Abstract:
A new amperometric cholesterol biosensor was prepared by immobilizing cholesterol oxidase by a glutaraldehyde crosslinking procedure on polypyrrole-polyaniline (ppy-pani) composite film on the surface of a platinum electrode. In order to prepare a biosensor for the determination of cholesterol, electropolymerization of pyrrole and aniline on Pt surface was performed with an electrochemical cell containing pyrrole and aniline in sulphuric acid by cyclic voltammetry between 0.0 and 0,7 V (vs.Ag/AgCl) at a scan rate of 50 mV upon Pt electrode. The amperometric determination is based on the electrochemical detection of H2O2, which is generated in enzymatic reaction of cholesterol. The cholesterol determined by the oxidation of enzymatically generated H2O2 at 0.7 V vs. Ag/AgCl. The optimized cholesterol oxidase biosensor displayed linear working range and a response time of 300 s. The effects of pH and temperature were investigated and optimum parameters were found to be 7.0, 25°C, respectively. In addition to this, the stability and reproducibility of biosensor were tried. Operational stability of the proposed cholesterol biosensor was obtained by periodical measurements of the biosensor response. Biosensor at optimum activity conditions was used in 30 activity assays in one day to determine the operational stability. The results show that 82% of the response current was retained after 30 activity assays. The electrode was stored in a refrigerator at 4 °C after the measurements. The storage stability of the biosensor was determined by performing activity assays within 23 days. The results demonstrate that 60% of the response current was retained after 23 days. Preparing biosensor is used for the analysis of cholesterol in serum.
INTRODUCTION
Cholesterol has increased considerably in recent years because it is a crucial parameter in clinical diagnosis. The clinical analysis of cholesterol in serum samples is crucial in the diagnosis and prevention of a great deal of clinical disorders such as hypertension, cerebral thrombosis, heart attack and artheriosclerosis [Citation1,Citation2].
Cholesterol and its fatty acid esters are one of the important constituents of mammalian cell membrane and are precursors of other biological materials, such as bile acid and steroid hormones. Cholesterol oxidase, which is industrially important for applications in bioconversions, is adventageous for clinical determination of cholesterol [Citation3].
Many methods have been expressed for the attachment of enzymes to supporting structures. The activity of an immobilized enzyme relates on a number of factors, which can be summarized as size and shape of the carrier material, the nature of the immobilization method, the composition of the carrier material, and the specific conditions during. The success of an immobilized enzyme for practical applications depends strongly on the properties of the carriers employed. The conventional techniques for enzyme immobilization contain entrapment by ion-exchange polymers conducting polymers, and nonconducting polymers sol–gels and cross-linking in bovine serum albumin–glutaraldehyde [Citation4].
In general, the determination of cholesterol is based on a spectrophotometric technique. This method requires complicated procedures of the precipitation of lipoprotein fractions and then total cholesterol determination by an enzymatic method. Cholesterol determination is costly because the enzyme must be used in each assay procedure. An amperometric enzyme electrode has also been used for the determination of cholesterol but the disadvantage is the long-term stability of the enzyme. It has been mentioned in the literature that free cholesterol is determined by a free cholesterol electrode, which is immobilized on the conducting polymer matrix (polypyrrole or polyaniline) [Citation5,Citation6].
In recent years, mediators and conducting polymers have been used as a matrix for immobilizing the enzymes [Citation7,Citation8]. Intrinsically conducting polymers with conjugated double bonds have been regarded as attractive advanced materials for electronic devices electrochromic displays chemical and biochemical sensor drug release systems, rechargeable batteries and for modifying electrode surfaces for electrical wiring of biomolecules, following the conducting polymers used for the immobilization of biomolecules, such as polyacetylene, polythiophene, polypyr role, polyindole and polyaniline [Citation4].
Polyaniline (PANI) as a conducting polymer has demonstrated many practical applications, such as electrocatalysis, electrochromic devices and biosensors, due to its high conductivity and good redox reversibility [Citation9,Citation10,Citation11,Citation12].
Glutaraldehyde has been widely employed as an enzyme immobilizing agent for many years. The electrochemical immobilization of the enzyme together with the pyrrole and glutaraldehyde may increase the useful lifetime of the enzyme electrode by formation of cross-linkages between the functional reagent and amino groups from enzyme into the conducting polymer [Citation4].
The enzymatic reactions in the use of cholesterol oxidase [COx] as a receptor can be determined as follows: Cholesterol + O2 → Cholest-4-en-3-one + H2O2 [Citation12,Citation13]
H2O2 → O2+ 2H+ + 2e− [Citation14,Citation15]
The cholesterol is catalyzed by COx in the existence of oxygen and hydrogen peroxide is produced at the same time. The electrooxidation current of hydrogen peroxide is detected after applying an appropriate potential to the system. The major problem for amperometric detection is the overestimation of the response current due to interferences. Recently, many researchers have mentioned the inclusion of metal nanoparticles with a catalytic effect in polymer modified electrodes to develop biosensor sensibility and to reduce the overpotential applied to the amperometric biosensors [Citation16–22]. Pt is a well-known catalyst that has a high catalytic activity for hydrogen peroxide electrooxidation [Citation16,Citation23–26].
In this study, we report the immobilization of cholesterol oxidase [COx] on poly(pyrrole)- poly(aniline) [PPy-PANI] composite films. Storage and reuse number were investigated for the enzyme electrode. The optimum working conditions with respect to the substrate concentrations, the pH and temperature were investigated.
MATERIAL AND METHODS
Instrumentation and Reagents
All electrochemical experiments were carried out using an Epsilon EC electrochemical analyzer.
A conventional three-electrode system was performed with a Pt plate (0.5 cm2) as the working electrode, an Ag/AgCl (3M KCl) as the reference electrode, and a platinum wire as the counter electrode. The pH values of the buffer solutions are measured with ORION Model 720A pH-ionmeter. Temperature control was accomplished with the Grant W14 thermostat. Cholesterol oxidase (EC 1.7.3.3 from Aspergillus Niger purified from the microorganism and with an activity of 10 unit.ml−1) and cholesterol were purchased from Sigma. Pyrrole and aniline were supplied by Fluka. All other chemicals were obtained from Sigma. All the solutions were prepared with the use of distilled water.
Preparation of Cholesterol Solution
Cholesterol is soluble in alcohol and also in water in the presence of surfactants. Solutions were prepared daily by dissolving cholesterol in isopropanol, Triton X-100, and the phosphate buffer (pH.7.0). The isopropanol, Triton X-100, phosphate buffer ratio is 10:4:86 by weight [Citation27].
Preparation of Cholesterol Biosensor
The surface of the Pt plate electrode cleaned according to [Citation11] was covered by the electropolymerization of pyrrole and aniline [Citation9,Citation12]. COx solution was prepared by dissolving 100 µL of COx enzyme (25 U) in 100 µL of 0.1M phosphate buffer, 50 µL of glutaraldehyde, and 3 mg Bovine serum albumin (BSA). The solution was purged with nitrogen in order to remove the oxygen. The electropolymerization of pyrrole-aniline upon the electrode surface was performed by the cyclic voltammetric scans between 0.0 and 1.0V at a scan rate of 50 mV/s. The electrode was washed with water and then buffer solution after the coating process. 50 µL of preparing cholesterol oxidase (COx) solution was dropped upon each surface of platinium-polypyrrole-polyaniline (Pt/PPy-Pani) electrode. The film was taken out of the enzyme solution, was completely wiped off from the film, and was dried under ambient conditions. The electrode was kept in the refrigerator at the phosphate buffer when it was not in use [Citation14].
Amperometric Biosensor Measurements
Amperometric response studies were performed in phosphate buffer. Operational, storage, pH and temperature stabilities were defined via application of +0.7V with respect to Ag/AgCl electrode to detect oxidation current of H2O2 After the background current reached stable value, cholesterol solution was added to the cell using a micropipette and stirred for 10 min; then the resulting current difference was recorded. The current values were calculated towards constant concentrations (1.10−5 M) of cholesterol.
RESULT AND DISCUSSION
In this article, we prepared a new cholesterole biosensor with the immobilization of cholesterol oxidase (COx) on poly(pyrrole)-poly(aniline) (PPy-PANI) composite films. The paramaters effecting the performance of the biosensor were examined.
Effects of pH and Temperature
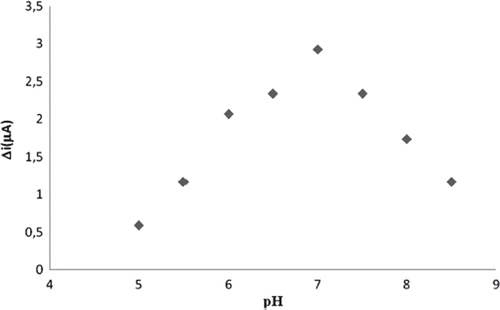
The enzyme activity drastically depends on temperature and pH since too high or low values may inactivate the enzyme. Value of pH depends on the charge of the enzyme and/or of the matrix. The pH change is benefical in understanding the association between the structure and functional group of the enzyme. Extreme value of pH cause enzyme denaturation, thereforethe optimum pH values should be defined. The effect of the pH and temperature on response current of the biosensor was determined. Cholesterol oxidase is optimally efficient at 37 °C and pH 7.0 [Citation26]. To determine optimum pH, an assay was applied by changing the pH between 5 and 8.5 at a constant temperature.
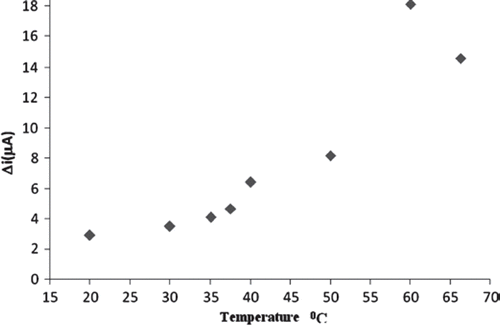
The biosensor response was increased as the pH was increased up to pH 7.0, after which it starts decreasing (). Immobilized cholesterol oxidase enzyme showed maximum activity at pH 7.0. This value was possible. At extreme pH values the enzyme was denaturated. Temperature is an important factor that has a significant effect on enzyme activity. The biosensor response was evaluated at different incubation temperature from 20 to 65 °C. The biosensor response increased up to 20 °C (). As illustrated in , current response gradually increased with increasing temperature and reached a maximum at 60 °C. This value is too high. Enzyme can be denaturated at the longtime incubation temperature. Therefore, the temperature of 25 °C was chosen as a working temperature for all further experiments.
Effect of Substrate Concentration on Biosensor
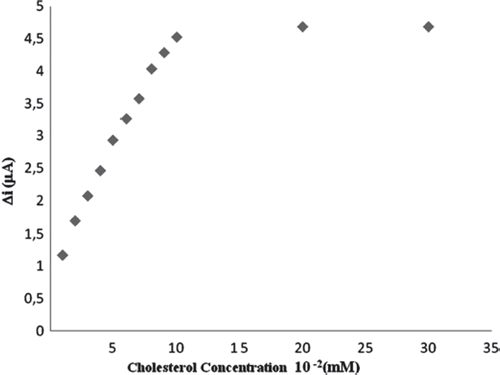
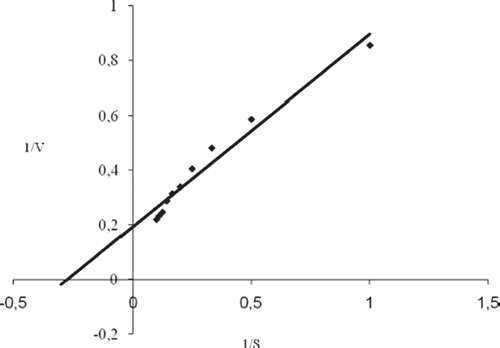
The value of the Michaelis–Menten kinetic parameter (Km), which shows the enzyme–substrate kinetics, was determined by the analysis of the slope of enzymatic reaction. Vmax is the maximum rate for enzymatic reaction. The effect of the substrate concentration on the reaction rate, catalyzed by immobilized ChOx, was studied using varying initial concentrations (0.01–0.3mM) of cholesterol substrate (). Vmax and Km were calculated from Lineweaver–Burk plots [Citation27]. With the increase in substrate concentration, there was an increase in amperometric current signal. This current reached a steady state after addition of 15 mM indicating saturation. Kinetic parameters Km and Imax for the enzyme biosensor were detected from the Lineweaver–Burk plot () at constant temperature (25 °C) and pH (pH 7) while varying the substrate concentration. Km and Imax were calculated as 3,3 mM, 5,26 mM/min, respectively. In the literature they have been presented as 9,8;0,41; 2,72; Km values mM for immobilized ChOx [Citation29–31]. The Km value of the system determines the affinity of enzyme for its substrate, with a smaller value of Km indicating increased affinity of enzyme for its substrate. For fabrication of biosensor, different matrices and methods of immobilization of enzymes were employed, and these could result in different conformational changes in the enzyme structures given that the enzyme kinetics is environment sensitive. Hence, the variation in value of Km could be attributed to these facts [Citation32].
Operational Stability and Storage Stability
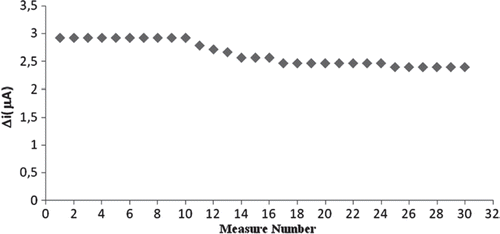
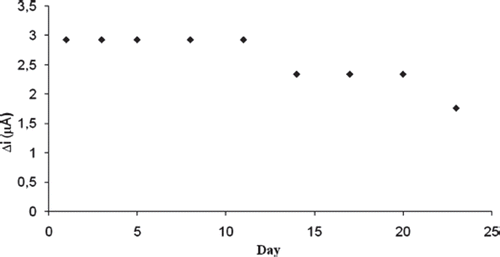
The biosensor was used at optimum activity conditions in 30 activity assays in one day to determine the operational stability. Storage stability of the biosensor was determined by performing activity assays within 23 days. The operational stability was studied by applying activity assay (under optimum conditions) for 30 times in the same day at constant temperature, pH and substrate concentration. At the end of the 30 measurements, the biosensor lost 18% of its initial activity. The activity assay was applied within 23 days to display the storage stability of immobilized enzyme. The stability of an enzyme is of significant importance for scheduling its application in a particular reaction. In general, an enzyme is not stable in aqueous solution during storage and the activity is gradually reduced [Citation28]. The reusability was tested because of its importance for repeated applications in a batch reactor. The main advantage of reusability is to reduce the cost of the treatment. As shown in the amperometric response remained constant for 30 days, and then an activity loss of 18% was observed on the 30th day (). The biosensor lost 40% of its initial activity during its regular use for 9 times over a period of 23 days when stored in 0.1M sodium phosphate buffer, pH 7.0 at 4 °C ().
Scanning Electron Microscope (SEM) Studies
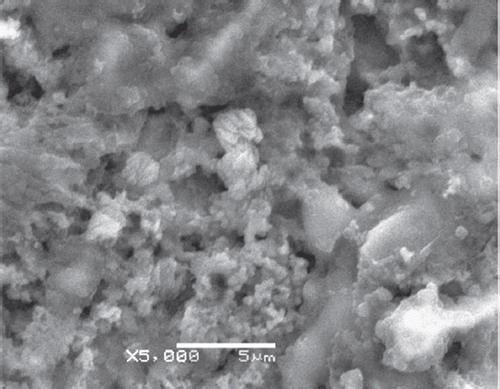
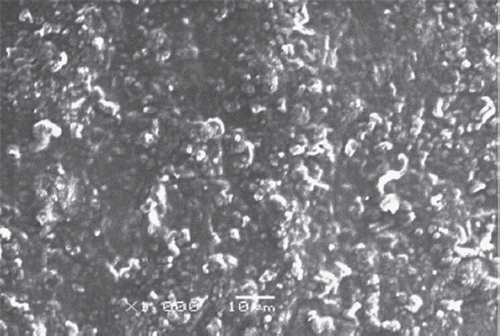
In these experiments, Scanning Electron Microscope JEOL Model 6060 LV instrument was used. The morphology of electrodes was characterized by SEM ( and ). The SEM images of Pt-Polypyrrole-Polyaniline and Pt-Polypyrrole-Polyaniline-cholesterole oxidase-glutaraldehyde-albumin are shown in and , respectively. Various works have studied the morphology of PPy and reported that the Ppy surface has a continuous structure with a rough and cauliflower-like shape [Citation33,Citation34]. The SEM image for Pt-Polypyrrole-Polyaniline-cholesterole oxidase-glutaraldehyde-albumin () also shows a very different morphology from that obtained for the Pt-Polypyrrole-Polyaniline layer, showing that cholesterole oxidase successfully immobilized to the surface of the Pt-Polypyrrole-Polyaniline layer.
Response Time
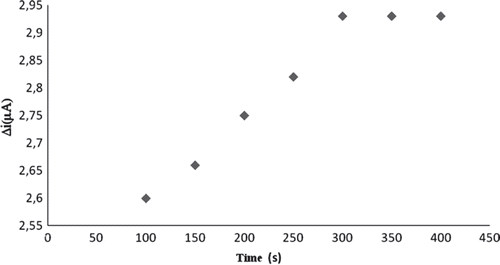
The amperometric response time of the biosensor to cholesterol was defined at constant cholesterol concentration (5.0.10−5 M). The current for 5.0.10−5 M cholesterol against time was plotted and given in . The response time can be taken as 300 seconds where the currents are approximately constant (). This time is highly suitable for biosensor response [Citation8].
Human Serum Sample Analysis
The cholesterol concentration of five serum pools was analyzed by the COx-biosensor. The cholesterol of these serum are analyzed also by the spectro-photometric method, which was used as a reference method.
The biosensor has been successfully applied to the determination of cholesterol in serum samples.
CONCLUSION
In this work, cholesterol oxidase was successfully immobilized poly(pyrrole)-poly(aniline) (PPy-PANI) composite film. The experimental results showed clearly that the biosensor perfectly exhibited performance for the determination of cholesterol. The cholesterol biosensor has high sensitivity and good selectivity. Operational stability and long-term storage stability are excellent. In addition, Polypyrrole-Polyaniline can supply a bio-compatible and electrochemical microenvironment for immobilization of the enzyme, making this material a good candidate for the fabrication of highly sensitive and selective cholesterol biosensors. This biosensor can be used for cholesterol determination in real samples.
Table 1. Comparison and determination with electrochemical method and cholesterol biosensor of cholesterol in human serum.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
REFERENCES
- Vidal, Juan-C., Javier Espuelas, Esperanza Garcia-Ruiz, Juan-R. Castillo (2004). Talanta 64:655–664.
- Cleeman, J.I., C. Lenfant (1998). JAMA-J. Am. Med. Assoc. 280, 2099–2104 (1998).
- Srisawasdi, P., P. Jearanaikoon, N. Wetprasit, B. Sri-wanthana, M.H. Kroll, P.H. Lolekha (2006). Clin. Chim. Acta 372:103–11.
- Çete, S., A. Yasar, F. Arslan (2007). Artificial Cells, Blood Substitutes, and Biotechnology. 35:607–620.
- Sunil, K., A.B. Arya, D.B. Monika, D. Bansi, A. Malhotra (2008). Biosensors and Bioelectronics. 23:1083–1100.
- Wang, H., S. Mu (1999). Sens. Act. B Chem. 56:22–30.
- Vidal, J.C., S. Mendez, J.R. Castillo (1999). Anal. Chim. Acta 385:203–211.
- Arslan, F. (2008). Sensors 8:5492–5500.
- Çete, S., A. Yaşar, F. Arslan (2006). Artificial Cells, Blood Substitutes, and Biotechnology. 34:367–380.
- Zhang, J., J. Lei, Y. Liu, J. Zhao, H. Ju (2008). Biosensors and Bioelectronics, (in press).
- Cochet, M., W.K. Maser, A.M. Benito, M.A. Callejas, M.T. Martínez, J.M. Benoit, J. Schreiber, O. Chauvet (2001). Chem. Commun. 1:1450–1451.
- Luo, X.L., A.J. Killard, A. Morrin, M.R. Smyth (2006). Anal. Chim. Acta. 575:39–44.
- Ma, Y.F., S.H. Ali, A.S. Dodoo, H.X. He (2006). J. Phys. Chem. B. 110:16359–16365.
- Zak, B. (1957). Am. J. Clin. Pathol. 27:583–588.
- Tsai, Y.C., S.Y. Chen, C.-A. (2008). Sens. Actuators B: Chem. 135:96–101.
- Karube, I., K. Hara, H. Matsuoka, S. Suzuki (1982). Anal. Chim. Acta 139:127–132.
- Yamato, H., T. Koshiba, M. Ohwa, W. Wernet, M. Matsumura (1997). Synth. Met. 87:231–236.
- Arjsiriwat, S., M. Tanticharoen, K. Kirtikara, K. Aoki, M. Somasundrum (2000). Electrochem. Commun. 2:441–444.
- Huang, H., Q. Yuan, X. Yang (2004). Colloid Surf. B: Biointerfaces. 39:31–37.
- Hrapovic, S., Y. Liu, K.B. Male, J.H.T. Luong (2004). Anal. Chem. 76:1083–1088.
- Ren, X., X. Meng, F. Tang (2005). Sens. Actuators B: Chem. 110:358–363.
- Becerik, I., F. Kadirgan (1997). J.Electroanal. Chem. 436:189–193.
- Vidal, J.C., E. Garcia-Ruiz, J.R. Castillo (2000). J. Pharm. Biomed. Anal. 24:51–63.
- Domínguez Sánchez, P., P. Tuñon Blanco, J.M. Fernández Alvarez, M.R. Smyth, R. O’Kennedy (1990). Electroanalysis 2:303.
- Hendji, A.N., P. Bataillard, N. Jaffrezic-Renault (1993). Sens. Actuators B: Chem. 15:127–134.
- Hall, S.B., E.A. Khudaish, A.L. Hart (2000). Electro-chim. Acta 45:3573–3579.
- Yapar, E., S.K. Kayahan, A. Bozkurt, L. Toppare. Carbohydrate Polymers (accepted manuscript).
- Akkaya, B., F. Sahin, G. Demirel, H. Tümtürk (2009). Biochemical Engineering Journal 43:333–337.
- Tan, X., M. Li, P. Cai, L. Luo, X. Zou (2005). Analytical Biochemistry. 337:111–120.
- Singh, S. (2004). Analytica Chimica Acta 502:229–234.
- Wang, H., S. Mu (1999). Sensors and Actuators B: Chemical, 56:22–30.
- Sunil, K., A.B. Arya, K. Arun, S.P. Prusty, A. Singh, Pratima R. Solanki, K.P. Manoj, M. Datta, Bansi D. Malhotra (2007). Analytical Biochemistry 363:210–218.
- Izaoumen, N., D. Bouchta, H. Zejli, M. El Kaoutit, A.M. Stalcup, K.R. Temsamani (2005). Talanta 66:111–117.
- El Kaoutita, M., A.H. Naggara, I. Ni. Rodrigueza, M. Dominguezc, J.H. Cisnerosa (2008). Synthetic Metals(in press).