Abstract
Although meloxicam (MX) is relatively safer than other NSAIDs, adverse effects relating to the gastro-intestinal tract are still a problem when administrated MX at high doses and on the long-term treatment. Drug delivery via skin provides an attractive alternative to oral administration, but is limited by the first layer of the skin-stratum corneum. Studies have been focused on developing effective methods to break the barrier of stratum corneum for enhancing delivery of MX to and across the skin. Strategies including formulation optimizing, chemical modification and physical enhancements to transiently reduce stratum corneum barrier function have been introduced. This article reviews the current state of the techniques in the delivery of MX to and across the skin, and it also includes the profiles of pharmacokinetic and safety related with skin delivery of MX.
Introduction
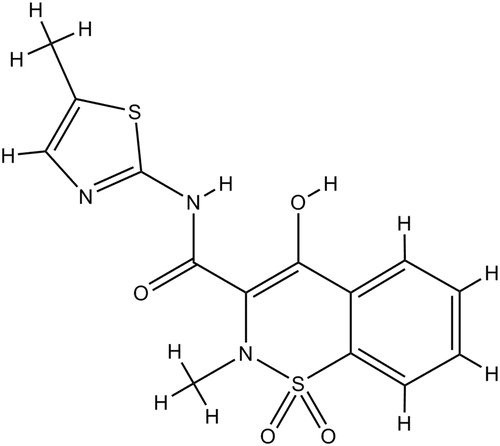
Meloxicam (MX) (), 4-hydroxy-2-methyl-N-(5-methyl-2-thiazoyl)-2H-1, 2-benzothiazine-3-carboxamide-1, 1-dioxide, is a non-steroidal anti-inflammatory drug (NSAID) belonging to the enolic acid class (Davies & Skjodt, Citation1999). MX was granted its first marketing authorization in 1995 and approved by the Food Drug Administration in 2000. It shows efficacy for reducing pain and inflammatory symptoms and has definite activity in treating osteoarthritis, rheumatoid arthritis and ankylosing spondylitis (Degner et al., Citation2000; Deeks et al., Citation2002). MX inhibits the cyclo-oxygenase-2 (COX-2) isozyme more potently than the COX-1isozyme, so it shows similar efficacy but lower toxicity than the other NSAIDs (Pairet et al., Citation1998; Senna et al., Citation2003). Additionally, it has been used as a potential drug for dealing with Alzheimer's disease and as a viable adjuvant therapeutic agent to treat different kinds of cancers including lung cancer, colorectal cancer, prostate cancer and urinary bladder cancer (Goldman et al., Citation1998; Tsubouchi et al., Citation1999; Montejo et al., Citation2010; Patel & Amin, Citation2011; Arantes-Rodrigues et al., Citation2013). Therefore, MX has great potential in clinical use.
MX is now commercially available as tablet, orally disintegrating tablet, dispersible tablet, suspension, capsule, granule, gel and injection. The detail information is shown in , and all data were obtained from the databases of United States Food and Drug Administration (US FDA), Japan Pharmaceuticals and Medical Devices Agency (PMDA) and China Food and Drug Administration (CFDA). MX gel is only commercially available in china. The other dosage forms are all administrated by oral except MX injection. Although MX has relatively low toxicity, the oral administration is associated with frequent gastrointestinal side effects, such as gastrointestinal perforation and ulceration and/or bleeding (Distel et al., Citation1996; Lanes et al., Citation2000). Administration of 15 mg MX for 14 and 28 days led to a difference in endoscopy scores and had higher endoscopy scores compared to 7.5 mg MX, suggesting that long-term therapy and high doses induce more significant gastrointestinal side effects (Patoia et al., Citation1996). So, it required an alternative route of MX which could avoid the disadvantages related with the existing oral medications.
Table 1. MX approved by US FDA, PMDA and CFDATable Footnotea.
One promising method is to administer the drug via the skin, because it offers the advantage of delivering a drug directly to the disease site in order to maximize local effects with minimum systemic activity. The skin represents the largest and most easily accessible organ of the body, and its use for topical and systemic delivery of drugs has been well documented (Prausnitz et al., Citation2004). Most topical NSAIDs have shown improved safety and tolerability compared with oral NSAIDs (Altman & Barthel, Citation2011). Topical NSAIDs are recommended in both US and international guidelines for the management of mild to moderate OA pain in superficial joints, and are recommended before oral NSAIDs in EULAR and NICE guidelines (Altman & Barthel, Citation2011). However, to the best of our knowledge, there are few formulation of MX via skin available in the market. In order to promote the development of MX dosage form, this review will focus on discussing the formulations, enhancement technologies, pharmacodynamics and potential safety aspects that may be involved with the use of MX to and across the skin.
Literature searching method
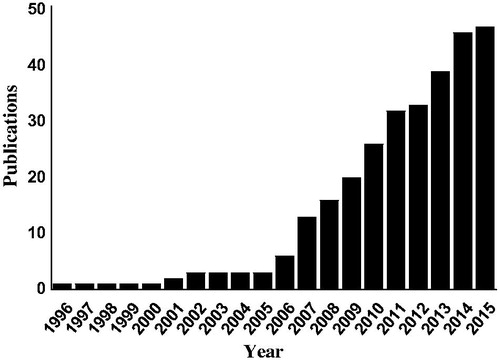
Articles cited in this article were collected via a search of PubMed (1 January 1990–16 November 2015). Search terms were as follows: “meloxicam” was combined with the terms “skin”, “percutaneous”, “topical”, “transdermal”, “dermal”, “patch”, “gel”, “cream”, “emulsion” and “liposome”. This search yielded 174 citations, of which 46 were chosen by checking the topic of articles manually. Since the first papers were published on MX delivery via skin in the late 1990s, research activity has grown rapidly. Cumulative number of articles associated with skin use of MX is shown in . Reference lists from articles identified in the formal search were examined for additional citations of interest. The remaining references cited in the review were identified by manual search using google scholar.
Figure 2. Cumulative number of publications on skin delivery of MX. The number of publications was determined by searching the PubMed database (http://www.ncbi.nlm.nih.gov/pubmed/) on 16 November 2015.
The skin as a delivery target
Taking the skin as a delivery target has various advantages compared with oral administration. In particular, it is used to avoid the first-pass hepatic metabolism for drugs with low oral bioavailability. It also has advantages over hypodermic injections, which cause pain, create dangerous medical rubbish and increase the risk of disease transmission by needle reuse (Miller & Pisani, Citation1999). Additionally, delivery systems via skin are noninvasive and can be easily self-administered. They can provide sustained and controlled delivery of drugs to provide a steady plasma profile and hence reduced systemic side effects (Prausnitz & Langer, Citation2008). Despite this, to date, there are only a few drugs that have been approved targeting the skin. This is due to the excellent barrier properties of the upper most layer of the skin, the stratum corneum (SC), which is the principal barrier to drug penetration (Wiechers, Citation1989). The delivery of MX to and across skin is difficult due to the physicochemical characteristic of MX, which do not meet the requirement of physicochemical properties of ideal drugs for skin delivery. The ideal drugs as candidate for skin delivery have the necessary physicochemical properties, which are compared to the physicochemical properties of MX in . Because MX as a zwitterionic drug has two pKa values (pKal = 1.09, pKa2 = 4.18) (Luger et al., Citation1996). Most of he zwitterionic drugs have relatively high melting points, low solubilities and poor lipophilicities (Mazzenga & Berner, Citation1991; Hatanaka et al., Citation2000). Therefore, the nature of MX makes it unsuitable for delivery through skin.
Table 2. Physicochemical properties of idea drug for skin delivery and MXTable Footnotea.
Approaches for MX delivery via skin
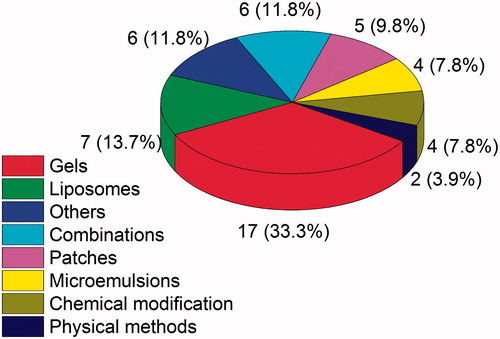
Many researchers have been studying to overcome the drawbacks of MX as a zwitterionic drug for skin delivery. Various penetration enhancement approaches have been introduced to break the skin barrier and enhance the permeation of MX across the skin. Formulation designs and chemical enhancement techniques are well documented, so they become the most extensive researched strategies to facilitate delivery of MX. Formulations such as patches, emulsions, nanoemulsions, gels, liposomes, etc. have all been used for enhance MX penetration through skin. In addition, physical approaches, like electroporation, iontophoresis, and sonophoresis, have been found suitable to create pathways for MX across the skin. The proportion of approaches is shown in .
Formulation strategies
The proliferation of traditional and new types of formulation strategies for skin permeation will expand the scope of drug administration via skin, which makes it feasible for a greater range of drugs that do not meet the ideal requirements for successful skin permeation. Thus, the development of new approaches based on formulation strategies to deliver MX through skin can be achieved.
Gels
Gels are defined as semisolid formulations, which are hydrophobic or hydrophilic in nature and immobilized within the spaces of a three-dimensional network structure (Rehman & Zulfakar, Citation2014). Among the various semisolid dosage forms, the gels are obtaining much more focus owing to simplicity of operation and their specific properties, such as biodegradability, biocompatibility, high water content and hydration of the skin (Vintiloiu & Leroux, Citation2008; Hoffman, Citation2012). Gels can be split into two categories: hydrogels and organogels. Hydrogels consist of an aqueous dispersion medium and a suitable hydrophilic gellant, such as hydroxypropyl methylcellulose (HPMC), hydroxypropyl cellulose (HPC), carbapol (Bachhav & Patravale, Citation2010; Sareen et al., Citation2011), sodium alginate, carboxymethylcellulose (CMC), poly(hydroxyethyl methacrylate) (Jain & Pathak, Citation2010), poloxamer (Inal & Yapar, Citation2013) and chitosan (Wang et al., Citation2008), which have been used as gel base for MX gels. Organogels are based on the gelation of organic solvents via low molecular agents or oil-soluble polymers that produce a three-dimensional, cross-linked network, which entraps a liquid solvent known as organogelators. MX is a water-insoluble drug, so organogels are suitable vehicles for transdermal delivery of this drug (Fetih, Citation2010).
MX release from gels can occur with various diffusion rates, which were partly influenced by the types of gelling agents. MX formulations were investigated using four kinds of hydrogel bases with various concentrations. The maximum flux was observed using 15% HPMC as gel bases compared to others, which included sodium alginate, CMC and chitosan. The result can be regarded as the difference in partitioning of the drug depending on the gel bases used (Abdul Rasool et al., Citation2011). A study was carried out to investigate the suitability and efficacy of various organogel formulations as vehicles for transdermal delivery of MX compared to hydrogel formulations. Results show that Mygliol 812 and Labrasol organogels were superior to carbopol hydrogels regarding in vitro drug release, skin permeation, and anti-inflammatory activity (Fetih, Citation2010). The release amount of MX from olive oil organogel is 1.5-fold higher than carbopol hydrogel, and it is supposed that the physicochemical affinity between the vehicle and drug may have impeded release of MX, and thus led to the lower release in hydrogel (Ruiz Martinez et al., Citation2007).
MX performs very poorly in aqueous solubility, which causes problems for designing gel formulations, so studies related to enhance the solubility of MX have been carried out. The solubility of MX increased linearly as a function of β-Cyclodextrin (β-CD) concentration, which suggested that hydrosoluble inclusion compounds were formed in the solution (Abdul Rasool et al., Citation2011). Gel consisting of 0.3% MX, 2.5% HPMC, propylene glycol, ethanol, and water (1:1:1) presented the highest drug release profile by improving the solubility of MX (Jantharaprapap & Stagni, Citation2007). To improve the solubility of MX, MX sodium formulations were prepared and evaluated. The solubility of MX sodium increased 7–18 times in buffer (pH = 7.4) with cosolvent such as propylene glycol and ethanol compared to buffer alone (Chang et al., Citation2007a).
Transdermal delivery systems based on gels would require the use of penetration enhancers. To enhance the penetration rate of MX in HPC gel, a series of terpenes were tested as enhancers. As a result, 3% menthol performed best with flux of 142.85 ± 6.45 μg/cm2/h (Chang et al., Citation2007b). 10% thymol added to HPMC gel as an enhancer showed the maximum flux of MX across mouse skin, and 1% oleic acid and 5% cineol are considered to be good candidates (Abdul Rasool et al., Citation2011). HPMC was chosen as the gel base to evaluate the effect of the penetration enhancers: dimethylsulfoxide, Tween20, oleic acid, and menthol, and 5% menthol presented the highest flux of 2.43 ± 0.47 μg/cm2/h (Jantharaprapap & Stagni, Citation2007).
In order to reach the therapeutic plasma level of MX, the application of penetration enhancer is necessary and valuable. The influences of combination of several enhancers in MX HPC gel on the percutaneous absorption were studied using response surface methodology. Gel consisted of 3% MX, 3% HPC, 6% azone, 3% SDS and 5% menthol performed best with the highest flux of 339.81 ± 18.24 μg/cm2/h, which could maintain a therapeutic concentration of approximately 400 μg/cm2/h. The diffusion rates of MX sodium from gel formulations composed of various penetration enhancers were investigated. The optimal formulation composed of 1.0% MX sodium, 2.5% HPC, 37.5% ethanol, 11% propylene glycol, 2.5% menthol and 4.0% azone had appropriate flux of 442.0 μg/cm2/h, which met the required flux of MX for maintaining the therapeutic plasma level (Chang et al., Citation2006, 2007a).
As shown in , 33% of approaches applied on skin delivery of MX are based on gels, which offer several advantages over other formulations. That is why the first approved dosage form in the Chinese market is MX gel, the details of which are listed in . Although the gel bases or types of gels would partially affect the permeation rate of MX, improving the solubility of MX and adding some penetration enhancers to the formulations are necessary to obtain the required permeation flux for maintaining therapeutic concentration. The extensive studies on MX gels will speed up the commercialization of MX for skin delivery.
Liposomes and liposome like vesicles
Liposomes have been first developed as vesicles for drug delivery to and across skin in 1980s (Mezei & Gulasekharam, Citation1980), because they are predominantly phospholipids bilayers similar to those found in biological membranes. Liposomes are promising vehicles for improving skin permeation rate of MX. However, conventional liposomes cannot penetrate skin deeply, but located to the upper layer of the stratum corneum, owing to their large size and lack of flexibility (Touitou et al., Citation2000). Therefore, in the last few years, more emphasis has been put on the development of liposome like vesicles including transfersomes, niosomes and menthosomes, which have been applied on skin delivery of MX.
Transfersomes are the first generation of elastic liposomes reported by Cevc & Blume (Citation1992) and mainly composed of phospholipids and an edge activator or a single-chain surfactant (Elsayed et al., Citation2006). Transfersomes show higher skin permeation rate of MX than conventional liposome and MX suspensions (Duangjit et al., Citation2010). Next, the influence of formulation factors such as cholesterol and cationic surfactants on the physicochemical properties of transfersomes, entrapment efficiency, stabilities of formulations and in vitro skin permeation of MX were investigated (Duangjit et al., Citation2013). Finally, the formulation consisted of 0.77% phosphatidylcholine, 0.04% cholesterol, 0.10% cetylpyridinium chloride and 0.07% MX provided the highest skin permeation flux, which was 2.69 times higher than that of a conventional liposome. The skin permeation rate of MX from liposomes was significantly enhanced possibly by the penetration-enhancing mechanism and the carrier adsorption to and/or fusion with the stratum corneum (Duangjit et al., Citation2014b).
Niosomes are the second generation of elastic liposomes introduced by van den Bergh et al. (Citation1999) and mainly consisted of cholesterol and nonionic surfactant. The effects of the combination of cyclodextrin and niosome on improving the skin permeation rates of MX have been investigated. The results demonstrated that noisome-bearing MX-cyclodextrin complex obtained the highest flux (12.48 ± 0.9 μg/cm2/h), which was approximately 1.4- and 9.1-fold higher than noisome-bearing MX and MX suspension, respectively (Jain et al., Citation2008). Niosomes composed of Span60 and cholesterol at 6:4 molar ratio have the highest entrapment efficiency (> 55%). There was a marked increase in the percentage inhibition of edema in animals treated with MX niosome gel compared to those treated with free MX and piroxicam gels (El-Menshawe & Hussein, Citation2013).
Menthosomes, novel ultra-deformable vehicles, are composed of phospholipids, menthol and edge activator. MX-loaded menthosomes were firstly introduced by Duangjit et al., and the physicochemical characteristics and skin permeation profile were investigated. The optimal formulation composed of 0.773% phosphatidylcholine, 0.077% menthol, 0.077% MX, 0.082% cholesterol and 0.224% cetylpyridinium chloride was screened using response surface methodology, which performed best with flux 0.31 μg/cm2/h (Duangjit et al., Citation2012). In a further study, the effects of various carriers including menthosomes, transfersomes and liposomes on increasing skin permeability of MX were evaluated. The 12 h cumulative amount of MX across hairless mouse skin from menthosomes, transfersomes and liposomes were 3.5, 3.0 and 1.5 μg/cm2, respectively (Duangjit et al., Citation2014a).
Compared to traditional liposomes, transfersomes have more flexibility and permeability to delivery MX through skin. Niosomes offer several advantages over traditional liposomes using nonionic surfactants, which significantly enhance the solubility of MX. The combination of transfersomes and menthol produces menthosomes, which take menthol as a penetration enhancer. In summary, liposomes and liposome like vesicles including aqueous solution core and a lipid bilayer (Cevc & Richardsen, Citation1999) improve the skin permeation of MX by increasing the solubility of MX and overcoming the barrier property of stratum corneum.
Patches
Patch generally consisted of a backing, a porous membrane, an adhesive layer, the drug and a liner. Duan et al. developed patches of MX by taking chitosan, HPMC or polyvinyl alcohol as hydrophilic components, polyamido-amine dendrimer as a permeation enhancer, and dibutyl phthalate as a plasticizer (Duan et al., Citation2015). MX patches were prepared using pectin as polymeric matrix, which is nontoxic and biodegradable (Kumar et al., Citation2010). Low solubility of MX would be the best challenge of developing patch containing MX. The mass ratio of MX in the adhesive layer was up to 15% without crystal due to combination of several solubility modulators, which resulted in great skin permeation of 400 μg/cm2. In the animal study, MX patch showed better significant efficacy than a commercially available piroxicam patch in adjuvant arthritis model (Ah et al., Citation2010). In another study, MX patch also has been proved to be more effective on relieving of edema and swelling compared to lornoxicam patch (Yener et al., Citation2010). A comparative study between electret and chemical enhancers using patch as carrier was carried out. The 10 hours cumulative permeation amount of MX achieved 30.15 μg/cm2 for electret group, which showed the highest penetration effect (Cui et al., Citation2008).
Patch provides several advantages such as controlled release and self-administrated over formulations mentioned above. As one of the most potential dosage forms, MX patch presented more effective and better compatibility with skin when compared to commercially available piroxicam patch. Although the increase in skin hydration occurred by using MX patch would improve the skin permeation of MX to some extent, application of chemical enhancers is still necessary. It is notable that researchers have been attaching importance to improvement of solubility of MX for designing MX patch without crystal.
Microemulsions
Microemulsions are clear, optically isotropic, thermodynamically stable liquid systems with droplet range of 10–100 nm (Tenjarla, Citation1999). Microemulsions have been developed to enhance the skin permeability of MX. Yuan et al. (Yuan et al., Citation2006; Yue et al., Citation2007) demonstrated the optimum formulation with the highest flux (5.40 μg/cm2/h) are composed of 0.375% MX, 5% isopropyl myristate (IPM), 50% Tween85/ethanol (1:1) and water. They also found surfactant showed more excellent solubility than oil phase for MX, which is according with the results reported by Dong et al. (Dong et al., Citation2011). Ultra-fine selfnanoemulsifying drug delivery system for skin delivery of MX has been prepared and evaluated. The cumulative permeated amount of MX in optimal formulation (77.60 μg/cm2) was increased up to 11.89 times when compared to control (6.62 μg/cm2) (Badran et al., Citation2014). As compared to conventional formulations, microemulsions are really superior and found to be advantageous with improved solubility, absorption and diffusion. One of the interesting findings was that MX revealed higher solubility in surfactant than in oil phase, which improved the skin permeation of MX.
Other formulations
Other formulations including solid dispersions (El-Badry & Fathy, Citation2006), films (Elmotasem, Citation2008), electrospun polyvinyl alcohol mats (Ngawhirunpat et al., Citation2009), creams (Mohammadi-Samani et al., Citation2014), microsponges (Nief & Hussein, Citation2014) and solid lipid nanoparticles (Khalil et al., Citation2014) have been utilized as carriers for skin delivery of MX. It has been proved that solid dispersions of MX in polyvinylpyrrolidone K-30 (PVP) prepared by freeze-drying was superior in dissolving MX than that prepared by solvent evaporation, which led to higher skin permeability of MX (El-Badry & Fathy, Citation2006). The skin permeation flux of the MX permeated from nanofiber mats was significantly increased compared to from films (Ngawhirunpat et al., Citation2009). Recently, the attention of different research groups has focused on nanoparticles made from solid lipids known by the name solid lipid nanoparticles (SLNs) (Muller et al., Citation2000). SLNs possessed various advantages compared to other colloidal carriers are aqueous colloidal dispersions with a size in the range of 50–1000 nm, the matrix of which consisted of biodegradable and biocompatible solid lipids. SLNs are considered as potential carrier systems for topical delivery of MX (Khalil et al., Citation2014). It can be seen from the above data that the making process plays an important role in improving the permeation rate of MX. The physical forms of MX, like SLNs, microsponges, nanofibers and films would generate various transdermal diffusion rates. Both the making process and the physical forms affect the permeability possibly by adjusting the particle size of MX for improvement of solubility, which provides another approach to design formulations for MX.
Combined formulations
There are many carrier systems, such as emulsions, niosomes, solid lipid nanoparticles and nanostructured lipid carriers, using for skin delivery of MX. However, these carrier systems are not convenient when applied on skin due to the relatively low viscosity, which brings disadvantages such as need to shake well before use, difficult to transport drug to skin from container without loss and hard to control the doses. Therefore, the combination of these carrier systems and gel has been proved to be more convenient and enhance the duration of drug on skin. A nanoemulsion (NE) gel containing MX for skin delivery was developed by Khurana et al., and in vitro transdermal researches showed skin permeation rate of MX from NE gel was higher than MX solution (Khurana et al., Citation2013c). Nanostructured lipid carriers (NLC) gel was proposed by the same group to delivery MX through skin, which demonstrated sustained release and enhanced the skin permeation and deposition of MX, especially into the dermis in comparison to MX gel (Khurana et al., Citation2013b), and followed by studies of mechanistic, in-vivo and stability evaluation (Khurana et al., Citation2015). In addition, they reported solid lipid nanoparticles (SLN)-based gel for skin delivery of MX, and the flux of the MX-loaded SLN gel was lower than NE gel (Khurana et al., Citation2013a). Gels prepared by poloxamer-407, chitosan or carbopol-934 were converted into niosomal gels used for transdermal delivery of MX, and the niosomal poloxamer-407 gel showed the superior drug release over the other formulations (El-Badry et al., Citation2014). MX nanoethosomes converted into carbopol gel has also been proved to have more effective anti-inflammatory activity (Ahad et al., Citation2014). Combined formulations designed for skin delivery of MX are based on the complementary advantages in those carriers, which mainly include better convenience, longer duration, higher permeability, stronger effect and greater stability. And it is worth noting that the types of gel may affect the diffusion rate of MX through skin.
Chemical modification
The penetration rate of MX through skin is limited by the low solubility of MX. Therefore, many strategies have been introduced to improve the solubility of MX. Among these strategies, the formation of an ion-pair between MX and oppositely charged species offers significant potential for enhancing solubility of MX. Ki & Choi (Citation2007) reported the formation of three kinds of ion-pair, two of which presented higher solubility and skin permeation rate through hairless mouse skin than MX alone in various carriers. After that, six kinds of ion-pair provided higher skin permeation were prepared by using six organic bases as oppositely charged species, including diethylamine, triethylamine, ethanolamine, diethanolamine, triethanolamine and N-(2′-hydroxyethanol)-piperidine (Zhang et al., Citation2009). MX sodium and potassium salts were also used to screen an optimal transdermal formulation (Chang et al., Citation2007a; Barhate, Citation2011; Huang et al., Citation2011). The effects of β-cyclodextrin, hydroxypropyl-β-cyclodextrin, polyvinyl pyrrolidone and urea on enhancing the solubility and influencing the percutaneous absorption of MX were evaluated (Saleem et al., Citation2010). Compared to other methods, chemical modification has the capacity to greatly improve the solubility of MX actually by generating new compounds called ion-pair, which are suitable for designing formulations for percutaneous absorption. However, this technique should be carefully selected because a great deal of data associated with the safety and clinical efficacy need to be collected.
Physical enhancement methods
There has been increasing interest in development of physical enhancement methods, which can easily break the barrier properties of the skin by using an energy source. Among these techniques, iontophoresis and electroporation have been paid more attention as these technologies reached commercialization levels. Iontophoresis resulted in 19.56-fold of 12 h cumulative amount of MX salts, when compared with those of passive controls. Iontophoresis and its combination with electroporation could significantly enhance the in vitro permeation of MX salts (Ren-Jiunn, Citation2008). Another notable method is the application of ultrasound, which overcomes the barrier of skin mainly by mechanical function and cavitation. The skin permeation of MX has been great improved by using low-frequency sonophoresis (Kim et al., Citation2009). The reports related to enhancement of skin permeation for MX are relatively rare. It is because that it is easy to obtain the required flux for maintaining the therapeutic concentration by the optimization of formulations, so it is not necessary to use relatively complicated physical enhancement methods. However, physical enhancement methods demonstrated the good prospect in controlling dosage and rapid administration.
Analysis of in vitro skin delivery of MX
Summary of the key findings of studies involving in vitro skin delivery of MX is shown in . Forming MX salts with basic compounds, like sodium hydroxide, potassium hydroxide, ethanolamine and other organic bases, have been used to enhance the solubility or skin permeation of MX, as discussed previously in the section of chemical modification. However, the most frequently used compound is still MX, because making salts of it requires extensive safety and efficacy data for regulatory approval. Fourteen kinds of delivery systems loading MX with various doses from 1.35 to 280.63 mg have been utilized to enhance penetration rate of MX. Human cadaver skin are relatively rare and expensive, so the common used skin models are rat skin and hairless mouse skin, which have similar permeability to human skin. As reported earlier (Busch et al., Citation1998; Jantharaprapap & Stagni, Citation2007), the required flux of MX to maintain a therapeutic concentration was approximately 400 μg/h across rat skin and 281 μg/h across human skin. Assuming that a topical application area is 100 cm2 (10 cm × 10 cm), the flux should be up to 4 μg/cm2/h through rat skin and 2.81 μg/cm2/h through human skin. The optimal fluxes affected by the types of MX, delivery systems, drug loading and skin models in each article mostly meet the requirement to reach a therapeutic concentration.
Table 3. Summary of the key findings of studies involving in vitro skin delivery of MX.
Pharmacokinetics studies of MX delivery via skin
Pharmacokinetic properties of drugs are likely to be influenced by a few of factors, such as the route of administration and the dosage of administered drug. Therefore, the pharmacokinetic profiles of MX in the plasma or in target tissues after drug delivery via skin must be evaluated to help distinguish between local and systemic drug effect, determine the recommended dose of MX for skin delivery and investigate the mechanism of dermal delivery of MX. Pharmacokinetic characteristics in plasma and synovial fluid were studied after administration of MX by the oral and transdermal route in beagle dogs. The higher drug level in synovial fluid than plasma suggested MX was directly delivered to the site of administration, which demonstrated that drug delivery of MX via skin had great potential to reduce the systemic adverse effects (Yuan et al., Citation2009).The pharmacokinetic profiles were evaluated by applying 0.5 g MX gel on rat skin, which reached the highest plasma concentration (Cmax) of 48.48 ± 6.57 μg/ml at 2 h (Tmax) (Gupta et al., Citation2002). Three female rabbits received according to a crossover design 0.135 g/cm2 of gel applied to a 7.5 × 7.5 cm area of their shaved back and intravenous infusion (i.v.) of 1 mg. 3.93 ± 0.85 mg of MX were delivered after applying MX gel containing 5% menthol onto the dorsal skin in rabbits, indicating that an area of approximately 10 × 10 cm might be able to deliver the necessary 7.5 mg/day of MX recommended for the treatment of arthritis and osteoarthritis in humans (Patel et al., Citation2011). MX concentration in skin was also determined to study dermis availability and to explore the mechanisms of MX through skin (Patel et al., Citation2011; Gao et al., Citation2007).
Pharmacokinetic properties of MX administrated via skin in human have also been observed. A comparative study between local and i.v. administration had been conducted by administration of 7.5 mg MX after herniorrhaphy, and lower plasma concentration of MX was observed in local administration but no differences in pain scores and analgesic consumption during the first 24 h, which indicated local administration might provide an advantage over i.v. administration by decreasing systemic adverse effects (Rømsing et al., Citation2001). The AUC0 − t values was appropriately 1061 ± 1141 ng h/ml after application of 30 mg MX on 10 volunteers through skin and only 1.21% compared with that (44 ± 7 μg h/ml) of reported after an oral dose of 15 mg MX to 17 volunteers, which demonstrated that the skin delivery of MX could avoid gastrointestinal irritation and decrease the systemic adverse effects in human (Yuan et al., Citation2007).
Pharmacokinetic characteristics of MX administrated via skin were evaluated in beagle dogs, rabbits, patients and healthy volunteers, the results of which demonstrated that local administration of MX could obtain the therapeutic concentration. The local concentration of MX was higher than the plasma concentration, which was helpful to maximize the local effects and reduce the systemic adverse effects. This is the biggest advantage provided by skin delivery of MX.
Safety
The side effects such as arterial thrombotic events, a functional renal failure, liver dysfunction and skin reactions accompanied by administration of MX, particularly at high doses and on long-term treatment are drawing more and more attention (Fenn & Morant, Citation1997). So, it is necessary to perform safety assessments for skin delivery of MX against the local and systemic toxic effects. Various formulations of MX including intravenous, intramuscular and subcutaneous injections, eye-drops, gel and suppositories have been assessed for safety and the results presented good local tolerance of MX, which indicated MX may be suitable for skin administration (Stei et al., Citation1996). Khurana et al. demonstrated that the prepared MX gel not only has good compatibility with skin but also nontoxic to the internal organs at dose levels of 8, 16 and 32 mg/kg in mice. They also studied the skin tolerability of the MX gel, which showed no erythema or edema on the rabbit skin (Khurana et al., Citation2013a, 2015). Histopathological examination of rat skin treated with the selected formulations concluding control, hydrogels and organogels showed normal skin histology (Fetih, Citation2010). The results of primary skin irritation study illustrated that the primary irritation index (PII) of MX-patch was 0.3, which below 0.5 is nonirritating level (Ah et al., Citation2010). Although MX delivery via skin has been proved good skin tolerability, the studies of systemic toxicities, especially in human lack the data.
Conclusions
Administration of NSAIDs is always accompanied with the side effects such as gastrointestinal, cardiovascular and renal adverse events, which are related to dose, duration and age (Altman & Barthel, Citation2011). To decrease these adverse effects, National Institute for Health and Clinical Excellence guidelines (Conditions, Citation2008) for the management of OA and American Geriatrics Society guidelines (Persons, Citation2009) for the treatment of chronic pain in the elderly patients suggest avoiding oral NSAIDs as well as possible, because local administrations of NSAIDs apply the drug directly onto the disease site to enhance local effects and decrease systemic activity. Therefore, it can be speculated that NSAIDs delivering through skin has a growing market. In addition, it was reported MX has better compatibility with skin than piroxicam, ketoprofen, indomethacin, diclofenac, and ibuprofen (Stei et al., Citation1996). So, developing the skin delivery system of MX has great potential.
However, to date, only one formulation of MX is available in Chinese market for use via skin, as shown in . The development of MX pharmaceutics for skin delivery moves slowly because of the excellent barrier effects of stratum corneum and the physical-chemical characteristics of MX, which induce low skin permeation rate of MX. Therefore, extensive studies have focussed on enhancing the permeate rate of MX through skin. A variety of drug carriers, such as gels, nanoethosomes, transfersomes, patches, microemulsions and nanoemulsion based gels, have been developed to enhance the skin permeation of MX. In addition, physical enhancement methods including iontophoresis, electroporation and sonophoresis have been proved to break the barrier of skin effectively for MX delivery. The data shown in demonstrated that the required flux of MX to reach a therapeutic concentration can be obtained by various delivery systems and enhancement methods. Looking to the future, among these delivery systems, patches would be better choices to be used for delivery of MX, because they can offer more accurate dosage and better compliance. In contrast, the formulations such as gels, transfersomes and microemulsions have weaknesses in controlling the time and dosage of administration.
The mechanism of the skin delivery of MX is still under investigating. It has been demonstrated that MX administrated through skin could maintain required concentrations in the targeting tissues while keeping a low plasma concentration, which avoided the irritation of MX in gastrointestinal tract and decreased the systemic adverse effects. Besides, local administration of MX also induced the same pharmacological effects as i.v. administration but with lower plasma concentration of MX, which confer an advantage by reducing systemic adverse effects. Many studies have proved that delivery of MX via skin has good skin tolerability without inducing erythema or edema. However, whether the local administration of MX induced systemic toxicity is still unclear. So, extensive developmental efforts, mechanistic studies of MX–skin interactions, further systemic toxicity studies, and pharmacological effects in human are all required to ensure the safety and effectiveness of delivering MX through skin. Our group has been developing microneedle-based delivery system for skin delivery of MX, and the further study will focus on the mechanism and safety.
Declaration of interest
This work was supported by the Natural Science Foundation of Fujian Province (Grant No. 2015J05167), the Education Department of Fujian Province (Grant No. JA14276), Putian Science and Technology Bureau (Grant No. 2014S02 (1)) and Putian University (Grant No. 2014052).
References
- Abdul Rasool KB, Gareeb RH, Fahmy SA, et al. (2011). Meloxicam β-cyclodextrin transdermal gel: physicochemical characterization and in vitro dissolution and diffusion studies. Curr Drug Deliv 8:381–91
- Ah YC, Choi JK, Choi YK, et al. (2010). A novel transdermal patch incorporating meloxicam: in vitro and in vivo characterization. Int J Pharm 385:12–19
- Ahad A, Raish M, Al-Mohizea AM, et al. (2014). Enhanced anti-inflammatory activity of carbopol loaded meloxicam nanoethosomes gel. Int J Biol Macromol 67:99–104
- Altman RD, Barthel HR. (2011). Topical therapies for osteoarthritis. Drugs 71:1259–79
- Arantes-Rodrigues R, Pinto-Leite R, Ferreira R, et al. (2013). Meloxicam in the treatment of in vitro and in vivo models of urinary bladder cancer. Biomed Pharmacother 67:277–84
- Bachhav YG, Patravale VB. (2010). Formulation of meloxicam gel for topical application: in vitro and in vivo evaluation. Acta Pharm 60:153–63
- Badran MM, Taha EI, Tayel MM, et al. (2014). Ultra-fine self nanoemulsifying drug delivery system for transdermal delivery of meloxicam: dependency on the type of surfactants. J Mol Liq 190:16–22
- Barhate SD. (2011). Development of meloxicam sodium transdermal gel. Int J Pharm Res Dev 2:1–4
- Busch U, Schmid J, Heinzel G, et al. (1998). Pharmacokinetics of meloxicam in animals and the relevance to humans. Drug Metab Dispos 26:576–84
- Cevc G, Blume G. (1992). Lipid vesicles penetrate into intact skin owing to the transdermal osmotic gradients and hydration force. Biochim Biophys Acta 1104:226–32
- Cevc G, Richardsen H. (1999). Lipid vesicles and membrane fusion. Adv Drug Deliv Rev 38:207–32
- Chang J-S, Huang Y-B, Hou S-S, et al. (2007a). Formulation optimization of meloxicam sodium gel using response surface methodology. Int J Pharm 338:48–54
- Chang J, Wu P, Huang Y, et al. (2006). In-vitro evaluation of meloxicam permeation using response surface methodology. J Food Drug Anal 14:236--41
- Chang JS, Tsai YH, Wu PC, et al. (2007b). The effect of mixed-solvent and terpenes on percutaneous absorption of meloxicam gel. Drug Dev Ind Pharm 33:984–9
- Conditions NCCfC. Osteoarthritis: national clinical guideline for care and management in adults. 2008. London (UK): Royal College of Physicians
- Cui L, Hou X, Jiang J, et al. (2008). Comparative enhancing effects of electret with chemical enhancers on transdermal delivery of meloxicam in vitro. J Phys Conf Ser 142:1–4
- Davies NM, Skjodt NM. (1999). Clinical pharmacokinetics of meloxicam. A cyclo-oxygenase-2 preferential nonsteroidal anti-inflammatory drug. Clin Pharmacokinet 36:115–26
- Deeks JJ, Smith LA, Bradley MD. (2002). Efficacy, tolerability, and upper gastrointestinal safety of celecoxib for treatment of osteoarthritis and rheumatoid arthritis: systematic review of randomised controlled trials. BMJ 325:619
- Degner F, Sigmund R, Zeidler H. (2000). Efficacy and tolerability of meloxicam in an observational, controlled cohort study in patients with rheumatic disease. Clin Ther 22:400–10
- Distel M, Mueller C, Bluhmki E, et al. (1996). Safety of meloxicam: a global analysis of clinical trials. Br J Rheumatol 35:68–77
- Dong X, Ke X, Liao Z. (2011). The microstructure characterization of meloxicam microemulsion and its influence on the solubilization capacity. Drug Dev Ind Pharm 37:894–900
- Duan X-D, Ji C-J, Nie L. (2015). Formulation and development of dendrimer-based transdermal patches of meloxicam for the management of arthritis. Trop J Pharm Res 14:583–90
- Duangjit S, Obata Y, Sano H, et al. (2012). Menthosomes, novel ultradeformable vesicles for transdermal drug delivery: optimization and characterization. Biol Pharm Bull 35:1720–8
- Duangjit S, Obata Y, Sano H, et al. (2014a). Comparative study of novel ultradeformable liposomes: menthosomes, transfersomes and liposomes for enhancing skin permeation of meloxicam. Biol Pharm Bull 37:239–47
- Duangjit S, Opanasopit P, Rojanarata T, et al. (2010). Characterization and in vitro skin permeation of meloxicam-loaded liposomes versus transfersomes. J Drug Deliv 2011:1–9
- Duangjit S, Opanasopit P, Rojanarata T, et al. (2013). Evaluation of meloxicam-loaded cationic transfersomes as transdermal drug delivery carriers. AAPS PharmSciTech 14:133–40
- Duangjit S, Pamornpathomkul B, Opanasopit P, et al. (2014b). Role of the charge, carbon chain length, and content of surfactant on the skin penetration of meloxicam-loaded liposomes. Int J Nanomedicine 9:2005–17
- El-Badry M, Fathy M. (2006). Enhancement of the dissolution and permeation rates of meloxicam by formation of its freeze-dried solid dispersions in polyvinylpyrrolidone K-30. Drug Dev Ind Pharm 32:141–50
- El-Badry M, Fetih G, Fathalla D, et al. (2014). Transdermal delivery of meloxicam using niosomal hydrogels: in vitro and pharmacodynamic evaluation. Pharm Dev Technol 20:820–6
- El-Menshawe SF, Hussein AK. (2013). Formulation and evaluation of meloxicam niosomes as vesicular carriers for enhanced skin delivery. Pharm Dev Technol 18:779–86
- Elmotasem H. (2008). Chitosan–alginate blend films for the transdermal delivery of meloxicam. Asian J Pharm Sci 3:12–29
- Elsayed MM, Abdallah OY, Naggar VF, et al. (2006). Deformable liposomes and ethosomes: mechanism of enhanced skin delivery. Int J Pharm 322:60–6
- Fenn GC, Morant SV. (1997). Safety of meloxicam: a global analysis of clinical trials [British Journal of Rheumatology, 1996;35(suppl. 1):68-77]. Br J Rheumatol 36:817–19
- Fetih G. (2010). Meloxicam formulations for transdermal delivery: hydrogels versus organogels. J Drug Deliv Sci Technol 20:451–6
- Gao QZ, Yang LY, Ding PT, et al. (2007). [Percutaneous absorption of meloxicam patches in hairless mouse]. Yao Xue Xue Bao 42:1320–2
- Goldman AP, Williams CS, Sheng H, et al. (1998). Meloxicam inhibits the growth of colorectal cancer cells. Carcinogenesis 19:2195–9
- Gupta SK, Bansal P, Bhardwaj RK, et al. (2002). Comparison of analgesic and anti-inflammatory activity of meloxicam gel with diclofenac and piroxicam gels in animal models: pharmacokinetic parameters after topical application. Skin Pharmacol Appl Skin Physiol 15:105–11
- Hatanaka T, Kamon T, Morigaki S, et al. (2000). Ion pair skin transport of a zwitterionic drug, cephalexin. J Control Release 66:63–71
- Hoffman AS. (2012). Hydrogels for biomedical applications. Adv Drug Del Rev 64:18–23
- Huang CT, Tsai CH, Tsou HY, et al. (2011). Formulation optimization of transdermal meloxicam potassium-loaded mesomorphic phases containing ethanol, oleic acid and mixture surfactant using the statistical experimental design methodology. J Microencapsul 28:508–14
- Inal O, Yapar EA. (2013). Effect of mechanical properties on the release of meloxicam from poloxamer gel bases. Indian J Pharm Sci 75:700–6
- Jain D, Pathak K. (2010). Design, characterization, and evaluation of meloxicam gel prepared by suspension and solution polymerization using solubility parameter as the basis for development. AAPS PharmSciTech 11:133–42
- Jain SK, Gupta Y, Jain A, et al. (2008). Elastic liposomes bearing meloxicam-beta-cyclodextrin for transdermal delivery. Curr Drug Deliv 5:207–14
- Jantharaprapap R, Stagni G. (2007). Effects of penetration enhancers on in vitro permeability of meloxicam gels. Int J Pharm 343:26–33
- Khalil RM, Abd-Elbary A, Kassem MA, et al. (2014). Nanostructured lipid carriers (NLCs) versus solid lipid nanoparticles (SLNs) for topical delivery of meloxicam. Pharm Dev Technol 19:304–14
- Khurana S, Bedi P, Jain N. (2013a). Preparation and evaluation of solid lipid nanoparticles based nanogel for dermal delivery of meloxicam. Chem Phys Lipids 175:65–72
- Khurana S, Jain NK, Bedi PM. (2013b). Development and characterization of a novel controlled release drug delivery system based on nanostructured lipid carriers gel for meloxicam. Life Sci 93:763–72
- Khurana S, Jain NK, Bedi PM. (2013c). Nanoemulsion based gel for transdermal delivery of meloxicam: physico-chemical, mechanistic investigation. Life Sci 92:383–92
- Khurana S, Jain NK, Bedi PM. (2015). Nanostructured lipid carriers based nanogel for meloxicam delivery: mechanistic, in-vivo and stability evaluation. Drug Dev Ind Pharm 41:1368–75
- Ki HM, Choi HK. (2007). The effect of meloxicam/ethanolamine salt formation on percutaneous absorption of meloxicam. Arch Pharm Res 30:215–21
- Kim T, Kim Y, Seo S, et al. (2009). Anti-hyperalgesic effects of meloxicam hydrogel via phonophoresis in acute inflammation in rats; comparing systemic and topical application. Biomol Ther (Seoul) 17:305–10
- Kumar M, Chauhan AK, Kumar S, et al. (2010). Design and evaluation of pectin based metrics for transdermal patches of meloxicam. JPRHC 2:244–7
- Lanes SF, Rodrigeuz LA, Hwangg E. (2000). Baseline risk of gastrointestinal disorders among new users of meloxicam, ibuprofen, diclofenac, naproxen and indomethacin. Pharmacoepidemiol Drug Saf 9:113–17
- Luger P, Daneck K, Engel W, et al. (1996). Structure and physicochemical properties of meloxicam, a new NSAID. Eur J Pharm Sci 4:175–87
- Mazzenga GC, Berner B. (1991). The transdermal delivery of zwitterionic drugs I: the solubility of zwitterion salts. J Control Release 16:77–88
- Mezei M, Gulasekharam V. (1980). Liposomes-a selective drug delivery system for the topical route of administration I. Lotion dosage form. Life Sci 26:1473–7
- Miller MA, Pisani E. (1999). The cost of unsafe injections. Bull World Health Organ 77:808–11
- Mohammadi-Samani S, Yousefi G, Mohammadi F, et al. (2014). Meloxicam transdermal delivery: effect of eutectic point on the rate and extent of skin permeation. Iran J Basic Med Sci 17:112–18
- Montejo C, Barcia E, Negro S, et al. (2010). Effective antiproliferative effect of meloxicam on prostate cancer cells: development of a new controlled release system. Int J Pharm 387:223–9
- Muller RH, Mader K, Gohla S. (2000). Solid lipid nanoparticles (SLN) for controlled drug delivery - a review of the state of the art. Eur J Pharm Biopharm 50:161–77
- Ngawhirunpat T, Opanasopit P, Rojanarata T, et al. (2009). Development of meloxicam-loaded electrospun polyvinyl alcohol mats as a transdermal therapeutic agent. Pharm Dev Technol 14:70–9
- Nief RA, Hussein AA. (2014). Preparation and evaluation of meloxicam microsponges as transdermal delivery system. Iraqi J Pharm Sci 23:62–74
- Pairet M, van Ryn J, Schierok H, et al. (1998). Differential inhibition of cyclooxygenases-1 and -2 by meloxicam and its 4'-isomer. Inflamm Res 47:270–6
- Patel M, Joshi A, Hassanzadeth H, et al. (2011). Quantification of dermal and transdermal delivery of meloxicam gels in rabbits. Drug Dev Ind Pharm 37:613–17
- Patel MM, Amin AF. (2011). Formulation and development of release modulated colon targeted system of meloxicam for potential application in the prophylaxis of colorectal cancer. Drug Deliv 18:281–93
- Patoia L, Santucci L, Furno P, et al. (1996). A 4-week, double-blind, parallel-group study to compare the gastrointestinal effects of meloxicam 7.5 mg, meloxicam 15 mg, piroxicam 20 mg and placebo by means of faecal blood loss, endoscopy and symptom evaluation in healthy volunteers. Rheumatology (Oxford) 35:61–7
- Persons AGSPoPMoPPiO (2009). Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 57:1331--46
- Prausnitz MR, Langer R. (2008). Transdermal drug delivery. Nat Biotechnol 26:1261–8
- Prausnitz MR, Mitragotri S, Langer R. (2004). Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov 3:115–24
- Rømsing J, Mysager S, Vilmann P, et al. (2001). Postoperative analgesia is not different after local vs systemic administration of meloxicam in patients undergoing inguinal hernia repair. Can J Anaesth 48:978–84
- Rehman K, Zulfakar MH. (2014). Recent advances in gel technologies for topical and transdermal drug delivery. Drug Dev Ind Pharm 40:433–40
- Ren-Jiunn W, Pao-Chu W, Huang Y-B, Yi-Hung T. (2008). The effects of iontophoresis and electroporation on transdermal delivery of meloxicam salts evaluated in vitro and in vivo. J Food Drug Anal 16:41–8
- Ruiz Martinez MA, Lopez-Viota Gallardo J, de Benavides MM, et al. (2007). Rheological behavior of gels and meloxicam release. Int J Pharm 333:17–23
- Saleem M, Bala S, Liyakat AA. (2010). Effect of different carriers on in vitro permeation of meloxicam through rat skin. Indian J Pharm Sci 72:710--18
- Sareen R, Kumar S, Gupta GD. (2011). Meloxicam carbopol-based gels: characterization and evaluation. Curr Drug Deliv 8:407–15
- Senna GE, Passalacqua G, Dama A, et al. (2003). Nimesulide and meloxicam are a safe alternative drugs for patients intolerant to nonsteroidal anti-inflammatory drugs. Eur Ann Allergy Clin Immunol 35:393–6
- Stei P, Kruss B, Wiegleb J, et al. (1996). Local tissue tolerability of meloxicam, a new NSAID: indications for parenteral, dermal and mucosal administration. Br J Rheumatol 35:44–50
- Tenjarla S. (1999). Microemulsions: an overview and pharmaceutical applications. Crit Rev Ther Drug Carrier Syst 16:461–521
- Touitou E, Dayan N, Bergelson L, et al. (2000). Ethosomes – novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release 65:403–18
- Tsubouchi Y, Mukai S, Kawahito Y, et al. (1999). Meloxicam inhibits the growth of non-small cell lung cancer. Anticancer Res 20:2867–72
- van den Bergh BA, Bouwstra JA, Junginger HE, et al. (1999). Elasticity of vesicles affects hairless mouse skin structure and permeability. J Control Release 62:367–79
- Vintiloiu A, Leroux J-C. (2008). Organogels and their use in drug delivery – a review. J Control Release 125:179–92
- Wang Y, Chen M, Li X, et al. (2008). A hybrid thermo-sensitive chitosan gel for sustained release of Meloxicam. J Biomater Sci Polym Ed 19:1239–47
- Wiechers JW. (1989). The barrier function of the skin in relation to percutaneous absorption of drugs. Pharm Weekbl Sci 11:185–98
- Yener G, Uner M, Gonullu U, et al. (2010). Design of meloxicam and lornoxicam transdermal patches: preparation, physical characterization, ex vivo and in vivo studies. Chem Pharm Bull (Tokyo) 58:1466–73
- Yuan Y, Chen X, Zhong D. (2007). Determination of meloxicam in human plasma by liquid chromatography-tandem mass spectrometry following transdermal administration. J Chromatogr B Analyt Technol Biomed Life Sci 852:650–4
- Yuan Y, Chen XY, Li SM, et al. (2009). Pharmacokinetic studies of meloxicam following oral and transdermal administration in Beagle dogs. Acta Pharmacol Sin 30:1060–4
- Yuan Y, Li SM, Mo FK, et al. (2006). Investigation of microemulsion system for transdermal delivery of meloxicam. Int J Pharm 321:117–23
- Yue Y, LI S-m, YU L-m, et al. (2007). Physicochemical properties and evaluation of microemulsion systems for transdermal delivery of meloxicam. Chem Res Chinese U 23:81–6
- Zhang JY, Fang L, Tan Z, et al. (2009). Influence of ion-pairing and chemical enhancers on the transdermal delivery of meloxicam. Drug Dev Ind Pharm 35:663–70