Abstract
Context: Nanoparticles (NPs) have been widely used as carriers to deliver siRNA and chemotherapeutic agents. Bcl-2 siRNA has been widely used to induce cancer cell apoptosis, and doxorubicin (Dox) can destroy cancer cells by binding with cancer cell DNA.
Objective: To investigate the therapeutic effect on lung cancer of simultaneously delivering Dox and Bcl-2-siRNA using epidermal growth factor (EGF) modified monomethoxy (polyethylene glycol)-poly (D, L-lactide-co-glycolide)-poly(L-lysine) (mPEG-PLGA-PLL, PEAL) NPs (EGF-PEAL).
Methods: EGF-PEAL NPs were characterized with respect to size, zeta potential and morphology. Cytotoxicity and drug (or siRNA) loading capacity of EGF-PEAL NPs were analyzed. Cellular uptake, drug release profile, cell killing effects of Dox and Bcl-2-siRNA-loaded EGF-PEAL NPs were assessed. Biodistribution and therapeutic effects of Dox and Bcl-2-siRNA EGF-PEAL NPs were evaluated in H1299 tumor-bearing mice.
Results and discussion: EGF-PEAL NPs or PEAL NPs had nearly negligible cytotoxicity toward H1299 cells. Dox and Bcl-2-siRNA gradually released from EGF-PEAL NPs and exhibited sustained release patterns. Dox and Bcl-2-siRNA-loaded NPs were taken up by cells and induced the apoptosis of H1299 cells more effectively than using Dox or Bcl-2 siRNA alone. With the intravenous injection of PEAL NPs into H1299 xenografted mice, we found that combination treatment suppressed lung cancer growth and reduced Bcl-2 expression in tumor tissue, and EGF-PEAL NPs concentrated in lung tumor much more than non-targeted PEAL NPs.
Conclusion: We conclude that co-delivery of Dox and Bcl-2-siRNA by tumor-targeted EGF-PEAL NPs could significantly inhibit lung cancer growth.
Introduction
Lung cancer, which has a high rate of incidence, has been one of the most lethal tumors in recent years (Siegel et al., Citation2012,Citation2013; Lwin et al., Citation2013; Zarogoulidis et al., Citation2013). Non-small cell lung cancer (NSCLC) accounts for approximately 75% of all lung cancers, and it also has a high expression of EGFR (Sridhar et al., Citation2003). The EGFR family has four members, including EGFR, Her2, ErbB3 and ErbB4. Previous studies have proven that only EFGR was expressed in NSCLC. Therefore, a monoclonal antibody, such as cetuximab, can greatly inhibit the proliferation of lung cancer cells while sensitizing them to apoptosis. The drug resistance due to the EGFR gene mutation establishes a barrier for the treatment of NSCLC (Brand et al., Citation2013; Chougule et al., Citation2013).
Bcl-2, which is an anti-apoptosis protein, is mainly distributed in the outer membrane of the mitochondrion and prevents cytochrome-c from releasing from the mitochondrion (Yang et al., Citation1997). The antisense oligonucleotide sequence, which targets the Bcl-2 gene in lung cancer cells, can knockdown Bcl-2 protein expression and effectively induce lung cancer cell apoptosis (Ziegler et al., Citation1997; Koty et al., Citation1999). Increased expression of Bcl-2 protein is associated with drug-resistant NSCLC (Wang et al., Citation2013a; Wu et al., Citation2013). In contrast, knockdown of Bcl-2 gene expression can sensitize A549 cancer cells to chemotherapeutic drugs. Doxorubicin (Dox) is an anthracycline, which kills some cancer cells (including lung cancer cells) by damaging the DNA of the cancer cells. However, the use of Dox is limited because of its extensive cytotoxicity, especially irreversible cardiotoxicity, and the development of drug resistance during treatment in lung cancer, especially NSCLC (Singhal et al., Citation2006; Rathos et al., Citation2013). These problems require an effective drug carrier to deliver Dox into cancer cells in order to decrease its side effects and even reverse drug resistance.
Nanoparticles (NPs) have been widely used as carriers for the delivery of genes and drugs. NPs can decrease severe side effects of chemotherapy drugs when compared to conventional drug delivery and protect siRNA from degradation (Farokhzad et al., Citation2006; Davis et al., Citation2010; Jain & Stylianopoulos, Citation2010). Meanwhile, NPs can effectively target lung cancer via enhanced permeability and retention effects and the use of targeted moieties such as the epidermal growth factor (EGF) group on NPs (Liu et al., Citation2012; Wang et al., Citation2013b).
Therefore, we propose to develop a nano drug delivery system with high specificity to carry chemotherapeutic drugs and siRNA to enhance treatment effects on tumors. Using polyethylene glycol-polylactic glycolic acid (PEG-PLGA) as a carrier to deliver a drug or siRNA has a significant influence on cancer therapy. However, the NPs cannot be enriched for a long time in the tumor microenvironment. The mPEG-PLGA-PLL (PEAL) synthesized in this assay is an amphiphilic copolymer, which is biodegradable and has great biocompatibility. Moreover, the poly(L-lysine) (PLL) cationic polypeptide can bind to the negatively charged cell membrane or siRNA using electrostatic absorption (Liu et al., Citation2012).
Using PEAL as a carrier to load drugs and genes has increased the therapeutic effect of some tumor treatments, and the use of modified NPs with EGF increased NP accumulation in tumors (Wang et al., Citation2013b). However, there are no reports on using EGF-PEAL NPs to co-deliver drugs and siRNA to cure lung tumors. We designed and prepared EGF-PEAL NPs, which not only have good solubility and enhanced drug-loading capacity but also avoid being endocytosed by mononuclear phagocytes. These NPs also have active targeting, which enables them to concentrate in lung cancer.
In our experiment, EGF-PEAL NPs were used to entrap Dox and Bcl-2 siRNA separately. The therapeutic effects of the co-delivery of Dox-EGF-PEAL NPs and Bcl-2 siRNA-EGF-PEAL NPs on H1299 cell line and lung cancer xenografts were observed. The synergistic antitumor mechanism of Dox-EGF-PEAL NPs and Bcl-2-EGF-PEAL NPs was explored. We expect this treatment will be an effective and specific lung cancer therapy method.
Materials and methods
Materials
BALB/c nu/nu female mouse, weighing 16–18 g, were provided by the Animal Experiment Center of Shanghai Cancer Institute. H1299 lung cancer cells were provided by the State Key Laboratory of Oncogenes and Related Genes, Shanghai Cancer Institute (Shanghai, China). Bcl-2 siRNA and FAM siRNA (FAM, Ex: 499 nm; Em: 522 nm) were purchased from Guangzhou RiboBio Co. Ltd (Guangzhou, China). Dox was purchased from Dalian Meilun Biology Technology Co. Ltd (Dalian, China). 1-(3-Dimethylamino-propyl)-3-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS) were purchased from Sigma-Aldrich Co. Ltd (Shanghai, China). EGF was purchased from Sino Biological Inc. (Beijing, China). All animal procedures were performed according to the research protocol approved by the Animal Care and Use Committee of Shanghai Cancer Institute.
Preparation of Dox-loaded EGF-PEAL NPs (Dox-EGF-PEAL) and Bcl-2 siRNA-loaded EGF-PEAL NPs (Bcl-2-EGF-PEAL)
PEAL polymer (MW 12 000 Da) was synthesized according to the method described previously (Liu et al., Citation2012). Dox-NPs or siRNA-NPs were prepared using the W/O/W double emulsion-solvent evaporation method. Briefly, the Dox solution (2 mg/ml, 40 μg dissolved in 20 μl of water) or siRNA solution (20 μm, 20 μl of siRNA dissolved in TE buffer) was emulsified in PEAL solution (25 mg/ml, 5 mg in 200 μl of dichloromethane) by sonication (350 W, 1 min) using the JY92-II ultrasonic cell disruptor from NingBo Scientz Biotechnology Co. Ltd. (Ningbo, China). Then, 2 ml of PluronicTM 188 solution (1 mg/ml) was added to the W/O emulsion and sonicated (350 W, 1 min), then evaporated to obtain Dox-NPs or siRNA-NPs. EGF-Dox NPs or EGF-siRNA-NPs were prepared by incubating Dox-NPs or siRNA-NPs with EDC (4 mg/ml, 40 μg dissolved in 10 μl of DMSO) and NHS (4 mg/ml, 40 μg dissolved in 10 μl of DMSO) at room temperature with gentle stirring under nitrogen protection. Then, 50 μl of EGF protein solution (1 mg/ml) was added into the solution (the molar ratio of EGF:NPs is 2:1), followed by stirring at room temperature for 6 h. Finally, EGF-Dox NPs or EGF- siRNA-NPs were purified by centrifugation to remove unreacted protein and a crosslinking agent. We used orthogonal design to optimize the NP preparation parameters (supporting materials Tables 1–4). The siRNA integration in NPs was also studied using a gel retardation assay (supporting materials Figure S1).
Study on physical and chemical properties of nanoparticles
The size and surface potential of NPs were evaluated using the Zetasizer IV analyzer (Malvern Zetasizer Nano ZS90, Malvern, UK). The morphology and size of NPs were evaluated using a transmission electron microscope (H-800; Hitachi, Japan).
The Dox or siRNA encapsulated in NPs was assessed using UV spectrophotometry. Briefly, 4 ml of EGF-Dox NPs or EGF- siRNA-NPs was centrifuged (15 000 rpm × 30 min) at 4 °C, and Dox or siRNA concentration in the supernatant was determined by UV spectrophotometry. The drug encapsulation efficiency (EE%) was expressed as the percentage of the amount of Dox or siRNA encapsulated in the NPs in relation to the total amount of Dox or siRNA initially added. The drug loading (DL%) was expressed as the percentage of the amount of Dox or siRNA encapsulated in the NPs with regards to the total amount of NPs.
W1 is the amount of total drug, W2 is the amount of drug in supernatant and W0 is the total amount of material used:
W1 is the total amount of Bcl-2 siRNA and W2 is the amount of Bcl-2 siRNA in the supernatant.
In vitro release of Dox and Bcl-2 siRNA
The release profile of NPs in phosphate-buffered saline (PBS) or TE buffer solution was evaluated using a dialysis method. Briefly, 2 ml of NPs loaded with Dox or siRNA was kept in a dialysis bag (GreenBird Inc., Shanghai, China) and gently shaken at 37 °C. Aliquots 2 ml in volume were drawn and replaced with an equal volume of fresh media. The concentration of Dox or siRNA was measured using UV spectrophotometry at a wavelength of 480 nm or 260 nm, respectively.
Cell viability analysis by MTT assay
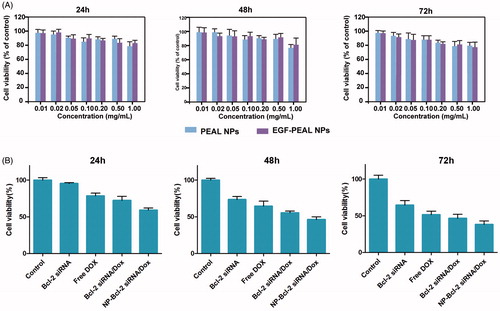
The cytotoxicity of PEAL NPs and EGF-PEAL NPs on H1299 cells was evaluated with the MTT assay. The H1299 cells were seeded into 96-well plates at a concentration of 5 × 103 cells/well and incubated for 24 h, and then, the cells were incubated for 24, 48 and 72 h with different concentrations of PEAL NPs and EGF-PEAL NPs. Then, an MTT-water solution (100 μl, 5 mg/ml) (Li et al., Citation2012) was added into each well and incubated for 4 h. After incubation, the culture medium was completely replaced with 100 μl of DMSO and incubated for 10 min. The absorbance was measured at 490 nm with a microplate reader from Bio-Rad Laboratories (Hercules, CA). We also tested the cytotoxicity of Dox-EGF-PEAL NPs and Bcl-2 siRNA-EGF-PEAL NPs against H1299 cells (Dox 1 μg/ml, siRNA 50 nm).
Cellular uptake of NPs
The H1299 cells were seeded into 6-well plates (4 × 105 cells/well) and then incubated for 24 h. DOX-EGF-PEAL and FAM-siRNA-EGF-PEAL were simultaneously added to the wells and incubated for 1 or 6 h at 37 °C, FAM is a fluorescent dye, carboxyfluorescein, Ex and Em is 470–505 nm and 523–543 nm, respectively. The cells were incubated for 1 h with free Dox and free FAM-siRNA as negative controls. Then, the H1299 cells were washed, trypsinized and harvested. NP uptake into cells was measured by FACScan flow cytometry from Becton Dickinson (Franklin Lakes, NJ). We also observed non-EGF modified NPs loaded DOX and FAM-siRNA cellular uptake using a confocal laser scanning microscope from Olympus Optical Company, Ltd (Tokyo, Japan), the results are in supporting materials Figure S3. We also used an EGF competition assay to evaluate the targeted moiety EGF effects on NP cellular uptake processes (supporting materials Figure S2).
Assay on the efficiency of gene silencing
Lung cancer H1299 cells were seeded in 12-well plates at a density of 2 × 105 cells/well and cultured for 24 h (37 °C, 5% CO2). After 24 h, the culture medium was changed to RPMI-1640 (free of fetal serum). Free Bcl-2 siRNA, Bcl-2-PEAL and Bcl-2-EGF-PEAL (0.4 μg siRNA per well) were added to the wells. After incubation for 48 h, the gene silencing effect of siRNA was quantitated using real-time PCR. The primers used in this study are shown in . Data analysis was performed using the ΔΔCt method, with the Ct value of GAPDH as the standard, ΔCt=the Ct value of Bcl-2 gene – Ct value of GAPDH. The expression of Bcl-2 gene was reported as 2-ΔΔCt.
Table 1. Primers used in this study.
Evaluation of apoptosis
Cell apoptosis was assessed using Annexin V-PI (Biotime Co. Ltd., Haimen, China). Briefly, the H1299 cells were seeded into a 6-well plate at a concentration of 4 × 105 cells/well and incubated for 24 h. Then, H1299 cells were incubated with Free Bcl-2 siRNA, Free Dox, Dox-EGF-PEAL, Bcl-2-EGF-PEAL, Dox-EGF-PEAL & Bcl-2-EGF-PEAL (v:v = 1:1, 0.5 μg/ml + 50 nm) at an equivalent concentration of Dox (1 μg/ml) or siRNA (100 nm) for 24 h. Then, cells were collected and centrifuged (1500 rpm ×5 min), resuspended in PBS and incubated with 195 μl of binding solution. The cells were incubated with 5 μl of Annexin V-FITC < and 10 μl of PI solution. After incubation for 15 min at room temperature, the cells were analyzed with FACScan flow cytometry from Becton Dickinson (New York). Cells that were not treated with Annexin V-PI were used as a control.
Tumor targeting effects
The H1299 lung cancer-bearing cells were established in nude female BALB/c mice by subcutaneous inoculation of a suspension of H1299 cells (2 × 106 cells/100 μl) into the armpit. Animal experiments were not carried out until the volume of the tumor grew to approximately 100 mm3. The cancer-bearing mice were divided into two groups randomly (n = 4). Cy5-siRNA-PEAL and Cy5-siRNA-EGF-PEAL were administered by intravenous injection when the volume of the cancer reached 100 mm3. Then, at 4, 24 and 48 h post-injection, the nude mice were anesthetized with 7% hydrate chloramine, and fluorescence was observed using In Vivo Imaging Apparatus (LB983, Berthold Technologies Gmbh & Co. KG, Bad Wildbad, Germany). After the experiment, the nude mice were separately sacrificed. Their heart, liver, spleen, lung, kidney, brain and tumor were harvested and also observed with the In Vivo Imaging Apparatus (Ex was 649 nm, Em was 666 nm).
Therapeutic effects
The H1299 lung cancer-bearing cells were established in nude female BALB/c mice by subcutaneous inoculation of a suspension of H1299 cells (2 × 106 cells/100 μl) into the armpit. Animal experiments were not carried out until the volume of the tumor grew to approximately 100 mm3. H1299 cancer-bearing mice described above were randomly divided into six groups (five mice per group). Saline, free Dox, free siRNA, Dox-EGF-PEAL, siRNA-EGF-PEAL, Dox-EGF-PEAL & siRNA-EGF-PEAL were injected into the mice via the tail vein. The nude mice were treated every 3 d for 3 weeks. During tumor therapy, the animals were weighed every 3 d, and the surviving mice were monitored throughout the experiment. The tumor size was measured by a Vernier caliper, and its volume (V) was calculated as V = d2*D/2, where d and D correspond to the shortest and the longest diameter of the tumor in mm, respectively. After the experiment, the tumors were harvested to detect the expression of Bcl-2 proteins in the tumors via western blotting.
Statistical analysis
SPSS version 13.0 statistical software (SPSS Inc., Chicago, IL) was utilized to analyze the relevant data. (x ± s) was used to represent the experimental data. The t-test was used to compare the means between two independent samples; one-way ANOVA was used to measure the differences between the means of multiple samples. The comparison among groups was carried out by the SNK method, and p < 0.05 indicated that the differences had statistical significance.
Results
Characterization of NPs
The size and zeta potential of PEAL NPs were 141.4 nm and 10.9 mV, respectively. The polydispersity index (PDI) was 0.198. The PEAL NPs were spherical with a smooth surface without conjugation (). The size and zeta potential of EGF-PEAL NPs were 189.7 nm and 2.9 mV, respectively. They also had a similar polydispersity (PDI = 0.198) (). EGF protein had little effect on DL and EE of NPs because no obvious difference was found between PEAL NPs and EGF-PEAL NPs ().
Figure 1. PEAL NPs were spherical and well distributed, with no aggregation (A, left) and positively charged (A, right). EGF-PEAL NPs were also spherical (B, left) and positively charged (B, right).
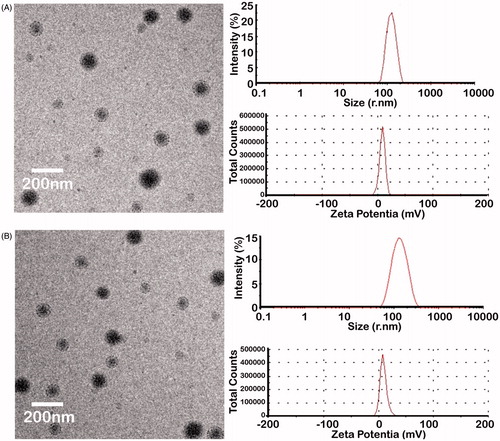
Table 2. The characterization of PEAL and EGF-PEAL.
In vitro release profile of Dox and siRNA
PEAL NPs and EGF-PEAL NPs loaded with Dox or siRNA showed a biphasic release pattern, with an initial burst release followed by a sustained release. The cumulative release amounts of Dox from free Dox, Dox-PEAL and Dox-EGF-PEAL groups were 78.6%, 40.1% and 38.5%, respectively, in the first 24 h, while the cumulative release amounts of Dox from both Dox-PEAL and Dox-EGF-PEAL groups were no more than 60% within 48 h ().
Figure 2. Dox and siRNA release profile in vitro. (A) Dox release from free Dox, Dox-PEAL NPs and Dox-EGF-PEAL NPs. (B) Bcl-2-siRNA release from free Bcl-2-siRNA, Bcl-2-PEAL NPs and Bcl-2-EGF-PEAL NPs. Data were expressed as mean ± SD (n = 3).
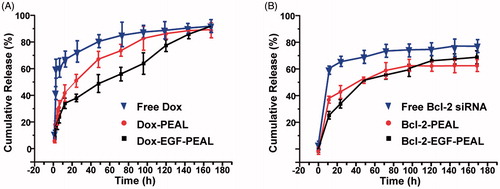
Similar to the drug-loaded NPs, Bcl-2-PEAL NPs and Bcl-2-EGF-PEAL NPs also could gradually release siRNA. The cumulative release amount of siRNA from free Bcl-2 siRNA, Bcl-2-PEAL NPs and Bcl-2-EGF-PEAL NPs was 67.8%, 43.1% and 26.8%, respectively, in the first 24 h, while the cumulative release amount of siRNA from both Bcl-2-PEAL NPs and Bcl-2-EGF-PEAL NPs was no more than 65% within 48 h (). As time passed, the Bcl-2-siRNA, which was entrapped in NPs, was gradually released via the erosion of the nanomaterial matrix, and the sustained release continued for 4–7 d. This indicated that PEAL NPs had, to some extent, the ability of controlled release, and EGF modification did not change their properties ().
Cytotoxicity
The MTT method was used to test the relative proliferation of H1299 cells incubated with PEAL NPs and EGF-PEAL NPs of different concentrations (0.01, 0.02, 0.05, 0.1, 0.2, 0.5 and 1.0 mg/ml) for 24, 48 and 72 h. The results indicated that all the cells, which were incubated with NPs of different concentrations, presented a survival rate above 80%. Good living statues were shown by all those cells. Non-toxicity is required for drug delivery materials. This result established a basis for further experiments because it was determined that PEAL NPs and EGF-PEAL NPs had a negligible toxicity toward H1299 cells ().
Uptake of Bcl-2 siRNA and Dox by H1299 cells
H1299 cells were incubated with Dox-EGF-PEAL and FAM-siRNA-EGF-PEAL (v:v = 1:1) for 1 and 6 h, and cell uptake was detected by flow cytometry. According to , the percentage of the double-positive area of siRNA and Dox, co-delivered by EGF-PEAL, was 5.87% after incubation for 1 h. There were slight improvements when compared to controls. The cell uptake of Dox and siRNA by H1299 cells greatly improved after incubation for 6 h. The percentage of double positive area of siRNA and Dox co-delivered by EGF-PEAL increased to 12.2%, which increased significantly when compared to controls ().
Figure 4. The uptake of Dox and FAM-Bcl-2 siRNA which were co-delivered by EGF-PEAL NPs, by H1299 at different points (1 and 6 h). At 1 h, compared to control group (free Dox and free FAM-Bcl-2 siRNA, 1 h), the drug and gene co-delivery group increased the uptake rate of Dox and siRNA. As the time of incubation increased (6 h), the enhancing effect became more obvious.
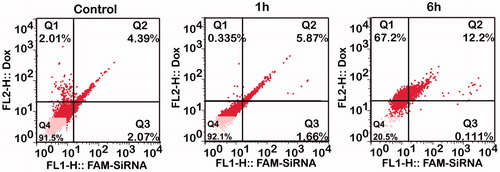
Due to the stability of EGF-PEAL NPs and its ease of uptake by cells, the uptake of Dox and siRNA delivered by EGF-PEAL NPs was significantly increased, and the aim of co-delivery of drug and siRNA was efficiently achieved.
Gene silencing efficiency
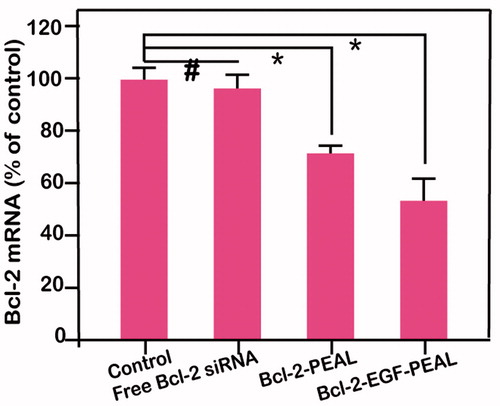
We learned from the above experiments that EGF-PEAL NPs could increase the cell uptake of siRNA. We compared the silencing effects of free Bcl-2-siRNA, Bcl-2- PEAL NPs, and Bcl-2-EGF-PEAL NPs using real-time PCR. The gene silencing effect toward Bcl-2 gene by using Bcl-2-PEAL NPs or Bcl-2-EGF-PEAL NPs was better than using free Bcl-2-siRNA, while the Bcl-2-EGF-PEAL NPs had a much better knockdown effect when compared to Bcl-2-PEAL NPs.
After incubation for 24 h, the expression of Bcl-2 gene in H1299 cells treated with EGF-PEAL NPs was 53.7%; however, in the PEAL NPs treatment group, it was 71.3%. This result had statistical significance (p < 0.001), which indicated that the genes silencing effect of targeting PEAL NPs was greater than that of non-targeting PEAL NPs. We also found the control group has almost no silencing effect (). Knockdown of expression in the antiapoptotic gene bcl-2 is beneficial to induce cancer cell apoptosis.
Co-delivery of Dox and Bcl-2 siRNA inducing apoptosis of H1299
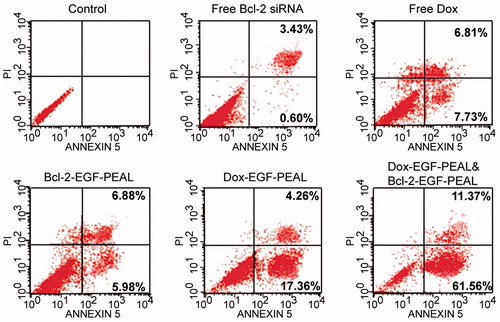
An Annexin V/PI double staining assay was used to evaluate the apoptotic effect of treatment with free Bcl-2 siRNA, free Dox, Dox-EGF-PEAL NPs, siRNA-EGF-PEAL NPs and siRNA & Dox-loaded EGF-PEAL NPs on H1299 cells. We found that the early and late apoptosis ratios of H1299 cells were 5.98 ± 0.76% and 6.88 ± 1.07%, respectively, after incubation with Bcl-2-EGF-PEAL for 24 h; the respective ratios were 0.60 ± 0.17% and 3.43 ± 0.58% with free Bcl-2 siRNA. The early and late apoptosis rates were 17.36 ± 3.64% and 4.26 ± 1.87%, respectively when H1299 cells were incubated with Dox-EGF-PEAL for 24 h. In comparison, the ratios for free Dox were 7.33 ± 1.24% and 6.81 ± 2.15%, respectively. Free Dox could induce the apoptosis of tumor cells, to some extent, but was apparently less effective than Dox-EGF-PEAL. This observation indicated that the NPs could enhance Dox uptake by H1299 cells. Furthermore, co-delivery of siRNA and Dox had the best ability to induce apoptosis when compared with single delivery of Dox or siRNA by EGF-PEAL NPs, which indicated that co-delivery of Dox and Bcl-2-siRNA had a synergistic effect in inducing apoptosis of H1299 cells ().
In vivo biodistribution
Bcl-2-siRNA labeled by Cy5 was applied in living imaging in vivo. After injection of Cy5-siRNA-PEAL and Cy5-siRNA-EGF-PEAL NPs into nude mice via tail veins, PEAL and EGF-PEAL were mainly distributed in the abdominal cavity, the thoracic cavity and the tumors (). According to , the fluorescent intensity of Cy5-siRNA-EGF-PEAL inside the tumors was greater than that of Cy5-siRNA-PEAL. Along with the time prolongation, the fluorescent intensity inside the tumor for Cy5-siRNA-PEAL group decreased, while it increased for Cy5-siRNA-EGF-PEAL, indicating that the tumor-targeting effect of Cy5-siRNA-EGF-PEAL was better than Cy5-siRNA-PEAL.
Figure 7. Distribution of Cy5-siRNA-PEAL NPs and Cy5-siRNA-EGF-PEAL NPs in vivo. (A) Images of Cy5-siRNA-PEAL NPs and Cy5-siRNA-EGF-PEAL NPs at 6, 24 and 48 h after being injected from tail veins in vivo. (B) The fluorescent images of hearts, livers, spleens, kidneys and tumors after 48 h. (C) Cryostat sections of tumor tissues. The fluorescent intensity of Cy5-siRNA-EGF-PEAL NPs in the tumor was obviously stronger than Cy5-siRNA-PEAL NPs group.
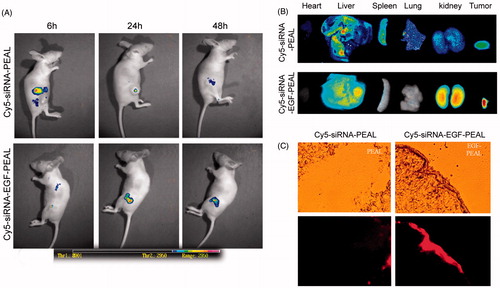
After injection with NPs for 48 h, NP biodistribution in major organs and tumors was evaluated with in vivo living imaging. Similar to the results in , the fluorescent intensity of the Cy5-siRNA-EGF-PEAL group in tumor was significantly stronger than that of the Cy5-siRNA-PEAL group (). Cy5-siRNA-EGF-PEAL NPs concentrated much more in the tumors than Cy5-siRNA-PEAL NPs.
A laser confocal microscope was utilized to observe the cryostat sections of tumors and found that the fluorescence of the Cy5-siRNA-EGF-PEAL group mainly distributed toward tumor tissue when compared to the Cy5-siRNA-PEAL group. Meanwhile, the fluorescence intensity of Cy5-siRNA-EGF-PEAL was much higher than the Cy5-siRNA-PEAL group (), which was consistent with the living imaging results. The results indicated that EGF-PEAL NPs could enhance the uptake ability by tumor cells through the EGFR, which is located on the surface of H1299 cells.
Therapeutic efficiency
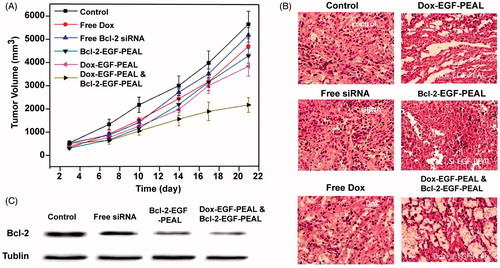
The anticancer efficiency of co-delivery Dox and Bcl-2-siRNA via EGF-PEAL NPs was evaluated in the H1299 cancer-bearing mice. The tumors in the free Dox and free Bcl-2 groups had sizes of 4832 ± 41.5 mm3 and 5102 ± 35.7 mm3, respectively, compared to 5800 ± 30.9 mm3 in the saline-treated control group after a treatment period of 21 d, which indicated that the free Dox or free Bcl-2 treatment had some tumor regression effect. However, the tumor volume of the Dox-EGF-PEAL NPs treated group and Bcl-2-EGF-PEAL NPs treated group reached 3861 ± 39.4 mm3 and 4259 ± 30.2 mm3, respectively, which was much smaller than the free Dox or siRNA group. The tumor volume of the co-delivery Dox and Bcl-2-siRNA group was 1849 ± 42.3 mm3, which grew the least among all groups (). Histological changes evaluated by HE staining showed that there was obvious cancer cell death in the co-delivery treated group, which was consistent with the above results (), and the expression of Bcl-2 protein in tumor tissues in the co-delivery treated group decreased (). Therefore, it is assumed that the growth inhibition of tumors of the co-delivery group was realized via promotion of tumor tissue necrosis and preventing the Bcl-2 gene from expression.
Figure 8. Therapeutic effect of EGF-PEAL co-delivery Dox and Bcl-2-siRNA on H1299 transplanted tumors. (A) The growth curve of tumors in different groups. (B) The morphological features of H1299 transplanted tumors in different groups. (C) The expression of Bcl-2 protein in H1299 transplanted tumors.
Discussion
Lung cancer has one of the highest incidences of cancer in China (Zhao et al., Citation2010). Chemotherapy is still the main treatment for lung cancer, especially NSCLC (Molina et al., Citation2008); however, conventional drug administration lacks targeted effects and has severe side effects, resulting in suboptimal therapy effects. The advent of using siRNA for cancer therapy has attracted more attention, as RNA interference is more specific to the target gene and can knockdown some oncogene expression for cancer therapy (Su et al., Citation2012). The effective, targeted and safe delivery of siRNA across the cell membrane to the cytoplasm in vivo remains a main obstacle, and therefore, the carriers used for siRNA delivery are crucial.
Recently, NPs have been extensively used as drug or gene delivery carriers for cancer therapy; NPs have the properties of controlled drug or gene release, long-time blood circulation and good biocompatibility (Beija et al., Citation2012). Furthermore, when the NPs are modified with a target moiety, they can be much better concentrated in the tumor, which was consistent with our results (Brannon-Peppas & Blanchette, Citation2012). When we modified the PEAL NPs with EGF, we found much more targeted PEAL NPs were concentrated in tumor, perhaps due to EGF-EGFR interaction, as EGFR is highly expressed in NSCLC. Previously, we successfully prepared a novel mPEG-PLGA-PLL NP to effectively deliver siRNA or Dox (Liu et al., Citation2012), and here, we used EGF to modify NPs for lung cancer study.
We optimized the NP preparation conditions to prepare the NPs using the orthogonal design. The NPs had an average particle size of 180 nm and were positively charged. The positive charge could promote binding of the NPs with negatively charged cell membrane via electrostatic interactions. In addition, the NPs could gradually release Dox or Bcl-2-siRNA to ensure their continuous effect in the tumor, which would not be disturbed by EGF modification. When we evaluated the NPs stability, we found ultrasonic intensity and storage period would not affect the integrity of siRNA in the NPs and that increasing the percentage of polymer could improve the carrying capacity of siRNA. Moreover, PEAL NPs can also protect siRNA from degradation during blood circulation. We prepared effective targeted EGF-PEAL NPs for siRNA or Dox delivery.
Previous research found close correlations between the Bcl-2 gene and apoptosis prevention in tumors (Sartorius & Krammer, Citation2002; Sheng & Liu, Citation2011). Some studies have shown that Bcl-2 protein silencing with siRNA can sensitize cancer cells to anti-cancer drugs such as paclitaxel (Beh et al., Citation2008). Previous work showed that empty PEAL NPs had no therapeutic effects on SW620 colon cancer-bearing mice (Yin et al., Citation2012). In this work, we found that co-delivery of Dox and Bcl-2 siRNA through EGF-PEAL NPs exhibited a better therapeutic effect than delivering Dox or Bcl-2 siRNA alone. This phenomenon may be due to the synergistic effect of inducing tumor apoptosis and death, despite the different antitumor mechanisms of Dox and Bcl-2 siRNA (Heo & Lim, Citation2014; Stigliano et al., Citation2014). These results indicate that knockdown of Bcl-2 gene expression sensitizes H1299 cells to Dox.
Cellular uptake results show that Dox or siRNA encapsulated by EGF-PEAL NPs can be effectively internalized by H1299 cells. We chose 1 and 6 h time intervals, as previous studies have shown that huh-7 cells could internalize great amounts of PEAL NPs for 4 h incubation times, effectively delivering drug or siRNA into cells (Liu et al., Citation2012). Furthermore, we found when Bcl-2 siRNA was delivered by EGF-PEAL NPs, it exerted gene knockdown activities much more efficiently than free siRNA or PEAL NPs. This phenomenon occurs because free siRNA is unstable and carries negative charges, and thus has difficulty binding to negatively charged cellular membrane (Du et al., Citation2012). Therefore, apoptosis assay and xenograft tumor therapeutics results demonstrated that co-delivery of Bcl-2 siRNA and Dox via EGF-PEAL NPs hold the most potent antitumor effects. The in vitro and in vivo experiments indicate that EGF-PEAL can effectively deliver Bcl-2 siRNA and Dox, and exert synergistic antitumor effects.
Active targeting has also been used to enhance NPs delivery of drugs into lung cancer (Tseng et al., Citation2007), and in vivo biodistribution results tell us that EGF-PEAL NPs distribute in transplanted tumors much more than PEAL NPs. However, EGF-PEAL NPs still are present at some concentrations in liver, spleen and kidneys, but much less than the PEAL NPs group. This observation indicates that EGF-modified NPs exhibit a higher drug or siRNA concentration when compared to non-modified NPs, leading to better therapeutic effects. Previous studies show that EGF modified NPs are seldom phagocytosed by reticuloendothelial system and mononuclear phagocytic system (Wang et al., Citation2013b). The presence of EGF on NPs may facilitate their accumulation in cancers, via interaction with EGFR highly expressed in lung cancer cells, and will aid NPs in achieving therapeutic effects by inducing apoptosis, proliferation regression of tumor cells more effectively than PEAL NPs.
Conclusion
In our work, we successfully developed a novel EGF-PEAL NPs formulation, and co-delivery of Dox and Bcl-2-siRNA using EGF-PEAL NPs could inhibit cancer proliferation and induce apoptosis of H1299 cells and had an excellent synergistic therapeutic effect on H1299 tumor-bearing nude mice. These results indicate that EGF-PEAL NPs have the potential to be utilized as a targeted drug for lung cancer. The advantage of EGF introduction into the PEAL NPs was obvious when investigating its biodistribution in H1299 xenograft tumors. It also provided enlightenment for lung tumor targeting therapy than using drug and siRNA simultaneously, which can get much better results, possibly even preventing the development of drug resistance. However, the concrete mechanism needs to be further researched.
Declaration of interest
This research was supported through grants from the National Natural Science Foundation of China (No. 81472841, 81272568, 81301973 and 81172078) and Science and Technology Commission of Shanghai Municipality (No. 14JC1492500) and the State Key Laboratory of Oncogenes and Related Genes (No. 91-14-01). The authors declare that there are no potential competing financial interests.
Supplementary material available on line
References
- Beh CW, Seow WY, Wang Y, et al. (2008). Efficient delivery of Bcl-2-targeted siRNA using cationic polymer nanoparticles: downregulating mRNA expression level and sensitizing cancer cells to anticancer drug. Biomacromolecules 10:41–8
- Beija M, Salvayre R, Lauth-de Viguerie N, et al. (2012). Colloidal systems for drug delivery: from design to therapy. Trends Biotechnol 30:485–96
- Brand TM, Iida M, Luthar N, et al. (2013). Nuclear EGFR as a molecular target in cancer. Radiother Oncol 108:370–7
- Brannon-Peppas L, Blanchette JO. (2012). Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev 64:206–12
- Chougule A, Prabhash K, Noronha V, et al. (2013). Frequency of EGFR mutations in 907 lung adenocarcioma patients of Indian ethnicity. PLoS One 8:e76164
- Davis ME, Zuckerman JE, Choi CHJ, et al. (2010). Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 464:1067–70
- Du J, Sun Y, Shi QS, et al. (2012). Biodegradable nanoparticles of mPEG-PLGA-PLL triblock copolymers as novel non-viral vectors for improving siRNA delivery and gene silencing. Int J Mol Sci 13:516–33
- Farokhzad OC, Cheng J, Teply BA, et al. (2006). Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci USA 103:6315–20
- Heo MB, Lim YT. (2014). Programmed nanoparticles for combined immunomodulation, antigen presentation and tracking of immunotherapeutic cells. Biomaterials 35:590–600
- Jain RK, Stylianopoulos T. (2010). Delivering nanomedicine to solid tumors. Nat Rev Clin Oncol 7:653–64
- Koty PP, Zhang H, Levitt ML. (1999). Antisense bcl-2 treatment increases programmed cell death in non-small cell lung cancer cell lines. Lung Cancer 23:115–27
- Li HY, Hu J, Zhao S, et al. (2012). Comparative study of the effect of baicalin and its natural analogs on neurons with oxygen and glucose deprivation involving innate immune reaction of TLR2/TNFα. J Biomed Biotechnol 2012:Article ID 267890
- Liu P, Yu H, Sun Y, et al. (2012). A mPEG-PLGA-b-PLL copolymer carrier for adriamycin and siRNA delivery. Biomaterials 33:4403–12
- Lwin Z, Riess JW, Gandara D. (2013). The continuing role of chemotherapy for advanced non-small cell lung cancer in the targeted therapy era. J Thorac Dis 5:S556–64
- Molina JR, Yang P, Cassivi SD, et al. (2008). Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clinic Proc 83:584–94
- Rathos MJ, Khanwalkar H, Joshi K, et al. (2013). Potentiation of in vitro and in vivo antitumor efficacy of doxorubicin by cyclin-dependent kinase inhibitor P276-00 in human non-small cell lung cancer cells. BMC Cancer 13:29
- Sartorius UA, Krammer PH. (2002). Upregulation of bcl-2 is involved in the mediation of chemotherapy resistance in human small cell lung cancer cell lines. Int J Cancer 97:584–92
- Sheng Q, Liu J. (2011). The therapeutic potential of targeting the EGFR family in epithelial ovarian cancer. Br J Cancer 104:1241–5
- Siegel R, Naishadham D, Jemal A. (2012). Cancer statistics. Cancer J Clin 62:10–29
- Siegel R, Naishadham D, Jemal A. (2013). Cancer statistics, 2013. Cancer J Clin 63:11–30
- Singhal SS, Wickramarachchi D, Singhal J, et al. (2006). Determinants of differential doxorubicin sensitivity between SCLC and NSCLC. FEBS Lett 580:2258–64
- Sridhar SS, Seymour L, Shepherd FA. (2003). Inhibitors of epidermal-growth-factor receptors: a review of clinical research with a focus on non-small-cell lung cancer. Lancet Oncol 4:397–406
- Stigliano C, Aryal S, de Tullio MD, et al. (2014). siRNA-chitosan complexes in poly(lactic-co-glycolic acid) nanoparticles for the silencing of aquaporin-1 in cancer cells. Mol Pharm 10:3186–94
- Su WP, Cheng FY, Shieh DB, et al. (2012). PLGA nanoparticles codeliver paclitaxel and Stat3 siRNA to overcome cellular resistance in lung cancer cells. Int J Nanomedicine 7:4269–83
- Tseng CL, Wang TW, Dong GC, et al. (2007). Development of gelatin nanoparticles with biotinylated EGF conjugation for lung cancer targeting. Biomaterials 28:3996–4005
- Wang JQ, Du ZW, Gao XF, et al. (2013a). [The effect of Bcl-2 gene silencing on the sensitivity of cell line A549 to chemotherapeutic drugs]. Zhonghua Jie He Hu Xi Za Zhi 36:191–7
- Wang Y, Liu P, Qiu L, et al. (2013b). Toxicity and therapy of cisplatin-loaded EGF modified mPEG-PLGA-PLL nanoparticles for SKOV3 cancer in mice. Biomaterials 34:4068–77
- Wu DW, Wu TC, Wu JY, et al. (2013). Phosphorylation of paxillin confers cisplatin resistance in non-small cell lung cancer via activating ERK-mediated Bcl-2 expression. Oncogene 33:4385–95
- Yang J, Liu X, Bhalla K, et al. (1997). Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275:1129–32
- Yin P, Wang Y, Qiu YY, et al. (2012). Bufalin-loaded mPEG-PLGA-PLL-cRGD nanoparticles: preparation, cellular uptake, tissue distribution, and anticancer activity. Int J Nanomedicine 7:3961–9
- Zarogoulidis K, Zarogoulidis P, Darwiche K, et al. (2013). Treatment of non-small cell lung cancer (NSCLC). J. Thorac Dis 5:S389–96
- Zhao P, Dai M, Chen W, et al. (2010). Cancer trends in China. Jpn J Clin Oncol 40:281–5
- Ziegler A, Luedke GH, Fabbro D, et al. (1997). Induction of apoptosis in small-cell lung cancer cells by an antisense oligodeoxynucleotide targeting the Bcl-2 coding sequence. J Natl Cancer Inst 89:1027–36