Abstract
Epirubicin (EPI) elicits poor-oral bioavailability hence commercially available as injection for intravenous administration which follows a rapid increase and fast decay in plasma drug concentration often needs a frequent dosing that may lead to serious side effects. Aim of the present study is to develop a nanoparticulate system which could deliver epirubicin effectively via oral administration and could eventually promote new concept “chemotherapy at home.” In this perspective, epirubicin loaded Poly-lactide-co-glycolic acid nanoparticles (EPI-NPs) were developed by double emulsion evaporation techniques and evaluated for its safety and efficacy against Ehrlich’s Ascites (EAT) induced tumor in balb/c mice. In vivo fate of nanoparticles after oral administration in Albino wistar rats was also studied. EPI-NPs showed marked reduction in tumor size ∼40% while tumor size was increased 3.55 and 3.28 folds in control as well as in group treated orally with free epirubicin solution (EPI-S), respectively. Furthermore, toxicological evaluation demonstrated insignificant difference in levels of biomarkers including MDA, CAT, SOD, LDH, CK-MB, AST and ALT when EPI-NPs-oral treatment was compared with control group while levels of these biomarkers were found extremely significant in group treated with EPI-S (i.v). and demonstrated increment in LDH (p < 0.001), CK-MB (p < 0.001), AST (p < 0.001), ALT (p < 0.001) and MDA levels (p < 0.001) and reduction in SOD (p < 0.001) and CAT levels (p < 0.001) thus confirmed better safety profile of EPI-NPs oral than EPI-S i.v. Biodistribution study demonstrated the presence of NPs in different body organs and blood which suggests probability of NPs translocation across intestine thus at the tumor site.
Introduction
Epirubicin is an anthracycline analog and safer alternative to doxorubicin with comparable clinical activities and lower adverse effects at equimolar dose (Ormrod et al., Citation1999). It acts by intercalating into DNA strands, forms a complex which results into inhibition of DNA and RNA synthesis (Plosker & Faulds, Citation1993). In addition, it induces DNA breakage by inhibiting topoisomerase II leading to cell death. Epirubicin has diverse clinical utility in various malignancies like cancer of breast, lungs, gastric, ovary, and pancreas as well as in non-Hodgkin’s lymphomas and acute leukemia. Commercially existing formulation of epirubicin (Ellence, Pfizer) is administered through intravenous route that leads to rapid increase and fast decay in plasma drug concentration; often to sub-therapeutic level hence necessitate frequent dosing that may lead to serious side effects. Clinical postulation suggested that extended exposure of cancer cells to anti-cancer agents at modest concentration could be effective and safer than current intermittent chemotherapy (Zhang & Feng, Citation2006). Apart from this, dose dependent hematological and cardiological toxicity are other concern (Plosker & Faulds, Citation1993; Ormrod et al., Citation1999). Hence, a number of delivery systems like pullulan acetate NPs (Zhang et al., Citation2009), self-assembled cholesterol-conjugated carboxymethyl curdlan NPs (Li et al., Citation2010), liposomes (Yang et al., Citation2013), immunoliposome (Mamot et al., Citation2005) and polymeric micelles (Harada et al., Citation2011) have been investigated to improve intravenous administration of epirubicin.
At present, proclivity towards oral chemotherapy is increasing as it offers number of fascinating opportunities including better quality of life, good treatment advantages and low healthcare cost. In addition, it would also obviate the need of hospitalization, medical and nursing care as well as infusion equipments thus can eventually promote new concept “chemotherapy at home” (Peltier et al., Citation2006; Zhang & Feng, Citation2006). However, epirubicin elicits poor oral bioavailability as it is a substrate for both P-glycoprotein (P-gp) and Cytochrome P450 (CYP450) which are abundantly distributed in the intestine thus oral delivery of epirubicin is highly challenging (Nelson, Citation2011). CYP 450/P-gp suppressors can make its oral delivery feasible however their clinical use are not encouraged owing to unintended interaction, non-selective inhibition, and immune suppression which may lead to long-term medical complications (Jain et al., Citation2011a).
Recently, nanoformulations like nanoemulsion (Verma et al., Citation2015), self nanoemulsifying drug-delivery system (Akhtar et al., Citation2013; Negi et al., Citation2013), solid lipid nanoparticles (Yuan et al., Citation2013) polymeric micelles (Yao et al., Citation2011), Dendrimer (Ke et al., Citation2008), layersomes (Jain et al., Citation2012), polymeric NPs (Jain et al., Citation2011b; Fatma et al., Citation2014; Katiyar et al., Citation2015), protein NPs (Golla et al., Citation2013) etc. have gained considerable attention for successful oral delivery of anticancer agents. Polymeric NPs have demonstrated additional advantages over other nano systems with respect to their greater stability, protection of encapsulated drugs and feasibility to modulate physicochemical characteristics like surface properties and release behavior (Galindo-Rodriguez et al., Citation2005; He et al., Citation2012). The most recognized and extensively investigated class is the biodegradable NPs. Poly-lactic-co-glycolic acid (PLGA) is a food and drug administration (FDA) approved biocompatible and biodegradable polymer and widely investigated for the delivery of anticancer drugs (Astete & Sabliov, Citation2006; Kalaria et al., Citation2009; Khuroo et al., Citation2014; Householder et al., Citation2015). PLGA NPs like other colloidal system are also taken up by enterocytes and M cells of Peyer’s patches in the small intestine (Joshi et al., Citation2014) which may turn into bypassing of CYP 450 mediated metabolism and P-gp mediated efflux. Joshi et al., (Citation2014) reported a better oral pharmacokinetic profile for gemcitabine-loaded oral PLGA NPs over plain oral solution. Jain et al., (Citation2011a) also confirmed the improved efficacy of doxorubicin through oral PLGA NPs over free solution against chemically-induced breast cancer. Similar observation was reported by Bhardwaj et al., (Citation2009) for oral paclitaxel PLGA NPs when compared with paclitaxel solution in Cremophor EL given via oral/i.v. route. Encouraging outcomes of other studies suggested that nanoparticulate system could be an alternative for the oral delivery of pharmaceutically challenging drug molecules.
In our previous report, we demonstrated a significant reduction in IC50 value against MCF-7 cell line, significantly improved permeation across caco-2 cell line and rat ileum, furthermore approximately 4.0-fold improvement in oral bioavailability (Tariq et al., Citation2015). However, developed formulation needs a thorough evaluation in terms of pharmacodynamic and toxicity aspects. Therefore, this work was further extended to evaluate the actual potential of EPI-NPs. The present work is aimed to evaluate in-vivo antitumor efficacy and safety profile of EPI-NPs against Ehrlich Ascites-induced tumor in Balb/c mice.
Material and methods
Materials
Epirubicin hydrochloride was provided ex-gratia by Fresenius Kabi Pvt. Ltd, India. PLGA 50:50 (Resomer 503 H, acid end group) was provided as a gift sample by Evonik industries, India. PLGA 50:50 (PURASORB PDLG 5004, ester end group) was procured from Purac Carbion. Dichloromethane (AR grade), acetonitrile (HPLC grade), o-phosphoric acid (HPLC Grade), sodium dihydrogen phosphate dihydrate (NaH2PO4.2H2O), sodium hydroxide (NaOH) were purchased from Merck, India. Water was obtained from Milli-Q water purification system (Millipore, MA). Lactate dehydrogenase (LHD) and creatine kinase (CK-MB) kit were procured from Span Diagnostics Ltd. Gujarat, India. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) kit were obtained from Reckon Diagnostics P. Ltd. Gujarat, India. Ehrlich’s Ascites cell line (EAT) was provided by Dr. B.S. Dwarakanath, Institute of nuclear medicine and allied sciences, New Delhi, India. Coumarin-6 was purchased from Sigma Aldrich (Saint Louis, MO). The study protocols for in-vivo biodistribution (protocol no. 947, 2013) and In vivo anti-tumor studies (protocol no. 1051, 2014) were reviewed and approved by Institutional Animal Ethics Committee of Jamia Hamdard. Animals were kept under standard laboratory conditions (temperature 25 ± 2 °C and relative humidity 55 ± 5%) with free access to standard laboratory diet (Lipton feed, Mumbai, India) and water ad libitum.
Preparation of epirubicin-loaded PLGA NPs
Epirubicin-loaded poly-lactide-co-glycolic acid nanoparticles (EPI-NPs) were prepared by earlier reported method (Tariq et al., Citation2015). Briefly, drug was dissolved in internal aqueous phase and PLGA was dissolved in 2 ml of dichloromethane. Drug solution was emulsified in polymeric solution under sonication over an ice bath for 60 s (25 W, 40% duty cycles, Sonopuls, Bandelin, Germany). Formed primary emulsion (w/o) was added into external aqueous phase under sonication over an ice bath at 25% amplitude and resultant dispersion was subjected to solvent evaporation under mild magnetic stirring (400 rpm) at room temperature followed by centrifugation at 18 000 rpm for 20 min. The obtained pellet was washed and dispersed then lyophilized for 24 h at –50 °C and 0.015 millibar pressure (Lab Conco., Kansas City, MO, LPYH, Lock 6, freeze dryer) .
Preparation of fluorescence labeled NPs
Coumarin-6 (CM6) was used as a fluorescence probe for the preparation of fluorescent NPs (CM6-NPs). Here, 2 mg of CM6 was dissolved in dichloromethane and rest of the procedure was followed as described for the preparation of EPI-NPs.
HPLC analysis
Quantitative analysis of EPI was done by using HPLC. Water separation module, e2695 HPLC system with Empower software (Milford, MA) coupled to point to point resolution PDA detector was used for the purpose. Separation was done by using mobile phase comprising of 0.16% v/v o-phosphoric acid aqueous solution (60% v/v) and organic phase (40% v/v); organic phase consisting of acetonitrile and methanol mixture (80:20 v/v). The separation was achieved by using C18 analytical column (4.6 × 250 mm, 5 μm, Purosphere, Merck, Kenilworth, NJ) at the flow rate of 1mL/min and column temperature 30 °C. Detection was made at 233.5 nm (in house developed method).
Fluorescence analysis
Quantitative analysis of CM6 was done by using spectrofluorometer at λex (440 nm) and λem (500 nm) wavelength (Cary Eclipse fluorescence spectrophotometer, Varian, CA).
In vivo fate of NPs after oral administration
Animals (Albino Wistar Rats, weight 200–220 g) were randomly divided into six groups, each group comprising of three animals. All rats were starved for 12 h before experimentation but allowed free access to water. One group was kept as a control and treated per-orally with phosphate buffer saline (PBS) and rest of the five groups were treated per-orally with CM6-NPs, 4 mg in 1ml.
At different time interval (1, 2, 4, 8, 24 h) blood was collected and animals were scarified by cervical dislocation methods under excessive anesthesia. Incision was made into abdomen and different body organs like stomach, jejunum, ileum, colon, spleen, liver, kidney, heart and lung were excised out immediately. Stomach, small intestine and colon were cut open; luminal sides were thoroughly rinsed with PBS to clear away any residual contents and NPs at the surface. Tissues were patted dried, weighed, wrapped in aluminum foil and stored in dark at –20 °C till analysis. Blood was allowed to clot and serum was stored at –20 °C till analysis.
Quantitative analysis of NPs in different tissue and blood was done as per the previous report with slight modification (Zhao et al., Citation2007). Tissues were homogenized in distilled water followed by extraction in acetonitrile (for 18 h at 25 °C). Resultant samples were centrifuged at 10 000 rpm for 10 min; obtained supernatants were subjected to measurement of fluorescence intensity at 440 nm (λex) and 500 nm (λem) wavelengths using spectrofluorometer. Serum samples were also subjected to same treatment and analyzed as described above. Auto-fluorescence of each sample was determined by subjecting same treatment to the samples obtained from control group. Final fluorescence of test samples was calculated by deducting auto fluorescence of control with fluorescence of test.
In vivo antitumor efficacy and toxicological studies
Tumor inhibition study
Ehrlich’s Ascites tumor (EAT) cells suspension, 100 μl (1 × 106) was injected subcutaneously into the hind right flank of Balb/c mice (weight, 20–25 g, age, 6–8 weeks) to induce a solid tumor, when tumor volume (500–600 mm3) was accomplished; animals were randomly divided into four groups, each group comprising of six animals. Group I was kept as control, i.e. treated with PBS, Group II was subjected to 8.5 mg/kg EPI-S though intravenous route while group III and IV were treated orally with EPI-S (8.5 mg/kg) and EPI-NPs (equivalent to 8.5 mg/kg epirubicin), respectively. Each group was subjected to four doses on alternate day. The day, on which treatment was begun, designated as a day zero and study was terminated on 21st day; tumor volume and weight of animal was measured alternatively. Tumor volume was measured by using digital caliper and calculated according to following equation;
At the end of study, blood and vital body organs were collected and subjected to blood count, biomarkers analysis and histopathological examinations (Negi et al., Citation2014; Lale et al., Citation2015).
Hematological and biomarkers analysis
Hematological parameters were done at approved commercial laboratory (Dr. Lal PathLabs, New Delhi, India). The level of biochemical markers like creatine phosphokinase (CK-MB), lactate dehydrogenase (LDH), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in blood serum were estimated spectrophotometrically by using methods provided by manufacturer along with commercial kits. Malondialdehyde (MDA), superoxide dismutase (SOD), and catalase (CAT) were estimated in heart homogenate by using previous reports, MDA (Ohkawa et al., Citation1979), SOD (Marklund & Marklund, Citation1974) and CAT (Claiborne, Citation1985).
Histopathological examinations
Excised tissues were fixed in 10% formalin solution at room temperature and preserve for further analysis. Fixed tissues were embedded in paraffin blocks and sectioned (5μm) by using a rotary microtome (Leica, Germany) followed by staining with hematoxyline and eosin stains. Sections were observed under light microscope (Motic) and images were captured by Motic software (Causeway Bay, Hong Kong) version 2.
Statistical analysis
All the data was expressed as mean ± standard deviation. Statistical analysis was performed with GraphPad Prism version 5 (GraphPad Software, Inc. Fay Avenue, CA) using one-way analysis of variance (one-way ANOVA) followed by Tukey: compare all pairs of column. The value < 0.05 was considered as statistically significant.
Results and discussion
In our previous work, we successfully developed and optimized EPI-NPs by double emulsion solvent evaporation method and obtained the particles size 235.3 ± 15.12 nm with narrow polydispersity index, 0.116 ± 0.01 (Tariq et al., Citation2015). EPI-NPs demonstrated significantly high cytotoxicity against MCF-7 cell line and superior uptake in caco-2 cell line when compared with free solution. Moreover, they showed significantly higher transport across caco-2 cell line monolayer as well as across the rat ileum. Encouraging in vitro findings were further supported by in vivo pharmacokinetic study which demonstrated approximately 4.0-folds higher improvement in oral bioavailability in comparison to free solution (Tariq et al., Citation2015). In the present work, an attempt was made to establish an actual potential of EPI-NPs by using Ehrlich Ascites-induced tumor model. Furthermore, in vivo fate of NPs after oral administration was established by using CM6- NPs since these dyes either do not release or release very poorly from the particles (Kulkarni & Feng, Citation2011). We also observed similar findings (data not shown) thus can be considered appropriate for biodistribution of NPs after oral administration.
In vivo fate of NPs after oral administration
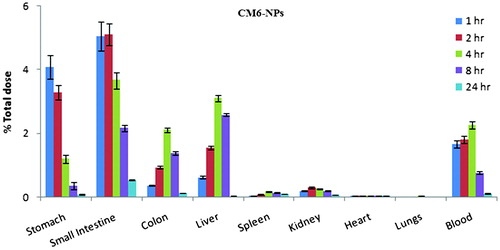
summarizes the biodistribution of CM6-NPs at 1, 2, 4, 8, and 24h of post oral administration in Wistar rat. At 1st hour of post administration, particles dominantly distributed in stomach (4.78 ± 0.37%) and intestine (6.04 ± 0.45%) followed by blood > liver > colon > Kidney > heart > spleen > lungs while distribution was shifted to other body organs with time (). Stomach and intestine showed continuous reduction in fluorescence with time while liver and blood showed their maximum fluorescence at 4h followed reduction similarly spleen and kidney showed their maximum fluorescence at 8th hour. Reduction of NPs level in stomach and intestine with time can be attributed to clearance of NPs from the intestine via endocytic uptake through enterocytes and M cell of Peyer’s patches and subsequently reached to blood and other body organs (Araujo et al., Citation1999; Yin et al., Citation2007; Lalatsa et al., Citation2012). After stomach and intestine, highest distribution of nanoparticles in liver can be explained by phagocytic uptake of particles due its hydrophobic surface while presence of particles in the renal tissue indicates their renal clearance (Semete et al., Citation2010,Citation2012). From the observation it can be concluded that noticeable quantity of NPs distributed in different body organs and blood when given orally indicated the translocation of intact NPs across the GI tract. Availability of intact NPs in blood suggests the probability of tumor targeting after oral administration via enhanced permeability and retention (EPR) effect.
In vivo antitumor activity and toxicological studies
The efficacy of EPI-NPs was assessed against EAT cell-induced tumor in Balb/c mice and In vivo anti-tumor activity of different treatment was summarized in . When animals gained tumor volume 500–600 mm3, treatments were started and at this stage tumor volume was considered 100%. Four doses of different treatment (EPI, 8.5mg/kg) were given on alternate day and tumor volume was monitored for next 21 days. Control group (treated orally with PBS) demonstrated a very fast and progressive tumor growth and gained the tumor volume, 1956.36 ± 186.57 mm3 (). A similar trend was seen in a group treated orally with EPI-S and insignificant difference in tumor volume (1805.93 ± 176.5 mm3) was noticed when compared with control group (p > 0.05) while a significant suppression in tumor size was observed in the animals treated orally and intravenously with EPI-NPs (∼40%) and EPI-S (∼59%), respectively when compared with control group (p < 0.0001). Though, oral EPI-NPs suppressed the tumor growth however it was found lower when compared with EPI-S treatment given intravenously.
Figure 2. In vivo antitumor activity against Ehrlich Ascites-induced tumor bearing balb/c mice, after oral administration of EPI-S oral, EPI-NPs oral and EPI-S intravenous administration; (a) change in tumor with time course; (b) change in animal weight with time course. The data presented as mean ± SD (n = 6), †p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, (a), in comparison to control, (b), in comparison to EPI-S oral, (c) in comparison to EPI-NPs oral).
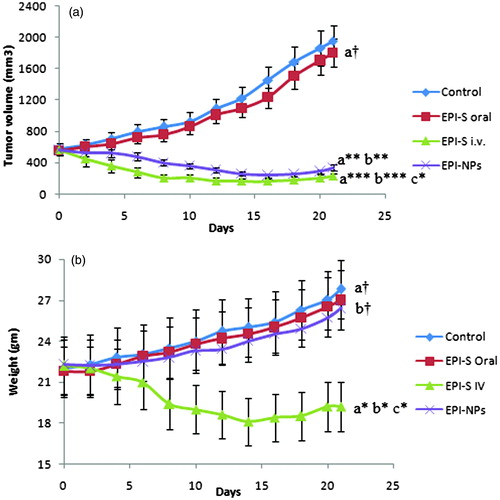
Insignificant difference between tumor volume of control group and animal treated orally with EPI-S can be attributed to poor-oral bioavailability of epirubicin while a significant suppression of tumor through oral EPI-NPs can be explained by improved oral bioavailability as observed in our previous work (Tariq et al., Citation2015) and sustained release of epirubicin which would maintained the therapeutic concentration for prolonged period of time. Moreover, biodistribution study demonstrated the probability of translocation of intact NPs into the blood thus tumor specific localization of epirubicin through enhanced permeability retention effect (EPR) of EPI-NPs could also be possible hence can be attributed to improved efficacy (Maeda, Citation1992; Bhardwaj et al., Citation2009; Jain et al., Citation2011a). EPI-S administered through intravenous route demonstrated maximum tumor suppression in the following way; initially, tumor suppression was rapid which become static followed by mild tumor increment. It can be attributed to high-drug concentration in tumor vicinity owing to high initial plasma drug concentration which followed a rapid clearance due to rapid decay in plasma drug level as well as P-gp mediated efflux (Jain et al., Citation2011a, 2012). It is noteworthy that tumor suppression achieved through EPI-S i.v. was significantly high when compared with oral EPI-NPs (p < 0.05). The plausible reason for above observations is that oral administration has to go through the different barriers of per-oral route while the drug is directly available when administered through intravenous route thus more bioavailable at the target site (Jain et al., Citation2012). In order to get rid of these barriers, either dose or dosing frequency of oral EPI-NPs can be increased to achieve tumor suppression equivalent to intravenous route. As epirubicin exhibits dose-dependent toxicity therefore increasing dosing frequency would be a better option. Jain et al. (Citation2012) also reported similar observation.
The weight of tumor bearing mice was regularly monitored and change in weight was depicted in . All the treatment groups demonstrated higher weight with respect to their own initial weight (p < 0.05) except group treated with EPI-S intravenously where a weight reduction was observed with respect to their own weight as well as weight of other groups (p < 0.05). Observations are in agreement with previous findings and can be attributed toxicity of drug (Yao et al., Citation2011).
Similar to other cytotoxic agents, epirubicin may produce myelosuppression. A reversible leukopenia/neutropenia is one of the major dose-limiting hematologic toxicity associated with epirubicin. It is usually transient; generally regain normal value by day 21 after drug administration. In addition, cardiotoxicity is a well known adverse effect of anthracyclines chemotherapeutics. It may be manifestated by early or late events. Hepatobiliary system is a major route of its elimination therefore it may cause hepatotoxicity. Therefore, hematological parameters, cardiac and hepatic biomarkers were estimated to identify any adverse effect.
Hematological toxicity was assessed by monitoring blood parameters and observations were summarized in . Animals treated with EPI-S (oral) and EPI-NPs (oral) showed insignificant difference in blood parameters (RBCs count, TLC, DLC, Hb content and platelets count) when compared with control group while these parameters were found reduce significantly in animals treated with EPI-S (i.v). For the evaluation of cardiac toxicity, the levels of different cardiac markers like MDA, SOD, CAT (in heart homogenate), LDH) and CK-MB (in serum) were determined while for the evaluation of hepatotoxicity, AST and ALT were estimated after the treatment with EPI-S (i.v.), EPI-S (oral), and EPI-NPs (oral). The effect on different biochemical markers are summarized in . Animals treated with EPI-S (oral) and EPI-NPs (oral) demonstrated no significant difference in biomarkers like MDA (), CAT (), SOD (), LDH (), CK-MB (), AST () and ALT () when compared with control group, while EPI-S (i.v.) treated animals demonstrated significant increment in LDH (p < 0.001), CK-MB (p < 0.001), AST (p < 0.001), ALT (p < 0.001) and MDA levels (p < 0.001) and significant reduction in SOD (p < 0.001) and CAT levels (p < 0.001). Superoxide dismutase and catalase are enzymes which are widely distributed in body organs and facilitate quenching of superoxide and peroxide radicals thus provide protection from oxidative damages. Reduced level of SOD and CAT indicates oxidative damage while increased level of MDA indicates lipid peroxidation. Epirubicin causes oxidation induced cardiotoxicity hence the level of SOD, CAT and MDA was measured in heart homogenate. LDH is also widely distributed in different body organs and its increased level is indicative of cellular breakdown including cardiac cell. CKMB is a specific cardiac biomarker as its primary source is myocardium thus increased level indicates the myocardial damage. AST and ALT are the enzymes which catalyze the reversible transfer of α-amino group to amino acids thus regulate their metabolism. AST and ALT are the biomarkers measured clinically for hepatic toxicity. Increased level of both the enzymes gives the sign and symptom of hepatotoxicity
Figure 3. The level of biochemical markers in heart homogenate and serum after oral administration of EPI-S, EPI-NPs and EPI-S intravenous administration in Ehrlich Ascites-induced tumor bearing balb/c mice; (a) MDA; (b) SOD; (c) CAT; (d) LDH; (e) CK-MB; (f) AST; (g) ALT. The data presented as mean ± SD (n = 3), † p > 0.05, ***p < 0.001, [(a), in comparison to control, (b), in comparison to EPI-S oral, (c) in comparison to EPI-NPs oral].
![Figure 3. The level of biochemical markers in heart homogenate and serum after oral administration of EPI-S, EPI-NPs and EPI-S intravenous administration in Ehrlich Ascites-induced tumor bearing balb/c mice; (a) MDA; (b) SOD; (c) CAT; (d) LDH; (e) CK-MB; (f) AST; (g) ALT. The data presented as mean ± SD (n = 3), † p > 0.05, ***p < 0.001, [(a), in comparison to control, (b), in comparison to EPI-S oral, (c) in comparison to EPI-NPs oral].](/cms/asset/3c1e674d-4449-4522-9d7e-3e2d9f2fcd45/idrd_a_1136713_f0003_b.jpg)
Table 1. Blood parameters in Ehrlich Ascites-induced tumor bearing balb/c mice after administration of EPI-S oral, EPI-NPs oral and EPI-S i.v. (mean ± SD, n = 3).
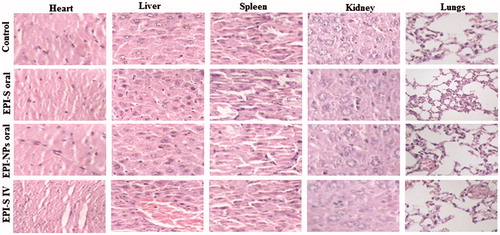
Histopathological examination of body organs obtained from different treatment group was also carried out to assess any possible toxicity. Normal striated cells with centrally located one or two nuclei were observed in heart tissue of animals treated with PBS (control), EPI-S oral, and EPI-NPs oral () while a marked degeneration of cardiac muscles was observed in case of animals treated with EPI-S i.v. which was characterized by the interstitial edema, disorganization and vacuolization of cardiac muscle cells (). Liver also showed mild fibrosis manifested by the occurrence of multiple focal cellular granulomatous lesions and foci of spotty necrosis in case of animal treated with EPI-S i.v. Interestingly, no substantial difference was observed in the histology of other tissues like spleen, lung and kidney weather treated with EPI orally or intravenously (). Lungs tissue showed bronchioles with normal epithelium and no emphysema, edema, inflammation or alveolar collapse. Similarly, spleen showed white pulp with trabecular architecture and normal sinusoids. In addition, tissue section of kidney demonstrated normal renal glomerula, proximal ducts with normal nucleus.
Figure 4. Histological analyzes of heart, liver, kidney, lungs and spleen of Ehrlich Ascites-induced tumor bearing balb/c mice, after treatment with; (a) oral PBS; (b) oral EPI-S; (c) Oral EPI-NPs; (d) EPI-S intravenous.
Observations of toxicological evaluation clearly indicated that the safety profile of EPI-NPs oral is better when compared with EPI-S i.v. High hematological, cardiac and hepatotoxicity after intravenous administration EPI-S can be attributed to very-high plasma drug concentration of EPI while lower toxicity of EPI-S oral can be explained by very-low plasma drug concentration owing poor oral absorption. However EPI-NPs demonstrated good oral bioavailability even though showed reduced toxicity which can be attributed to significantly low but therapeutically effective plasma drug concentration in comparison to EPI-S i.v. (p < 0.0001).
Conclusion
EPI-NPs were successfully developed and optimized for its oral delivery. EPI-NPs demonstrated highly significant anti-tumor activity against EAT induced tumor bearing mice when compared with control group (p < 0.0001). In addition, hematological, biochemical marker and histopathological analysis revealed a marked reduction in toxicity when compared with EPI-S i.v. at equimolar dose. EPI-NPs showed significant tumor suppression but comparatively lower than EPI-S intravenous administration. However, it could be achieved equivalent to EPI-S i.v. through increasing oral-dosing frequency. Thus, multiple oral dosing with EI-NPs can be a lucrative approach for effective tumor suppression and reduced toxicity over EPI-S i.v.
Acknowledgements
The authors acknowledge the contribution of Fresenius Kabi and Evonik Degussa Pvt. Ltd. for providing gift samples. The authors are indebted to Jamia Millia Islamia, and SAIF (AIIMS), New Delhi India to carry out SEM and TEM analysis. Assistance rendered by Dr. A K Tiwari and Mr. Mohammad Ibrahim in animal studies is also duly acknowledged.
Declaration of interest
There is no academic, financial, personal and commercial conflict of interest of authors. Authors are thankful to Jamia Hamdard, for facilitating research activities and Department of Biotechnology (DBT) for financial support.
References
- Akhtar N, Talegaonkar S, Khar RK, Jaggi M. (2013). Self-nanoemulsifying lipid carrier system for enhancement of oral bioavailability of etoposide by P-glycoprotein modulation: in vitro cell line and in vivo pharmacokinetic investigation. J Biomed Nanotechnol 9:1216–29
- Araujo L, Sheppard M, Löbenberg R, Kreuter J. (1999). Uptake of PMMA nanoparticles from the gastrointestinal tract after oral administration to rats: modification of the body distribution after suspension in surfactant solutions and in oil vehicles. Int J Pharm 176:209–24
- Astete CE, Sabliov CM. (2006). Synthesis and characterization of PLGA nanoparticles. J Biomater Sci Polym Ed 17:247–89
- Bhardwaj V, Ankola DD, Gupta SC, et al. (2009). PLGA nanoparticles stabilized with cationic surfactant: safety studies and application in oral delivery of paclitaxel to treat chemical-induced breast cancer in rat. Pharm Res 26:2495–503
- Claiborne A. (1985). Catalase activity. In: RA, G. ed. Handbook of methods for oxygene radical research. Boca Raton (FL): CRC Press. p 283–4
- Fatma S, Talegaonkar S, Iqbal Z, et al. (2014). Novel flavonoid-based biodegradable nanoparticles for effective oral delivery of etoposide by P-glycoprotein modulation: an in vitro, ex vivo and in vivo investigations. Drug Deliv 21:1–12
- Galindo-Rodriguez SA, Allemann E, Fessi H, Doelker E. (2005). Polymeric nanoparticles for oral delivery of drugs and vaccines: a critical evaluation of in vivo studies. Crit Rev Ther Drug Carrier Syst 22:419–64
- Golla K, Reddy PS, Bhaskar C, Kondapi AK. (2013). Biocompatibility, absorption and safety of protein nanoparticle-based delivery of doxorubicin through oral administration in rats. Drug Deliv 20:156–67
- Harada M, Bobe I, Saito H, et al. (2011). Improved anti-tumor activity of stabilized anthracycline polymeric micelle formulation, NC-6300. Cancer Sci 102:192–9
- He C, Yin L, Tang C, Yin C. (2012). Size-dependent absorption mechanism of polymeric nanoparticles for oral delivery of protein drugs. Biomaterials 33:8569–78
- Householder KT, Diperna DM, Chung EP, et al. (2015). Intravenous delivery of camptothecin-loaded PLGA nanoparticles for the treatment of intracranial glioma. Int J Pharm 479:374–80
- Jain AK, Swarnakar NK, Das M, et al. (2011a). Augmented anticancer efficacy of doxorubicin-loaded polymeric nanoparticles after oral administration in a breast cancer induced animal model. Mol Pharm 8:1140–51
- Jain AK, Swarnakar NK, Das M, et al. (2011b). The effect of the oral administration of polymeric nanoparticles on the efficacy and toxicity of tamoxifen. Biomaterials 32:503–15
- Jain S, Patil SR, Swarnakar NK, Agrawal AK. (2012). Oral delivery of doxorubicin using novel polyelectrolyte-stabilized liposomes (layersomes). Mol Pharm 9:2626–35
- Joshi G, Kumar A, Sawant K. (2014). Enhanced bioavailability and intestinal uptake of Gemcitabine HCl loaded PLGA nanoparticles after oral delivery. Eur J Pharm Sci 60:80–9
- Kalaria DR, Sharma G, Beniwal V, Ravi Kumar MN. (2009). Design of biodegradable nanoparticles for oral delivery of doxorubicin: in vivo pharmacokinetics and toxicity studies in rats. Pharm Res 26:492–501
- Katiyar SS, Muntimadugu E, Rafeeqi TA, et al. (2015). Co-delivery of rapamycin- and piperine-loaded polymeric nanoparticles for breast cancer treatment. Drug Deliv 3:1–9
- Ke W, Zhao Y, Huang R, et al. (2008). Enhanced oral bioavailability of doxorubicin in a dendrimer drug delivery system. J Pharm Sci 97:2208–16
- Khuroo T, Verma D, Talegaonkar S, et al. (2014). Topotecan-tamoxifen duple PLGA polymeric nanoparticles: investigation of in vitro, in vivo and cellular uptake potential. Int J Pharm 473:384–94
- Kulkarni SA, Feng SS. (2011). Effects of surface modification on delivery efficiency of biodegradable nanoparticles across the blood-brain barrier. Nanomedicine (Lond) 6:377–94
- Lalatsa A, Garrett NL, Ferrarelli T, et al. (2012). Delivery of peptides to the blood and brain after oral uptake of quaternary ammonium palmitoyl glycol chitosan nanoparticles. Mol Pharm 9:1764–74
- Lale SV, Kumar A, Naz F, et al. (2015). Multifunctional ATRP based pH responsive polymeric nanoparticles for improved doxorubicin chemotherapy in breast cancer by proton sponge effect/endo-lysosomal escape. Polym Chem 6:2115–32
- Li L, Gao FP, Tang HB, et al. (2010). Self-assembled nanoparticles of cholesterol-conjugated carboxymethyl curdlan as a novel carrier of epirubicin. Nanotechnol 21:265601
- Maeda H. (1992). The tumor blood vessel as an ideal target for macromolecular anticancer agents. J Control Release 19:315–24
- Mamot C, Drummond DC, Noble CO, et al. (2005). Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res 65:11631–8
- Marklund S, Marklund G. (1974). Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–74
- Negi LM, Talegaonkar S, Jaggi M, et al. (2014). Surface engineered nanostructured lipid carriers for targeting MDR tumor: Part II. In vivo biodistribution, pharmacodynamic and hematological toxicity studies. Colloids Surf B Biointerfaces 123:610–15
- Negi LM, Tariq M, Talegaonkar S. (2013). Nano scale self-emulsifying oil based carrier system for improved oral bioavailability of camptothecin derivative by P-Glycoprotein modulation. Colloids Surf B Biointerfaces 111:346–53
- Nelson DR. (2011). Progress in tracing the evolutionary paths of cytochrome P450. Biochim Biophys Acta 1814:14–18
- Ohkawa H, Ohishi N, Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–8
- Ormrod D, Holm K, Goa K, Spencer C. (1999). Epirubicin: a review of its efficacy as adjuvant therapy and in the treatment of metastatic disease in breast cancer. Drugs Aging 15:389–416
- Peltier S, Oger JM, Lagarce F, et al. (2006). Enhanced oral paclitaxel bioavailability after administration of paclitaxel-loaded lipid nanocapsules. Pharm Res 23:1243–50
- Plosker GL, Faulds D. (1993). Epirubicin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in cancer chemotherapy. Drugs 45:788–856
- Semete B, Booysen L, Kalombo L, et al. (2012). Effects of protein binding on the biodistribution of PEGylated PLGA nanoparticles post oral administration. Int J Pharm 424:115–20
- Semete B, Booysen L, Lemmer Y, et al. (2010). In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems. Nanomedicine 6:662–71
- Tariq M, Alam MA, Singh AT, et al. (2015). Biodegradable polymeric nanoparticles for oral delivery of epirubicin: In vitro, ex vivo, and in vivo investigations. Colloids Surf B Biointerfaces 128:448–56
- Verma P, Meher JG, Asthana S, et al. (2015). Perspectives of nanoemulsion assisted oral delivery of docetaxel for improved chemotherapy of cancer. Drug Deliv 21:1–10
- Yang Q, Ma Y, Zhao Y, et al. (2013). Accelerated drug release and clearance of PEGylated epirubicin liposomes following repeated injections: a new challenge for sequential low-dose chemotherapy. Int J Nanomed 8:1257–68
- Yao HJ, Ju RJ, Wang XX, et al. (2011). The antitumor efficacy of functional paclitaxel nanomicelles in treating resistant breast cancers by oral delivery. Biomaterials 32:3285–302
- Yin Y, Chen D, Qiao M, et al. (2007). Lectin-conjugated PLGA nanoparticles loaded with thymopentin: ex vivo bioadhesion and in vivo biodistribution. J Control Release 123:27–38
- Yuan H, Chen CY, Chai GH, et al. (2013). Improved transport and absorption through gastrointestinal tract by PEGylated solid lipid nanoparticles. Mol Pharm 10:1865–73
- Zhang HZ, Gao FP, Liu LR, et al. (2009). Pullulan acetate nanoparticles prepared by solvent diffusion method for epirubicin chemotherapy. Colloids Surf B Biointerfaces 71:19–26
- Zhang Z, Feng SS. (2006). Nanoparticles of poly(lactide)/vitamin E TPGS copolymer for cancer chemotherapy: synthesis, formulation, characterization and in vitro drug release. Biomaterials 27:262–70
- Zhao J, Liu CS, Yuan Y, et al. (2007). Preparation of hemoglobin-loaded nano-sized particles with porous structure as oxygen carriers. Biomaterials 28:1414–22