Abstract
Platelets are enriched with Transforming Growth Factor-ß (TGF-ß). However, information is limited concerning TGF-ß's effects at the molecular level. Nevertheless, it has been demonstrated that TGF-ß activates cell proliferation and its postitive influence on cartilage formation has been proven within the field of Tissue Engineering (TE). As Platelet Rich Plasma (PRP) contains TGF-ß, it was the purpose of this study to optimize PRP-isolation for further TGF-ß extraction. Red blood cell count (RBC) was separated from whole blood by centrifugation. From the supernatant PRP and platelet poor plasma (PPP) layer, the latter supernatant was re-centrifuged to extract PRP. Various experimental series were run to investigate influences concerning anticoagulating alternatives, different amounts of buffer, various centrifugal forces, or substituting centrifugation for sedimentation. TGF-ß levels were determined using ELISA. The technique of platelet-/ TGF-ß-extraction described here proves to be more effective than other methods, is easily repeatable and nottime-consuming, which predisposes it for TE requirements.
INTRODUCTION
Autologous cartilage generation for reconstructive surgery of congenital or acquired cartilage defects is decisive in tissue engineering. The use of autogenous tissue components reduces the risk of rejection and disease transmission. First, chondrocytes are isolated from a patient's cartilage-biopsy for in vitro proliferation. During cell expansion, chondrocytes dedifferentiate and lose their cartilage phenotype. However, as soon as proliferated cells are added to a three-dimensional scaffold, re-expression of the cartilage phenotype is required and cells are expected to redifferentiate: certain conditions have been detected under which extracellular cartilage matrix production is triggered [Citation2, Citation3, Citation11, Citation12, Citation16, Citation20, Citation21, Citation25]. Redifferentiation of cartilage cells and proliferation, plus formation of a cartilage matrix, may additionally be stimulated by growth factors. There is a large variety of growth factors used for this purpose, such as transforming growth factor-ß1 (TGF-ß1), insulin-like growth factor (IGF), fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), epithelial cell growth factor (ECGF), and others [Citation10]. Platelets contain several of these growth factors [Citation26], thus it has been confirmed by Marx et al. [Citation15] that using platelet-rich-plasma (PRP) as a source of autologous growth factors increases bone formation and density after autologous bone grafting. PRP may be defined as a platelet concentrate within the leukocyte fraction of centrifuged blood (buffy coat). An alternative description defines the supernatant product of blood centrifugation as a combination of PRP (platelet containing part) and Platelet Poor Plasma (PPP). The composites may be further separated from each other after decantation from the red blood cell (RBC) fraction.
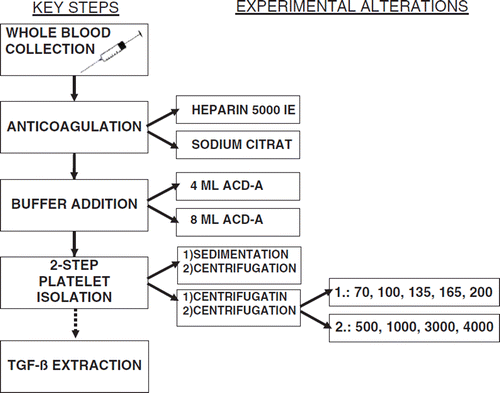
In transfusion medicine PRP is commonly obtained by the discontinuous plasmapheresis method [Citation19]: the patient's blood is supplemented with an anticoagulant and intermittently pumped into a rotating unit. Cell components are separated and packed erythrocytes are taken out of the unit to be finally reinfused into the patient. The high extracorporeal blood volume is generally well tolerated; however, the clinical use of PRP as a source of endogenous growth factors obtained by discontinuous cell separation is limited since it bears the risk of cardiovascular stress, especially for elderly people [Citation31], and remains relatively expensive due to high production costs [Citation22] for transfusion institutes [Citation29]. Nonetheless, the demand for autologous PRP preparations continues to increase, for example from surgery units for bone augmentation. Currently, there are several commercial options available to produce small amounts of ready-to-use PRP, which are more convenient for the patient at the clinic, less expensive, and allow clinicians to prepare the PRP needed in the operating room [Citation27, Citation28, Citation30]. For example, there is the Curasan PRP Kit (Kleinostheim, Germany) and the PCCS system (3i Implant Innovations, Palm Beach Gardens, FL, USA), which differ in PRP yield efficiency: the correlation between the platelet count in the PRP and the platelet count in the whole blood was greater for the PCCS than for the Curasan PRP [Citation29]. Thus far it has not been studied whether the PCCS is able to produce a PRP comparable to PRP products of blood banks [Citation28]. The aim of this study was to optimize PRP isolation for the purpose of autologous TGF-ß1 extraction (). For economical and biological reasons, PRP preparation was fractionated from plasma of patients and used as an autologous source of one of the prominent growth factors stored in platelets. It is considered a multifunctional cytokine with mitogenic and motogenic qualities, which regulates extracellular matrix formation and cell differentiation, among other things, in cartilage tissue engineering [Citation5, Citation12]. The method is based on techniques for pelleted PRP generation, which contains a higher concentration of platelets compared to PRP applied in transfusion medicine. Evaluation of the method was accomplished by determination of platelet counts as well as the quantification of TGF-ß. Important criteria concerning the fabrication process were:
– patient convenience;
– adequate pricing;
– minimal material consumption;
– easy handling;
– PRP yield with adequate amounts of TGF-ß using acceptable quantities of whole blood.
MATERIALS AND METHODS
Patients and Blood Samples
Blood samples were collected from 105 donors (aged 18–70 years), 53 males and 52 females, without any relevant diseases (HIV, HBV, treponema pallidum, etc.). All donors gave written informed consent and samples were taken while donors underwent routine operative treatment (e.g. septorhinoplasty) at the Department of Otolaryngology, Head and Neck Surgery, University of Regensburg, Germany. The study was also approved by the institute's local ethics board.
Vein puncture was performed with an 18 G needle (Microlance 1 1/2, Becton-Dickinson, Franklin Lakes, NJ, USA) and 20 ml whole blood was slowly collected from every donor into a commercial disposable syringe (Discardit II, Becton-Dickinson, Franklin Lakes, NJ, USA).
Anticoagulation
Experimental alteration of the anticoagulant-form was performed as follows: syringes were charged with 2 ml of heparin (5000 IE) and 4 ml sodium citrate (32 %, ph 8) prior to vein puncture. In total, 14 whole blood samples supplemented with 4 ml sodium citrate were investigated, which underwent further centrifugation steps at 200 × g and 4000 × g before PRP yield efficiency analysis. All other samples (n = 91) were charged with heparin alone, since sodium citrate was found to have no significant positive influence on PRP extraction. Blood-filled syringes were inverted manually five or six times to ensure that the anticoagulant was evenly dispersed. Samples were maintained at room temperature (22 ± 2 °C) (RT) and processed immediately to avoid irreversible conversion of platelets. The anticoagulated blood was then drawn into a 50 ml polypropylene (PP) test-tube (Cellstar®, Frickenhausen, Germany) under sterile conditions.
ACD-A Addition
Anticoagulant adenosin-citrate-dextrose-acid (ACD-A) aqueous buffer (pH-value of 4.7 to 5.3) (Baxter, Maurepas, France) was added. Buffer concentration was determined according to standardized treatment protocols for processing blood components by cell separators [Citation16]. In addition, several of our own investigations were carried out as follows: 6 heparinized blood samples underwent 2 parallel test runs differing in terms of the amount of ACDA added: 4 ml in tube A versus 8 ml in tube B. All 12 test tubes further underwent identical PRP isolation procedures before final efficiency measurements were performed: centrifugation at 200×g followed by centrifugation at 4000×g. Based on these results, 4ml of ACDA aqueous buffer were used for all subsequent test series.
Platelet Isolation: Two-step Centrifugation
Centrifugation steps, each with a duration of 15 minutes at RT, were performed with either an Eppendorf centrifuge 5810 R (Eppendorf, Hamburg, Germany) or a Biofuge Stratos centrifuge (Heraeus® Instruments, Kendro Laboratory Products, Langenselbold, Germany). Various settings were adjusted in order to analyze the effects of different revolution rates on PRP isolation efficiency during each centrifugation step.
The impact of varying velocities - 70, 100, 135, 165 and 200×g - during the first centrifugation step was investigated by quantification of the remaining platelets in the RBC fraction. In total, 57 heparinized blood-buffer samples were included. The first centrifugation step led to separation of PRP and PPP from the RBC fraction visualized in three layers: an upper light serum layer passing into an intermediate phase containing large quantities of cells (buffy coat) and the highly viscous RBC fraction. The serum portion, including the buffy coat, was entirely transfused and filled into another PP tube using a disposable pipette. Based on these results, 4ml of ACDA aqueous buffer and the centrifuge velocity of 200×g (first centrifugation step) were used for all further test series.
The subsequent second centrifugation step was performed to isolate as many pelleted platelets as possible; for this phase, higher numbers of revolutions per minute were required and tests were run at 500, 1000, 3000 and 4000×g. Supernatant PPP was finally decanted except for 1 ml, which was retained for re-suspension of the pellet in an Eppendorf tube ().
Figure 2. Test procedure for pellet fabrication from patient's whole blood: after addition of ACD-A aqueous buffer and first centrifugation with 200 ×g for 15 minutes, a second centrifugation with 4000 ×g for 15 minutes is performed → platelet pellet is obtained. [WB: whole blood, S: serum, RS: remaining serum, C: cruor, PL: platelets].
![Figure 2. Test procedure for pellet fabrication from patient's whole blood: after addition of ACD-A aqueous buffer and first centrifugation with 200 ×g for 15 minutes, a second centrifugation with 4000 ×g for 15 minutes is performed → platelet pellet is obtained. [WB: whole blood, S: serum, RS: remaining serum, C: cruor, PL: platelets].](/cms/asset/c09e82f2-8778-4489-a5fe-cf6b57d24a73/ianb19_a_435822_f0002_b.gif)
Since a revolution rate of 4000 ×g turned out to yield the highest PRP amounts, final isolation tests were run re-varying the revolution numbers of the first centrifugation step (100 ×g versus 200 ×g). The efficiency of each of these combinations was evaluated by detection of the platelet quantity in the following media: whole blood, RBC, PRP and PPP ( and ).
Platelet Isolation: Gravity-sedimentation Centrifugation
Alternative experiments were run with modified first step isolation: instead of centrifugation, 14 whole blood samples were left in the refrigerator (4°C for at least 8 hours) to undergo gravity-sedimentation. Resulting supernatant PRP plus PPP composition (including the cell-enriched buffy coat) was collected with a disposable pipette and transferred to a PP test tube. Regular second centrifugation at 4000 ×g followed for PRP extraction.
Quantification of Platelet Yield
Platelet density was determined in each liquid/medium (whole blood, RBC, PPP, and PRP) obtained from all test runs: all samples were filled into safe-lock 2 ml tubes and processed using standard blood count analysis in our hospital's central laboratory (Scholz-Nimbus 2000).
Quantification of Platelet-derived Growth Factor TGF-ß
According to preliminary results, 8 whole blood specimens were processed by two-step centrifugation (200/ 4000 ×g). In total, 64 data were measured: each of the 8 processed samples was scanned eight-fold for TGF-ß. To quantify the TGF-ß concentration, the resuspended pellet was diluted 10x3x10. TGF-ß levels were determined by a sandwich ELISA method (Quantikine®-human TGF-ß1, R&D Systems, Wiesbaden-Nordenstadt, Germany) according to the manufacturer's protocol. Briefly, the 200 µl samples were applied to a plate precoated with TGF-ß receptor II, incubated at RT for 3 hours, probed with 200 µl horseradish peroxidase-conjugated polyclonal antibody against TGF-ß1 for 1.5 hours at RT and finally combined with substrate for 20 minutes. Reaction was then terminated with 50 µl substrate. Evaluation was performed based on color development using a micro-plate reader at the wavelength of 450 nm with compatible software (Softmax 2.34, Molecular Devices, Munich, Germany). TGF-ß1 levels were determined based on a standard curve (0 to 2000 pg/ml).
RESULTS
Anticoagulation
Charging 14 whole blood samples with 32% sodium-citrate instead of heparin 5000 IE led to PRP efficiency of 75% (sum of platelets in whole blood in relation to sum of isolated platelets in PRP and PPP, respectively) with 1.2% of platelets remaining in the PPP (after centrifugations of 200 ×g and 4000 ×g). This data showed no significant advantage for platelet isolation compared to experiments performed identically with heparin alone, where PRP efficiency was determined to be 77% with 1.8 % of platelets remaining in the PPP.
ACD-A Addition
Comparison of two different ACD-A volumes (4 ml and 8 ml) added to 12 heparinized whole blood samples taken from 6 patients showed no significant differences regarding the final PRP yield (). Consequently, 4 ml of ACD-A aqueous buffer were used for all further test series.
Platelet Isolation: Two-step Centrifugation
The impact of different revolution rates, ranging from 70, 100, 135, 165 to 200 ×g during the first isolation step, was evaluated by comparing the amounts of all remaining platelets in the RBC-fraction: increased centrifuge velocity minimized the quantity of remaining platelets (maximum: 114×106; minimum: 5×106) in the RBC fraction, while elevating the PRP yield (). As a result of this statistically significant finding, 200 ×g was defined as the standard centrifugation velocity of the first isolation step for all subsequent experiments.
When the second step of centrifugation was performed at ranges 500 to 1000 ×g, analysis of the PPP showed an unacceptably high amount of remaining platelets. Only full capacity centrifugation at 4000 ×g enabled nearly complete platelet extraction from the serum in most cases.
Final platelet isolation tests were run re-varying the revolution rates of the first centrifugation step (100 ×g versus 200 ×g), while the revolution rate of the second centrifugation step was kept at 4000 ×g. Efficiency of each combination (100 ×g/ 4000 ×g vs. 200 ×g/ 4000 ×g) was evaluated by detection of the platelet quantity in the diverse media: whole blood, PRP and PPP (and ).
Figure 6. Centrifugation combination 200×g/4000×g: Ratio of the platelet quantities in whole blood to concentrate and Remaining serum by using a centrifugation velocity of 200×g/4000×g. [Data represent the average + SD].
![Figure 6. Centrifugation combination 200×g/4000×g: Ratio of the platelet quantities in whole blood to concentrate and Remaining serum by using a centrifugation velocity of 200×g/4000×g. [Data represent the average + SD].](/cms/asset/2d9057fe-92b4-45c7-8f29-545d3a6a7ef5/ianb19_a_435822_f0006_b.gif)
Centrifugation series at 100 ×g followed by 4000 ×g showed a platelet isolation efficiency of 51%, and a cell rate of 0.5% in the PPP. However, a PRP isolation efficiency of 77% and a remaining platelet portion of 1.8% was achieved by centrifugation at 200 ×g and 4000 ×g. Missing platelets were either damaged during the procedure or could be detected in the RBC fraction.
Platelet Isolation: Gravity-sedimentation Centrifugation
Using this well-established technique, gravity-sedimentation was performed in 14 samples (using heparinized whole blood) instead of first step centrifugation. Evaluation of this modification showed that 66% of platelets could be isolated from whole blood, whereas 1.3%, and 1.5% of the platelets remained in the PPP and RBC fraction, respectively.
Altogether, more serum (up to 17.5 ml) was obtained by sedimentation compared to first step centrifugation (up to 13.5 ml), although without containing higher quantities of platelets.
Quantification of Platelet-derived Growth Factor TGF-ß
After optimizing the platelet isolation procedure, 8 separate specimens were processed to determine the amount of PRP-derived TGF-ß. Results ranged from 273 ng/ml to 579.6 ng/ml TGF-ß harvested from PRP containing cell numbers from 843×106 to 1929×106 ().
Table 1. Listing of 8 separate specimens that were investigated according to volume (after first step centrifugation) and platelet amount of various media (whole blood, supernatant, PRP, and RBC fraction) during the TGF-β/platelet isolation process: the amount of PRPderived TGF-β resulting ranged from 273 ng/ml to 579.6 ng/ml.
DISCUSSION
The knowledge about growth factors in connection with tissue regeneration was significantly advanced by the development of platelet rich plasma (PRP).
The use of PRP is based on the theoretical premise that by concentrating platelets, the effect of the growth factors released will be increased. Today, PRP plays a vital role in a variety of surgical fields, such as maxillofacial surgery, cardiac surgery and periodontal therapy [Citation14, Citation15], particularly in support of wound healing [Citation6]. In 1998, Marx et al. [Citation15] reported increased bone formation and density after autologous bone grafting with additional PRP as a physiological source of autologous growth factors. Two years later it was shown that PRP contains substantial amounts of transforming growth factor-ß1 (TGF-ß), insulin-like growth factor-1 (IGF-1) and platelet-derived growth factor (PDGF-AB) [Citation10]. Furthermore, Blunk et al. identified the importance of TGF-ß in tissue-engineered cartilage formation compared to other regulatory factors [Citation4]. Specifically, an influence of TGF-ß on chondrocyte proliferation, metabolism and quantity of extracellular matrix components could be shown in vitro and in vivo [Citation9, Citation13, Citation24, Citation33]. Therefore, TGF-ß can be considered one of the most important factors in cartilage formation by supporting the re-differentiation of chondrocytes, which is a decisive step during cartilage matrix production [Citation12]. In cartilage tissue engineering, TGF-ß is employed in concentrations ranging from 5 to 50ng/ml [Citation4] as an additive for cell culture medium and three-dimensional formation.
A wide range of studies have been reported in the literature on platelet isolation techniques [Citation7,Citation8, Citation17, Citation18]. However, the use of PRP from transfusion institutes as an autologous source of growth factors bears the risk of cardiovascular stress for the patient and high costs due to a complex production process [Citation26].
Currently, there are several commercial kits available for PRP isolation: the Platelet-Concentrate-Collection-System (PCCS), the Curasan system (analogous to the PRP kit, Curasan, Kleinostheim, Germany), the Smart PReP™ system of Harvest Technologies Corporation (Munich, Germany) and the Friadent-Schuetze method (PRP kit; Friadent-Schuetze, Vienna, Austria). Weibrich et al. [Citation28] proved that PCCS leads to a significantly higher platelet concentration harvest compared to the Cusaran PRP kit: PCCS collection efficiency was 68.5% (+/- 22.1), whereas the Curasan PRP kit led to a 17.6% (+/- 9.9) harvest. Further analysis of the PCCS showed that 4.9% (+/- 3.3) and 2.9% (+/-2.3) of the initial platelet amount ultimately remained in the RBC and PPP fraction, respectively.
Moreover, easy handling of the PCCS system was convincing. Other studies done by Weibrich et al. report a PCCS collection efficiency up to 69.0% (+/- 22). However, it should be mentioned that various other records showed efficiency rates far below 50%, 18%, 29% and 34% in this study [Citation27].
In contrast to the presented experiment, both the PCCS and Curasan PRP kit recommend a first centrifugation step at considerably higher revolutions (1120×g and 1750×g); the following step is then centrifuged at 1120×g (PCCS) and 2520×g (Curasan PRP kit). The data presented here show that 1% and 1.9% of the initial platelet amount ultimately remained in the RBC and PPP fraction, respectively.
A further study compared the Smart PReP™ System to the Friadent-Schuetze method. Platelet collection efficiency was 63.4% (+/- 7.9) and 49.6% (+/- 13.6), respectively, with significant differences in final TGF-ß concentrations [Citation29].
All commercially available isolation methods produce more or less adequate amounts of platelet concentrations and TGF-ß levels. However, comparing various studies with respect to the mean TGF-ß level obtained shows that levels in other studies are lower than those obtained in the present study (). Although this fact highlights the potential prospects of the presented technique, it should be kept in mind that each system maintains a certain failure rate and differs in terms of laboratory technician training required to achieve profitable results [Citation29]. In the present study, 77% collection efficiency could be obtained by combining centrifugation steps at 200 ×g and 4000 ×g. Centrifugation steps at 100 ×g and 4000 ×g only led to a 51% collection efficiency rate. Thus, the data show that the amount of platelets remaining in the RBC fraction can be greatly reduced by using higher revolutions. The first centrifugation step appears to be an important determining factor since only platelets that could be collected in the serum after the first step can be later sedimented by second centrifugation.
Table 2. Mean TGF-β levels obtained by other researchers and cited in the literature.
Similar to Marx [Citation15] and Whitman [Citation32], who achieved platelet concentrates using cell separators, citrated blood was used in this investigation. Thorn et al. [Citation23] published a method that is similar to the centrifugation procedure described here. However, in their study different equipment was used (standard triple packs) and PRP was isolated unpelleted by single centrifugation for 15 minutes at 327×g (37 ○C) from 200 ml whole blood collected from one patient. In contrast to our results, they report about 90% to 95% collection efficiency, but since they focused on autologous fibrin fabrication including PDGF, no analyses were done with respect to TGF-ß extraction. Another article on autologous platelet gel production including different blood components describes a 65% efficiency rate achieved by the Medtronic cell separator [Citation1].
Compared to the considerable expense, effort and patient stress with conventional PRP fabrication by transfusion medicine cell separators, the method proposed here appears to be much more cost-effective, easier to handle, and more patient friendly. Regarding collection efficiency, higher levels may be obtained by the presented method compared to commercially available isolation systems: the 77% collection efficiency rate appears to be realistic, but still allows for even further improvement. Furthermore, the content of the growth factor TGF-ß in the isolated platelet concentrates proves to be above adequate for conventional tissue engineering purposes.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Altmeppen, J., Hansen, E., Bonnlander, G.L., Horch, R.E., and Jeschke, M.G. (2004). J Surg Res. 117:202–207.
- Arevalo-Silva, C.A., Cao, Y., Vacanti, M., Weng, Y., Vacanti, C.A., and Eavey, R.D. (2000). Arch Otolaryngol Head Neck Surg. 126:1234–1238.
- Bhanot, S., and Alex, J.C. (2002). Facial Plast Surg. 18:27–33.
- Blunk, T., Sieminski, A.L., Gooch, K.J., Courter, D.L., Hollander, A.P., Nahir, A.M., Langer, R., Vunjak-Novakovic, G., and Freed, L.E. (2002). Tissue Eng. 8:73–84.
- Boettner, M., Krieglstein, K., and Unsicker, K. (2000). J Neurochem. 75:2227–2240.
- De Obarrio, J.J., Arauz-Dutari, J.I., Chamberlain, T.M., and Croston, A. (2000). Int J Periodont Rest. 20:486–497.
- Dugrillon, A., Eichler, H., Kern, S., and Kluter, H. (2002). Int J Oral Maxillofac Surg. 31:615–619.
- Graham, J.M. (2002). Scientific Word Journal. 12:1607–1609.
- Hill, D.J., and Logan, A. (1992). Prog Growth Factor Res. 4:45–68.
- Kiuru, J., Viinikka, L., Myllyla, G., Pesonen, K., and Perheentupa, J. (1991). Life Sciences. 49:1997–2003.
- Kropf, J., Schurek, J.O., Wollner, A., and Gressner, A.M. (1997). Clin Chem. 43:1965–1974.
- Lee, D.K., Choi, K.B., Oh, I.S., Song, S.U., Hwang, S., Lim, C.L., Hyun, J.P., Lee, H.Y., Chi, G.F., Yi, Y., Yip, V., Kim, J., Lee, E.B., Noh, M.J., and Lee, K.H. (2005). Tissue Eng. 11:310–318.
- Malemud, D.J. (1993). Rheum Dis Clin N Am. 19:569–580.
- Man, D., Plosker, H., and Winland-Brown, J.E. (2001). Plast Reconstr Surg. 107:238–239.
- Marx, R.E., Carlson, E.R., Eichstaedt, R.M., Schimmele, S.R., Strauss, J.E., and Georgeff, K.R. (1998). Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 85:638–646.
- Mow, V.C., Ratcliffe, A., and Poole, A.R. (1992). Biomaterials. 13:67–97.
- Okuda, K., Kawase, T., Momose, M., Murata, M., Saito, Y., Suzuki, H., Wolff, L.F., and Yoshie, H. (2003). J Periodontol. 74:849–857.
- Pacifici, L., Casella, F., and Maggiore, C. (2002). Minerva Stomatol. 51:341–350.
- Reimann, P.M., and Mason, P.D. (1990). Intensive Care Med. 16:3–10.
- Shapiro, F., Koide, S., and Glimcher, M.J. (1993). J Bone Joint Surg Am. 75:532–553.
- Silver, F.H., and Glasgold, A.I. (1995). Otolaryng Clin N Am. 28:847–864.
- Singbartl, G., and Schleinzer, W. (1999). Anasthesiol Intensivmed Notfallmed Schmerzther. 34:350–358.
- Thorn, J.J., Sorensen, H., Weis-Fogh, U., and Andersen, M. (2004). Int J Oral Maxillofac Surg. 33:95–100.
- Trippel, S.B. (1995). J Rheumatol Suppl. 43:129–132.
- van Osch, G.J., Mandl, E.W., Marijnissen, W.J., van der Veen, S.W., Verwoerd-Verhoef, H.L., and Verhaar, J.A. (2002). Biorheology. 9:215–220.
- Weibrich, G., Buch, R.S., Kleis, W.K., Hafner, G., Hitzler, W.E., and Wagner, W. (2002). Growth Factors. 20:93–97.
- Weibrich, G., Buch, R.S., Kleis, W.K., and Hitzler, W.E. (2001). Zahnärztl Implantol. 17:116–123).
- Weibrich, G., and Kleis, W.K. (2001). Clin Oral Implants Res. 13:437–443.
- Weibrich, G., Kleis, W.K., Buch, R., Hitzler, W.E., and Hafner, G. (2003). Clin Oral Implan Res. 14:233–239.
- Weibrich, G., Kleis, W.K., Kunz-Kostomanolakis, M., Loos, A.H., and Wagner, W. (2001). Int J Oral Maxillofac Implants. 16:693–699.
- Westphal, R.G. (1984). Clin Haematol. 13:289–301.
- Whitman, D.H., Berry, R.L., and Green D.M. (1997). J Oral Maxillofac Surg. 55:1294–1299.
- Yeh, L.A., Augustine, A.J., and Lee, P. (1995). J Rheumatol. 22:1740–1746.
![Figure 4. Ratio of remaining platelet quantities in the cruor compared to the total quantity of platelets after the first centrifugation step. [Data represent the average + SD].](/cms/asset/dab72fda-00e7-4091-b908-6cec7fa57fd1/ianb19_a_435822_f0003_b.gif)
![Figure 5. Centrifugation combination 100×g/4000×g: Ratio of the platelet quantities in whole blood to concentrate and Remaining serum by using a centrifugation velocity of 100×g/4000×g. [Data represent the average + SD].](/cms/asset/9ed3dd9a-d9d1-4294-8b44-e5c17a4b06c1/ianb19_a_435822_f0004_b.gif)
![Figure 3. Variation of buffer quantity: Isolation efficacy after addition of 4ml (a) and 8ml (b) of ACDA aqueous buffer using whole blood of the same patient with the same platelet concentration. [Data represent the average + SD].](/cms/asset/b0e2d3bf-dcde-493c-a87e-19e5437d0c43/ianb19_a_435822_f0005_b.gif)