSM showed a controlled release without any initial burst release followed and increased the cytotoxicity on NSCLC in vitro. BLSM could also enhance the cellular uptake significantly by the improvement of membrane permeability and reduction of efflux phenomenon. BLSM showed markedly higher antitumor efficacy than baohuoside I in in vivo studies. In this study, we showed that lecithin/Solutol HS 15 mixed micelles significantly increased the antitumor and targeting efficacy, and they may be a potential delivery system to maximize the use of baohuoside I as a therapeutic agent for humans.
Declaration of interest
The authors report that they have no conflicts of interest. This work was supported by the National Natural Science Foundation of China (81303275 and 81274088).
References
- Abidin L, Mujeeb M, Imam SS, et al. (2015). Enhanced transdermal delivery of luteolin via non-ionic surfactant-based vesicle: quality evaluation and anti-arthritic assessment. Drug Deliv. [Epub ahead of print]. DOI: 10.3109/10717544.2014.945130
- Ballinger AB1, Forget D, Le Brun A, et al. (1996). An evaluation of the safety of mixed micelles in healthy subjects. J Parenter Enteral Nutr 20:110–2
- Buckingham LE, Balasubramanian M, Emanuele RM, et al. (1995). Comparison of solutol HS 15, cremophor EL and novel ethoxylated fatty acid surfactants as multidrug resistance modification agents. Int J Cancer 62:436–42
- Buckingham LE, Balasubramanian M, Safa AR, et al. (1996). Reversal of multi-drug resistance in vitro by fatty acid PEG fatty acid diesters. Int J Cancer 65:74–9
- Chen LC, Sha X, Jiang X, et al. (2013). Pluronic P105/F127 mixed micelles for the delivery of docetaxel against Taxol-resistant non-small cell lung cancer: optimization and in vitro, in vivo evaluation. Int J Nanomed 8:73–84
- Chen Y, Jia X, Tan X, et al. (2009). Absorption and metabolism of flavonoids in Herba epimedii via rat intestinal perfusion model. Zhongguo Zhong Yao Za Zhi 34:2928–31
- Chen Y, Zhao YH, Jia XB, et al. (2008). Intestinal absorption mechanisms of prenylated flavonoids present in the heat-processed Epimedium koreanum Nakai (Yin Yanghuo). Pharm Res 25:2190–9
- Cheng CY, Oh H, Wang TY, et al. (2014). Mixtures of lecithin and bile salt can form highly viscous wormlike micellar solutions in water. Langmuir 30:10221–30
- Choi HJ, Eun JS, Kim DK, et al. (2008). Icariside II from Epimedium koreanum inhibits hypoxia-inducible factor-1alpha in human osteosarcoma cells. Eur J Pharmacol 579:58–65
- Coon JS, Knudson W, Clodfelter K, et al. (1991). Solutol HS 15, nontoxic polyoxyethylene esters of 12-hydro-xystearic acid, reverses multidrug resistance. Cancer Res 51:897–902
- Duan Y, Wang J, Yang X, et al. (2015). Curcumin-loaded mixed micelles: preparation, optimization, physicochemical properties and cytotoxicity in vitro. Drug Deliv 22:50–7
- Fang J, Nakamura H, Maeda H. (2011). The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev 63:136–51
- Hu KL, Li JW, Shen YH, et al. (2009). Lactoferrin-conjugated PEG-PLA nanoparticles with improved brain delivery: in vitro and in vivo evaluations. J Control Release 134:55–61
- Jeong EJ, Liu X, Jia X, et al. (2005). Coupling of conjugating enzymes and efflux transporters: impact on bioavailability and drug interactions. Curr Drug Metab 6:455–68
- Ji X, Gao Y, Chen L, et al. (2012). Nanohybrid systems of non-ionic surfactant inserting liposomes loading paclitaxel for reversal of multidrug resistance. Int J Pharm 422:390–7
- Jin X, Zhang ZH, Sun E, et al. (2012a). Preparation of a nanoscale baohuoside I-phospholipid complex and determination of its absorption: in vivo and in vitro evaluations. Int J Nanomed 7:4907–16
- Jin X, Zhang ZH, Sun E, et al. (2012b). A novel drug–phospholipid complex loaded micelle for baohuoside I enhanced oral absorption: in vivo and in vitro evaluations. Drug Dev Ind Pharm 39:1421–30
- Kim BS, Won M, Lee KM, et al. (2008). In vitro permeation studies of nanoemulsions containing ketoprofen as a model drug. Drug Deliv 15:465–9
- Kim SH, Ahn KS, Jeong SJ, et al. (2011). Janus activated kinase 2/signal transducer and activator of transcription 3 pathway mediates icariside II-induced apoptosis in U266 multiple myeloma cells. Eur J Pharmacol 1:10–16
- Kumar BS, Saraswathi R, Kumar KV, et al. (2014). Development and characterization of lecithin stabilized glibenclamide nanocrystals for enhanced solubility and drug delivery. Drug Deliv 21:173–84
- Liu YQ, Yang QX, Cheng MC, et al. (2014). Synergistic inhibitory effect of Icariside II with Icaritin from Herba epimedii on pre-osteoclastic RAW264.7 cell growth. Phytomedicine 21:1633–7
- Magee GA, French J, Gibbon B. (2003). Bile salt/lecithin mixed micelles optimized for the solubilization of a poorly soluble steroid molecule using statistical experimental design. Drug Dev Ind Pharm 29:441–50
- Makhlof A, Tozuka Y, Takeuchi H. (2009). PH-sensitive nanospheres for colon-specific drug delivery in experimentally induced colitis rat model. Eur J Pharm Biopharm 72:1–8
- Martinez-Perez C, Ward C, Cook G, et al. (2014). Novel flavonoids as anti-cancer agents: mechanisms of action and promise for their potential application in breast cancer. Biochem Soc Trans 42:1017–23
- Mouri A, Diat O, Lerner DA, et al. (2014). Water solubilization capacity of pharmaceutical microemulsions based on Peceol®, lecithin and ethanol. Int J Pharm 475:324–34
- Orlikova B, Menezes JC, Ji S, et al. (2014). Methylenedioxy flavonoids: assessment of cytotoxic and anti-cancer potential in human leukemia cells. Eur J Med Chem 84:173–80
- Özcan I, Azizoğlu E, Senyiğit T, et al. (2013). Comparison of PLGA and lecithin/chitosan nanoparticles for dermal targeting of betamethasone valerate. J Drug Target 21:542–50
- Panyama J, Sahoo SK, Prabha S, et al. (2003). Fluorescence and electron microscopy probes for cellular and tissue uptake of poly(d,l-lactide-co-glycolide) nanoparticles. Int J Pharm 262:1–11
- Potluri P, Betageri GV. (2006). Mixed-micellar proliposomal systems for enhanced oral delivery of progesterone. Drug Deliv 13:227–32
- Qian Q, Li SL, Sun E, et al. (2012). Metabolite profiles of icariin in rat plasma by ultra-fast liquid chromatography coupled to triple-quadrupole/time-of-flight mass spectrometry. J Pharm Biomed Anal 66:392–8
- Rajebahadur M, Zia H, Nues A, et al. (2006). Mechanistic study of solubility enhancement of nifedipine using vitamin E TPGS or solutol HS-15. Drug Deliv 13:201–6
- Ravishankar D, Rajora AK, Greco F, et al. (2013). Flavonoids as prospective compounds for anti-cancer therapy. Int J Biochem Cell Biol 45:2821–31
- Singh MS, Lamprecht A. (2015). P-glycoprotein inhibition of drug resistant cell lines by nanoparticles. Drug Dev Ind Pharm. [Epub ahead of print]. DOI: 10.3109/03639045.2015.1054396
- Song J, Shu L, Zhang Z, et al. (2012). Reactive oxygen species-mediated mitochondrial pathway is involved in Baohuoside I-induced apoptosis in human non-small cell lung cancer. Chem Biol Interact 199:9–17
- Tong SW, Xiang B, Dong DW, et al. (2012). Enhanced antitumor efficacy and decreased toxicity by self-associated docetaxel in phospholipid-based micelles. Int J Pharm 434:413–9
- Wachter C, Vierl U, Cevc G. (2008). Adaptability and elasticity of the mixed lipid bilayer vesicles containing non-ionic surfactant designed for targeted drug delivery across the skin. J Drug Target 16:611–25
- Wen Z, Yan Z, He R, et al. (2011). Brain targeting and toxicity study of odorranalectin-conjugated nanoparticles following intranasal administration. Drug Deliv 18:555–61
- Xiao HH, Fung CY, Mok SK, et al. (2014). Flavonoids from Herba epimedii selectively activate estrogen receptor alpha (ERα) and stimulate ER-dependent osteoblastic functions in UMR-106 cells. J Steroid Biochem Mol Biol 143:141–51
- Yu X, Tong Y, Han XQ, et al. (2013). Anti-angiogenic activity of Herba epimedii on zebrafish embryos in vivo and HUVECs in vitro. Phytother Res 27:1368–75
- Zhai YK, Guo X, Pan YL, et al. (2013). A systematic review of the efficacy and pharmacological profile of Herba epimedii in osteoporosis therapy. Pharmazie 68:713–22