Abstract
The objective of the study was to develop, optimize and evaluate a nanoemulsion (NE) of Amphotericin B (AmB) using excipients with inherent antifungal activities (Candida albicans and Aspergillus niger) for topical delivery. AmB-loaded NE was prepared using Capmul PG8 (CPG8), labrasol and polyethylene glycol-400 by spontaneous titration method and evaluated for mean particle size, polydispersity index, zeta potential and zone of inhibition (ZOI). NE6 composed of CPG8 (15%w/w), Smix (24%w/w) and water (61%w/w) was finally selected as optimized NE. AmB-NE6 was studied for improved in vitro release, ex vivo skin permeation and deposition using the Franz diffusion cell across the rat skin followed with drug penetration using confocal laser scanning microscopy (CLSM) as compared to drug solution (DS) and commercial Fungisome®. The results of in vitro studies exhibited the maximum ZOI value of NE6 as 19.1 ± 1.4 and 22.8 ± 2.0 mm against A. niger and C. albicans, respectively, along with desired globular size (49.5 ± 1.5 nm), zeta potential (−24.59 mV) and spherical morphology. AmB-NE6 revealed slow and sustained release of AmB as compared to DS in buffer solution (pH 7.4). Furthermore, AmB-NE6 elicited the highest flux rate (22.88 ± 1.7 μg/cm2/h) as compared to DS (2.7 ± 0.02 μg/cm2/h) and Fungisome® (11.5 ± 1.0 μg/cm2/h). Moreover, the enhancement ratio and drug deposition were found to be highest in AmB-NE6 than DS across the stratum corneum barrier. Finally, CLSM results corroborated enhanced penetration of the AmB-NE6 across the skin as compared to Fungisome® and DS suggesting an efficient, stable and sustained topical delivery.
Introduction
Amphotericin B (AmB) is a potent polyene macrolide as broad-spectrum antibiotic to treat life-threatening systemic and local fungal infections with restricted therapeutic efficacy owing to poor water solubility and severe nephrotoxicities (Kleinberg, Citation2006; Nahar et al., Citation2008; Shafaat et al., Citation2013). The conventional commercial dosage form (Fungizone®) is available as a micellar suspension using the sodium deoxycholate as bile salt (Wong-Beringer et al., Citation1998). However, the clinical use of this micellar formulation is challenging due to fever, chills, nausea, vomiting, anemia and nephrotoxicity after injection (Espuelas et al., Citation1997). Lipid-based vesicular and nanoemulsion (NE) formulations of AmB have been reported with reduced toxicity, drug-related side effects and improved therapeutic potential. However, liposomal drug delivery was challengeable due to the physical and chemical instability on storage for the formulation scientists and designers (Glavas-Dodov et al., Citation2005; Hussain et al., Citation2013). The major challenge of topical delivery is the stratum corneum (SC) strata as a barrier that could lead to limited percutaneous permeation for numerous drug molecules. However, the SC barrier may be modified using suitable surfactants in NE which make the SC layer more loosened and permeable (Hussain et al., Citation2013). Moreover, topical delivery of AmB offered several advantages over conventional delivery to achieve site-specific and targeted delivery to the infected site in order to maximize therapeutic efficacy and reduce undesirable effects. In our preceding research articles, we extensively reported sefsol-218-based stable NE formulations for topical delivery of AmB and reported enhanced permeation of the drug with minimum systemic toxicity assessed by acute and sub-acute toxicity studies. Further, the mechanistic evaluation of penetration by NE was corroborated by confocal laser scanning microscopy (CLSM) study of treated rat skin followed with long-term stabilities studies at varied temperature and pH (Hussain et al., Citation2013, Citation2014a,Citationb).
NE is a thermodynamically stable carrier and has been exploited for topical delivery because of enhanced permeation, high drug loading efficiency and cost-effective formulation. Lipophilic drugs can be loaded and stabilized in NE as reported with sefsol-218 NE for AmB and rifampicin (Ahmed et al., Citation2008; Hussain et al., Citation2013). Extensive literature review dictated that various reports have been published to claim the lipidic NE as evidence for bactericidal activities which could produce a more detrimental effect when laden with antibiotic in such carrier (Singh et al., Citation2015). Moreover, Joe et al. (Citation2012) has highlighted the antibacterial potential of NE against clinical pathogens such as Haemphilus influenzae, Bacillus cereus, Neisseria gonorrhoeae, Streptococcus pneumonia and Vibrio cholera. This approach could be used by developing NE with lipid and surfactants holding inherent antifungal activities. This may lead to synergistic activities with AmB and reduced drug-related toxicity owing to a reduction in the unnecessary introduction of AmB and excipients into the patient body. Furthermore, low-cost mineral oil-based NE had extremely high bactericidal activity against both pathogens (Chang et al., Citation2012). Kolhapure et al. (Citation2015) has been highlighted for enhanced delivery of antibiotics using nanoengineering approach as drug delivery.
In this study, an attempt has been made to develop NE formulation using capmul PG8 (CPG8) as lipid, labrasol (LAB) as a surfactant with inherent antifungal activities by slow spontaneous titration method. NE formulations were then characterized and evaluated to select the optimized NE so that AmB could be loaded. Furthermore, AmB-loaded NE was evaluated for particle size, zeta potential, morphology, antifungal activities and in vitro drug release as compared to the drug solution (DS) and Fungisome®. Ex vivo permeation and CLSM studies ensured sufficient localization and penetration through the rat skin for augmented effect as compared to the Fungisome® and the dye solution, respectively. This study addressed a fundamental inspiration to confer a concerted antifungal activity of lipid NE and AmB laden NE working in tandem against fungal strains.
Materials and methods
Materials
Both strains Candida albicans (MTCC 4748) and Aspergillus niger (MTCC 282) were obtained from IMTECH Chandigarh, India. AmB (86.04% pure) was generously gifted from Kwality Pvt. Ltd., (Amritsar, India). Polyethylene glycol 400 (PEG-400) and Tween®-80 (97% pure) were procured from Himedia (Mumbai, India). LAB and CPG8 were a kind contribution from Gattefosse (Saint-priest, France) and ABITEC Corporation (Janesville, Germany), respectively. Fungisome® liposomal cream (0.01% w/w) (Lifecare Innovation Private Ltd., Gurgaon, India) was procured from a local medical shop. All reagents and solvents were of the analytical grade used in the experiments.
Methods
Solubility study
The solubility of AmB in various lipids (Capmul MCM C8, Peceol, Captex 355 and Miglyol-829) and surfactants (LAB, Tween-80, Transcutol-P, Cremophor-EL, Acconon-C80 and PEG-400) was carried out quantitatively. The drug in excess was added to 2 ml of excipient (oils and surfactants) using stopper glass vial (10 ml) individually with a vortex mixer for 10 min. Then, it was placed in shaker water bath (an isothermal shaker) at 40 ± 1 °C for 48 h for maximum solubility. Now, vials were removed and centrifuged at 3000 rpm for 15 min to separate the supernatant for filtration using the membrane filter (0.45 μm). Finally, the content of AmB was estimated quantitatively using the UV-Vis Spectrophotometer (Shimadzu, U-1800 Spectrophotometer, Tokyo, Japan) at 382 nm by diluting with methanol (Hussain et al., Citation2014b).
In vitro antifungal activities assessment by the zone of inhibition (ZOI)
Antifungal assessment of various lipids and surfactants was determined using the agar well diffusion method with slight modification (Kadimi et al., Citation2007; Hussain et al., Citation2014b). The C. albicans (MTCC 4748) and A. niger (MTCC 282) were employed to culture into the Sabouraud Dextrose (SD) broth media at pH 6.8 for antifungal activities in term of the ZOI. Next, the nutrient agar media was prepared in distilled water and sterilized using autoclave at 121 °C for 15 min. The nutrient agar media was then allowed to cool at room temperature aseptically. One milliliter of culture (C. albicans, 4 × 105 CFU/ml) was mixed with the 25 ml of SD agar media. The mixture was slowly poured into the sterile Petri-plates under the laminar chamber to get solidified. The wells were created using the sterilized borer and filled with the test sample followed by incubation for 48 h at 37 ± 1 °C. A similar procedure and protocol were adopted for A. niger (1 × 105 CFU/ml) and incubated for 48 h at the same temperature. The ZOI around the well was measured in mm using vernier caliper scale and expressed as in mean value. The excipients were tested using 10% solution (water soluble excipients) or 10% emulsion (lipid emulsified with inactive surfactant).
Preparation of nanoemulsion and dye (Rhodamine-123)-loaded NE
Different NE formulations were developed by the slow spontaneous titration method with slight modification as reported (Azeem et al., Citation2009; Hussain et al., Citation2013). Based on the maximum solubility and ZOI values, CPG8 (as oil) and LAB as well as PEG-400 (as co-surfactant) were eventually selected for NE preparation. It is a well-established fact that the minimum concentration of surfactant is desired for the selection of optimized formula from the phase diagram. Hence, CPG8 was selected as oil phase in the present study due to maximum ZOI, drug solubility and oil considered as generally regarded as safe category. LAB and PEG-400 were used as the prime surfactant and co-surfactant respectively in developing the NEs in the present investigation. Different weight ratios combination (1:2, 1:3 and 2:1) of LAB and PEG-400 as (Smix) were attempted. Then, CPG8 and a particular Smix ratio were mixed completely with increasing concentration of one with respect to the other and vice versa (from 1:9 to 9:1) in individual stopper glass vials. Best 12 varied combinations (oil:Smix) were obtained to delineate the precise boundary of pseudo-ternary phase diagram (TPD). Each combination was slowly titrated against aqueous phase to obtain clear and stable oil in water NE (visually inspected). One axis for aqueous phase, the second axis for the Smix and third for oil phase of TPD is separately represented in phase diagram for specific Smix ratio. Oil and LAB showed maximum drug solubility and antifungal activities (ZOI) but PEG-400 (water-soluble alcohol) had no any antifungal activity which was selected as complementary co-surfactant in this study. A fixed amount of AmB (100 mg) was completely dissolved in 1 ml of the CPG8 and dimethyl sulfoxide (DMSO) combination (1:1). Finally, an optimized AmB-NE6 was developed by taking suitable Smix ratio and oil with slow titration against aqueous phase. The appearance of turbidity was the end point in the titration procedure and consumed volume was recorded. The final pH of the prepared NE was adjusted to 7.4 for maximum drug stability and compatible application topically.
For AmB and Rhodamine 123 dye (R123)-loaded NE preparation, a constant concentration of R123 (0.01% w/v) was incorporated into the oil phase following the same preceding procedure as described earlier in this section. Control R123 dye solution (0.01% w/v) was prepared in distilled water.
Characterization NE and AmB-NE6
Blank NE and drug-loaded formulations were subjected to characterize for particle size and size distribution followed with zeta potential measurement using the dynamic light scattering (DLS) technique (Malvern Zetamaster, ZEM 5002, Worcestershire, UK) as reported method (Attwood et al., Citation1992; Hussain et al., Citation2015; Hussain & Singh, Citation2016). DLS is frequently employed method working on population-based particle size analysis. NEs were freshly prepared to analyze the particle size after dilution with distilled water (100-folds) and then monitored at 25 °C with a scattering angle of 90°. Each ml of the test sample was diluted with distilled water (99 ml) in a glass beaker under constant stirring with a glass rod to obtain homogeneously distributed NE globules in bulk. The mixture obtained so was transferred to sample holder using a syringe for analysis. The run conditions were He-Ne Red laser, at a power of 4.0 mW, followed with absorption wavelength of 633 nm and the refractive index of 1.333. Finally, the zeta potential (mV) of the formulations was determined using the same instrument following similar experimental procedure without dilution. All experimental runs were performed in triplicate to obtain a mean data.
Morphological study using transmission electron microscopy (TEM)
The morphological assessment (for their size and shape) of blank and drug-loaded NE formulation was investigated using TEM technique (Tecnail 2, 120 KV, FEI Company, Eindhoven, The Netherlands). All formulations to be tested (1 ml) were diluted with Millipore water (100-folds) followed with constant stirring to ensure homogeneous mixing. Then, a drop of the formulation was placed on a copper grid previously coated with carbon film. The excess sample was removed from the grid using a non-shedding filter paper. Finally, a drop of negative staining agent (Phosphotungstic acid solution, 2% w/v) was dropped on the copper grid for ease of scanning while analysis (Utreja et al., Citation2011). After 2 min of the contact period, the excess agent was removed with filter paper. The grid was allowed to dry in open air at ambient temperature (25 °C) before under scanning and image analysis.
In vitro drug release study
The in vitro drug release profile of the AmB solution, AmB-NE6 and the commercial Fungisome® (0.01% w/w) was performed using dialysis membrane (molecular weight cut off 14 000 Dalton, Himedia Labs, Mumbai, India) followed with the procedure described earlier in our previous report (Hussain et al., Citation2013). Poor solubility of the drug in water and buffer (phosphate buffer, pH 7.4) required 2% v/v DMSO in the medium to maintain the sink condition throughout the study that improves the diffusion rate. A constant quantity of the drug (∼ 0.1 mg) from each formulation was placed on the dialysis membrane and suspended in the beaker containing phosphate buffer media (100 ml, pH 7.4). The release study was carried out under constant stirring (100 rpm) at 32 ± 1 °C for 12 h. Sampling (1 ml) was carried out at 0.5, 1, 2, 4, 6, 8, 10 and 12 h time points followed by replacement with equal volume of fresh buffer solution. Filtered samples were analyzed using UV-Vis Spectrophotometer at λmax 382 nm (U-2800, Hitachi, Tokyo, Japan) in triplicate. Dilution was made (if needed) with the same buffer solution.
Ex vivo skin permeation and drug deposition studies
Ex vivo skin permeation and drug deposition potential of the developed NE (AmB-NE6) were determined using the Franz diffusion apparatus with a specified diffusion area (3.104 cm2) and receptor volume (22.5 ml) across the abdominal albino rat skin (Hussain et al., Citation2014a,b, 2015). The DS (1 mg/ml) was used as the control and the Fungisome® as the commercial sample in this study for comparison. The abdominal section of the rat skin was surgically excised followed with washing using saline solution. The prepared skin was then placed between the chambers (donor and acceptor) of the Franz diffusion cell with the dermis layer facing the receptor medium of the chamber. The formulation (100 mg) with an equivalent amount of AmB (1 mg) was consistently applied on the epidermal layer of the skin in the donor compartment. The receptor chamber was filled with the buffer solution (PBS, pH 7.4) containing DMSO (2% v/v) under constant stirring with magnetic bead and maintained at 32 ± 1 °C to simulate the skin temperature in the experiment. Sampling was performed as described in “In vitro Drug Release” section at time intervals of 0.5, 1, 2, 4, 6, 8, 10, 12, 16, 20 and 24 h. The amount of the drug was estimated using previous validated HPLC method as reported earlier by us (Hussain et al., Citation2013). In order to determine the drug content deposited into the skin, the formulation adhered to the skin surface was removed by washing with the PBS after the commencement of the experiment (24 h).
Minimum inhibitory concentration (MIC) determination using resazurin assay
The MIC value of the drug-loaded NE was determined using resazurin-microtiter plate method (96-well plate techniques) with slight modification as reported earlier (Singh et al., Citation2015). The method was adopted owing to its precise and sensitive results to compare the earlier results. The study was performed by adding 50 μl of AmB and AmB-NE6 in first three (1, 2, 3) and last three (4, 5, 6) rows respectively with 50 μl of NB (nutrient broth) previously added to each well. Similar procedure was applied for blank NE6 in first three rows. Serial dilution was carried out to contain 50 μl of the sample in each well followed by the addition of 10 μl of the dye and 10 μl culture (∼105 CFU/mL for both strains). The decreasing concentrations of the drug in each well were obtained which were different for different formulations such as NE6, AmB-NE6 and AmB solution in DMSO. Last two horizontal rows were served as a positive and negative control confirming the growth of fungal for its viability without sample and absence of any fungal growth for sterility of working conditions (including samples), respectively. The outermost each wells were filled with sterilized water to avoid dryness of the plate under incubation time or evaporation of moisture. The plates were properly wrapped and sealed with paraffin film to prevent any cross contamination. Each plate was taken in triplicate and incubated at 37 °C for 48 h. The change of color from the blue to pink indicates the growth of fungal strain while persistent of a blue color indicates the absence of any fungal strains. The lowest concentration showing the complete absence of pink color was considered as MIC value against that strain and expressed in μg/ml (or mg/ml).
Penetration across the skin layer (an in vivo study)
To corroborate the findings of the enhanced permeation and drug deposition studies of the developed NE, a prime requirement of penetration study using CLSM was needed in albino rats (∼200–300 g) using either sex. All the grouped animals were housed in respective cages under standard laboratory conditions with free access to food and water ad libitum. The protocol of the study was approved by the animal ethical committee as reported earlier (Hussain et al., Citation2013).
The oil loaded with AmB in NE formulations has been investigated with effective drug and probe Rhodamine 123 distribution across the various strata of the skin by CLSM. In this study, Rhodamine 123 (0.5 mg/ml) was loaded as probe-dye in each formulation for visualization of formulation into the deeper layer of the rat skin in penetration study. Animals were randomly grouped into three groups containing three rats in each group. The dorsal skin (portion) was made free of hairs by shaving without any injury or cut marks. The penetration potential of the optimized NE containing probe (AmB-NE Rh123) was studied and compared against the dye solution and blank NE when topically applied in defined area (1 cm2). The group A, B and C were served for the dye solution, NE and AmB-NE Rh123, respectively. Formulations were applied to the marked area and observed for 24 h. Rats were ethically sacrificed by cervical dislocation to obtain the excised skin followed by washing the skin thrice using ethanol on foil paper. The excised area of the skin was then removed and sliced into the pieces of 1 mm2 size for evaluation of the probe penetration (depth) using the CLSM (Fluorescence Correlation Microscope-Olympus FluoView FV1000, Olympus, Melville, NY) with an argon laser beam with excitation at 488 nm and emission at 590 nm (Hussain et al., Citation2014b).
Results and discussion
Solubility studies
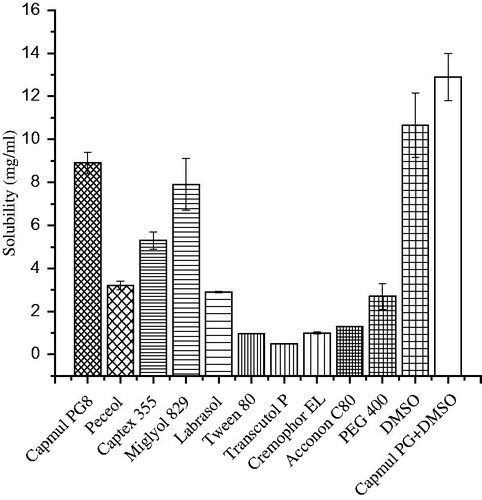
The solubility of AmB in various lipids and surfactants was carried out to select the excipients with maximum solubilizing capacity. Solubility (mg/ml) of the AmB in excipients has been portrayed in . The drug is poor water-soluble and less permeation across the biological membrane due to a long chain hydrophobic portion in its molecular structure and high molecular weight (924 Da), respectively, leading to extremely low oral bioavailability (0.3%) (Ouellette et al., Citation2004). These two properties of the drug rendered a challenge for formulation scientist to deliver efficiently and economically. Among lipids and surfactants, CPG8 and LAB showed 8.8 ± 0.5 and 2.9 ± 0.03 mg/ml as maximum solubility values. However, the combined mixture of CPG8 and DMSO in 3:1 ratio revealed additive effect in solubility (12.9 ± 1.1 mg/ml). The solubility of AmB in Tween-80 and Peceol (3.2 ± 0.5 mg/ml) was found to be comparable to reported value (Hussain et al., Citation2013). Overall result depicted that lipophilic nature of the drug assisted its solubility in lipids as compared to hydrophilic surfactants with a higher value of HLB (>10).
In vitro antifungal activities by the ZOI
In vitro antifungal activities of lipids and surfactants were determined using agar well diffusion method for the selection of the effective excipients with inherent antifungal activity against both strains. The results of ZOI values have been illustrated in and (for selected excipients). Lipids were found to be less diffusible into the growth media and, therefore, emulsified with inactive surfactant (cremophor-EL) to make it easily diffusible (Hussain & Singh, Citation2016). The greater the inhibitory circle around the well, the more effective the excipient worked and expressed in mm. The results showed that the maximum ZOI values was found in CPG8 with 17.75 ± 1.2 and 22.25 ± 1.8 mm against A. niger and C. albicans, respectively suggesting more susceptibility against the Candida strain as compared to Aspergillus. Moreover, LAB demonstrated the maximum ZOI values as 11.0 ± 0.4 and 12.2 ± 0.6 mm against the A. niger and C. albicans respectively indicating comparative effectiveness in both strains. Chemically, CPG8 and LAB are constituted of capric (C10:0) as well as caprylic content (C8:0) and a mixture of several compounds as reported in our previous publication (Hussain & Singh, Citation2016). Antifungal activities of these potent excipients may be due to capric and caprylic content as major constituents in the lipid and surfactant which could be a reasonable reason to select the excipients in NE formulation. Therefore, we employed the particular amalgamation of this lipid (CPG8) and surfactant (LAB) that holds an innate antifungal activity which could be transformed into NE for hosting and ferrying the AmB. This fundamental inspiration was to confer a concerted antifungal activity of lipid NE and the AmB (if ladened) working in tandem. A similar concept was reported against bacterial strain using cationic and non-cationic nanoemusion (Singh et al., Citation2015). This approach could a suitable alternative to reduce the dose of AmB and dose-related side effects. Moreover, there is no need to introduce an unnecessary amount of AmB and excipients into the patient body using this carrier with inherent antifungal activities.
Figure 2. Photographs of the zone of inhibition: (A–C) the Petri plates revealing control, capmul PG8 (CPG8) and capmul MCM C8 (C8), respectively against C. albicans. (D–F) the Petri plates showing control, CPG8 and C-8, respectively against A. niger. The data represents the mean ± SD, (n = 3).
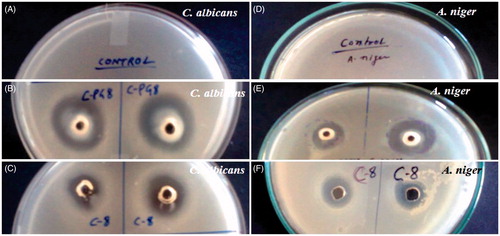
Table 1. Antifungal activities (zone of inhibition) against C. albicans and A. niger.
Preparation of nanoemulsion formulations
Based on the maximum drug solubility and ZOI values, CPG8 and LAB were selected as final excipients for NE fabrication using inactive PEG-400 as co-surfactant. Several pseudo-TPD were delineated using varied Smix ratio (surfactant to co-surfactant) to obtain series of formulations (NE1-NE6) by slow spontaneous titration method as reported earlier (Hussain et al., Citation2013). Thus, TPD were constructed using the PEG-400 as co-surfactant and the LAB (surfactant) in the various ratio of Smix. The optimized TPD was constructed with Smix ratio of 1:2 () and 1:3 () as shown in for developing formulations (NE5-NE6). The detail of composition of developed NE has been presented in . The optimized formulation was finally screened based on the minimum content of Smix content and other responsible characteristics factors from the in vitro evaluations. The developed formulations were stored at ambient temperature for further investigations.
Figure 3. Pseudo-ternary phase diagrams of the optimized nanoemulsions delineated in different combinations of Smix as (A) and (B) reveal Smix ratio of 1:2 and 1:3, respectively.
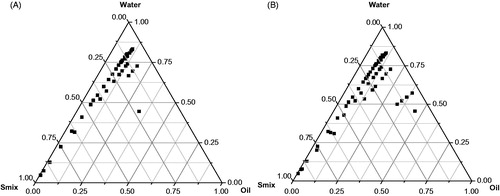
Table 2. Composition and characterization of developed nanoemulsion with their size, zeta potential, polydispersity index and ZOI (zone of inhibition) values.
In vitro characterizations
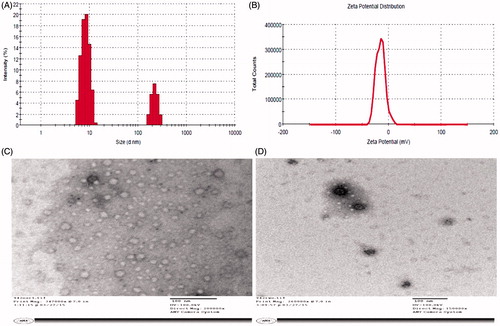
Various formulations (NE1-NE6) were developed and characterized for particle size polydispersibility index (PDI) and zeta potential as shown in . revealed that NE1, NE5 and NE6 had the maximum ZOI values as compared to NE2, NE3 and NE4 which may be owing to the higher content of lipid (CPG8). The optimized NE6 was selected due to minimum globular size (49.5 ± 1.5 nm), least PDI value (0.33) and optimum negative zeta potential (−24.59 mV) as shown in . The negative surface charge of the globule could be due to lipid-based formulation (fatty acids and esters) which further improves the stability of nanoglobules of NE (repulsion due to similar charge) against Oswald ripening (globules coalescence leading to phase separation). NE is thermodynamically stable formulation with nano-scale globular size and transparent system. In the present investigation, the particle size of the formulation is of prime importance for effective activity against the fungal strains. It is, therefore, made a hypothesis that reduced the globular size of NE renders increased surface area which further leads to enhanced contact of the NE globules around the fungal hyphae and spores for maximum detrimental effect. Thus, globular size and size distribution and zeta potential are considered as essential parameters for the formulation stability and efficacy. Moreover, the results of in vitro antifungal activities (ZOI) against both strains showed that NE6 was found to have efficient activity (19.1 ± 1.4 and 22.6 ± 2.0 mm against A. niger and C. albicans respectively). Thus, the ZOI values are prudent to correlate with the minimum globular size amongst them for enhanced antifungal activity in NE6 without drug content.
Morphological study using transmission electron microscopy (TEM)
TEM is an essential asset and the most popular method to characterize the NEs for their morphology and particle size estimation in pharmaceutical technology to serve the purpose. Single globular-based scanning and imaging technique represent a complementary device to the regular size analysis such as dynamic light scattering (DLS) method (). DLS method provides population-based results of the hydrodynamic radius of dispersed globular droplets in the bulk of NE. In this TEM method, negative staining was applied to improve the contrast of the globular morphology. It is well-known fact that the theoretical concept of fungal killing involves maximum adherence of the nanoglobules on the fungal cell surface (Hussain & Singh, Citation2016). A significant effort has been made to develop uniform and small size of NE (<100 nm) which is likely to predict the efficient in vivo performance of the NE or AmB-NE6 when topically applied to treat cutaneous fungal infections. The approximate spherical nanoglobules of the optimized NE6 and AmB-loaded AmB-NE6 have been illustrated in . The representative microphotograph of drug-loaded formulation showed deep black globule due to loaded drug in dissolved form. The size of these globules was estimated which further confirmed the results evaluated using light scattering techniques (DLS method).
In vitro drug release assessment
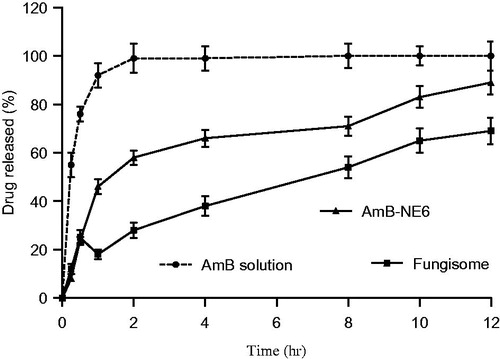
In vitro drug release behavior of the developed AmB-NE6 formulations was carried out and compared with the DS (AmB in DMSO) and commercial cream Fungisome® (0.01%w/w) in a buffer solution containing 5% v/v DMSO solution to create sink condition. The release pattern of the formulation has been exhibited in . The result of release study showed that 92.3 ± 5.4% drug was released within 60 min from the DS as compared to AmB-NE6 (46.1 ± 3.7%) which suggests that there is no any interaction of the drug with the membrane. However, slow release of the drug from encapsulated NE may be due to loaded drug into the nanoglobules to the buffer medium. It is quite important to observe that there was no any burst effect in AmB-NE6 as compared to Fungisome® cream that exhibited a burst release effect at 30 min due to free drug available outside the vesicle as shown in . The drug release rate from the AmB-NE6 and Fungisome® was found to be apparently slow as compared to DS which was statistically significant (p < 0.05). The reasonable reason for the slow and sustained release of the drug from the AmB-NE6 might be its viscosity as well as poor partitioning of the drug from the oil droplets to the buffer medium. However, NE exhibited 2.1 and 1.28-folds augmented drug release as compared to the Fungisome® within initial 2 h and over a period of 12 h, respectively. The data of the release study was subjected to the mathematical models (zero-order, first-order, Higuchi and Korsmeyer–Peppas mathematical models) to determine the release pattern and release mechanism from the carrier. AmB-NE6 and Fungisome® exhibited zero-order release kinetics with r2 value 0.998 and 0.999, respectively as compared to DS (first-order release kinetics, r2 = 0.997) within initial 2 h.
Ex vivo skin permeation and deposition study
The permeated amount (cumulative amount) versus time of AmB from various formulations has been depicted in . The flux rate of the AmB-NE6 and Fungisome® were 22.88 ± 1.7 and 11.05 ± 1.0 μg/cm2/h respectively as compared to DS (2.73 ± 0.02 μg/cm2/h) which suggested us that the permeation flux of AmB-NE6 were obviously higher by 8.38 and 2.08 times as compared to DS and Fungisome®, respectively. Moreover, the enhancement ratio (ER2) of Amb-NE6 and Fungisome® was 8.73 and 4.04, respectively as compared to DS while ER3 was 2.02 for AmB-NE6 as compared to Fungisome® (). These improved values of permeation parameters dictated us that the permeation of the drug from the NE has been significantly enhanced which might be due to several combined effect of the formulations. The possible rationale could be drawn such as wetting properties of oil in water NE after topical application leading to increased value of transepidermal water loss and nanoscale formulation capable of permeating across the skin. Furthermore, surfactant-based extraction of SC lipid constructs it a path for permeation. According to the earlier report, it has been suggested that permeation increases with the decrease of the mean particle size of nanostructured lipid carrier (Wissing et al., Citation2001). From , it can be seen that the permeated amount of the drug from the DS was extremely low as compared to other formulation which may be due to the absence of any surfactant and facilitating carrier. The results were found to be consistent with our reported studies suggesting that NE had enhanced topical transport with improved solubilization properties (Hussain et al., Citation2013). Similar finding was investigated for the consistency as consequence of the hydrophilic and lipophilic components of the microemulsion with enhanced activity that the microemulsion was capable of reducing the interfacial surface tension between carrier and the skin due to of their contact with the lipids of the skin resulting in the faster permeation across the skin (Teichmann et al., Citation2007). Moreover, PEG-400 and LAB have been shown as an effective permeation enhancer by extracting the SC lipids to disrupt the hydrophobic SC barrier leading to facilitated permeation. The exerted enhanced permeation rate for the topical release of AmB formulation from the AmB-NE6 may be due to obviously possible mechanisms (Teichmann et al., Citation2007; Hussain et al., Citation2015, 2016).
Figure 6. The cumulative amount of Amphotericin B across the albino rat skin using various formulations.
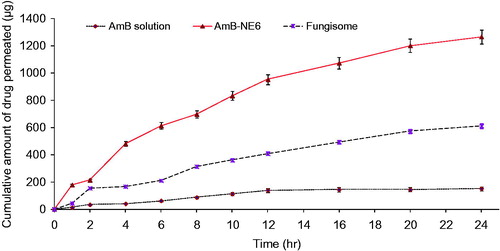
Table 3. Ex vivo permeation profile of different Amphotericin B formulations across the albino rat skin after 24 h of study.
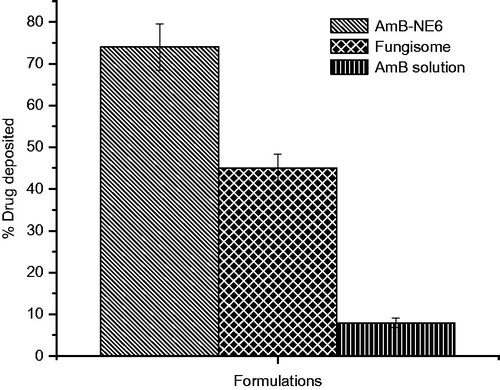
Furthermore, amount of the drug deposited into the skin was portrayed in suggesting maximum drug deposition by AmB-NE6 (74 ± 5.6%) as compared to DS (8.8 ± 1.2%) and Fungisome® (45 ± 3.4%) which could be due to formulation-mediated enhanced permeation flux (Jain et al., Citation2008). Generally, the maximum the flux rate, the maximum the drug deposition occurs. Thus, the drug deposition achieved by the AmB-NE6 was found to be 8.4 and 1.64-folds higher as compared to the DS and Fungisome® respectively.
MIC of the developed nanoemulsion
The variation in color of the resazurin is the result of oxidation–reduction reaction involved after incubation of the culture with the dye for the evaluation of cell growth and cytotoxicity assay (Sarker et al., Citation2007). A weak blue fluorescent (non-toxic dye) turns into the pink color after reduction of the dye to the resorufin by oxidoreductase within the viable cells. The MIC was determined at the lowest concentration without color change. The MIC values of the blank NE6, AmB and AmB-NE6 were found to be as 12.5 mg/ml, 0.1 μg/ml and 0.01 μg/ml against C. albicans respectively, signifying enhanced antifungal activity of AmB after loading into the NE6 as compared to free AmB. Similarly, the MIC values of the blank NE6, AmB and AmB-NE6 were found as 18.25 mg/ml, 0.12 μg/ml and 0.04 μg/ml against A. niger respectively, indicating increased antifungal activity of AmB after loading into the NE6 as compared to free AmB. Furthermore, C. albicans was observed to be more sensitive to the developed NE6 and AmB-NE6 which could be correlated with the value of MIC exerting significantly (p < 0.01) greater antifungal activity in comparison with the reference free AmB solution in DMSO (5% v/v). This may be due to the inherent antifungal activity of LAB and CPG8 against the C. albicans and A. niger. The present result is in accordance with the report recently when the cationic and non-cationic NE was used to treat against the bacteria for augmented lethal activity (Singh et al., Citation2015). Loaded drug in nanoglobules is quite accessible to the ergosterol composition of the fungal hyphae which may be the reasonable reason for the growth inhibition and similar finding has been investigated using lipid nanoparticle (LNPs) to ergosterol-rich fungal cells due to its higher affinity for ergosterol in fungal cell membrane than to cholesterol in LNPs (Fukui et al., Citation2003a,Citationb).
Visualization of the skin penetration in vivo
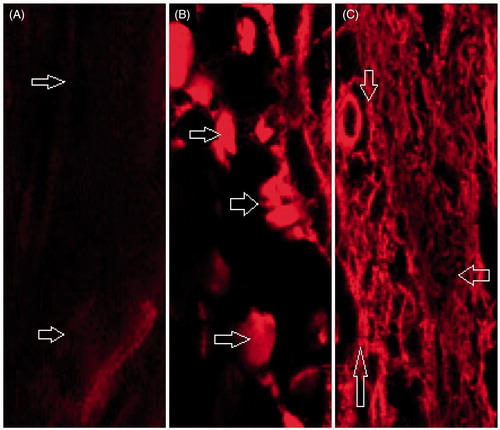
In order to corroborate the results of the ex vivo permeation across the rat skin, the penetration of probe-loaded AmB-NE6 and NE6 across the rat skin was studied and compared against the dye (Rhodamine 123 solution) using the CLSM method. Recently, the CLSM is a well-established technique to find the drug distribution into the various strata of the skin (Chen et al., Citation2006). illustrated the fluorescence intensity of the treated skin section with NE6, AmB-NE6 and NE6 loaded with Rhodamine-123 at 24 h. It was evidently depicted that the dye intensity increases with increase in lipid and surfactant-mediated drug penetration from the NE into the skin. The dye solution showed very limited fluorescent intensity as compared to NE6 and AmB-NE6 which could be due to less perturbation caused by the aqueous dye solution (). On the other hand, NE6 and AmB-NE exhibited enhanced drug penetration as a consequence of the deep green fluorescent intensity into the viable epidermis and dermis while the dye solution was restricted to the SC of the viable epidermis () after 24 h. In general, the drug content released from the NE depends on the amount of oil loaded in the NE (Hussain et al., Citation2014a). Thus, the surface acting property of LAB and lipophilic CPG8-loaded with the drug greatly influence the globular penetration across the SC which represents a considerable obstacle to the permeation of high molecular weight AmB. Importantly, the nanosize of the NE could also another potential reason for enhanced penetration as described earlier. The findings were consistent with the ex vivo drug permeation results.
Conclusion
AmB is poor water-soluble and less permeation antibiotic leading to limited clinical therapeutic efficacy owing to severe side effects (nephrotoxicity) when administered using conventional dosage form to treat cutaneous fungal infections. The objective of the study was to develop and optimize NE for the topical delivery of AmB in nanocarrier system with concerted inherent antifungal activity. Further, the investigation demonstrated the antifungal activities (for NE and drug-loaded NE) and the permeation rate by the in vitro ZOI and the Franz diffusion apparatus respectively. Moreover, the findings of permeation flux were corroborated by performing the penetration study (CLSM) suggesting the enhanced drug penetration into the various strata of the rat skin. Improved drug solubility and inherent antifungal activities of NE (drug-free) exhibited increased AmB release and effective drug therapeutic potential as compared to free DS and commercial product respectively. Furthermore, the impact of nanocarrier has facilitated the permeation flux and penetration across the SC barrier resulting into the augmented drug permeation rate and drug deposition. Conclusively, the increased antifungal and enhanced permeation of the drug-loaded NE was inferred to deliver the AmB that could be reassessed using lipids possessing inherent antifungal properties.
Declaration of interest
No financial funding agency. Authors report no conflict of interest.
References
- Ahmed M, Ramadan W, Rambhu D, Shakeel F. (2008). Potential of nanoemulsions for intravenous delivery of rifampicin. Pharmazie 63:806–11
- Attwood D, Mallon D, Taylor CJ. (1992). Phase studies on oil in water microemulsions. Int J Pharm 8:R5–8
- Azeem A, Rizwan M, Ahmad FJ, et al. (2009). Nanoemulsion components screening and selection: a technical note. AAPS PharmSciTech 10:69–76
- Chang SC, Lin SJ, Chen TW, Lin YT. (2012). A biocide-free mineral oil nanoemulsion exhibiting strong bactericidal activity against Mycobacterium immunogenum and Pseudomonas aeruginosa. Int Biodeter Biodegr 70:66–73
- Chen H, Chang X, Du D, et al. (2006). Podophyllotoxin-loaded solid lipid nanoparticles for epidermal targeting. J Control Release 110:296–306
- Espuelas MS, Legrand P, Irache JM, et al. (1997). Poly(ɛ-caprolacton) nanospheres as an alternative way to reduce amphotericin B toxicity. Int J Pharm 158:19–27
- Fukui H, Koike T, Nakagawa T, et al. (2003a). Comparison of LNS-AmB, a novel low-dose formulation of amphotericin B with lipid nano-sphere (LNS), with commercial lipid-based formulations. Int J Pharm 267:101–12
- Fukui H, Koike T, Saheki A, et al. (2003b). Evaluation of the efficacy and toxicity of amphotericin B incorporated in lipid nano-sphere (LNS). Int J Pharm 263:51–60
- Glavas-Dodov M, Fredro-Kumbaradzi E, Goracinova K, et al. (2005). The effects of lyophilization on the stability of liposomes containing 5-FU. Int J Pharm 291:79–86
- Hussain A, Haque MW, Singh SK, Ahmed FJ. (2015). Optimized permeation enhancer for topical delivery of 5-fluorouracil-loaded elastic liposome using Design Expert: part II. Drug Deliv. [Epub ahead of print]. DOI: 10.3109/10717544.2015.1124473
- Hussain A, Samad A, Nazish I, Ahmed FJ. (2013). Nanocarrier-based topical drug delivery for an antifungal drug. Drug Dev Ind Pharm 40:527–41
- Hussain A, Samad A, Singh SK, et al. (2014a). Enhanced stability and permeation potential of nanoemulsion containing sefsol-218 oil for topical delivery of amphotericin B. Drug Dev Ind Pharm 41:780–90
- Hussain A, Samad A, Singh SK, et al. (2014b). Nanoemulsion gel-based topical delivery of an antifungal drug: in vitro activity and in vivo evaluation. Drug Deliv 23:652–67
- Hussain A, Samad A, Ramzan M, et al. (2016). Elastic liposome-based gel for topical delivery of 5-fluorouracil: in vitro and in vivo investigation. Drug Deliv . [Epub ahead of print]. DOI: 10.3109/10717544.2014.976891
- Hussain A, Singh SK. (2016). Evidences for anti-mycobacterium activities of lipids and surfactants. World J Microbiol Biotechnol 32:7. doi: 10.1007/s11274-015-1965-4
- Jain SK, Gupta Y, Jain A, Rai K. (2008). Enhanced transdermal delivery of acyclovir sodium via elastic liposomes. Drug Deliv 15:141–7
- Joe MM, Bradeeba K, Parthasarathi R, et al. (2012). Development of surfactin-based nanoemulsion formulation from selected cooking oils: evaluation for antimicrobial activity against selected food associated microorganisms. J Taiwan Inst Chem Eng 43:172–80
- Kadimi US, Balasubramanian DR, Ganni UR, et al. (2007). In vitro studies on liposomal amphotericin B obtained by supercritical carbon dioxide-mediated process. Nanomedicine 3:273–80
- Kalhapure RS, Suleman N, Mocktar C, et al. (2015). Nanoengineered drug delivery systems for enhancing antibiotic therapy. J Pharm Sci 104:872–905
- Kleinberg M. (2006). What is the current and future status of conventional amphotericin B? Int J Antimicrob Agents 27S:S12–6
- Nahar M, Mishra D, Dubey V, Jain NK. (2008). Development, characterization and toxicity evaluation of amphotericin B-loaded nanogelatin nanoparticles. Nanomedicine 4:252–61
- Ouellette M, Drummelsmith J, Papadopoulou B. (2004). Leishmaniasis: drugs in the clinic, resistance and new developments. Drug Resist Updat 7:257–66
- Sarker SD, Nahar L, Kumarasamy Y. (2007). Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 42:321–4
- Shafaat K, Kumar B, Das SK, et al. (2013). Novel nanoemulsion as vehicle for transdermal delivery of clozepine in vitro and in vivo studies. Int J Pharm Pharm Sci 5:126–34
- Singh N, Verma SM, Singh SK, Verma PR. (2015). Evidence for bactericidal activities of lipidic nanoemulsions against Pseudomonas aeroginosa. Antonie Van Leeuwenhoek 107:1555–68
- Teichmann A, Heuschkel S, Jacobi U, et al. (2007). Comparison of stratum corneum penetration and localization of a lipophilic model drug applied in an o/w microemulsion and an amphiphilic cream. Eur J Pharm Biopharm 67:699–706
- Utreja P, Jain S, Tiwar AK. (2011). Localized delivery of paclitaxel using elastic liposomes: formulation development and evaluation. Drug Deliv 18:367–76
- Wissing SA, Lippacher A, Müller RH. (2001). Investigations on the occlusive properties of solid lipid nanoparticles (SLN). J Cosmet Sci 52:313–23
- Wong-Beringer A, Jacobs RA, Guglielmo BJ. (1998). Lipid formulations of amphotericin B: clinical efficacy and toxicities. Clin Infect Dis 27:603–18