Abstract
Surfactin, a natural lipopeptide produced by Bacillus, is gaining attention for potentially biomedical and pharmaceutical applications. Here, surfactin was assayed for oral delivery of insulin (INS) by its ability to bind to and promote protein to penetrate through the cell membrane. Analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, surfactin was found to form co-precipitates with INS to protect it from acidic and enzymatic attack in the gastrointestinal tract. Further analysis by non-reductive electrophoresis showed surfactin could bind to INS forming heteropolymers. Analysis with circular dichroism, we found this binding significantly influenced the INS structure with decreased rigid α-helix and β-turn, but with increased flexible β-sheet and random coil. The change with more flexible structure was favorable for INS to penetrate through the cell membrane. Fluorescence spectra analysis also showed surfactin could lead Phe and Tyr in the inner of INS exposed outside, further promoting INS permeabilization by improving the hydrophobic-lipophilic interactions between INS and cell membrane. As a result, the effective permeability (Peff) of INS plus surfactin was 4.3 times of that of INS alone. In vivo assay showed oral INS with surfactin displayed excellent hypoglycemic effects with a relative bioavailability of 12.48% and 5.97% in diabetic mice and non-diabetic dogs, respectively. Summary, surfactin is potential for oral delivery of INS by its role as an effective protease inhibitor and permeability enhancer.
Introduction
Insulin (INS) is a peptide drug administered subcutaneously (s.c.), and two or three injections are required for the control of diabetes. This frequent injection remains a painful treatment leading to the patient non-compliance and unsatisfactory metabolic regulation (Shi et al., Citation2015). Repeated injections also causes lipodystrophy and damage to tissues over time. In addition, the injected INS goes into general circulation causing peripheral hyperinsulinemia, which is associated with peripheral hypertension, hypoglycemia, atherosclerosis, cancer and other harmful metabolic side effects (Nordestgaard et al., Citation1997). Therefore, oral delivery of INS is proposed for decades.
The oral route of INS administration not only improves the overall well-being of diabetic patients but also takes INS directly to the liver through portal circulation, which causes rapid hepatic insulinization (Li et al., Citation2014). However, the oral bioavailability (BA) of INS is negligible («1%) when administered in the form of a conventional solution, mainly limited by acid inactivation in the stomach, enzymatic degradation in the gastrointestinal (GI) tract, as well as the poor permeability of the intestinal membranes (Li et al., Citation2014; Shi et al., Citation2015). Therefore, the development of an efficient oral INS delivery presents an interesting challenge.
Surfactin, a bacterial lipopeptide produced by Bacillus, has a structure consisting of a cyclic heptapeptide headgroup (Glu-Leu-D-Leu-Val-Asp-D-Leu-Leu) linked to a C13-15 β-hydroxy fatty acid by lactone bond (Figure 1 in Supplementary Materials). By special cyclic peptide structure, surfactin is resistant to pepsin and trypsin digestion (Shaligram & Singhal, Citation2010). This structure also endows surfactin with a powerful biosurfactant activity by its amphiphilic nature, a polar amino acid head and a hydrocarbon chain (Carrillo et al., Citation2003; Shaligram & Singhal, Citation2010).
Recently, surfactin is gaining attention for potentially biomedical and pharmaceutical applications. Surfactin can bind to proteins (e.g. hemoglobin) to form a fractal structure representing a “necklace model” of micelle-like clusters randomly distributed along the protein polypeptide chain (Zou et al., Citation2010). In the interaction of surfactin with proteins, the hydrophobic side chain penetrates into the proteins and makes a non-covalent complex that aims to ensure proteins stability (Zou et al., Citation2010). Surfactin can also penetrate through the cell membrane. The penetration process is mainly governed by the hydrophobic interactions between fatty acid chain of surfactin and phospholipid chains of cell membrane (Shaligram & Singhal, Citation2010).
Previously, we found surfactin exhibiting excellent oral immunoadjuvant activity by protecting protein antigens from pepsin and trypsin degradation in the GI tract, and improving the uptake of antigens by the intestinal epithelial cells (Gao et al., Citation2013). Otherwise, surfactin is verified safety for oral administration in animals (Hwang et al., Citation2009). To combine all of the aforementioned advantages, we hypothesized that surfactin could be used for oral delivery of INS by its ability to bind to and improve INS to penetrate through cell membrane after oral administration.
Materials and methods
Materials and reagents
Surfactin (C15) was purified from Bacillus amyloliquefaciens WH1 culture as described previously (Qi et al., Citation2010). Recombinant human INS was a gift from JS Bioway Pharmaceutical Co., Ltd (Changzhou, China). Fluorescein isothiocyanate (FITC), NHS-Rhodamine B, Streptozotocin, pepsin and trypsin were purchased from Sigma-Aldrich (St. Louis, MO). Somatostatin for injection was purchased from Wuhan Hualong BioPharmaceutical Co., Ltd (Wuhan, China). Human INS enzyme-linked immunosorbent assay (ELISA) Kits and Canine C-Peptide ELISA kits were purchased from RayBio (Norcross, GA). All other chemicals were of analytical grade supplied by Sinopharm Chemical Reagent (Shanghai, China).
Analysis of surfactin to protect INS from pepsin and trypsin degradation in vitro
Protection of INS against enzymatic degradation by surfactin was evaluated in vitro (Zou et al., Citation2010; Li et al., Citation2013). Briefly, INS (1 mg/ml) mixed with or without surfactin (1 mg/ml), was incubated at 37 °C with 0.1% (w/v) pepsin at pH 1.2, or with 0.1% (w/v) trypsin at pH 6.8 for 30 min. Following incubation, the samples were analyzed by 15% sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE).
INS was also labeled with fluorescein (FITC-INS) (Ramkissoon-Ganorkar et al., Citation1999), then FITC-INS (1 mg/ml) with or without surfactin (1 mg/ml) was treated with pepsin at pH 1.2 as described above. After digestion, the residual precipitated particles were examined by a confocal laser scanning microscopy (CLSM) (Carl Zeiss 510-META, Oberkochen, Germany).
Determination of co-precipitate efficiency of INS and surfactin
Seventy-five micrograms of INS plus surfactin (50, 100, 200, 300 or 500 μg, respectively) were dissolved in 200 μl phosphate buffer (PB) (0.01 mol/l, pH 6.8), then the pH value was adjusted to 1.2 by 1 mol/l HCl to form the co-precipitate of surfactin and INS. The supernatant was collected by centrifugation, then the pH value was adjusted to 6.8 by 1 mol/l NaOH. Un-precipitated INS in the supernatant was determined by Human INS ELISA kits for calculating the co-precipitate efficiency of INS and surfactin according to Equation (Equation1(1) ):
(1)
where CE indicates the co-precipitate efficiency, Tins is total INS in the formulation and Wins is the quantity of INS in the supernatant.
Analysis of surfactin to bind with INS by non-reductive PAGE
Non-reductive PAGE was used to detect surfactin to bind with INS. INS (1 mg) was mixed with surfactin (1 mg) in 1 ml PB at pH 6.8, or first at pH 1.2 to form co-precipitate following to 6.8 for redissolving, then analyzed by 15% non-reductive PAGE without SDS in electrophoresis buffer (Gaofu et al., Citation2008).
Determining INS structure influenced by surfactin
The secondary structure of INS influenced by surfactin was probed by Jasco J-810 circular dichroism (CD) spectropolarimeter at 25 °C. The CD spectra of INS (0.025 mg/ml) alone, surfactin (0.0125, 0.025 or 0.05 mg/ml, respectively) alone, or INS (0.025 mg/ml) plus surfactin (0.0125, 0.025 or 0.05 mg/ml, respectively) firstly at pH 1.2 to form co-precipitate then to pH 6.8 for redissolving, were recorded with a spectral resolution of 0.1 nm. The scan speed was 100 nm/min, and the bandwidth of 1 nm and quartz cell with an optical path of 10 mm was conducted. The final spectra were obtained by subtracting the sample spectra from the related concentrations of surfactin in solution, then converted to molar ellipticity (Zou et al., Citation2010). Also, the fluorescence spectra of the above-mentioned samples were recorded to determine the microenvironment around aromatic amino acid residues like Phe and Tyr in INS in the wavelength range from 200 to 420 nm when excited at 275 nm with RF-4600 fluorescence spectrophotometer (Hitachi, Japan) (Liu et al., Citation2010).
Determination of INS absorbed by intestine ex vivo and in vivo
For ex vivo assay, INS transport in the mouse jejunum was monitored by the everted gut sacs experiment as described previously (Yin et al., Citation2009). Briefly, 0.5 ml INS (4 mg/ml) plus surfactin (4 mg/ml) or INS (4 mg/ml) alone was syringed into a 5-cm jejunum for transport study, which was incubated in oxygenated Kreb’s–Ringer buffer at 37 °C with smooth shaking. INS concentration in the incubation buffer was quantified by ELISA and the effective permeability (Peff) was calculated by the methods described previously (Yin et al., Citation2009; Zhang et al., Citation2012).
For in vivo assay, FITC-labeled surfactin (FITC-surfactin) and NHS-Rhodamine B labeled INS were synthesized and purified in the laboratory (Ramkissoon-Ganorkar et al., Citation1999). Prior to oral administration, diabetic mice fasted for 12 h and then orally given with 200 μg NHS-Rhodamine B-INS plus 200 μg FITC-surfactin or 200 μg NHS-Rhodamine B-INS alone. The animals fasted for another 0.5 h, then the small intestine (jejunum) was recovered and frozen in liquid nitrogen in the presence of tissue-freezing medium OCT (VWR, Atlanta, GA). The frozen tissues were sectioned into 10 μm thick specimens using a Leica 3050 microtome, then observed by CLSM (Jintapattanakit et al., Citation2007; Zou et al., Citation2010; Sonia et al., Citation2011).
Assessment of INS bioactivity influenced by surfactin
Assessment of INS bioactivity influenced by surfactin was described in Supplementary Materials.
Determination of hypoglycemic effects of oral INS plus surfactin in diabetic mice
Balb/C mice (5–6 weeks old, 18–20 g body weight) were purchased from Slaccas Experimental Animal Co, Ltd. (Shanghai, China) following the University Ethics Committee’s guidelines. Streptozotocin was injected intraperitoneally at a dose of 70 mg/kg in 0.1 M citrate buffer (pH 4.0). Blood samples were collected from the tail for detecting blood glucose by a glucometer of ACCU-CHEK Active (Roche, Switzerland). Animals showing blood glucose levels more than 25 mmol/l were used for the subsequent studies.
The diabetic mice (25–33 mmol/l of blood glucose, six mice per group) fasted overnight but were allowed access to water prior to and throughout the course of the experiments. First, INS (180 IU/kg) was mixed with serial doses of surfactin (100, 500 or 1000 μg/mouse, respectively) in 200 μl PB for oral administration of diabetic mice by an oral feeding needle. After the dose of surfactin was selected, serial doses of INS (180, 90 or 45 IU/kg, respectively) were mixed with surfactin in 200 μl PB for oral administration of mice as above. Control animals were s.c. injected with INS (2 IU/kg) as a positive control. Blood samples were collected for detecting blood glucose prior to oral administration as fasting glucose levels, and at different times intervals (1, 2, 4, 6, 8, 10, 12, 14, 16 and 24 h, respectively) post oral administration. Results are presented as the % reduction from the initial value. The reduction in blood glucose concentration was obtained from the blood glucose concentration-time curves (% change of initial) of each mouse by Equation (Equation2(2) ):
(2)
where F is the fasting blood glucose level, Pt is the blood glucose level at time (t) post-administration (Rekha & Sharma, Citation2009; Sonaje et al., Citation2009; Sajeesh et al., Citation2010; Makhlof et al., Citation2011).
For determination of oral BA of INS plus surfactin, 24 diabetic mice were randomly divided into two groups for 12 mice per group and fasted as described above. One group of mice were orally administered with 90 IU/kg of INS plus 100 μg/mouse of surfactin, and another group were s.c. injected with INS (2 IU/kg) as a positive control (Damgé et al., Citation2007). Blood samples were collected prior to oral administration as the fasting glucose levels, and at different times intervals (1, 2, 4, 6, 8, 10, 12, 14, 16 and 24 h, respectively) post oral administration. The samples were detected by a glucometer for blood glucose, as well by ELISA kits for serum INS concentrations. The relative oral BA of INS plus surfactin was calculated by Equation (Equation3(3) ):
(3)
The AUC0–24 is the area under the reduction of blood glucose levels from 0 to 24 h; weight (kg) is the body weight of mice and dose (IU) is the amount of INS administered to the animals (Carino et al., Citation2000; Pan et al., Citation2002; Ma et al., Citation2006; Damgé et al., Citation2007; Furtado et al., Citation2008; Kim et al., Citation2010b).
For oral glucose tolerance test, 24 diabetic mice were randomly divided into two groups for 12 mice per group, and then fasted as described above. One group of mice was orally administered with 90 IU/kg of INS plus 100 μg/mouse of surfactin, and another group was orally treated with PB as a placebo control. One hour after oral administration, all mice were orally given with 200 μl glucose solution at a dose of 2 g/kg body weight. Then, the blood glucose was determined at each time interval (Carino et al., Citation2000).
Hypoglycemic effects of oral INS plus surfactin in non-diabetic dogs
It is difficult to set up diabetic models, so health dogs are used for the assessment of INS bioactivity in many studies (Surendrakumar et al., Citation2003; Cherrington et al., Citation2004; Katsuma et al., Citation2006). Nine healthy Beagle dogs (male, 3–4 years old, 10–12 kg body weight) were purchased from Slaccas Experimental Animal Co, Ltd. The dogs were randomly divided into three groups with three dogs per group, and fasted overnight but allowed access to water prior to and throughout the experiments. The dogs were orally administered with INS (9 IU/kg) as a negative control group or s.c. injected with INS (2 IU/kg) as a positive control group, or orally administered with 9 IU/kg of INS plus 0.5 mg/kg of surfactin (Sonaje et al., Citation2010). All dogs were treated with intravenous drip of somatostatin (0.8 µg/kg/min) to inhibit the production of endogenous INS (Cherrington et al., Citation2004).
Blood samples were taken via the cephalic vein as the initial before oral administration, and at different times intervals (20, 40, 60, 80, 100, 120, 140, 160, 180, 200, 220, 240, 260, 280, 300, 320, 340, 360, 390, 420, 450 and 480 min, respectively) post oral administration. Blood glucose levels were determined by a glucometer. Serum human INS and canine C-Peptide concentrations were detected by ELISA kits, respectively. Results are presented as the % reduction from the initial value by Equation (Equation2(2) ), and the oral BA of INS by Equation (Equation3
(3) ).
Statistical analysis of data
Data from different groups were compared by ANOVA with a significant level of *p < 0.05 and **p < 0.01.
Results
Surfactin protecting INS from proteases degradation
We investigated the possible role of surfactin to protect INS from acidic and proteases degradation in vitro. In the presence of surfactin, INS was not completely degraded by pepsin at pH 1.2 or by trypsin at pH 6.8 (Lane 3, ), while it was completely degraded by pepsin or partially digested by trypsin in the absence of surfactin (Lane 2, ). Moreover, in the presence of surfactin, INS can bind to surfactin showing polymers (smeary bands) on the gel (>5 kDa) after digestion by pepsin (Lane 3, ).
Figure 1. Surfactin co-precipitating with INS to protect it from proteolytic degradation. (A) SDS-PAGE analysis of surfactin to protect INS from proteolytic degradation. INS with or without surfactin digested by pepsin at pH 1.2 or trypsin at pH 6.8. Lane M: molecular weight marker; Lane 1: INS alone; Lane 2: INS without surfactin digested by pepsin or trypsin; Lane 3: INS digested by pepsin or trypsin with surfactin. It was clearly shown that INS has been completely digested by pepsin (left panel, Lane 2) and partially digested by trypsin (right panel, Lane 2) in the absence of surfactin. (B) Fluorescent analysis of co-precipitates containing INS and surfactin after digestion by pepsin. FITC-INS (with or without surfactin) was treated with (+) or without pepsin (−) at pH 6.8 or 1.2, then the pepsin-digested precipitates were observed by CLSM. Surfactin forms co-precipitates with INS at pH 1.2 (top). Further analysis by CLSM verified that FITC-INS (indicated by dashed arrows) co-precipitates with non-fluorescent surfactin (indicated by solid arrows) at acidic pH values (bottom). (C) Co-precipitate efficiency (CE) of INS with surfactin. CE values are more than 86.08%, indicating that most of INS are precipitated in the co-precipitates.
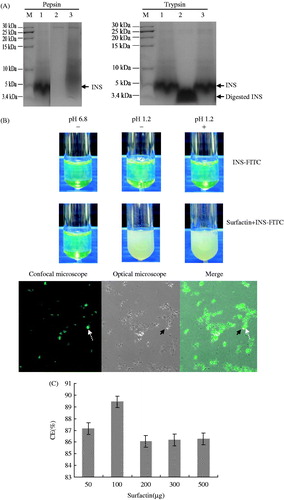
The protective effect of surfactin might be due to its ability to form co-precipitates with INS at acidic pH values. To investigate this possibility, FITC-INS was incubated with pepsin in the presence of surfactin, and the results showed surfactin could form co-precipitates with INS to protect it from pepsin degradation. As shown in , FITC-INS was found co-precipitated with surfactin at pH 1.2. After digestion by pepsin, the residual precipitates were observed by CLSM. The results clearly showed FITC-INS co-precipitates together with non-fluorescent surfactin at pH 1.2 (). The co-precipitate efficiency (CE) of INS was more than 86.08% (), indicating that most of INS were precipitated in the co-precipitates.
Surfactin binding to INS to form polymers
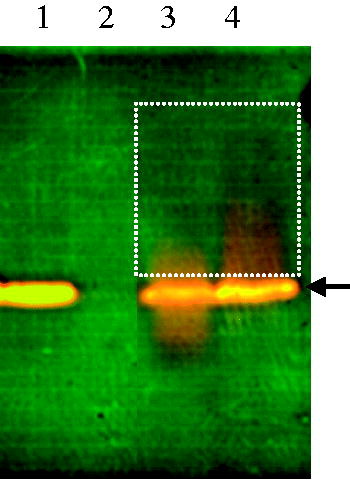
We detected surfactin binding to INS to form polymers by non-reductive PAGE. Because of low molecular weight (∼1 kDa), surfactin was not visible on the gel (Lane 2, ); however, it could bind to INS to form heteropolymers showing smeary bands on the gel (Lane 3 and 4 labeled with a dashed rectangle, ). The results clearly showed that surfactin could bind to INS either at pH 6.8 (Lane 3, ) or first at pH 1.2 then to 6.8 (Lane 4, ).
Figure 2. Non-reductive PAGE for analysis of INS binding with surfactin. INS was mixed with 1 mg/ml of surfactin. After mixing, smeary bands were found on the gel with larger molecular weights than INS (Lane 3 and 4, labeled with dashed rectangle), indicating that surfactin binds to INS to form heteropolymers. Lane 1: INS alone; lane 2: surfactin; lane 3: INS mixed with surfactin at pH 6.8; lane 4: INS mixed with surfactin at pH 1.2, then to 6.8 for redissolving.
CD and fluorescence spectra of INS/surfactin system
The CD experiments were conducted to study the INS structural variation induced by surfactin. The results showed the rigid structure of α-helix in INS decreased with the increase of surfactin concentrations in solution. In contrast, the flexible structure of β-sheet and random coil in INS increased with the increase of surfactin concentrations in solution. β-turn disappeared in the presence of surfactin while it was 27.0% in INS without surfactin (, ). The results showed surfactin strongly influenced the second structure of INS with a more flexible structure such as β-sheet and random coil, and less rigid structure of α-helix. This variation of INS structure is favorable for improving the INS permeability.
Figure 3. INS structure influenced by surfactin. INS was mixed with surfactin at pH 1.2 to form co-precipitates following to pH 6.8 for re-dissolving, then the CD or fluorescence spectrum was detected for analysis of INS structure influenced by surfactin. (A) CD spectrum of INS in the presence of surfactin. The ratio of surfactin to INS is 0.5:1, 1:1 and 2:1 (w/w), indicated by solid red, black and yellow line, respectively. (B) The fluorescence spectrum of INS in the presence of surfactin. P1: Surfactin; P2: INS; P3: INS mixed with surfactin at pH 1.2, then to 6.8; P4: INS mixed with surfactin at pH 6.8. The x-axis is wavelength (nm), and the y-axis is fluorescence intensity.
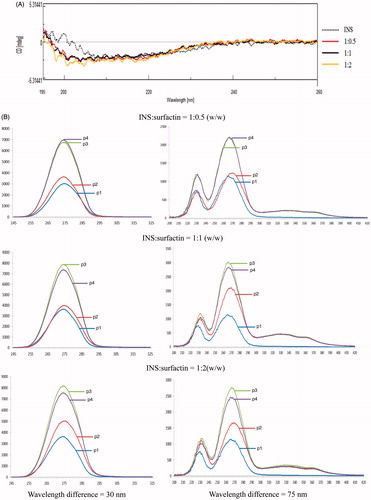
Table 1. INS structure influenced by surfactin.
The fluorescence spectrum reflects the microenvironment around aromatic amino acid residues like Phe and Tyr in proteins (Liu et al., Citation2010; Zou et al., Citation2010). The fluorescence spectrum of INS was scanned from 200 to 420 nm and two strong fluorescent intensity peaks were found around 268 nm (Wavelength difference = 75 nm) and 275 nm (Wavelength difference = 30 nm), respectively (). The results showed the fluorescent intensity of INS increased with the increase of surfactin concentrations in solution. Moreover, the fluorescent intensity of INS in the solution at pH 1.2 then to 6.8 was stronger than that at pH 6.8 only. The results indicated that the interaction between surfactin and INS resulted in the microenvironment changes around Phe and Tyr in INS, and these hydrophobic amino acid residuals in the inner of INS were exposed outside after being influenced by surfactin.
Surfactin promoting INS uptake by intestine
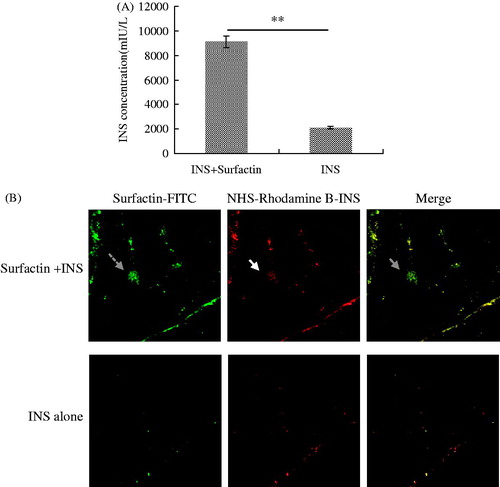
We first monitored INS transport in the mouse jejunum by the everted gut sacs experiment. The results showed INS concentration was 9104 mIU/l in the presence of surfactin while only 2098 mIU/l in the absence of surfactin (). The Peff value of INS with surfactin is about 4.3 times of the control. We further determined surfactin to improve INS uptake by the intestine in vivo. Diabetic mice were orally given with NHS-Rhodamine-INS with or without FITC-surfactin, then the intestines were sectioned into specimens for analysis by CLSM. As shown in , the labeled INS was present in much higher quantity in the intestinal tissues of mice co-administered with surfactin than the control. The labeled INS (directed by solid arrow) co-localized with FITC-surfactin (directed by dashed arrow) in the intestinal villi of mice orally administered with NHS-Rhodamine B-INS plus FITC-surfactin (directed by gray arrow), but not in the control. These data showed that surfactin could promote the INS uptake by the intestine after oral administration.
Figure 4. Surfactin improving INS permeation across intestine epithelial cells. (A) ELISA analysis of INS absorbed by the jejunum in the everted-gut sacs experiment. The Peff value of INS plus surfactin was 4.3 times of that of INS alone. N=6 per group. (B) Intestines of mice orally treated by NHS-Rhodamine B-INS with or without FITC-surfactin. Diabetic mice were orally treated with NHS-Rhodamine B-INS with or without FITC-surfactin, then the intestines of mice were sectioned and observed by CLSM (100 ×). INS with red fluorescence (directed by solid arrow) is co-located with surfactin (green fluorescence, directed by dashed arrow) in the intestinal villi of mice orally treated with NHS-Rhodamine B-INS plus FITC-surfactin (directed by gray arrow), while not in the intestinal villi of mice orally administered with NHS-Rhodamine B-INS alone.
Hypoglycemic effects of oral INS plus surfactin in diabetic mice
Surfactin had no obvious influence on INS bioactivity (Figure 2 in Supplementary Materials). We detected the hypoglycemic effects of oral INS promoted by surfactin in diabetic mice. As illustrated in , INS (180 IU/kg) plus different doses of surfactin (100, 500 or 1000 μg/mouse, respectively) all led to significant hypoglycemic effects when compared to INS alone, but the surfactin dose of 100 μg/mouse showed higher hypoglycemic effects than the other two doses of 500 and 1000 μg/mouse. The surfactin dose of 100 μg/mouse in the formulation displayed the best hypoglycemic effects, with the blood glucose depression of 18%, 25%, 35%, 42%, 49%, 46%, 42%, 34%, 25% and 33% post-administration for 1, 2, 4, 6, 8, 10, 12, 14, 16 and 24 h, respectively (). Surfactin was given as 100 μg/mouse, then mixed with different doses of INS for oral administration. It was found 90 IU/kg of INS plus 100 μg/mouse of surfactin resulted in the best hypoglycemic effects when compared to other two INS doses (45 and 180 IU/kg, respectively), with the blood glucose depression of 47%, 49%, 60%, 51%, 56%, 54%, 50%, 53%, 55% and 71% post-administration for 1, 2, 4, 6, 8, 10, 12, 14, 16 and 24 h, respectively ().
Figure 5. Oral hypoglycemic effects of INS plus surfactin in diabetic mice. (A) Mice orally treated with INS (180 IU/kg) plus different doses of surfactin (100, 500 and 1000 μg per mouse, respectively). (B) Mice orally treated with different doses of INS (180, 90 or 45 IU/kg) plus surfactin (100 μg/mouse). (C) Serum INS levels over time after oral INS (90 IU/kg) plus surfactin (100 μg/mouse) in diabetic mice. (D) OGGT for orla INS (90 IU/kg) plus surfactin (100 μg/mouse). Reported results are means ± SEM of 6 (A & B) or 12 (C & D) mice.
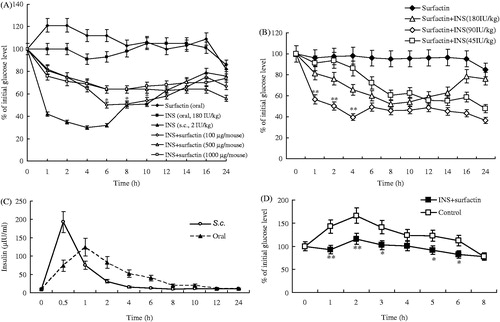
Ninety IU/kg of INS plus 100 μg/mouse of surfactin was used for oral administration of diabetic mice, then the blood INS concentrations at different timepoints were determined by ELISA. is the serum INS concentrations (μIU/ml) versus time curves. The peak INS concentration of INS plus surfactin (124.278 μIU/ml) occurred at the 1 h timepoint, then gradually decreased until the end of the experiment. The peak INS concentration of s.c. injection occurred at the 0.5 h timepoint as 192.622 μIU/ml, then decreased to 10.257 μIU/ml at the 6 h timepoint. The serum INS concentrations (μIU/ml) versus time curves were used to calculate the relative oral BA of INS as 12.48% according to equation (Equation3(3) ).
shows the comparison of the blood glucose levels in diabetic mice fed with glucose. The blood glucose levels of mice orally treated with INS plus surfactin were significantly lower than the control animals at most of the timepoints (1, 2, 3, 5 and 6 h post oral administration with glucose, respectively). The results indicated that oral INS formulated with surfactin is useful in the everyday control of glucose levels in the face of the continuous influx of glucose and possibly other carbohydrates.
Hypoglycemic effects of oral INS plus surfactin in non-diabetic dogs
Oral INS (9 IU/kg) plus surfactin (0.5 mg/kg) resulted in obvious hypoglycemic effects in non-diabetic dogs when compared to the controls, with the blood glucose depression of 6%, 10%, 8%, 16%, 2%, 8%, 15%, 6%, 2% and 4% post-administration for 80, 100, 120, 140, 260, 280, 300, 320, 390 and 450 min, respectively (). There were two periods (directed by dashed rectangles) with significant hypoglycemic effects when compared to the controls: (1) from the timepoint of 100 min to 140 min and (2) from the timepoint of 300 min to the end of the experiment.
Figure 6. Oral hypoglycemic effects of INS plus surfactin in non-diabetic dogs. (A) Reduction of blood glucose concentrations. Two periods (directed by dashed rectangles) with significant hypoglycemic effects for oral INS plus surfactin when compared to the control. (B) Serum C-peptide concentrations in dogs. (C) Serum INS levels in dogs. The peak INS concentration of oral INS plus surfactin occurred at the 140 min timepoint as 101.842 μIU/ml. Reported results are means ± SEM of three dogs.
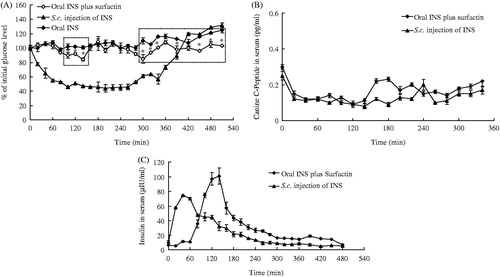
All dogs were given with somatostatin to inhibit the endogenous INS production. The results showed the concentrations of canine C-peptide at different timepoints were lower than the initial value in all groups (), indicating the endogenous INS production was successfully inhibited by somatostatin. Thus, the blood INS was mainly from the administered one. is the serum INS concentrations (μIU/ml) versus time curves. The peak INS concentration of oral INS plus surfactin occurred at the 140 min timepoint as 75.261 μIU/ml, then gradually decreased until the end of the experiment. The peak INS concentration of s.c. injection occurred at the 40 min timepoint as 101.842 μIU/ml, then decreased to the initial value at the 260 min timepoint. The relative oral BA of INS was calculated as 5.97% according to Equation (Equation3(3) ).
Discussion
To develop oral INS, different types of drug carriers have been employed to protect INS from the harsh environment of the GI tract and deliver it to the target side in the body (Mansourpour et al., Citation2015). For example, chemical modification, absorption enhancers, mucoadhesive polymers, protease inhibitors and permeation enhancers as well as encapsulation in drug delivery systems, or even a combination of the aforementioned strategies have been adopted for improving the BA of oral INS (Li et al., Citation2014; Jain & Jain, Citation2015). In this study, surfactin was used to protect INS from the harsh environment of the GI tract, and improve the INS absorption by the intestine epithelial cells.
Surfactin can form co-precipitates with INS at acidic pH values to protect it from acidic and enzymatic attack. When re-dissolved at pH 6.8, surfactin binds to INS to form heteropolymers, protecting INS from enzymatic attack and promoting it through the intestinal epithelium cell membrane. Interestingly, heteropolymers were easily formed when the pH value was firstly at 1.2 to form co-precipitates, then to 6.8 for redissolving. In this progress, INS (pI = 5.6) is loaded with positive charges while surfactin (pI = 2) with negative charges. Thus, INS can bind to surfactin by electrostatic interaction. Once binding, surfactin further interacts with INS by hydrophobic interaction to form mosaic polymers. Surfactin is reported with “detergent-like” activity on cell membrane with unspecific mechanisms of membrane permeabilization (Shaligram & Singhal, Citation2010; Deleu et al., Citation2013), and possesses excellent chain flexibility and mobility to inter-diffuse across the mucosal barriers (Carrillo et al., Citation2003). Thus, surfactin is favorable for promoting INS across the epithelial cell membrane after binding.
Surfactin fulfills several roles including proteases inhibitor, permeabilization enhancer and pH-sensitive system for oral delivery of INS in this study. Compared to other proteins/peptides, e.g. variant-specific surface protein for resisting acidic pH and proteolytic degradation in the GI tract, or aprotinin, soybean trypsin inhibitor and ovomucoid as protease inhibitors (Kanzarkar et al., Citation2015), surfactin acts as protease inhibitor with different mechanisms for protecting INS from protease digestion. Surfactin can form co-precipitates with INS to resist pepsin degradation in the stomach, and bind to INS to form heteropolymer for inhibiting trypsin attack in the intestine.
As a permeability enhancer, surfactin can bind to INS by non-covalent interactions, then promote INS to permeate across the intestine layer by changing the INS structure with more flexible β-sheet and random coil but less rigid α-helix. Surfactin is also reported to reduce α-helix in other proteins such as hemoglobin or lysozyme (Liu et al., Citation2010; Zou et al., Citation2010). The flexible structure of a protein is favorable for permeation across the cell membrane. For example, both human ribonuclease 7 and piscidin 1 (a novel cytotoxic peptide) analog with flexible structure are found with a marked increase in membrane permeability to incorporate into the bacterial membrane for a high antibacterial activity (Huang et al., Citation2007; Kim et al., Citation2010a). Therefore, we suggested that a flexible INS is favorable for permeating through the intestine epithelial cell membrane.
The fluorescence spectrum of INS is also influenced by surfactin with stronger fluorescence intensity observed, indicating the microenvironment is changed around Phe and Tyr in INS. These hydrophobic amino acid residues located in the inner are exposed outside of INS in the presence of surfactin, which can further increase INS permeability by enhancing the hydrophobic interactions between INS and phospholipid bilayer of the membrane. Moreover, surfactin can promote INS to permeate the intestine layer by its “detergent-like” action on the cell membrane (Zou et al., Citation2010; Deleu et al., Citation2013). Therefore, the biomolecule of surfactin is different from other peptide permeability enhancers such as zonula occludens toxin receptor agonist, which promotes INS permeability by modulating the tight junctions of the epithelial cell membrane (Kanzarkar et al., Citation2015).
The high molecular weight of INS, along with the lack of lipophilicity, inhibits this hormone from permeating the intestinal epithelium. Additionally, 50% of the INS are degraded inside the liver via the first-pass hepatic INS extraction (Sonia & Sharma, Citation2013), resulting in a poor BA of INS in the systemic circulation. Here, surfactin provides a possibility for solving these problems by its characteristics as a protease inhibitor and permeability enhancer. Hypoglycemic effects are observed for this surfactin-based oral INS delivery, with a relative BA of 12.48% in diabetic mice and a relative BA of 5.97% in dogs, which is similar to the level of INS inhalation (Exubera, Pfizer, NY) (Cherrington et al., Citation2004).
In this study, high doses of surfactin (1000 and 500 μg/mouse, respectively) did not show higher hypoglycemic effects than the low dose of 100 μg/mouse in the formulation (). This might be due to the reason that surfactin can form micelle at high concentrations (Zou et al., Citation2010). By this reason, the high concentration of surfactin influences its binding to INS. Similarly, the INS dose of 90 IU/kg is not only more efficient than the low dose of 45 IU/kg but also more efficient than the high dose of 180 IU/kg. We deduced that INS can efficiently bind with surfactin at an appropriate ratio. If more INS used, many INS molecules cannot be bound with an appropriate number of surfactin molecules. Thereby, these INS molecules cannot be efficiently absorbed by the intestine. As a result, the higher dose of INS did not show more hypoglycemic effects than the 90 IU/kg dose. We also found hypoglycemic effects of oral INS in non-diabetic dogs () are lower than that in diabetic mice (). This might be due to different animals used in the study. This is also observed in the groups with injected INS, the hypoglycemic effects in non-diabetic dogs are also lower than that in diabetic mice.
Conclusion
Surfactin is potential for developing novel oral INS delivery by its roles as: (1) pH-sensitive system to form co-precipitates with INS at acidic pH values, and re-dissolving at neutral pH values; (2) protease inhibitor with resistance to acidic and pepsin degradation in the stomach, and trypsin attack in the intestine; (3) permeability enhancer by binding to and changing INS with more flexible but less rigid structures, exposing hydrophobic amino acid residuals outside, and carrying INS across the intestine by “detergent-like” activity.
Supplementary material available online
Supplementary_materials.doc
Download MS Word (32 KB)Declaration of interest
The authors report no declarations of interest. The authors are thankful for the Project SRF150 supported by the National Natural Science Foundation of China.
References
- Carino GP, Jacob JS, Mathiowitz E. (2000). Nanosphere based oral insulin delivery. J Control Release 65:261–9
- Carrillo C, Teruel JA, Aranda FJ, Ortiz A. (2003). Molecular mechanism of membrane permeabilization by the peptide antibiotic surfactin. Biochim Biophys Acta 1611:91–7
- Cherrington AD, Neal DW, Edgerton DS, et al. (2004). Inhalation of insulin in dogs: assessment of insulin levels and comparison to subcutaneous injection. Diabetes 53:877–81
- Damgé C, Maincent P, Ubrich N. (2007). Oral delivery of insulin associated to polymeric nanoparticles in diabetic rats. J Control Release 117:163–70
- Deleu M, Lorent J, Lins L, et al. (2013). Effects of surfactin on membrane models displaying lipid phase separation. Biochim Biophys Acta 1828:801–15
- Furtado S, Abramson D, Burrill R, et al. (2008). Oral delivery of insulin loaded poly(fumaric-co-sebacic) anhydride microspheres. Int J Pharm 347:149–55
- Gao Z, Zhao X, Lee S, et al. (2013). WH1fungin a surfactin cyclic lipopeptide is a novel oral immunoadjuvant. Vaccine 31:2796–803
- Gaofu Q, Shiqing M, Fayin Z, et al. (2008). In vitro assessment of plant lectins with anti-pinwood nematode activity. J Invertebr Pathol 98:40–5
- Huang YC, Lin YM, Chang TW, et al. (2007). The flexible and clustered lysine residues of human ribonuclease 7 are critical for membrane permeability and antimicrobial activity. J Biol Chem 282:4626–33
- Hwang YH, Kim MS, Song IB, et al. (2009). Subacute (28 day) toxicity of Surfactin C, a lipopeptide produced by Bacillus subtilis, in rats. J Health Sci 55:351–5
- Jain A, Jain SK. (2015). L-Valine appended PLGA nanoparticles for oral insulin delivery. Acta Diabetol 52:663–76
- Jintapattanakit A, Junyaprasert VB, Mao S, et al. (2007). Peroral delivery of insulin using chitosan derivatives: a comparative study of polyelectrolyte nanocomplexes and nanoparticles. Int J Pharm 342:240–9
- Kanzarkar M, Pathak PP, Vaidya M, et al. (2015). Oral insulin delivery system for diabetes mellitus. Pharm Pat Anal 4:29–36
- Katsuma M, Watanabe S, Kawai H, et al. (2006). Effects of absorption promoters on insulin absorption through colon-targeted delivery. Int J Pharm 307:156–62
- Kim JK, Lee SA, Shin S, et al. (2010a). Structural flexibility and the positive charges are the key factors in bacterial cell selectivity and membrane penetration of peptoid-substituted analog of Piscidin 1. Biochim Biophys Acta 1798:1913–25
- Kim SK, Lee S, Jin S, et al. (2010b). Diabetes correction in pancreatectomized canines by orally absorbable insulin-deoxycholate complex. Mol Pharm 7:708–17
- Li X, Guo S, Zhu C, et al. (2013). Intestinal mucosa permeability following oral insulin delivery using core shell corona nanolipoparticles. Biomaterials 34:9678–87
- Li P, Tan A, Prestidge CA, et al. (2014). Self-nanoemulsifying drug delivery systems for oral insulin delivery: in vitro and in vivo evaluations of enteric coating and drug loading. Int J Pharm 477:390–8
- Liu J, Zou A, Mu B. (2010). Toluidine blue: aggregation properties and distribution behavior in surfactin micelle solution. Colloids Surf B Biointerfaces 75:496–500
- Ma E, Ma H, Liu Z, et al. (2006). In vitro and in vivo evaluation of a novel oral insulin formulation. Acta Pharmacol Sin 27:1382–8
- Makhlof A, Tozuka Y, Takeuchi H. (2011). Design and evaluation of novel pH-sensitive chitosan nanoparticles for oral insulin delivery. Eur J Pharm Sci 42:445–51
- Mansourpour M, Mahjub R, Amini M, et al. (2015). Development of acid-resistant alginate/trimethyl chitosan nanoparticles containing cationic β-cyclodextrin polymers for insulin oral delivery. AAPS PharmSciTech 16:952–62
- Nordestgaard BG, Agerholm-Larsen B, Stender S. (1997). Effect of exogenous hyperinsulinaemia on atherogenesis in cholesterol-fed rabbits. Diabetologia 40:512–20
- Pan Y, Li Y, Zhao H, et al. (2002). Bioadhesive polysaccharide in protein delivery system: chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int J Pharm 249:139–47
- Qi G, Zhu F, Du P, et al. (2010). Lipopeptide induces apoptosis in fungal cells by a mitochondria-dependent pathway. Peptides 31:1978–86
- Ramkissoon-Ganorkar C, Liu F, Baudys M, Kim SW. (1999). Modulating insulin-release profile from pH/thermosensitive polymeric beads through polymer molecular weight. J Control Release 59:287–98
- Rekha MR, Sharma CP. (2009). Synthesis and evaluation of lauryl succinyl chitosan particles towards oral insulin delivery and absorption. J Control Release 135:144–51
- Sajeesh S, Bouchemal K, Marsaud V, et al. (2010). Cyclodextrin complexed insulin encapsulated hydrogel microparticles: an oral delivery system for insulin. J Control Release 147:377–84
- Shaligram NS, Singhal RS. (2010). Surfactin – a review on biosynthesis, fermentation, purification and applications. Food Technol Biotechnol 48:119–34
- Shi K, Fang Y, Kan Q, et al. (2015). Surface functional modification of self-assembled insulin nanospheres for improving intestinal absorption. Int J Biol Macromol 74:49–60
- Sonaje K, Lin YH, Juang JH, et al. (2009). In vivo evaluation of safety and efficacy of self-assembled nanoparticles for oral insulin delivery. Biomaterials 30:2329–39
- Sonaje K, Chen YJ, Chen HL, et al. (2010). Enteric-coated capsules filled with freeze-dried chitosan/poly(gamma-glutamic acid) nanoparticles for oral insulin delivery. Biomaterials 31:3384–94
- Sonia TA, Sharma CP. (2013). In vitro evaluation of quaternized polydimethylaminoethylmethacrylate sub-microparticles for oral insulin delivery. J Biomater Appl 28:62–73
- Sonia TA, Rekha MR, Sharma CP. (2011). Bioadhesive hydrophobic chitosan microparticles for oral delivery of insulin: in vitro characterization and in vivo uptake studies. J Appl Polym Sci 119:2902–10
- Surendrakumar K, Martyn GP, Hodgers EC, et al. (2003). Sustained release of insulin from sodium hyaluronate based dry powder formulations after pulmonary delivery to beagle dogs. J Control Release 91:385–94
- Yin L, Ding J, He C, et al. (2009). Drug permeability and mucoadhesion properties of thiolated trimethyl chitosan nanoparticles in oral insulin delivery. Biomaterials 30:5691–700
- Zhang J, Liu D, Huang Y, et al. (2012). Biopharmaceutics classification and intestinal absorption study of apigenin. Int J Pharm 436:311–17
- Zou A, Liu J, Garamus VM, et al. (2010). Interaction between the natural lipopeptide [Glu1,) Asp5)] surfactin-C15 and hemoglobin in aqueous solution. Biomacromolecules 11:593–9
